Abstract
Objective:
To determine whether breast MRI-guided vacuum-assisted biopsy (MRI-VAB) high-risk lesion histology influences surgical or long-term imaging follow-up outcomes.
Methods:
Patients with imaging-concordant high-risk findings on 9-gauge breast MRI-VAB between January 2007 and July 2012 who had surgical histopathology or 2 year imaging follow-up were retrospectively reviewed.
Results:
90 patients with 99 lesions were included. Lesions were atypical ductal hyperplasia (ADH) (n = 21), lobular neoplasia [n = 36; atypical lobular hyperplasia (ALH) (n = 22), lobular carcinoma in situ (LCIS) (n = 6), and ALH plus LCIS (n = 8)], and other high-risk lesion (n = 42; papillary lesions, radial scar, flat epithelial atypia, atypia unspecified). Of 53 excised lesions, 6 (11%) were upgraded to invasive cancer or ductal carcinoma in situ (DCIS). 4 of 21 (19%) ADH lesions were upgraded to DCIS. 2 of 36 (6%) lobular neoplasia lesions, both combined ALH and LCIS, were upgraded to DCIS, and invasive lobular carcinoma, respectively. The remaining 46 lesions were managed conservatively with imaging follow-up: 17 (37%) had mammography only, while 29 (63%) had mammography and MRI follow-up. There was no evidence of breast cancer development at the site of MRI-VAB in the cases with only imaging follow-up.
Conclusion:
We conclude that the upgrade rate for high-risk lesions at MRI-VAB at surgical excision is low. Surgical excision is warranted for ADH and combined ALH-LCIS lesions. For other lesions, a multidisciplinary approach to decide on personalized management may be appropriate.
Advances in knowledge:
Surgical excision is warranted for ADH lesions and combined ALH-LCIS lesions identified at breast MRI-VAB. A multidisciplinary approach to patient management of other high-risk lesions may be appropriate.
Introduction
Utilization of MRI of the breast for the detection and evaluation of breast cancer is increasing.1, 2 The sensitivity of breast MRI for the detection of breast lesions is as high as 100%3, 4 however, the reported specificity ranges from 37 to 78%, and there is considerable overlap in the MRI appearance of benign and malignant lesions.3–5 A significant proportion of the suspicious lesions detected on breast MRI are not identified on MRI-directed ultrasonography and are occult on mammography. For such lesions, MRI-guided breast biopsy is the only available method to obtain a tissue diagnosis.3, 4,6,7
Some benign lesions detected on MRI-guided breast biopsy are considered high risk—i.e. they are associated with increased risk for future development of malignancy. The high-risk lesions include atypical ductal hyperplasia (ADH), lobular neoplasia [atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS)], radial scar, papillary lesions, flat epithelial atypia and mucocele-like lesions. Surgical excision of high-risk lesions is often recommended because of the possibility of upgrade to malignancy when additional tissue is sampled.8–11 However, the management guidelines for MRI-detected breast lesions with imaging-concordant high-risk histopathology results on MRI-guided vacuum-assisted biopsy (MRI-VAB) are not currently standardized. Specifically, it is not established whether such lesions should be surgically excised. Given that the frequency of breast MRI and MRI-VAB is increasing, knowledge of the rates of upgrade of MRI-VAB-detected high-risk lesions to malignancy is important for guiding patient management decisions.
The purpose of this study was to determine the rates of upgrade of MRI-VAB-detected high-risk breast lesions to carcinoma at surgery or on imaging follow-up.
Methods and materials
Lesion selection
With the approval of our institutional review board, consecutive patients who underwent 9-gauge MRI-VAB at The University of Texas MD Anderson Cancer Center from 1 January 2007, to 1 July 2012, were retrospectively identified. The patients’ electronic medical records, including radiology reports and pathology reports, were reviewed. MRI studies were interpreted in conjunction with mammograms and clinical history. MR images were evaluated and interpreted in accordance with the American College of Radiology BI-RADS MR lexicon.12 MRI-detected lesions with BI-RADS category 4 or 5 assessment underwent a targeted ultrasound before MRI biopsy at the discretion of the reporting radiologist. In our practice, if a sonographic correlate is identified, the biopsy is done with ultrasound guidance. MRI-detected lesions that lack sonographic correlates or that are better seen on MRI than sonography are subjected to MRI-guided biopsy.
MRI-VAB-detected lesions with imaging-concordant high-risk histopathology results on evaluation of the biopsy specimen and either (1) surgical histopathology results or (2) at least 2 years’ imaging follow-up were included. The mean mammography follow-up interval after the high-risk MRI biopsy was 5.9 years (range 1–9.8 years). The mean MRI follow-up was 3.3 years (range 1–10.6 years). Imaging-concordant high-risk histopathology results included the following: ADH, lobular neoplasia (ALH and LCIS), radial scar (radial sclerosing lesion, scleroelastic lesion, sclerosing papillary lesion, and complex sclerosing lesion), papillary lesion (benign and atypical), flat epithelial atypia, and mucocele-like lesion.9 The diagnostic pre-biopsy breast MRI and MRI-VAB images of the patients who met the inclusion criteria were reviewed by the authors.
MRI-VAB procedure
Early in our MRI-VAB experience, the radiologist who interpreted the pre-biopsy breast MRI images performed the MRI-VAB to ensure biopsy of the correct lesion. However, as our MRI-VAB volume grew, the radiologist who interpreted the initial breast MRI clearly annotated the MRI-suspicious lesion recommended for biopsy on orthogonal plane images, so that MRI-VAB could be performed by any scheduled breast radiologist. In addition, the series and image numbers were marked on both planes, and the lesion clock position and distance from the nipple were noted in the report. The above information was also sent via email to the radiologist performing the biopsy and to the biopsy technical and scheduling team.
MRI-VAB was performed on either a 1.5 T (GE Healthcare, Signa, Little Chalfront, UK) or 3.0 T (GE Healthcare, Signa Excite HDx, Little Chalfront, UK) MRI unit, depending on where the initial diagnostic breast MRI was performed and on unit availability on the day of biopsy. Bilateral 7-channel phased array breast coil (Open Breast Array Coil, Invivo, Gainesville, FL) and a fenestrated grid localization system were utilized. Biopsies were performed using a 9-gauge vacuum-assisted device (Hologic Inc, MRI ATEC, Marlborough, MA) with the standard 20 mm aperture in most cases and with the 12 mm aperture when the compressed breast tissue thickness was less than 2 cm.
Biopsies were performed by breast imaging fellowship-trained faculty radiologists and by trainees under their supervision.
Pre-biopsy sagittal images were obtained before and after intravenous administration of the same type of contrast agent used during the initial breast MRI examination (Gadavist or Multihance). Manual calculation from the sagittal images and grid was used to localize the lesion. After obturator advancement into the lesion, axial images were obtained to confirm appropriate positioning of the obturator. Biopsy was performed, and then axial and, if necessary, sagittal images were obtained to confirm adequate sampling and lesion removal.
After biopsy was completed, a marker clip was placed, and clip placement was confirmed with post-biopsy mammography. Any clip migration noted on mammography was documented in the biopsy report. After the pathologist reported the histopathology results, the radiologist who performed the MRI-VAB assessed histopathology–radiology concordance and issued an addendum stating whether the imaging and histopathology findings were concordant or discordant.
All high-risk lesions were reviewed in a multidisciplinary conference comprising a dedicated fellowship-trained breast radiologist, dedicated breast pathologist, breast surgeon, medical oncologist, radiation oncologist and a primary care provider. Personalized decisions were made regarding excision vs follow up based on patient’s clinical presentation (screening, symptomatic workup or known breast cancer), comorbidity, the adequacy of sampling, lesion size and level of radiological suspicion.9
Data analysis
Data collected included patient age and race/ethnicity, clinical indication for diagnostic breast MRI, enhancement characteristics and histopathology results for the lesion biopsied with MRI-VAB, follow-up imaging type and time after MRI-VAB, surgery in the same breast as MRI-VAB during the 24 months after MRI-VAB, upgrade of the MRI-VAB-detected lesion to carcinoma at surgery, and breast carcinoma development in the same breast as MRI-VAB during the 24 months after MRI-VAB. The location of subsequently detected breast carcinoma in relation to the site of MRI-VAB was determined on the basis of breast and surgical specimen radiology images and reports, with clip migration taken into account.
Results
A total of 425 lesions were biopsied with MRI-VAB during the study period, and 125 (29%) had high-risk histopathology results. Of these 125 lesions, 26 lesions did not satisfy the study criteria due to loss of follow-up. A total of 99 lesions in 90 patients met the inclusion criteria (imaging-concordant high-risk histopathology results on MRI-VAB and either surgical pathology results or at least 24 months of imaging follow-up).
Patient demographic and clinical characteristics for these 99 lesions are shown in Table 1.
Table 1.
Patient demographics, clinical and lesion characteristics in 90 patients with 99 high-risk lesions detected on MRI-VAB
| Characteristic | |
| Median age (range), yrs | 53 (28–79) |
| Race/ethnicity | |
| White | 71 (71) |
| Black | 10 (10) |
| Hispanic | 13 (13) |
| Asian | 3 (3) |
| Unspecified | 2 (2) |
| Indications for diagnostic breast MRI | |
| Screening | 25 (25) |
| Work-up of unresolved imaging findings | 12 (12) |
| Known breast cancer | 42 (42) |
| Ipsilateral breast cancer | 22 (22) |
| Contralateral breast cancer | 20 (20) |
| Assessment of residual cancer after surgical excision | 3 (3) |
| Assessment of tumor response to neoadjuvant chemotherapy | 2 (2) |
| Axillary metastasis with unknown primary tumor | 1 (1) |
| History of breast cancer | 1 (1) |
| Othera | 13 (13) |
| Lesion characteristics | |
| Non-mass enhancement | 54 (54) |
| Mass | 39 (39) |
| Focus | 6 (6) |
| Contrast enhancement kinetics | |
| Washout | 32 (32) |
| Persistent | 39 (39) |
| Plateau | 25 (25) |
| Unavailable | 3 (3) |
| Number of samples taken on MRI-VAB, median (range) | 12 (4−22) |
| VAB histopathology | |
| ADH | 21 (21) |
| Lobular neoplasia | 36 (36) |
| ALH | 22 (22) |
| LCIS | 6 (6) |
| Both ALH and LCIS | 8 (8) |
| Papillary lesion | 22 (22) |
| Radial scar | 11 (11) |
| Flat epithelial atypia | 1 (1) |
| Atypia unspecified | 8 (8) |
| Mucocele-like lesion | 0 (0) |
ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; LCIS, lobular carcinoma in situ; MRI-VAB, MRI-vacuum-assisted biopsy.
Numbers in parentheses are percentages.
Other refers to skin thickening, nipple discharge and palpable findings.
The median patient age was 53 years (range, 28–79). The median lesion size was 1.0 cm (range, 0.3–8.0 cm). The histopathology results on MRI-VAB were as follows: ADH, 21 lesions; lobular neoplasia (ALH and/or LCIS), 36 lesions; flat epithelial atypia, 1 lesion; radial scar, 11 lesions; papillary lesion, 22 lesions; and atypia unspecified, 8 lesions. Of the 36 lesions with lobular neoplasia, 22 were ALH, 6 were LCIS, and 8 were combined ALH and LCIS.
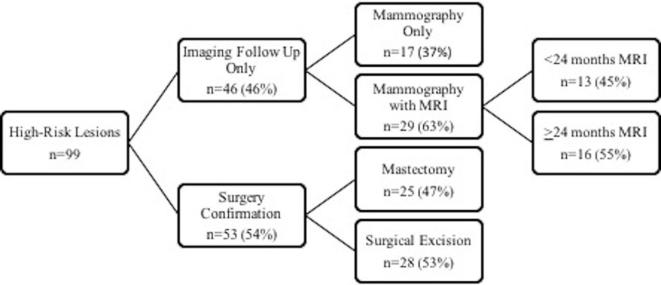
46 lesions included in our analysis had only imaging follow-up, 17 with mammography only, 16 with mammography and 24 months’ MRI follow-up, and 13 with mammography and less than 24 months’ MRI follow-up (Figure 1). The histopathology results on MRI-VAB of these lesions were as follows: ADH, 5 lesions; lobular neoplasia (ALH and/or LCIS), 15 lesions; flat epithelial atypia, 1 lesion; radial scar, 9 lesions; papillary lesion, 13 lesions; and atypia unspecified, 3 lesions. Of the 53 lesions with surgical correlation (Figure 1), histopathology correlation for the MRI biopsy site was not available for 6 lesions due to pathology slides being unavailable for review. However, none of the high-risk lesions that had surgical histopathology correlation demonstrated surgical discordance.
Figure 1.
Imaging follow-up and surgical management of 99 high-risk benign breast lesions detected on MRI-VAB. MRI = dynamic contrast-enhanced breast MRI. MRI-VAB, MRI-vacuum-assisted biopsy.
Of the 53 surgically excised lesions, 18 (34%) were excised per consensus recommendation at our institution’s multidisciplinary conference; 18 (34%) were excised at mastectomy for concurrent ipsilateral cancer; 7 (13%) were excised during prophylactic mastectomy for concurrent contralateral cancer; 5 (9%) were excised per the breast surgeon’s recommendation; 2 (4%) were excised per the pathologist’s recommendation; 1 (2%) was excised for alleviation of bloody nipple discharge; 1 (2%) was excised when a patient developed contralateral Paget’s disease 2 years after MRI-VAB and underwent bilateral mastectomy; and 1 (2%) was excised when a patient with a history of contralateral breast cancer and BRCA1 mutation chose to undergo bilateral prophylactic mastectomy after receiving her high-risk benign MRI-VAB result.
Overall, 6 of 53 (11.3%) lesions were upgraded to invasive cancer or ductal carcinoma in situ (DCIS) at surgical excision. Of these six lesions that upgraded to malignancy at surgery, four were excised per consensus recommendation at the multidisciplinary conference, one was excised per the breast surgeon’s recommendation, and one was removed during mastectomy for concurrent ipsilateral breast cancer. 4 of 21 ADH lesions (19%) were upgraded to DCIS. 2 of 36 lobular neoplasia (both combined ALH-LCIS) lesions (6%) were upgraded, to DCIS and invasive lobular carcinoma (Table 2). Imaging findings for a representative ADH lesion which upgraded to DCIS at surgical excision are shown in Figure 2.
Table 2.
Rate of underestimation of malignancy in 90 patients with 99 high-risk lesions detected on MRI-VAB
| Histopathologic diagnosis | Lesion frequency | No. excised | Underestimation rate | Cancer at surgery |
| ADH | 21 (21) | 16 | 4/21 (19) | 4 (DCIS) |
| Lobular neoplasia | 36 (36) | 21 | 2/36 (6) | 2 (1 DCIS, 1 ILC) |
| ALH | 22 (22) | 13 | 0/36 (0) | 0 |
| LCIS | 6 (6) | 2 | 0/36 (0) | 0 |
| ALH and LCIS | 8 (8) | 6 | 2/36 (6) | 2 (1 DCIS, 1 ILC) |
| Radial scar | 11 (11) | 2 | 0/11 (0) | 0 |
| Papillary lesion | 22 (22) | 9 | 0/22 (0) | 0 |
| Flat epithelial atypia | 1 (1) | 0 | 0/1 (0) | 0 |
| Atypia unspecified | 8 (8) | 5 | 0/8 (0) | 0 |
| Mucocele-like lesion | 0 (0) | 0 | 0/0 (0) | 0 |
ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; LCIS,lobular carcinoma in situ; MRI-VAB,MRI-vacuum-assisted biopsy.
Numbers in parentheses are percentages.
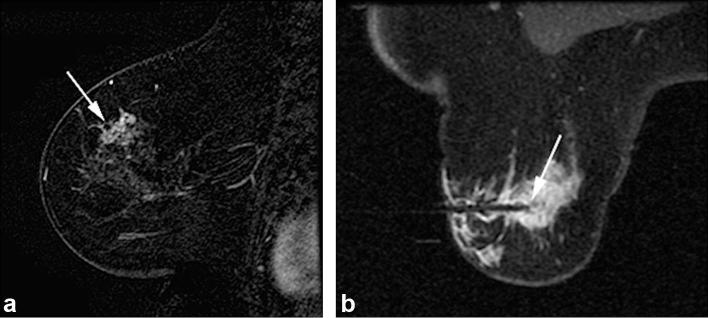
Figure 2. .
A 69-year-old female presented with palpable area of thickening in left superior breast with no correlate seen on mammography or sonography. (2A) Sagittal T1- post-contrast (subtraction) breast MRI image demonstrates clumped non-mass enhancement with segmental distribution at 12 o’clock position, corresponding to area of palpable finding (arrow). (2B) Axial T1 weighted post-contrast image obtained during MRI-guided vacuum-assisted needle biopsy confirms obturator position within suspicious lesion targeted for MRI-guided biopsy (arrow). Histopathology from VAB revealed ADH. Surgical excision was recommended and upgrade to DCIS, intermediate grade involving an area of approximately 2.5 cm, was revealed at segmental mastectomy. ADH, atypical ductal hyperplasia; DCIS,ductal carcinomain situ; VAB, vacuum-assisted biopsy.
The upgraded lesions consisted of the following types: one mass, four non-mass enhancement, and one focus of enhancement. The median lesion size of the upgraded lesions was 2.4 cm (range 0.4–6 cm). Of the lesions not upgraded, there were 38 masses, 50 non-mass enhancement and 5 foci of enhancement. The median lesion size was 1.7 cm (range 0.3–8 cm). Neither lesion type (p = 0.37) nor size (p = 0.64) is related to upgrade, however statistical power is limited by the number of lesion upgrades.
Of the 26 lesions that were excluded due to loss of follow-up, the distribution of pathologies was similar to the cases included in the study. The histopathology results on MRI-VAB of the 26 excluded lesions were as follows: ADH, 4 lesions; lobular neoplasia (ALH and/or LCIS), 4 lesions; flat epithelial atypia, 1 lesion; radial scar, 5 lesions; papillary lesion, 9 lesions; and atypia unspecified, 3 lesions.
Malignancy was diagnosed during imaging follow-up in the same breast as MRI-VAB in 2 of 90 patients (2 of 99 lesions). One patient had initial breast MRI for primary breast cancer in the contralateral breast and then underwent MRI-VAB which revealed ADH. More than 5 years after MRI-VAB, mammography revealed an invasive papillary carcinoma in a different quadrant in the same breast, 6 cm away from the MRI-VAB site. The second patient had initial breast MRI for high-risk screening and underwent MRI-VAB of a suspicious area of non-mass enhancement which revealed ADH. Surgical excision was performed and revealed DCIS, and the patient underwent adjuvant radiation therapy. Annual screening MRI 3 years later showed an abnormal area of enhancement adjacent to the post-surgical cavity, and MRI-VAB was performed and revealed recurrent DCIS.
Of the 99 lesions in our analysis, 33 were in patients who received chemotherapy and/or radiotherapy within 1 year after MRI-VAB.
Of the 53 lesions with surgical correlation, 21 lesions (40%) in 19 patients received breast cancer treatment within 1 year after MRI-VAB and 34 lesions in 30 patients were exposed to breast cancer risk-reducing treatment with selective estrogen receptor modulators (e.g. tamoxifen and raloxifene) or aromatase inhibitors (e.g. exemestane and anastrozole) within 1 year after MRI-VAB.
Of the 46 lesions with only imaging follow-up, 27 lesions(59%) in 26 patients were exposed to breast cancer risk-reducing treatment after MRI-VAB. Additionally, 12 (26%) out of the 46 lesions with only imaging follow-up, were exposed to breast cancer chemotherapy and/or radiation therapy.
Discussion
In our study, the high-risk breast lesions identified by MRI-VAB that were upgraded to malignancy at the time of surgery were ADH (4/21, 19%) and lobular neoplasia lesions with both ALH and LCIS (2/36, 6%). Our results indicate that surgical excision of such lesions is warranted. The lack of upgrade of the other high-risk lesions, including radial scar (radial sclerosing lesion, scleroelastic lesion, sclerosing papillary lesion, and complex sclerosing lesion), papillary lesions (benign and atypical), flat epithelial atypia and mucocele-like lesions, call into question whether surgical excision of those lesions is necessary.
Although ours is not the first study to determine the incidence of upgrade to malignancy for MRI-VAB-detected high-risk breast lesions, our study differs from the preceding studies. These previous studies had relatively small sample sizes with small numbers of each type of lesion.13–20 Furthermore, there were differences in definitions of high-risk lesions. The lesion whose classification most differs between studies is intraductal papilloma, which was classified as benign in several studies.6, 17,20,21 The frequency and upgrade rates of the high-risk lesions ADH, ALH, LCIS and radial scar have been most frequently assessed. Many studies have excluded flat epithelial atypia and mucocele-like lesions from review.
Studies have shown that ADH is the most common high-risk lesion identified by MRI-VAB as well as the lesion with the highest upgrade rate. Reported rates of upgrade to malignancy for ADH detected at MRI-guided biopsy range from 11 to 57%.19 In a study reported by Liberman et al15 in 2007 of 237 lesions successfully biopsied with 9-gauge MRI-VAB, ADH was found in 15 (6%) of the lesions. 13 of these 15 lesions were surgically excised, and surgery showed DCIS in 5 cases (38%). The authors concluded that ADH detected by MRI-VAB is an indication for surgical excision.15 Strigel et al16 identified ADH in 51 of 482 MRI-detected suspicious lesions and upgrade to malignancy in 11 of 34 surgically excised lesions (32%), but these lesions were not biopsied exclusively with MRI-VAB, and unlike in our study, there was no follow up of the lesions without surgical correlation to assess future development of cancer at the biopsy site. A recent study by Verheyden et al22 showed the highest frequency of ADH on MRI-VAB, with 72 ADH lesions in 1509 biopsies and an upgrade rate of 26% (17 of 66 lesions). Our rate of upgrade of ADH to carcinoma is at the lower end of the range of rates shown by prior studies. Of the 99 high-risk lesions in our study, 21 (21%) were ADH, and 4 of these cases of ADH (19%) were upgraded at surgery. DCIS is the most common malignancy that ADH diagnosed at biopsy has been reported to upgrade,15 similarly all four ADH lesions that were upgraded at surgery in our series were upgraded to DCIS.
At MRI-guided biopsy, diagnosis of ALH, LCIS, or combined ALH-LCIS is less common than diagnosis of ADH. The frequency of lobular neoplasia in prior studies assessing the rates of upgrade of MRI-VAB-detected high-risk lesions ranges from 4 to 30%.18, 22 The aforementioned study by Strigel et al16 identified 6 ALH lesions and 3 LCIS lesions among 61 high-risk lesions. One of the two cases of ALH (50%) and neither of the two cases of LCIS that were surgically excised were upgraded to malignancy. In 2014, Heller et al23 reported that 45 of 151 MRI-VAB-detected high-risk lesions (30%) were lobular neoplasia (30 LCIS and 15 ALH). 8 of the 30 cases of LCIS (27%) and 2 of the 15 cases of ALH (13%) that were surgically excised were upgraded to malignancy. We found a significantly lower rate of upgrade to malignancy for lobular neoplasia, 6% (2/36), with upgrade to carcinoma occurring only in lesions with combined ALH and LCIS (confirmed at surgery). No lesion in our study with either ALH or LCIS alone was upgraded to malignancy. Imaging findings from a case of LCIS diagnosed at MRI-VAB in our study are shown in Figure 3. These results suggest that lesions with both ALH and LCIS on MRI-VAB should be considered for surgical excision.
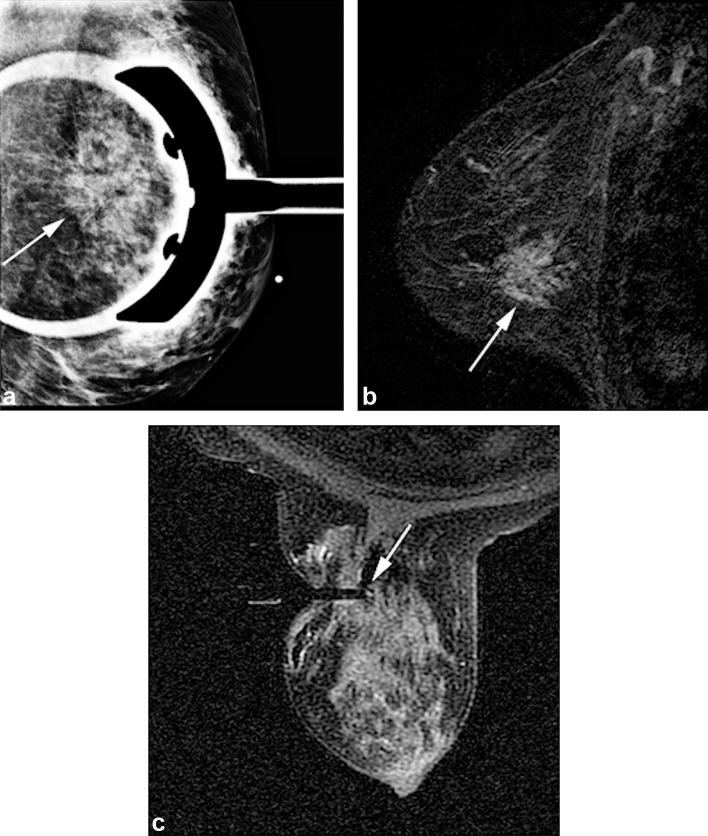
Figure 3. .
A 57-year-old female with abnormal screening mammogram. (3A) Diagnostic mammogram spot compression LM view shows architectural distortion (arrow) at left breast 3 o’clock position. No correlate was identified on sonography. MRI was performed. (3B) Sagittal T1 weighted post-contrast (subtraction) breast MRI image demonstrates 4.2 cm clumped non-mass regional enhancement (arrow) at 3 o’clock position, corresponding to area of concern on mammography. (3C) Axial T1 weighted post-contrast image obtained during MRI-guided vacuum-assisted needle biopsy confirms obturator position within the suspicious lesion targeted for MRI-guided biopsy (arrow). Histopathology review revealed LCIS focally involving complex sclerosing lesion. Surgical excision was recommended at multidisciplinary conference. Surgical pathology revealed no upgrade to malignancy. LCIS, lobular carcinoma in situ.
The frequency and rate of upgrade to malignancy of MRI-VAB-detected mucocele-like lesions has not previously been reported. There are limited data on the frequency and rates of upgrade to malignancy of flat epithelial atypia. Crystal et al14 found that the frequency of flat epithelial atypia was 1% (2/161) and no cases were upgraded. Tokazi et al21 found that the frequency of flat epithelial atypia was 5% (5/102), and 2 lesions were upgraded. Heller et al19 found that the frequency of flat epithelial atypia was 11% (16/151), and no cases were upgraded at surgery. Lamb et al24 identified 208 patients with pure flat epithelial atypia diagnosed by image-guided core needle biopsy and an upgrade rate to malignancy of 2.4% (5 of 208). Only 13 of these lesions, however, were biopsied with MRI-VAB. Our study identified a single lesion of flat epithelial atypia that was not upgraded to malignancy on the basis of imaging follow-up.
Frequencies and rates of upgrade to malignancy for MRI-VAB-detected radial scar have been evaluated in several previous studies. Liberman et al25 found a frequency of 1% (1/95 biopsies) with no upgrade. Crystal et al14 found radial scar in 2 of 26 high-risk lesions diagnosed at 9-gauge MRI-VAB that were subsequently surgically excised and demonstrated no upgrade. Strigel et al16 identified radial scar in 1 of 482 lesions biopsied and Rauch et al13 in 6 of 218 lesions biopsied. Neither study showed upgrade to malignancy of the lesions that were surgically excised. However, Lourenco et al18 reported an upgrade rate for radial scars of 23.1%. Their study identified radial scar in 20 of 446 (4%) MRI-guided biopsies and upgrade to malignancy in 3 of 13 surgically excised lesions. Our study identified 11 radial scars, none of which were upgraded to malignancy. The aforementioned study by Lourenco et al18 was performed using a 9-gauge vacuum-assisted device with a reported minimum of six core biopsy samples. In contrast, our study was performed using a 9-gauge VAB device with a median of 12 core biopsy samples. The number of core biopsy samples may account for the difference in upgrade rates. This possibility was suggested by a recent study by Ferreira et al26 that showed lower rates of upgrade of radial scar with greater number of fragments obtained at biopsy and lower rates of upgrade of radial scar in lesions subjected to VAB than in those subjected to core biopsy (rates of upgrade 19.5% overall, 4% for VAB, and 23.9% for standard core needle biopsy).
Some studies of MRI-VAB-detected high-risk breast lesions have excluded papillary lesions.6, 13,16,20,21 Previous studies have shown upgrade rates ranging from 0 to 6.7%.14, 18,19,23 The study with the largest number of papillary lesions identified at the time of biopsy, by Heller et al,23 revealed a frequency of papillary lesions diagnosed by MRI-VAB of 2.62% (30/1145) and an upgrade rate of 6.7% (2/30). The upgraded lesions were papillary lesions without atypia. In contrast, none of the papillary lesions in our study was upgraded to malignancy.
One limitation of our study is the lack of surgical correlation in 46 of 99 cases. However, all of the lesions lacking surgical correlation had at least 24 months of imaging and clinical follow-up, and of the lesions followed with MRI, 55% (16/29) had a minimum MRI follow-up of 24 months. There was no evidence of breast cancer development at the site of MRI-VAB in the cases with only imaging follow-up, although one patient did develop a malignancy in the same breast as MRI-VAB in a different quadrant, 6 cm away from the biopsy site.
Another limitation is the lack of histopathology correlation for 6 of the 53 lesions that were surgically excised. However, none of the high-risk lesions that had surgical histopathology correlation demonstrated surgical discordance.
A final limitation is that we did not exclude breast cancer patients who received chemotherapy and/or radiation therapy for breast cancer within 1 year after MRI-VAB or patients at high risk for developing breast cancer who received risk-reducing treatment. This treatment may represent a factor in the suppression of breast cancer development in these patients. This reflects true clinical practice, where many high-risk lesions are diagnosed concurrently in patients diagnosed with breast cancer or are diagnosed subsequent to chemotherapy or risk-reduction therapies.
Conclusions
We conclude that in patients with MRI-VAB-detected high-risk breast lesions, upgrade rate at surgical excision is low. Surgical excision is warranted for ADH and combined ALH-LCIS. For other high-risk lesions, a multidisciplinary approach may be appropriate to decide on personalized management which may include imaging surveillance or surgical excision based on patient risk factors, comorbid conditions, and their history of concurrent breast cancer.
Footnotes
Acknowledgements: We thank Stephanie Deming for assistance with the editing of this manuscript.
Disclosure: Dr Wei T Yang discloses is an advisory board member with Seno Medical and receives Elsevier Royalties.
IRB statement: IRB approval was obtained. A waiver of consent was granted due to the retrospective nature of this study.
Contributor Information
Megan E Speer, Email: mspeer@mdanderson.org.
Monica L Huang, Email: mlhuang@mdanderson.org.
Rosalind P Candelaria, Email: gmrauch@mdanderson.org.
Kenneth R Hess, Email: wyang@mdanderson.org.
Palita Hansakul, Email: p_hansakul@hotmail.com.
Wei T Yang, Email: khess@mdanderson.org.
Gaiane M Rauch, Email: rcandelaria@mdanderson.org.
REFERENCES
- 1.Swayampakula AK, Dillis C, Abraham J. Role of MRI in screening, diagnosis and management of breast cancer. Expert Rev Anticancer Ther 2008; 8: 811–7. doi: 10.1586/14737140.8.5.811 [DOI] [PubMed] [Google Scholar]
- 2.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw 2009; 7: 193–201. doi: 10.6004/jnccn.2009.0013 [DOI] [PubMed] [Google Scholar]
- 3.Han BK, Schnall MD, Orel SG, Rosen M. Outcome of MRI-guided breast biopsy. AJR Am J Roentgenol 2008; 191: 1798–804. doi: 10.2214/AJR.07.2827 [DOI] [PubMed] [Google Scholar]
- 4.Mahoney MC. Initial clinical experience with a new MRI vacuum-assisted breast biopsy device. J Magn Reson Imaging 2008; 28: 900–5. doi: 10.1002/jmri.21549 [DOI] [PubMed] [Google Scholar]
- 5.Menezes GL, Knuttel FM, Stehouwer BL, Pijnappel RM, van den Bosch MA. Magnetic resonance imaging in breast cancer: A literature review and future perspectives. World J Clin Oncol 2014; 5: 61–70. doi: 10.5306/wjco.v5.i2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orel SG, Rosen M, Mies C, Schnall MD. MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology 2006; 238: 54–61. doi: 10.1148/radiol.2381050050 [DOI] [PubMed] [Google Scholar]
- 7.Oxner CR, Vora L, Yim J, Kruper L, Ellenhorn JD. Magnetic resonance imaging-guided breast biopsy in lesions not visualized by mammogram or ultrasound. Am Surg 2012; 78: 1087–90. [PubMed] [Google Scholar]
- 8.Heller SL, Hernandez O, Moy L. Radiologic-pathologic correlation at breast MR imaging: what is the appropriate management for high-risk lesions? Magn Reson Imaging Clin N Am 2012; 21: 583–99. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy S, Bevers T, Kuerer H, Yang WT. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 2012; 198: W132–W140. doi: 10.2214/AJR.11.7799 [DOI] [PubMed] [Google Scholar]
- 10.Kohr JR, Eby PR, Allison KH, DeMartini WB, Gutierrez RL, Peacock S, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology 2010; 255: 723–30. doi: 10.1148/radiol.09091406 [DOI] [PubMed] [Google Scholar]
- 11.Mesurolle B, Perez JC, Azzumea F, Lemercier E, Xie X, Aldis A, et al. Atypical ductal hyperplasia diagnosed at sonographically guided core needle biopsy: frequency, final surgical outcome, and factors associated with underestimation. AJR Am J Roentgenol 2014; 202: 1389–94. doi: 10.2214/AJR.13.10864 [DOI] [PubMed] [Google Scholar]
- 12.Morris EA, Comstock CE, Lee CE. ACR BI-RADS magnetic resonance imaging. In: ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: The British Institute of Radiology.; 2013. [Google Scholar]
- 13.Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol 2012; 198: 292–9. doi: 10.2214/AJR.11.7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crystal P, Sadaf A, Bukhanov K, McCready D, O'Malley F, Helbich TH. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted? Eur Radiol 2011; 21: 582–9. doi: 10.1007/s00330-010-1949-6 [DOI] [PubMed] [Google Scholar]
- 15.Liberman L, Holland AE, Marjan D, Murray MP, Bartella L, Morris EA, et al. Underestimation of atypical ductal hyperplasia at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol 2007; 188: 684–90. doi: 10.2214/AJR.06.0809 [DOI] [PubMed] [Google Scholar]
- 16.Strigel RM, Eby PR, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. Frequency, upgrade rates, and characteristics of high-risk lesions initially identified with breast MRI. AJR Am J Roentgenol 2010; 195: 792–8. doi: 10.2214/AJR.09.4081 [DOI] [PubMed] [Google Scholar]
- 17.Perretta T, Pistolese CA, Bolacchi F, Cossu E, Fiaschetti V, Simonetti G. MR imaging-guided 10-gauge vacuum-assisted breast biopsy: histological characterisation. Radiol Med 2008; 113: 830–40. doi: 10.1007/s11547-008-0289-y [DOI] [PubMed] [Google Scholar]
- 18.Lourenco AP, Khalil H, Sanford M, Donegan L. High-risk lesions at MRI-guided breast biopsy: frequency and rate of underestimation. American Journal of Roentgenology 2014; 203: 682–6. doi: 10.2214/AJR.13.11905 [DOI] [PubMed] [Google Scholar]
- 19.Heller SL, Moy L. Imaging features and management of high-risk lesions on contrast-enhanced dynamic breast MRI. AJR Am J Roentgenol 2012; 198: 249–55. doi: 10.2214/AJR.11.7610 [DOI] [PubMed] [Google Scholar]
- 20.Perlet C, Heywang-Kobrunner SH, Heinig A, Sittek H, Casselman J, Anderson I, et al. Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer 2006; 106: 982–90. doi: 10.1002/cncr.21720 [DOI] [PubMed] [Google Scholar]
- 21.Tozaki M, Yamashiro N, Sakamoto M, Sakamoto N, Mizuuchi N, Fukuma E. Magnetic resonance-guided vacuum- assisted breast biopsy: results in 100 Japanese women. Jpn J Radiol 2010; 28: 527–33. doi: 10.1007/s11604-010-0464-7 [DOI] [PubMed] [Google Scholar]
- 22.Verheyden C, Pages-Bouic E, Balleyguier C, Cherel P, Lepori D, Laffargue G, et al. Underestimation rate at MR imaging-guided vacuum-assisted breast biopsy: A multi-institutional retrospective study of 1509 breast biopsies. Radiology 2016; 281: 708–19. doi: 10.1148/radiol.2016151947 [DOI] [PubMed] [Google Scholar]
- 23.Heller SL, Elias K, Gupta A, Greenwood HI, Mercado CL, Moy L. Outcome of high-risk lesions at MRI-guided 9-gauge vacuum- assisted breast biopsy. AJR Am J Roentgenol 2014; 202: 237–45. doi: 10.2214/AJR.13.10600 [DOI] [PubMed] [Google Scholar]
- 24.Lamb LR, Bahl M, Gadd MA, Lehman CD. Flat epithelial atypia: upgrade rates and risk-stratification approach to support informed decision making. J Am Coll Surg 2017; 225: 696–701. doi: 10.1016/j.jamcollsurg.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 25.Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol 2005; 185: 183–93. doi: 10.2214/ajr.185.1.01850183 [DOI] [PubMed] [Google Scholar]
- 26.Ferreira AI, Borges S, Sousa A, Ribeiro C, Mesquita A, Martins PC, et al. Radial scar of the breast: Is it possible to avoid surgery? Eur J Surg Oncol 2017; 43: 1265–72. doi: 10.1016/j.ejso.2017.01.238 [DOI] [PubMed] [Google Scholar]