Abstract
Objective:
To evaluate the correlation between preparative solid food status and the incidence of nausea, vomiting and aspiration symptoms in contrast-enhanced CT examination, and to provide direction for standardizing the preparative dietary policies.
Methods:
Patients who underwent routine enhanced CT examination at our hospital between June 2015 and June 2017 (110,836 cases) were enrolled and allocated into solid food fasting group (51,807 cases) and solid food non-fasting group (59,029 cases). Fluids ingestion was not restricted for any case. The differences in the incidence of nausea, vomiting and aspiration symptoms between the two groups of patients with various basic data were compared. The risk factors for the occurrence of nausea, vomiting and aspiration symptoms were analyzed.
Results:
The total incidence of nausea and vomiting was extremely low (0.071%), and no aspiration developed. There was no significant difference in the incidence of nausea and vomiting between the two groups in all respects (p > 0.05). The incidence of nausea and vomiting in patients with an iodine adverse drug reaction (ADR) history was higher than those with other ADR history (p = 0.008) and those without ADR history (p = 0.001).
Conclusion:
The occurrence of nausea and vomiting has no correlation with the preparative solid food status. Unless compulsory in clinical needs and constraints and gastrointestinal examination, solid food fasting is not a must in other examinations. Particular attention should be paid to the patients with an iodine ADR history in an effort to prevent possible ADRs.
Advances in knowledge:
The correlation between preparative solid food status and the incidence of nausea, vomiting and aspiration symptoms in contrast-enhanced CT examination were comprehensively analyzed in a large-scale population.
Introduction
With the rapid development of CT imaging equipments and image enhancement technology, the population undergoing contrast-enhanced CT examination has significantly increased in recent years.1Currently, fasting for hours is considered essential worldwide for patients prior to enhanced CT examination. Its main purpose is to accelerate gastrointestinal emptying, to reduce the risk of nausea, vomiting, and aspiration symptoms after injection of iodinated contrast media (ICM), and to avoid the interference with image quality by the gastrointestinal contents. The American College of Radiologyguideline mentions that hypotonic formulations can reduce the risk of ICMs-related pneumonia in patients who are prone to aspiration in gastrointestinal CT examination, and expresses concerns about aspiration that may occur in unconscious or severely trauma patients.2 The fluids ingestion restriction and solid food fasting policies in different international medical centers differ significantly, and the policies on the specified fasting time periods are considerably variable (from 0 h to overnight).3 Regrettably, there are still no pertinent large-scale clinical trials, standard guidelines and systematic reviews about the effect of preparative solid dietary regimes on the incidence of gastrointestinal symptoms.3, 4 At present, there is no standardized preparative dietary protocols for enhanced CT examination in Chinese medical care routine. The application guidelines for imaging techniques recommends a 4 h fasting preceding enhanced non-gastrointestinal examination and a 4–8 h fasting preceding enhanced gastrointestinal examination.5, 6 The American Society of Anesthesiologists Committee guidelines and the Enhanced Recovery After Surgery Society recommendations propose shortening the fasting time furthestly before operation, especially for transparent liquids.4, 7,8 In our own practice, some special fasted patients (e.g. diabetes) may experience dehydration or hypoglycemia symptoms following prolonged fasting, and even develop severe shock reactions. This is consistent with previous studies.9, 10 Therefore, it is urgent and important to reconsider the necessity of preparative solid food fasting prior to enhanced CT.11, 12
The purpose of this large-scale clinical observation is to evaluate the correlation between preparative solid food status and the incidence of nausea, vomiting and aspiration symptoms in enhanced CT examination, to analyze the risk factors for the occurrence of nausea, vomiting and aspiration symptoms, and to provide scientific evidence for establishing standardized preparative dietary regimes in enhanced CT examination.
Subjects and methods
Subjects
Data from patients who underwent routine enhanced CT examination consecutively at our hospital between June 2015 and June 2017 were analyzed prospectively. The patients scheduled for abdominal examination (liver, gallbladder, pancreas and gastrointestinal tract) were allocated into the solid food fasting group (51,807 cases), and those scheduled for non-abdominal examination were allocated into the solid food non-fasting group (59,029 cases). There was no fluids ingestion restriction for any case. Prior to examination, the indications and contraindications for all patients were determined according to ICM application guideline,13 and informed consent and study enroll agreement were signed. A record sheet self-designed by our group was used for screening assessment. The exclusion criteria included the following: critically ill patients requiring fasting (e.g. acute abdomen with unknown etiology, suspected or diagnosed with gastrointestinal bleeding, perforation, obstruction, and acute pancreatitis),14 emergency patients with observation time less than 30 min at night, and patients with existing nausea and vomiting symptoms prior to examination. The ICMs used included iopromide 370 (Bayer Healthcare, Leverkusen, Germany), iodixanol 270 (GE Healthcare, London, UK), iopamidol 350 (Bracco, Milan, Italy), ioversol 320 (Jiang Su Heng Rui Medicine CO., LTD, Jiangsu, China), iobitridol 350 (Guerbet, Paris, France;), and iohexol 350 (Yangzijiang Medicine CO., LTD, Jiangsu, China). All ICMs were injected intravenously at a speed of 3–6 ml s−1 using a high-pressure syringe (Ulrich medical, Ulm, Germany).1 The subjects in this study were routine examination cases, and the work was approved by the local institutional review board.
Observation method
Case report form (CRF)
A CRF (Supplemental Table 1) was designed for observation of ICM usage, including the date of examination; basic information of the patients (gender, age, body weight); the fasting status and fasting time; the source of patients; the ICMs-induced ADR history and other allergy history; risk factors; the examination parts; the name, dose and injection rate of ICMs; and the occurrence condition, occurrence time, accompanying symptoms, treatment, remission time and outcome of nausea and vomiting. Full-time nurses assessed and screened the patients item by item according to the CRF contents, completed and archived them. For special cases, the nurses communicated with radiologists, technicians, clinicians. The differences in the incidence of nausea, vomiting and aspiration between the two groups of patients with various basic data (gender, body mass index, source of patients, examination parts, ICMs used, injection rate and injection dose etc.) were compared, and the effect of various risk factors on the incidence of nausea and vomiting were analyzed.
Preparative dietary protocols
Full-time nurses read the description of the patients’ medical records when making the appointment before examination, and inform the patients on the dietary protocols according to the examination parts. For patients scheduled for gastrointestinal examination, preparative solid food fasting was performed for at least 4 h. For those scheduled for non-gastrointestinal examination, preparative solid food intake was not restricted. Fluids ingestion was not restricted in any patient. The patients were encouraged to drink fluids at 30 min before examination, immediately before examination and after examination.9 The patients can be appropriately supplemented with sugar brine, juice, soup and other fluids, while avoid drinking acid- and gas-producing fluids or soda. If the examination has to be postponed because of CT machine failure and schedule changes, nurses should inform the patients timely, and the patients could end fasting and resume eating normally until further notice.15, 16 When necessary, physicians should be notified to perform intravenous rehydration or oral administration of 20–40 ml of 50% glucose to unappetizing and debilitated patients. The nurses indicated the examination time section, the preparative dietary regimes and the exact duration of solid food fasting on the reservation list, so that the patients and their relatives could understand and perform the dietary preparation protocols strictly.
The assignment of responsibility of medical care
The radiologists, technicians, and nurses received systematic education and special training for clinical data collection, evaluation contents, observation points, treatment methods and follow-up, and cleared division of their own. The full-time nurses were responsible for the filling of CRF. The technicians were responsible for the filling of the injection dose and speed. The symptoms of patients during and after examination were reported to the radiologists by the technicians and nurses. Simple nausea and vomiting symptoms were diagnosed by the radiologists. For the patients with nausea and vomiting accompanied with other severe symptoms, the clinicians or the emergency doctors were informed for diagnosis and treatment.
Assessment indexes
The assessment indexes included the following: (i) incidence of nausea, vomiting, and aspiration: the number of cases with corresponding symptoms within 30 min after injection of ICMs/the total number of cases examined; (ii) time periods for the occurrence of symptoms: during, within 15 min, and 15–30 min after injection of ICMs; (iii) the degree of ADRs2, 13: mild, moderate, and severe; (iv) outcome: self-remission, need treatment, and remission time.
Statistical methods
SPSS 22.0 software package was used for statistical analysis. The count data were represented by frequency or percentage, and Χ2 was used for the comparison between groups. When p < 0.05, the difference was considered to be statistically significant.
Results
Incidence of nausea and vomiting in patients with different basic information
A total of 116,719 cases underwent routine enhanced CT examination at our hospital between June 2015 and June 2017. The exclusion criteria included the following: patients with incomplete CRF filling (952 cases), patients with acute abdomen with unknown etiology (132 cases), critically ill patients requiring fasting (e.g. suspected or diagnosed with gastrointestinal bleeding, perforation, obstruction, and acute pancreatitis, 1128 cases), emergency patients with observation time less than 30 min at night (3520 cases), and patients with existing nausea and vomiting symptoms prior to examination (151 cases). Therefore, 110,836 cases were enrolled in this study, which were allocated into the solid food fasting group (51,807 cases), and the solid food non-fasting group (59,029 cases). The total incidence of nausea and vomiting in patients was extremely low (79/110,836, 0.071%). The comparison of gender, body mass index, source of patients, examination parts, names of ICMs, injection rate and injection dose in the two groups are presented in Table 1. None of the differences was statistically significant (p > 0.05).
Table 1.
The occurrence of nausea and vomiting in patients with different basic information
| Types | Fasting group (%) | Non-fasting group (%) | p-value | |
| Total | 32/51807 (0.062) | 47/59029 (0.080) | 0.266 | |
| Gender | Male | 20/29428 (0.068) | 22/31536 (0.070) | 0.993 |
| Female | 12/22379 (0.054) | 25/27493 (0.091) | 0.128 | |
| BMI | <18.5 | 3/5337 (0.056) | 8/4504 (0.178) | 0.073 |
| 18.5–23.5 | 15/26444 (0.057) | 18/28515 (0.063) | 0.76 | |
| >23.5 | 14/20026 (0.070) | 21/26010 (0.081) | 0.676 | |
| Source of patient | Outpatient | 18/23961 (0.075) | 19/20726 (0.092) | 0.623 |
| Hospitalization | 14/27169 (0.052) | 26/37656 (0.069) | 0.376 | |
| Physical examination | 0/677 (0.00) | 2/647 (0.309) | 0.239 | |
| Parts of examination | Head and neck | 2/2289 (0.09) | 9/12395 (0.07) | 1.000 |
| Chest | 7/13648 (0.05) | 9/14532 (0.06) | 0.708 | |
| Abdomen | 14/30978 (0.05) | 3/10266 (0.03) | 0.682 | |
| Head neck CTA/CTP | 6/8354 (0.07) | 19/21537 (0.09) | 0.660 | |
| Heart CTA | 3/5413 (0.05) | 6/16217 (0.04) | 0.849 | |
| Others | 0/545 (0.00) | 1/1234 (0.08) | 1.000 | |
| Name of ICMs | Iopromide 370 | 4/7955 (0.050) | 10/8166 (0.122) | 0.120 |
| Ioversol 320(China) | 9/10436 (0.086) | 9/13792 (0.065) | 0.553 | |
| Iobitridol 350 | 2/2252 (0.089) | 4/2435 (0.164) | 0.754 | |
| Iodixanol 270 | 11/10018 (0.110) | 9/11031 (0.082) | 0.507 | |
| Iohexol 350 | 2/9925 (0.020) | 3/10722 (0.028) | 1.000 | |
| Iopamidol 350 | 3/10182 (0.04) | 11/11822 (0.099) | 0.062 | |
| Ioversol 350(Imported) | 1/1039 (0.1) | 1/1061 (0.09) | 1.000 | |
| Injection rate | <5 ml s−1 | 27/44558 (0.061) | 36/42719 (0.084) | 0.193 |
| ≥5 ml s−1 | 5/7249 (0.069) | 11/16310 (0.067) | 1.000 | |
| Injection dose | <100 ml | 30/46521 (0.064) | 37/48328 (0.077) | 0.484 |
| ≥100 ml | 2/5286 (0.038) | 10/10701 (0.093) | 0.368 |
BMI, body mass index; CTA, CT angiography; CTP, CT perfusion; ICMs, iodinated contrast medias.
The occurrence of nausea, vomiting and aspiration symptoms
The incidence of nausea and vomiting in the fasting group was 0.06% (32/51,807 cases), and that in the non-fasting group was 0.08% (47/59,029 cases). No aspiration symptom was noted. There was no statistical difference in the incidence of nausea and vomiting between the two groups of patients (p > 0.05). The results are presented in Table 2.
Table 2.
The occurrence of nausea, vomiting and aspiration symptoms
| Types | Fasting group (%) | Non-fasting group (%) | p-value | |
| Symptoms | Nausea | 7/32 (21.88) | 7/47 (14.89) | 0.425 |
| Nausea and vomiting | 25/32 (78.13) | 40/47 (85.11) | 0.425 | |
| Accidental aspiration | 0 | 0 | / | |
| Time of occurrence | During examination | 17/32 (53.13) | 25/47 (53.19) | 0.995 |
| Within 15 min post-exam | 14/32 (43.75) | 22/47 (46.81) | 0.789 | |
| 15–30 min post-examination | 1/32 (3.13) | 0/47 (0.00) | 0.405 |
Incidence of nausea and vomiting in patients with various risk factors and ADR history
No statistical significance was noted regarding the difference in the incidence of nausea and vomiting symptoms between the two groups of patients with various risk factors (p > 0.05). Among all patients, nausea and vomiting mainly occurred in those with risk factors, such as hypertension, coronary heart disease, heart failure, diabetes that require treatment, and age older than 70 years. Of them, nine events (seven cases) were noted in patients with risk factors in the fasting group, accounting for 22% (7/32). 23 events (18 cases) occurred in patients with risk factors in the non-fasting group, accounting for 38% (18/47). There was no statistical significance in the difference between the two groups (p = 0.123). The results are shown in Table 3.
Table 3.
Incidence of nausea and vomiting in patients with various risk factors and ADR history
| Types | Fasting group (%) | Non-fasting group (%) | P-value | |
| Risk factors | Hypertension | 3/8652 (0.03) | 9/15558 (0.06) | 0.635 |
| Coronary heart disease | 2/2039 (0.10) | 3/4821 (0.06) | 1.000 | |
| Diabetes require treatment | 1/2676 (0.04) | 1/4430 (0.02) | 1.000 | |
| Cardiac insufficiency | 0/66 (0.00) | 1/107 (0.93) | 1.000 | |
| Older than 70 years | 3/8964 (0.03) | 7/13154 (0.05) | 0.722 | |
| ADR history | Iodine ADR history | 2/132 (1.52) | 1/157 (0.64) | 0.880 |
| Other ADR history | 5/3866 (0.13) | 5/5455 (0.09) | 0.584 | |
| No ADR history | 25/47809 (0.05) | 41/53417 (0.08) | 0.128 |
ADR, adverse drug reaction.
Note: One patient may have multiple underlying diseases or multiple risk factors.
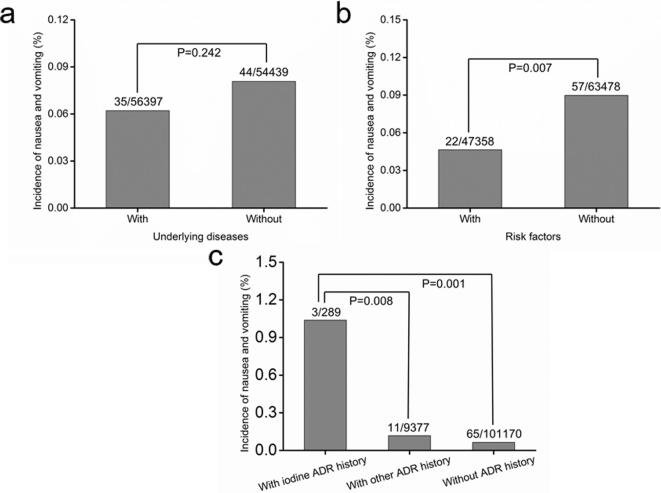
Given that none of the differences in the incidence of nausea and vomiting between the two groups exhibited statistical significance (Tables 1–3), the data of these two groups were combined to analyze the effect of various risk factors on the incidence of nausea and vomiting (Table 4). The results revealed no significant difference in the incidence of nausea and vomiting between patients without underlying diseases and those with underlying diseases (p = 0.242, Figure 1a). The incidence of nausea and vomiting was increased in patients without risk factors than those with risk factors (p = 0.007, Figure 1b). The incidence of nausea and vomiting symptoms was increased in patients with iodine ADR history compared with those with other ADR history (p = 0.008) and those without ADR history (p = 0.001, Figure 1c), respectively.
Figure 1.
Incidence of nausea and vomiting in the presence or absence of underlying diseases, risk factors, and ADR history. ADR, adverse drug reaction.
Treatment methods and outcome
32 cases in the fasting group experienced nausea and vomiting, and 28 of them had mild ADRs. 26 cases had only nausea and vomiting symptoms, and they improved without treatment. Two cases were treated in the emergency room of the radiology department. One had a headache and was intramuscularly injected with 25 mg phenergan (Southwest Pharmaceutical Co., Ltd, Chongqing, China). The other case had mild laryngeal edema, and improved after intravenous injection of 10 mg dexamethasone (Guangda Pharmaceutical Co., Ltd, Shenyang, China). Three cases had moderate ADRs accompanied with shivering, and they improved after intramuscular injection of 25 mg phenergan in the emergency room of the radiology department. One case had severe ADR accompanied with shivering, palpitation, and a sudden drop in blood pressure. This patient was transferred to the Emergency Department for treatment. Venous access was established, and oxygen was administered through an oxygen mask. After intramuscular injection of 25 mg phenergan, intravenous injection of 10 mg dexamethasone, and intramuscular injection of 1 mg of 1:1000 adrenaline, the patient recovered.
47 cases in the non-fasting group experienced nausea and vomiting, and 45 of them experienced mild ADRs. 41 cases experienced only nausea and vomiting symptoms and improved without treatment. Four cases were treated in the emergency room of the radiology department. One case experienced chest tightness, and the other three cases were accompanied with fatigue, dizziness, and wheezing. These symptoms improved after inhalation with constant low-flow oxygen. One case had moderate ADR accompanied with shivering, mild dyspnea, and mild eyelid edema. The symptoms improved after intramuscular injection of 25 mg phenergan and venous injection of 10 mg dexamethasone. One case had severe ADR accompanied with a sudden drop in blood pressure and numbness of limbs. This patient was transferred to the Emergency Department for treatment. Venous access was established, and oxygen was administered through an oxygen mask. After intramuscular injection of 25 mg phenergan and 1 mg of 1:1000 adrenaline, the symptoms subsequently improved.
Discussion
The traditional fasting concept preceding enhanced CT examination originates from past clinical experiences, which is mainly based on dogma rather than scientific evidences. Such a practice made sense in the past given that the incidence of gastrointestinal symptoms was fairly high after receiving ICMs (6.7%,17 4.58% for nausea,18 and 1.84% for vomiting.18 In recent years, with the extensive applications of hypotonic and non-ionic ICMs, the incidence of vomiting has decreased remarkably (1.4%,17 0.3%,19 0.33%,9 and 0.05%.1 Therefore, the requirement for dietary restriction decreased gradually. In fact, not every patient is required to fast before enhanced CT,3 particularly for those undergoing angiography or examination of body parts, such as the head, neck, chest, and limbs. Prolonged fasting would amplify the stress response of patients and result in prematurely started catabolism.20 This will damage the body's internal environment and metabolic balance, and induce a series of symptoms mainly manifested as thirst, hunger, and hypoglycemia,21 and even cause blood pressure decrease and severe shock reactions.22 In particular, for the patients who have had a long-term poor-eating history with weak physical condition caused by their own existing diseases, fasting is more prone to cause discomfort. It has been reported that preparative fasting in enhanced CT could increase the risk of nausea and vomiting.17 In addition, fasting could increase the gastric pH value, and cause more severe consequences in aspiration patients.9, 23 Oral ingestion of clear fluids at 4 h before examination can dilute the gastric acid, stimulate gastric emptying, and relieve dizziness, thirst, hypoglycemia, nausea and other symptoms. Therefore, we questioned the traditional practice of requiring all patients undergoing enhanced examination to receive preparative fasting. This prospective observational study makes up for the deficiency of the American College of Radiology and Chinese guidelines on preparative dietary protocols prior to enhanced CT examination, prompting us to adjust the traditional preparative dietary protocols and to develop standardized preparative dietary regimes based on the patients' examination body parts and the specific conditions of the individuals.
Nausea and vomiting mainly occurred during the injection of ICMs and within 15 min after injection (Table 2), which could represent the transient symptoms caused by the physiological reactions such as fever and oral metal smells. Alternatively, it could also represent the transient aggravated symptoms of underlying diseases.24 This feature was related, to a certain degree, to the physical and chemical properties of ICMs, while no correlation with the dietary preparation status. The vast majority of patients could improve spontaneously. There was no difference in the basic information in the two groups of patients. However, an unexpected phenomenon was identified after combining the data. The incidence of nausea and vomiting in patients with risk factors was lower than those without risk factors. Although it is difficult to explain, it can be determined that these risk factors will not increase the likelihood of nausea and vomiting symptoms. In addition, the incidence of gastrointestinal symptoms in patients with ICMs-induced ADR history was higher than those with other ADR history and without ADR history. This finding was consistent with the reports that the incidence of nausea and vomiting ranked in the top two positions among the ICMs-induced ADRs.2, 17 For the four cases with moderate ADRs and two cases with severe ADRs in the two groups, in addition to nausea and vomiting, the patients also experienced other allergic reactions, such as skin flush, urticaria, eyelid edema, anaphylactic shock, chills, and dyspnea. These events were more closely related to the anaphylactoid-like reactions induced by ICMs, suggesting that patients with ICMs-induced ADR history were more prone to the occurrence of gastrointestinal symptoms, which had no correlation with the dietary preparation status. The injection rate of head and neck CTA, CTP and coronary CTA is typically increased compared with that of other body parts, and the results suggested that the injection speed and dose did not correlate with nausea and vomiting. In addition, there was no significant difference in the incidence of nausea and vomiting between sub hypertonic and isotonic ICMs or different types of ICMs, suggesting that the osmotic pressure of ICMs was not a risk factor that contributed to nausea and vomiting.
In this study, fluids ingestion was not restricted for any patient. At 30–60 min and just before examination, the patients were encouraged to drink 300–600 ml water depending on their water-drinking ability. At 30 min after examination, the patients continued to drink 200–300 ml water. No case of aspiration was noted, suggesting that the fluids ingestion preceding enhanced CT examination was not correlated with the occurrence of aspiration pneumonia. Particular attention should be paid to the patients with ICMs-induced ADR history. For patients with a risk of aspiration, we recommend them to turn their head to one side during the injection of ICMs to prevent the vomit from accidentally entering the trachea and cause tracheal foreign body or aspiration pneumonia. Although the frequency of nausea and vomiting was extremely low in this study and no aspiration was noted, it does not mean these events will not occur in future work. Severe and life-threatening reactions may occur in the absence of any specific risk factors, although these reactions are rare.24 Therefore, before the examination, we need to know more about the patients' previous condition, obtain the patients' medical history, focus on the identification of the risk factors that may cause nausea, vomiting and aspiration, and try to provide the best dietary guidance to the patients as possible.
Regarding limitations, only routine examination subjects were observed in the present study. Despite cooperation between full-time nurses and medical team, the exact duration of fasting and the amount of dietary intake were not accurately monitored. The compliance of the patients' dietary preparation needs to be improved. Furthermore, this study did not explore the correlation between premedication before examination and the occurrence of gastrointestinal symptoms, and the mechanism of nausea and vomiting after injection of ICMs requires further investigations. Therefore, it is desirable for readers to pay attention to this limitation when interpreting this study.
Conclusion
This large-scale prospective observational study comprehensively and systematically observed the occurrence of nausea, vomiting and aspiration following different preparative dietary regimes in enhanced CT examination. The correlation between the occurrence of nausea, vomiting and multiple risk factors was analyzed in details. The results would provide reliable basis for establishing standardized preparative dietary protocols prior to enhanced CT examination. Unless compulsory in clinical needs and constraints and gastrointestinal examination, solid food fasting is not a must in other examinations.
Table 4.
Incidence of nausea and vomiting in the presence or absence of underlying diseases and risk factors
| Types | Total | Fasting group (%) | Non-fasting group (%) | p-value | |
| Underlying diseases | W/O | 44/54439 (0.08) | 17/27599 (0.06) | 27/26840 (0.10) | 0.109 |
| W | 35/56397 (0.06) | 15/24208 (0.06) | 20/32189 (0.06) | 0.994 | |
| Risk factors | W/O | 57/63478 (0.09) | 25/33654 (0.07) | 32/29824 (0.11) | 0.166 |
| W | 22/47358 (0.05) | 7/18153 (0.04) | 15/29205 (0.05) | 0.530 |
W/O, without; W, with; W/O, without.
Note: One patient may have multiple underlying diseases or multiple risk factors.
Footnotes
The authors Xue Li and Heng Liu contributed equally to the work.
Funding: This work was supported by the Fund of Chongqing Clinical Research Centre of Imaging and Nuclear Medicine (CSTC2015YFPT-gcjsyjzx0175) and Central government guide the development of local science and technology special fund (YDZX20175000004270). The authors would like to thank all nurses in our department for data collecting.
Contributor Information
Xue Li, Email: lixue928136@163.com.
Heng Liu, Email: liuheng0918@163.com.
Li Zhao, Email: 275398302@qq.com.
Junling Liu, Email: 373072057@qq.com.
Li Cai, Email: 849087005@qq.com.
Letian Zhang, Email: zletian_1982@126.com.
Lei Liu, Email: ttcrystalma@163.com.
Weiguo Zhang, Email: wgzhang01@163.com.
REFERENCES
- 1.Li X, Chen J, Zhang L, Liu H, Wang S, Chen X, et al. Clinical observation of the adverse drug reactions caused by non-ionic iodinated contrast media: results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br J Radiol 2015; 88: 20140491. doi: 10.1259/bjr.20140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Radiology. ACR manual on contrast media. Version 2013; 9. [Google Scholar]
- 3.Lee BY, Ok JJ, Abdelaziz Elsayed AA, Kim Y, Han DH. Preparative fasting for contrast-enhanced CT: reconsideration. Radiology 2012; 263: 444–50. doi: 10.1148/radiol.12111605 [DOI] [PubMed] [Google Scholar]
- 4.American Society of Anesthesiologists Committee Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2011; 114: 495–511. doi: 10.1097/ALN.0b013e3181fcbfd9 [DOI] [PubMed] [Google Scholar]
- 5.Shi MG, Wang MP, JM Y. A clinical guide for radiological technologist. People's Medical Publishing House(PMPH) 2013;: 246–347. [Google Scholar]
- 6.JM Y. Practice of Medical Imaging Technology. People's Medical Publishing House(PMPH) 2015;: 523–657. [Google Scholar]
- 7.Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013; 37: 285–305. doi: 10.1007/s00268-012-1787-6 [DOI] [PubMed] [Google Scholar]
- 8.Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Søreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2011; 28: 556–69. doi: 10.1097/EJA.0b013e3283495ba1 [DOI] [PubMed] [Google Scholar]
- 9.Siegel E. Strict NPO Rules May Be Counterproductive in Contrast CT-Medscape-Oct05. 2012;.
- 10.Liang Y, Qin X, Wei M, Qin M, Huang D. Research progress on influence of preoperative fasting time on flexible bronchofiberscope examination. Chinese Nursing Research 2012; 26: 870–1. [Google Scholar]
- 11.Wagner HJ, Evers JP, Hoppe M, Klose KJ. Must the patient fast before intravascular injection of a non-ionic contrast medium? Results of a controlled study. Rofo 1997; 166: 370–5. doi: 10.1055/s-2007-1015444 [DOI] [PubMed] [Google Scholar]
- 12.Roy C, Marcus C, Menanteau B, Blum A, Issenuth J, Raffaelli C, et al. Must patients fast before a radiologic examination with injection of an iodinated contrast media? J Radiol 1998; 79: 892–6. [PubMed] [Google Scholar]
- 13. Chinese Society of Radiology. Iodine contrast agents application guideline. 2nd Ed; 2013. [Google Scholar]
- 14.Li X, Zeng D. YIXUE YINGXIANGKE HULI GONGZUO SHOUCE. Beijing, China: The British Institute of Radiology.; 2014. [Google Scholar]
- 15.Yildiz H, Gunal SE, Yilmaz G, Yucel S. Oral carbohydrate supplementation reduces preoperative discomfort in laparoscopic cholecystectomy. J Invest Surg 2013; 26: 89–95. doi: 10.3109/08941939.2012.699998 [DOI] [PubMed] [Google Scholar]
- 16.Bopp C, Hofer S, Klein A, Weigand MA, Martin E, Gust R. A liberal preoperative fasting regimen improves patient comfort and satisfaction with anesthesia care in day-stay minor surgery. Minerva Anestesiol 2011; 77: 680–6. [PubMed] [Google Scholar]
- 17.Oowaki K, Saigusa H, Ojiri H, Ariizumi M, Yamagisi J, Fukuda K, et al. Relationship between oral food intake and nausea caused by intravenous injection of iodinated contrast material. Nihon Igaku Hoshasen Gakkai Zasshi 1994; 54: 476–9. [PubMed] [Google Scholar]
- 18.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990; 175: 621–8. doi: 10.1148/radiology.175.3.2343107 [DOI] [PubMed] [Google Scholar]
- 19.Gomi T, Nagamoto M, Hasegawa M, Katoh A, Sugiyama M, Murata N, et al. Are there any differences in acute adverse reactions among five low-osmolar non-ionic iodinated contrast media? Eur Radiol 2010; 20: 1631–5. doi: 10.1007/s00330-009-1698-6 [DOI] [PubMed] [Google Scholar]
- 20.Pimenta GP, de Aguilar-Nascimento JE. Prolonged preoperative fasting in elective surgical patients: why should we reduce it? Nutr Clin Pract 2014; 29: 22–8. doi: 10.1177/0884533613514277 [DOI] [PubMed] [Google Scholar]
- 21.Nygren J. The metabolic effects of fasting and surgery. Best Pract Res Clin Anaesthesiol 2006; 20: 429–38. doi: 10.1016/j.bpa.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Chen C, Ji D, Li Z, Nian G, Ni C. Preoperative fasting for solids and liquids program for liver cancer patients combined with diabetes. Nurs J Chin PLA 2015; 32: 6–9. [Google Scholar]
- 23.Sutherland AD, Maltby JR, Sale JP, Reid CR. The effect of preoperative oral fluid and ranitidine on gastric fluid volume and pH. Can J Anaesth 1987; 34: 117–21. doi: 10.1007/BF03015327 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Liu H, Zhao L, Liu J, Cai L, Liu L, et al. Clinical observation of adverse drug reactions to non-ionic iodinated contrast media in population with underlying diseases and risk factors. Br J Radiol 2017; 90: 20160729. doi: 10.1259/bjr.20160729 [DOI] [PMC free article] [PubMed] [Google Scholar]