Abstract
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and the second cause of cancer-related deaths worldwide. In most cases, it is diagnosed in patients with identified risk factors, mainly cirrhosis from all causes. These patients are candidates for a surveillance program that, depending on guidelines, involves regular liver ultrasound alone or combined with serum markers. These programs have been shown to improve the oncological outcome by detecting earlier stage tumors and providing patients with potentially curative treatment and improved survival. Yet, the level of evidence supporting these guidelines remains limited. This review article presents an overview of the evidence supporting surveillance programs for hepatocellular carcinoma, in particular the efficacy, cost-effectiveness, and consequences of this approach for patient survival. Western and Eastern guideline recommendations are described and discussed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and the second cause of cancer-related deaths worldwide with over 500,000 deaths per year.1–3 Like other cancers, outcome and long-term survival are better in patients diagnosed with early stage HCC. For example, in patients with preserved hepatic function, no evidence of portal hypertension, and single asymptomatic tumors <5 cm in diameter, surgical resection provides 5-year survival rates of 70%.4 Similarly, the survival rate following liver transplantation for tumors that meet the Milan criteria (solitary nodule <5 cm or three nodules each <3 cm in diameter) is nearly 75%.4, 5 Patients with a larger tumor burden are frequently not candidates for curative treatment, and therefore, their expected survival rate is lower. Unfortunately, fewer than 30% of patients are diagnosed early enough to meet criteria for tumor ablation, resection or transplantation.6 This supports the rationale for developing an effective surveillance program designed to improve patient management.
Surveillance programs involve closely monitoring patients with certain regularly scheduled examinations or tests. At first glance, HCC would seem to fulfill all criteria established by the World Health Organization for a surveillance program: the disease burden is an important health problem worldwide, there is a clearly identified target population, surveillance is accepted by both patients and health providers, surveillance accuracy is acceptable, there are standardized recall procedures, surveillance is affordable, there is an advantage of treating early-stage HCC, and finally, surveillance reduces mortality. However, a thorough review of the literature shows that the level of evidence supporting these criteria is quite heterogeneous. It is well established that HCC is a major health problem that occurs in 90% of patients with an identified risk factor, usually cirrhosis of various origins.1 Moreover, the diagnostic accuracy of contrast-enhanced cross-sectional imaging and biopsy—alone or in combination—in the characterization of focal liver lesions has been demontrasted.7 On the contrary, while liver ultrasound is considered as a valid first-line examination technique for tumor detection and has been adopted by Western and Asian guidelines,1,8–11 its efficacy as a surveillance method is mainly based on the results of a single Chinese randomized controlled trial.12 Thus, there is a lack of robust evidence to support its benefit in reducing mortality.
The aim of this review article is to provide an overview of the evidence supporting these surveillance programs, with, in particular, their efficacy, cost-effectiveness, and consequences for patient survival. This should improve understanding of and provide a discussion on the most important guidelines for HCC surveillance.
HIGH-RISK POPULATIONS
As stated above, around 90% of HCCs develop in patients with underlying risk factors, most frequently chronic viral hepatitis (types B and C), alcohol abuse and non-alcoholic fatty liver disease (NAFLD).6 The epidemiology of risk factors strongly depends upon geographical factors: while most HCC are associated with hepatitis B (60%) in Asian countries, chronic hepatitis C and alcohol appear to be the major risk factors is the Western countries,4 and NAFLD is increasing, especially in Northern America.2, 13
Patients with cirrhosis
Most patients with HCC are diagnosed at the stage of cirrhosis and approximately, one-third of the patients with cirrhosis will develop HCC during their lifetime,14 corresponding to a risk of approximately 1–8% per year. It is important to note that this risk varies depending on the etiology of cirrhosis and the possible control of its cause. It is around 2% in patients with hepatitis B virus (HBV)-related cirrhosis, and up to 3–8% in hepatitis C virus (HCV)-related cirrhosis in the absence of viral control or eradication, respectively.15 According to the Japanese guidelines, this justifies identifying patients with cirrhosis and HBV or HCV infection as “super-high-risk patients” (Table 1).10 However, successful viral eradication leading to a sustained virological response in HCV and HBV patients significantly decreases, but does not eliminate, the risk of HCC.16, 17 Other factors, such as male gender, age and the presence of portal hypertension have been shown to increase the risk of HCC in patients with cirrhosis. To date, these factors have not changed recommendations, and all patients with cirrhosis should be included in HCC surveillance programs, whatever the control or severity of the underlying cause of liver disease.
Table 1.
High-risk population candidate to surveillance programs according to Western and Eastern guidelines
| Western | Eastern | ||||
| AASLD9 | EASL1 | JSH10 | APASL8 | KLCSG11 | |
| Super-high-risk patients | Cirrhosis with HBV or HCV | ||||
| High-risk patients | Cirrhosis | Cirrhosisb | Cirrhosis of any cause | Cirrhosis with HBV or HCV | Cirrhosis of any cause |
| HBVa | HBVc | HBV | HBV | ||
| HCVa | HCV F3 | HCV | HCV | ||
AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; HBV, hepatitis B virus; HVC, hepatitis C virus; JSH, Japanese Society of Hepatology; APASL, Asian Pacific Association for the study of the Liver; KLCSG, Korean Liver Cancer Study Group.
Asian males (>40 years), Asian females (>50 years); African/American > 20 years.
Child Pugh A or B, and Chid Pugh C awaiting liver transplantation.
Active carrier or family history of HCC.
Non-cirrhotic patients
Although the actual yearly incidence has not been clearly identified, patients with chronic active HBV infection are at risk of HCC even in the absence of cirrhosis. It is even recommended to screen specific subgroups of inactive HBV carriers such as African males born in Africa because of aflatoxin-B1 exposure.18 There are fewer studies on HCV, but data suggest that patients with advanced fibrosis have a high risk of HCC.19, 20 Data on the incidence of HCC in patients with non-viral chronic liver disease without cirrhosis, such as NAFLD, autoimmune liver disease, hemochromatosis, or others are also limited.1, 13 The risk in patients with NAFLD is probably not limited to those with advanced fibrosis or cirrhosis.13, 21 However, the uncertainty regarding the actual incidence of HCC in NAFLD patients without cirrhosis, which is present in high rates in Western (and now even Eastern) populations, explains why guidelines on HCC surveillance recommend following current practices and only be performed in patients with cirrhosis.7, 13 Moreover, this population also has more competing causes of mortality than patients with viral hepatitis which may impact on the efficacy of surveillance.
SURVEILLANCE MODALITY
Tests to be used
Guidelines suggest using two different types of tests for HCC surveillance: serological and imaging examinations. The most widely accepted imaging modality, that is present in and recommended by all guidelines, is ultrasonography. It is inexpensive, accessible, well accepted by the patient and has an acceptable diagnostic accuracy. It is also, frequently, the only imaging method available in developing countries or remote rural areas. The reported sensitivity of this test ranges from 58 to 89%, with an excellent specificity (above 90%).22 A recent meta-analysis including 13 prospective studies showed that ultrasound surveillance was accurate for the detection of all types of HCC, before it was clinically detectable with a pooled sensitivity of 94% (Figure 1). However, ultrasound was less effective for detecting early-stage HCC, with a sensitivity of only 63%.23 This low sensitivity is explained by patient-related factors that may limit ultrasound exploration of the liver (obesity, marked steatosis, heterogeneous cirrhotic liver parenchyma, poor co-operation), tumor features (location, infiltrative forms, tumor echogenicity). The fact that obesity or steatosis compromise the completeness of an ultrasound examination of the liver is clinically important in the context of the increasing epidemic of NAFLD. It shows that results derived from populations with other chronic liver diseases may not be easily extrapolated to patients with NAFLD. Technical limitations and the radiologist’s skill and expertise also play an important role. Indeed, a recent Japanese study with ultrasound performed by highly skilled radiologists reported detection of tumors with a mean diameter of 1.6 ± 0.6 cm.24
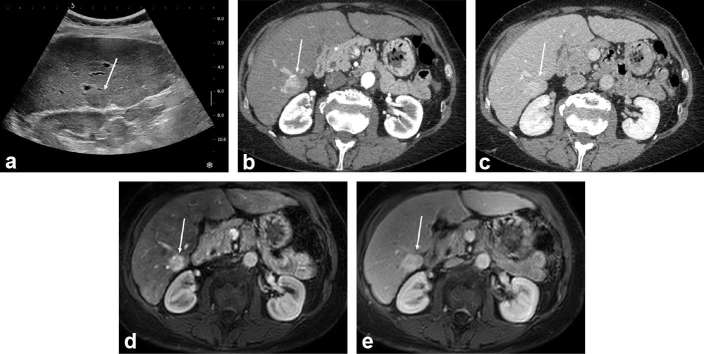
Figure 1.
HCC discovered during surveillance in a 56-year-old male with HCV-related cirrhosis. Systematic ultrasound depicted a mildly hypoechoic 25 mm subcapsular in Segment 6 (arrow in a). On contrast-enhanced CT (b, c) and gadoxetic-acid enhanced MRI (d, e) the lesion showed marked and homogeneous enhancement on arterial phase (b, d), and no washout on portal venous phase (c, e). The lesion was biopsied and proven to be a well-differentiated HCC. The patient underwent resection and remains recurrence-free at 23 months. HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
The role of other imaging techniques has not been thoroughly investigated. A recent randomized study comparing twice-a-year ultrasound to once-a-year triple-phase-contrast CT reported that ultrasound was slightly more sensitive and less expensive for the detection of early HCC.25 A recent meta-analysis reported a sensitivity of 84% with gadoxetic acid-enhanced MRI for the detection of all HCC.26 Nevertheless, despite its better results, MRI is not suitable for surveillance programs because a complete MRI examination takes between 30 and 45 min. Therefore, the use of short scanning protocols that take less than 10 min has been suggested, while reducing the number of acquired sequences and concentrating on the most sensitive sequences for tumor detection (T2 weighted, diffusion-weighted, and T1 weighted during the hepatobiliary phase). This was supported by recent retrospective studies showing that “simulated” fast MR protocols (by limiting interpretation to selected sequences) could be an acceptable alternative to ultrasound.27–29
The use of serological markers is more controversial. Alpha-fetoprotein (AFP) has been the most widely investigated. More recently, des-gamma-carboxy prothrombin—also known as prothrombin induced by Vitamin K Absence II—or the ratio of glycosylated AFP (L3 fraction) to total AFP, has been analyzed.30, 31 The results of AFP as a test for surveillance are poor because only 10–20% of early HCC express AFP. Although the combination of ultrasound and AFP has been estimated to increase the sensitivity for early HCC by 6% compared to ultrasound alone, the rate of false-positive results is also increased, with negative consequences on direct and indirect costs for each early HCC detected.32, 33 This explains why Western guidelines do not recommend dosing AFP for surveillance.1, 9 Eastern guidelines take a different approach and still propose a combination of ultrasound and serum markers.8, 10,11 (Table 2) The most extensive program is that of the Japanese Society of Hepatology, which recommends ultrasound and tumor markers (including AFP, AFP-L3 and prothrombin induced by Vitamin K Absence II) for both super-high-risk and high-risk populations.10 Interestingly, these guidelines also propose periodic imaging with dynamic CT or preferably dynamic gadoxetic-enhanced MRI for super-high-risk patients, or those with a very coarse background liver parenchyma because of cirrhosis and obesity resulting in a difficult evaluation by ultrasound.
Table 2.
Surveillance modality according to Western and Eastern guidelines
| Patient group | Western | Eastern | ||||
| AASLD9 | EASL1 | JSH10 | APASL8 | KLCSG11 | ||
| Modality | Super-high- and high-risk patients | Liver ultrasound | Liver ultrasound | Liver ultrasound + AFP/AFP-L3, PIVKA-IICT/EOB-MRI | Liver ultrasoundAFPa | Liver ultrasoundAFPb |
| Interval | Super-high-risk patients | 3–4 monthsCT/MRI 6–12 months | ||||
| High-risk patients | 6 months | 6 months | 6 monthsNo CT/EOB-MRI | 6 months | 6 months | |
AASLD, American Association for the Study of Liver Diseases; AFP, alpha-fetoprotein; EASL, European Association for the Study of the Liver; EOB, ethoxybenzyl; JSH, Japanese Society of Hepatology; APASL, Asian Pacific Association for the study of the Liver; KLCSG, Korean Liver Cancer Study Group; PIVKA-II, prothrombin induced by Vitamin K Absence II.
Accepted diagnostic cut-off value >200 ng ml–1, even though the dosage is not recommended.
For nodules < 1 cm.
Surveillance interval
Based on the expected doubling time of HCC, all guidelines recommend surveillance at 6 month intervals, except Japanese guidelines which also recommend a 3–4 month interval for super-high-risk populations (Table 2).17 In this setting, CT/MRI should be performed every 12 months. The recommended 6-month interval for ultrasound surveillance is based on the results of a large French randomized multicenter study that did not show any benefit to shorter surveillance intervals.34 This study showed that a 3-month interval did not significantly increase the likelihood of detecting small (≤3 cm) HCCs (79 vs 70%), or improve the amenability to curative treatment (62 vs 58%) or 5-survival (85 vs 86%). On the other hand, the 3 months interval was associated with a higher risk of detecting non-malignant lesions than the 6-month interval, leading to an increased cost of recall procedures. The results of comparisons of patient cohorts monitored every 6 or 12 months were either similar, or reported a lower survival rate with the 12 month intervals.35–37 Finally, the meta-analysis by Singal et al mentioned above has shown that the pooled sensitivity of ultrasound-based surveillance decreases to around 50% with the annual program.23
RESULTS OF SURVEILLANCE PROGRAMS: FROM EFFICACY TO EFFECTIVENESS
Benefits of surveillance programs
Analysis of the benefits of the efficacy of surveillance programs is mainly based on the results of a Chinese randomized controlled trial. This population-based cluster study randomized surveillance (AFP and liver ultrasound performed every 6 months) vs no surveillance in entire villages of patients with HBV and reported a 37% reduction in HCC-related mortality in the surveillance group, despite a low rate of participation (55%).12 Importantly, not all patients included in this study were cirrhotic. Therefore, the target population was different from the target population advocated in most programs. In fact, there are no randomized controlled trials on cirrhotic populations.
Yet, the results of a recent American retrospective cohort study found a similar (38%) decrease in mortality.38 Like other studies reporting the benefits of regular ultrasound surveillance,23, 35,36,39,40 the latter study should be interpreted with caution, since all of the reference studies suffer from methodological biases of cohort studies (not representative of cirrhotic populations as a whole, and subject to lead-time bias and length-time bias). Nevertheless, a recent meta-analysis by Singal et al including 47 studies with 15,158 patients and 6284 (41.4%) with HCC detected by surveillance, reported improved early stage detection [odds ratio (OR) 2.08, 95% CI (1.80–2.37)] and curative treatment rates [OR 2.24, 95% CI (1.99–2.52)], as well as significantly prolonged survival [OR 1.90, 95% CI (1.67–2.17)], which remained significant in the subset of studies adjusting for lead-time bias.41 New data are emerging based on a study of large prospective cohorts of patients with cirrhosis included in surveillance programs.42 Indeed, a benefit to survival related to compliance with HCC surveillance guidelines is expected, in particular after correction for lead-time bias and whatever the assumptions regarding tumor doubling time.42 The magnitude of increase in survival for populations undergoing ultrasound surveillance remains difficult to assess. Recently, Cadier et al reported a mean gain of 0.37 years.43 It may seem limited but has to be compared to the short median overall survival of patients with HCC (e.g. 9.4 months, i.e. 0.78 years, in the recent national French observational study by Goutté et al44).
Cost-effectiveness
Cost-effectiveness analysis is an economic analysis that compares the relative costs and outcomes (effects) of different courses of action. For HCC surveillance, it should compare the gain in quality-adjusted life expectancy obtained following surveillance to the total costs of the program. However, it is important to note that the former depends upon the incidence of HCC and the possibility of providing curative management to patients diagnosed with HCC and the latter includes the costs of surveillance tests, as well as false-positive cases, tumor diagnosis, staging, and of course, treatment.
Studies show that an incidence of HCC of at least 1.5% per year would justify surveillance in HCC in patients with cirrhosis,45 whatever the etiology. However, surveillance of Child–Pugh Class C patients—except those awaiting liver transplantation—is probably not cost-effective due to the difficulty of performing secondary curative treatment.39, 46 In 2012, Ruggeri published a systematic review of seven studies providing a full cost-effectiveness evaluation of different HCC surveillance techniques.47 These results showed that ultrasound alone or in association with AFP was likely to be the most cost-effective strategy. Indeed, although this strategy has been consistently reported to be the most effective program for early tumor diagnosis, it is also associated with increased costs compared to longer testing intervals (i.e. 12 months or more). This extra cost is compensated by the lower cost of secondary treatments. Indeed, the cost of surveillance and diagnosis only represents 10–20% of the total costs of cancer management.48, 49 This was recently confirmed in a study performed in France and the USA based on the assessment of Markov models and transition probabilities derived from academic and real-life data obtained in longitudinal cohorts.43
Finally, and importantly, previous results may not be easily extrapolated to patients with NAFLD. Indeed, in this setting, CT or MRI are more frequently performed after a first incomplete ultrasound examination of the liver. As a consequence, if surveillance based on regular ultrasound is considered cost-effective in patients at risk for HCC, it may no longer be the case if MRI or CT scans have to be performed. Whether or not above-mentioned short MRI scanning protocols may replace ultrasound in this context requires further large-scale investigation.
Uptake and limitations of surveillance programs
Several studies have shown that the rate of patients eligible for surveillance and who actually undergo the program as planned is low. Davila et al reported that no more than 28% of patients diagnosed with HCC had undergone at least one ultrasound surveillance test in the 3 years preceding diagnosis.50 The same group reported a study in a larger population of at risk patients and confirmed the low rate of surveillance with only 12 and 59% of patients undergoing consistent and inconsistent surveillance, respectively.51 As stressed by Giannini et al52 “efficacy” and “effectiveness” should be clearly differentiated. Efficacy is a measure of the degree to which one procedure obtains the expected result under a standardized condition, while effectiveness measures the extent of benefit when the procedure is applied to clinical practice.52 Therefore, the latter includes non-standardized factors corresponding to the “real-life” experience of both patients and health providers. In the present situation, these factors may include convincing and recommendations by physicians, patient acceptance and adherence, the organization of the healthcare system as well as economic, cultural and social influences. Several studies have tried to identify these factors. Davila et al demonstrated that being followed by a gastroenterologist/hepatologist or an academic physician was associated with a higher likelihood of receiving surveillance than being followed by a primary care physician.51 This was recently confirmed by Goldberg et al in a series of 26,577 patients.53 Farvardin et al tried to identify the association between surveillance and patient knowledge, attitudes, and perceived barriers in a racially diverse and socioeconomically disadvantaged cohort of patients with cirrhosis.54 They showed that around 50% of the patients believed that eating a healthy diet prevented the need for HCC surveillance, and that close to 35% believed that HCC surveillance was not necessary if they had a normal physical exam and/or lacked clinical symptoms. Overall, nearly half the patients were found to have barriers to receiving HCC surveillance, including difficulty with the scheduling process, costs, and transportation difficulties. Goldberg et al also reported an inverse relationship between ultrasound lead time (difference between the date, an ultrasound was ordered and requested examination date) and the odds of having the test performed [OR = 0.77, 95% CI (0.72–0.82), when ordered >180 days ahead of time; and OR = 0.90, 95% CI (0.85–0.94), if lead time 91–180 days].53 Atiq et al suggested that another explanation was the possible harm caused by surveillance programs—defined as any follow-up tests (CT, MRI, liver biopsy, angiogram) performed for false-positive or indeterminate surveillance results. In a series of 680 patients with cirrhosis who underwent surveillance by ultrasound and AFP dosage, surveillance-related physical harm was observed in over one-fourth of the patients, with a higher proportion of ultrasound-related than AFP-related harm.55 Even though harm was mild to moderate in most patients, these data clearly show that the use of surveillance is insufficient and suboptimal in the real world of healthcare and should stimulate educational policies to expand knowledge and for the correct use of this tool. Radiologists who frequently perform liver ultrasound need to better be aware of and help address these unmet needs. Thus, all initiatives to optimize compliance based on procedures to call patients enrolled in HCC surveillance programs (including traditional approaches such as mail outreach or newer communication strategies) are welcome, because they have been shown to increase the rate of patients who performed their next ultrasound surveillance examination.56
CONCLUSION
HCC surveillance based on regular ultrasound examination of the liver—alone or in combination with serum markers—is beneficial and cost-effective. It leads to the detection of earlier stage disease, a higher rate of curative treatment and improved survival. This strategy has been endorsed by all guidelines on HCC surveillance. Nevertheless, surveillance must be performed in selected high-risk patients—mostly patients with cirrhosis or with chronic HBV or HCV infection. To date, adoption of surveillance programs is still unacceptably low due to various factors including convincing and recommendations by physicians, patient acceptance and adherence, organization of the healthcare system but also economic, cultural and social influences. Collective efforts must be continued to improve the benefit of this strategy.
Contributor Information
Maxime Ronot, Email: maxime.ronot@aphp.fr.
Romain Pommier, Email: romain.pommier75@gmail.com.
Marco Dioguardi Burgio, Email: marco_dioguardi@hotmail.it.
Yvonne Purcell, Email: yvonne.purcell@gmail.com.
Pierre Nahon, Email: pierre.nahon@aphp.fr.
Valérie Vilgrain, Email: valerie.vilgrain@aphp.fr.
REFERENCES
- 1.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004; 127(5 Suppl 1): S27–S34. doi: 10.1053/j.gastro.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–76. doi: 10.1053/j.gastro.2007.04.061 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Early diagnosis and treatment of hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol 2000; 14: 991–1008. doi: 10.1053/bega.2000.0143 [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–700. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. . Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005; 41: 707–15. doi: 10.1002/hep.20636 [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts 2016; 9: 65–90. doi: 10.1159/000443344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. . Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010; 4: 439–74. doi: 10.1007/s12072-010-9165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–2. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. . Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis 2011; 29: 339–64. doi: 10.1159/000327577 [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea practice guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis 2014; 32: 764–77. doi: 10.1159/000368020 [DOI] [PubMed] [Google Scholar]
- 12.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130: 417–22. doi: 10.1007/s00432-004-0552-0 [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. . The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Am J Gastroenterol 2012; 107: 811–26. doi: 10.1038/ajg.2012.128 [DOI] [PubMed] [Google Scholar]
- 14.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. . The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006; 43: 1303–10. doi: 10.1002/hep.21176 [DOI] [PubMed] [Google Scholar]
- 15.Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007; 5: 938–45. doi: 10.1016/j.cgh.2007.02.039 [DOI] [PubMed] [Google Scholar]
- 16.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, et al. . Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007; 45: 579–87. doi: 10.1002/hep.21492 [DOI] [PubMed] [Google Scholar]
- 17.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2008; 28: 1067–77. doi: 10.1111/j.1365-2036.2008.03816.x [DOI] [PubMed] [Google Scholar]
- 18.European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57: 167–85. doi: 10.1016/j.jhep.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. . Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT study group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med 1999; 131: 174–81. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. . Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009; 136: 138–48. doi: 10.1053/j.gastro.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127(5 Suppl 1): S35–S50. doi: 10.1053/j.gastro.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med 2001; 20: 99–104. doi: 10.7863/jum.2001.20.2.99 [DOI] [PubMed] [Google Scholar]
- 23.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. . Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009; 30: 37–47. doi: 10.1111/j.1365-2036.2009.04014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Tateishi R, Yoshida H, Ohki T, Masuzaki R, Imamura J, et al. . Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int 2009; 3: 544–50. doi: 10.1007/s12072-009-9145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography - a randomised study. Aliment Pharmacol Ther 2013; 38: 303–12. doi: 10.1111/apt.12370 [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. . Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015; 275: 97–109. doi: 10.1148/radiol.14140690 [DOI] [PubMed] [Google Scholar]
- 27.Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, Cooper N, et al. . Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol 2017; 42: 179–90. doi: 10.1007/s00261-016-0841-5 [DOI] [PubMed] [Google Scholar]
- 28.Marks RM, Ryan A, Heba ER, Tang A, Wolfson TJ, Gamst AC, et al. . Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 2015; 204: 527–35. doi: 10.2214/AJR.14.12986 [DOI] [PubMed] [Google Scholar]
- 29.Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn Reson Imaging 2014; 32: 610–8. doi: 10.1016/j.mri.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 30.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. . Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009; 137: 110–8. doi: 10.1053/j.gastro.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, et al. . Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology 2003; 37: 1114–21. doi: 10.1053/jhep.2003.50195 [DOI] [PubMed] [Google Scholar]
- 32.Sherman M. Serological surveillance for hepatocellular carcinoma: time to quit. J Hepatol 2010; 52: 614–5. doi: 10.1016/j.jhep.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 33.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008; 6: 1418–24. doi: 10.1016/j.cgh.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. . Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011; 54: 1987–97. doi: 10.1002/hep.24545 [DOI] [PubMed] [Google Scholar]
- 35.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. . Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004; 126: 1005–14. doi: 10.1053/j.gastro.2003.12.049 [DOI] [PubMed] [Google Scholar]
- 36.Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, et al. . A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood 2003; 102: 78–82. doi: 10.1182/blood-2002-10-3310 [DOI] [PubMed] [Google Scholar]
- 37.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. . Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010; 53: 291–7. doi: 10.1016/j.jhep.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 38.Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, et al. . Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016; 65: 1148–54. doi: 10.1016/j.jhep.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevisani F, Santi V, Gramenzi A, Di Nolfo MA, Del Poggio P, Benvegnù L, et al. . Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterol 2007; 102: 2448–57. doi: 10.1111/j.1572-0241.2007.01395.x [DOI] [PubMed] [Google Scholar]
- 40.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. . Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res 2008; 38: 37–51. doi: 10.1111/j.1872-034X.2007.00216.x [DOI] [PubMed] [Google Scholar]
- 41.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. doi: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costentin CLR, Bourcier V, Corvi L, Petro-Sanchez V, Marcellin P. Compliance tohepatocellular carcinoma screening guidelines in patients with compensated viralcirrhosis increases the probability of curative treatment and survival taking into accountlead time bias (ANRS CO12 CIRVIR COHORT). Proceeding of the 10th International LiverCancer Association., 2016 September 9-11, Vancouver, Canada; 2016.
- 43.Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I, et al. . Early detection and curative treatment of hepatocellular carcinoma: a cost-effectiveness analysis in France and in the United States. Hepatology 2017; 65: 1237–48. doi: 10.1002/hep.28961 [DOI] [PubMed] [Google Scholar]
- 44.Goutté N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol 2017; 66: 537–44. doi: 10.1016/j.jhep.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 45.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101: 422–34. doi: 10.1016/S0002-9343(96)00197-0 [DOI] [PubMed] [Google Scholar]
- 46.Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, et al. . Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol 2012; 56: 1089–96. doi: 10.1016/j.jhep.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri M. Hepatocellular carcinoma: cost-effectiveness of screening. A systematic review. Risk Manag Healthc Policy 2012; 5: 49–54. doi: 10.2147/RMHP.S18677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. . Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001; 48: 251–9. doi: 10.1136/gut.48.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel D, Terrault NA, Yao FY, Bass NM, Ladabaum U. Cost-effectiveness of hepatocellular carcinoma surveillance in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol 2005; 3: 75–84. doi: 10.1016/S1542-3565(04)00443-4 [DOI] [PubMed] [Google Scholar]
- 50.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol 2007; 41: 777–82. doi: 10.1097/MCG.0b013e3180381560 [DOI] [PubMed] [Google Scholar]
- 51.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. . Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011; 154: 85–93. doi: 10.7326/0003-4819-154-2-201101180-00006 [DOI] [PubMed] [Google Scholar]
- 52.Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol 2013; 19: 8808–21. doi: 10.3748/wjg.v19.i47.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, et al. . Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017; 65: 864–74. doi: 10.1002/hep.28765 [DOI] [PubMed] [Google Scholar]
- 54.Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, et al. . Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017; 65: 875–84. doi: 10.1002/hep.28770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. . An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017; 65: 1196–205. doi: 10.1002/hep.28895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Adamson B, et al. . Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology 2017; 152: 608–15. doi: 10.1053/j.gastro.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]