Abstract
Objective:
To evaluate feasibility, image quality and accuracy of a reduced contrast volume protocol for pre-procedural CT imaging in transcatheter aortic valve implantation (TAVI) using a third generation wide array CT scanner.
Methods:
115 consecutive patients (51F, mean age 82.5 ± 6.2 y, mean BMI 26.7 ± 3.6) referred for TAVI were examined with wide-array CT scanner with a combined scan protocol and a total amount of 50 ml contrast agent. A 4-point visual scale (4-1) was used to assess image quality. Contrast attenuation values (HU) and contrast-to-noise ratio (CNR) were measured at the level of the aortic root, ascending/descending aorta, subrenal aorta and at the level of right and left common femoral arteries. Coronary tree was assessed and compared with invasive coronary angiography (ICA). Aortic annulus measurements were compared with final procedural results. Patients creatinine was monitored at the baseline and 72 h after procedure.
Results:
Median quality score value was >3. Mean CNR at the level of the aortic root, ascending/descending aorta, subrenal aorta and at the level of right and left common femoral arteries were 14.8 ± 2.3, 15.7 ± 1.7, 14.9 ± 3.1, 15.8 ± 4.7, 20.3 ± 9.9, 20.8 ± 6.9 respectively. Only 1 patient had moderate paravalvular regurgitation. In comparison with ICA for coronary assessment CTA showed in a segment based analysis sensitivity, specificity, negative predictive value, positive predictive value and accuracy of 97, 85, 99,62 and 88% respectively. Mean creatinine before CT and 72 h after procedure were 1.21 ± 0.52 and1.22 ± 0.49 mg dl−1. Mean DLP was 442.4 ± 21.2 mGy/cm.
Conclusion:
CT with low contrast volume is feasible and clinically useful, allowing precise pre-procedural TAVI planning with accurate assessment of coronary tree.
Advances in knowledge:
third generation CT scanner with whole heart coverage allows examinations for assessment of aorta and coronary arteries in TAVI planning using low dose of contrast medium maintaining good quality and high diagnostic accuracy.
introduction
Transcatheter aortic valve implantation (TAVI) has been put into practice for the care of patients suffering from severe symptomatic aortic stenosis and represents an accepted and widely validated option for patients considered at high surgical risk.1, 2 CT has consequently become the standard non-invasive imaging method for pre-procedural TAVI assessment for aortic root dimensions and access site.3 However the CT scan protocols require the use of contrast media (CM) and this may result in a potential risk for TAVI candidates who are frequently suffering from impaired renal function and considered to be at increased risk of contrast-induced nephropathy (CIN).4, 5 Thus a reduction of contrast agent volume used for CT planning should be welcome as far as reasonably achievable to reduce the risk of potential renal function worsening. Use of reduced contrast volume protocols for pre-procedural CT imaging for aortic root and peripheral arteries has been demonstrated in recent studies with good results, especially using third-generation scanners with fast wide volume coverage.6–8 However in most of these studies a small population has been enrolled and the study design did not include assessment of coronary tree. An holistic evaluation of both aorta and coronary arteries could lead to a reduction of not necessary invasive coronary angiography (ICA) in patients without coronary arteries disease (CAD) with a consequent reduction of cumulative CM volumes as well as the total cost for patients management.9 Thus aim of this study is to demonstrate feasibility and diagnostic accuracy of a low contrast CT scan protocol using new generation wide array CT scanner for pre-procedural TAVI planning with assessment of aortic root, coronary arteries and peripheral vessels. Moreover patients creatinine was monitored at the baseline and 72 h after procedure to check the impact of this protocol on renal function.
methods and materials
Study population
Between November 2016 and August 2017 we enrolled 115 consecutive inpatients referred to our hospital for CT and ICA before TAVI planning due to severe symptomatic aortic valve stenosis and considered at high surgical risk. In all patients, ICA was performed at least 3 days after CT. Exclusion criteria were previous severe adverse reactions to an iodinated contrast agent (n = 2), body mass index (BMI) ≥38 kg m−2 (due to the need for a different scan protocol) and severely impaired renal function with estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 (n = 3). Written informed consent was obtained from all patients and the study protocol was approved by the Institutional Ethics Committee.
CT scan protocol
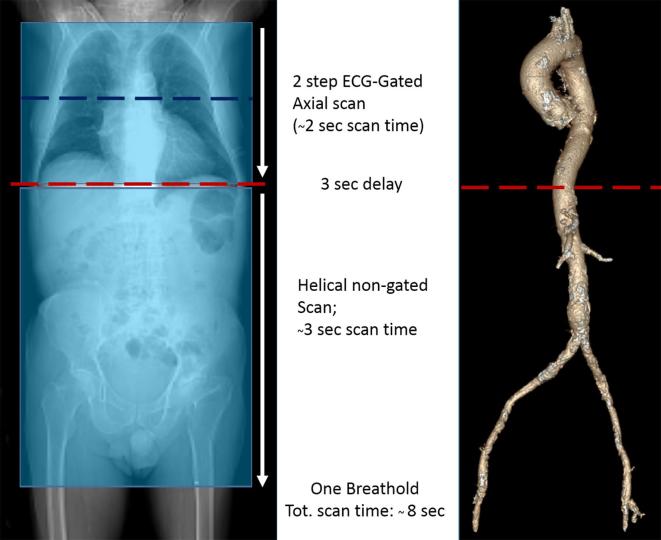
All CT examinations were performed using a 256-slices wide volume coverage CT scanner (Revolution CT; GE Healthcare, Milwaukee, WI). No premedication with beta-blockers or nitrates was added before CT acquisition. CT examinations were performed using the following parameters: peak tube voltage, 100 kV; Detector collimation: 160 mm using 256 rows by 0.625 mm on Z axis. Detector geometry: 256 rows by 832 detection elements per row. High contrast spatial resolution: 0.23 mm. Slice thickness: 0.625; gantry rotation time, 280 ms; prospective triggering; and iterative reconstruction algorithm (ASIR-V; GE Healthcare). A body mass index (BMI)-adapted protocol was used for the tube current with the following parameters: for a BMI ≤ 26 kg m−2, 500 mA; for a BMI of 27–30 kg m−2, 600 mA; for a BMI > 30 kg m−2, 650 mA. Retrospective ECG-gated with wide X-ray window of 500 ms in five distinct diastolic phases was used in all patients to assess the heart and the thoracic aorta with two axial-volumes (8 and 16 cm) from about the aortic arch to 2 cm below the heart base. A subsequent non – gated spiral scan was used for abdomen and pelvis to assess the abdominal aorta and iliac-femoral arteries (up to the proximal third of the thighs) with a delay of about 3 s (the minimum allowed by the scanner) (Figure 1). All patients received a 50 ml bolus of contrast medium (400 mg of iodine per milliliter, Iomeprol; Bracco, Milan, Italy) despite the BMI via an antecubital vein at an infusion rate of 5 ml s−1 followed by 50 ml of saline solution at 5 ml s−1. The bolus tracking technique was used to synchronize the arrival of contrast material at the aortic root with the start of acquisition.
Figure 1.
CT scanning protocol. Wide volume CT scan allows two-beat scan of the entire chest with whole heart coverage in one single volume, minimizing coronary tree and aortic root artefacts. Moreover subsequent fast helical scan of the abdomen (3 s) enables a very short overall scan time without need for prolonged breathold.
Invasive coronary angiography
All patients included in our study underwent ICA within 1 month before the TAVI procedure. ICA procedures were with standard technique by 2 operators with more than 15 years of clinical experience blinded to CT results. The coronary arteries were classified according to the American Heart Association Classification.10 Angiograms were examined using a quantitative coronary angiography software (QantCor, QCA; Pie Medical Imaging, Maastricht, Netherlands). The coronary stenosis was rated as significant in case of lumen diameter reduction >50%.
Renal function
For the evaluation of renal function estimated glomerular filtration rate (eGFR; ml/min/1.73 m2) and serum creatinine (μmol l−1) ≤1 week before the pre-TAVI CT scan were recorded for each patient according to the current hospital protocol. Renal safety of the protocol, in terms of after procedure incidence of CIN (defined as a 25% increase in serum creatinine from baseline or a 0.5 mg dl−1 increase in absolute value) was evaluated by monitoring the renal function of patients 72 h after CTA.11
CT images reconstruction and analysis
Data sets of CT examinations were post-processed on a dedicated workstation (Advantage Workstation VolumeShare 4.6, GE Healthcare) By twoexperienced readers that interpreted all studies for image quality evaluation. For each patient, objective assessment of CT number as indicator for contrast enhancement, expressed as mean Hounsfield Units (HU), and the corresponding standard deviation (SD) as indicator for image noise were measured by regions of interest (ROIs) placed on axial images at four different levels of the aorta (aortic root, ascending aorta, descending aorta, sub renal abdominal aorta) and at the level of the middle third of the right and left common femoral arteries. Additional circular ROIs were placed into the right lobe of the liver (liv) with no vascular structures to calculate the contrast-to-noise ratio (CNR). The CNRs were calculated for each arterial segment as the difference between the vessel enhancement (HU) minus liver tissue enhancement, divided by the vessel SD. Subjective image quality was graded on a 4-point scale: 4, excellent image quality, no artefacts; 3, good image quality; 2, fair; 1, poor image quality, not diagnostic. Detailed measures of the aortic root anatomy focused on aortic annulus (AoA) dimensions (minimum and maximum diameters, perimeter, and area) and the distance from the annulus to the coronary ostia were independently evaluated by two readers in systole in an orthogonal plane on the center line of the aorta as described by previous studies12 and recommended by the SCCT expert consensus document13 (Figure 2).
Figure 2.
MDCT AoAn evaluation: The center axis of left ventricle outflow tract and aortic root were chosen as reference with the 3 orthogonal planes. The longitudinal axis in coronal and sagittal views was aligned (A, B). The transverse plane was aligned at the lowest level of the valve until the hinge points of the aortic leaflets were depictable (C—transverse plane). Aortic annulus was defined as a virtual ring formed by joining the basal attachments of the aortic leaflets. Maximum diameter, minimum diameter, and area were measured at this level (D).
Coronary arteries evaluation
For coronary arteries assessment data sets of coronary CT were reconstructed using a dedicated vessel analysis software (CardIQ3 Package; GE Healthcare) by two radiologists (both with ≥10 years of clinical experience in coronary CT angiography performance and analysis) to evaluate the presence and the degree of coronary stenosis. Coronary arteries were segmented as suggested by the American Heart Association (AHA).14 An image quality score was classified for each native coronary artery segment, stented segment, and coronary artery bypass graft (CABG) using a 4-point Likert image quality score: Score 1 (non-diagnostic: severe artifacts, segment classified as “Poor /Not evaluable”), Score 2 (adequate: overall reduced image quality because of artefacts but sufficient to exclude significant stenosis), Score 3 (good: image impaired by mild artefacts but with fully preserved quality to evaluate stenosis degree), Score 4 (excellent: absence of artefacts). Image noise(defined as the SD of the CT density in a ROI placed in the aortic root immediately cranial to the left main coronary artery), was measured. The SNR was measured by dividing the mean attenuation within the ROI in the lumen of the proximal coronary arteries for the image noise (standard deviation) of the same ROI. The CNR was determined by dividing the difference between the contrast attenuation of the vessel lumen and the surrounding tissue for image noise.15 Transverse reconstructed images and multiplanar vessels reconstruction were used by the two readers to evaluate all the native coronary segments and grafts to rule out the presence of significant stenosis, defined as a narrowing of the vascular lumen more than 50%, by visual estimation. An additional evaluation for the presence of significant stenosis with a lumen narrowing exceeding 70% was performed with the same modality. Moreover, all the stented segments were evaluated for the presence of significant intra stent restenosis (ISR) (defined as a lumen diameter reduction more than 50%) by visual assessment. For any disagreement in CT data analysis between the 2 readers, consensus was achieved.
Tavi procedure outcome
TAVI procedure success was defined accordingly to correct device, annular rupture, evidence of prosthesis instability, and peri-procedural mortality. The CT area derived measurements were compared to the actual implanted size of the valve prosthesis. As proposed by reference guidelines in cases of annuli without calcification, the valve size should be at least ≥1 mm bigger than the annular size. However, in cases of moderate calcification, the valve size should be ≥0.5 mm bigger than annular size, and for severe or diffuse calcification, the chosen valve size could be nearly equal to the measured annular size.16, 17
Radiation dose
The effective dose (ED) of each CT was evaluated according to the European working Group for Guidelines on Quality Criteria in MDCT. The dose-length product (DLP), defined as total radiation energy absorbed by the patient’s body, was measured in mGy × cm for each patient. The ED (mSv) was calculated as the DLP multiplied for a conversion coefficient for the chest (K = 0.014 mSv/[mGy cm]) and for abdomen (0.015 mSv/[mGy cm]). Because of the scan protocol including both thorax and abdomen an average conversion factor (k = 0.015 mSv/[mGy.cm]) was used.18
Statistical analysis
Statistical analysis was performed with the SPSS v. 17.0 software (SPSS Inc, Chicago, IL). Continuous variables were expressed as mean ± SD, and discrete variables were expressed as absolute numbers and percentages. Cohen's kappa with its 95% confidence interval (CI) was used to quantify interobserver reliability for nominal variables. The Spearman correlation and Bland-Altman analysis were used to compare interobserver differences about the evaluation of AoA diameters and area. Student t test was used to evaluate the differences of continuous variables between the baseline and post-TAVI parameters. Diagnostic accuracy sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated on a segment-based model, vessel-based model and on a patient-based model, based on a 50 and 70% threshold in comparison with ICA findings as gold standard. On a patient-based analysis, patients with coronary arteries or Bypass Grafts with ≥1 detected stenosis >50% or>70% or with a stent with ≥1 detected significant ISR were classified as “positive.” The intraobserver and interobserver variability for the assessment of >50% or >70% coronary artery or graft stenoses and for the detection of significant ISR on was tested with Cohen κ. A p value < 0.05 was considered statistically significant.
results
Study population characteristics
The study population characteristics are listed in Table 1. The mean age and BMI were 82.5 ± 6.2 years and 26.7 ± 3.6 (range 18–35) kg m−2 respectively. A prevalence of patients with family history of CAD (73%) and hypertension (71%) was found. All CT were performed without complications. Mean average total scan time was 8 s. Mean heartrate during examinations was 67.7 ± 11.1 (15 patients with atrial fibrillation).
Table 1.
Study population characteristics
| Age (years) | 82.5 ± 6.2 |
| Male/female | 64/51 |
| BMI (kg m−2) | 26.7 ± 3.6 |
| BSA (m2) | 1.79 ± 0.16 |
| Baseline creatinine (mg dl−1) | 1.19 ± 0.43 |
| HR (beats/min) | 67.7 ± 11.1 |
| LVEF (%) | 49 ± 6.6 |
| Aortic valve gradient (max) | 74.6 ± 7.3 |
| Atrial fibrillation | 15/115 |
| Patients with previous coronary stenting, n | 17 |
| Stent number | 49 |
| Drug-eluting stent, n | 44 |
| Bare-metal stent, n | 5 |
| Patients with previous CABG, n | 7 |
| CABG n | 16 |
| Cardiovascular risk factors: | |
| Hypertension, n | 82 |
| Hypercholesterolemia, n | 79 |
| Diabetes mellitus, n | 21 |
| Current smoking, | 8 |
| Family history of CAD, n | 84 |
| Implanted valve (n) | 115 |
BMI, body mass index; BSA, body surface area; CABG, coronary arteries by pass grafting mark; HR, heart rate; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention.
Procedural outcome and aortic image quality
Procedural success was 100% without intraoperative mortality. A moderate paravalvular regurgitation occurred in 1 patient (1%) 2 days after valve implantation. Implanted prosthetic valves are listed in Table 2. No exams were classified as non-diagnostic and no additional contrast medium administration was needed. The overall mean attenuation values for each aortic segment and for the femoral arteries were above 500HU with a median Likert quality score of 3.5 for the aortic root and 3 for the other vascular segments (Table 3).
Table 2.
Implanted prosthesis
| Implanted prosthesis | Number |
| ACURATE Neo™ Symetis | |
| S 23 | 1 |
| M25 | 2 |
| CoreValve® | |
| 31 | 1 |
| CoreValve® Evolut™ R | |
| 23 | 3 |
| 26 | 6 |
| 29 | 3 |
| Edwards SAPIEN™ 3 | |
| 23 | 33 |
| 26 | 43 |
| 29 | 12 |
| Edwards SAPIEN™ XT | |
| 26 | 2 |
| Portico™ | |
| 23 | 2 |
| 25 | 3 |
| 27 | 2 |
| 29 | 1 |
Table 3.
Overall mean quantitative parameters for aorta and peripheral accesses
| Vessel segment | Mean HU | Mean noise | Mean CNR | Image quality score (median value) |
| Aortic root | 576.6 ± 54.5 | 29.1 ± 6.1 | 15.8 ± 2.3 | 3.5 |
| Ascending aorta | 580.2 ± 77.4 | 33.2 ± 6.2 | 15.7 ± 1.7 | 3 |
| Descending aorta | 552.1 ± 88.4 | 30.4 ± 5.8 | 14.9 ± 3.1 | 3 |
| Subrenal aorta | 525.2 ± 77.6 | 30.6 ± 4.5 | 15.8 ± 4.7 | 3 |
| R common femoral artery | 509.7 ± 78.4 | 23.7 ± 7.5 | 20.4 ± 8.9 | 3 |
| L common femoral artery | 502.3 ± 81.09 | 22.8 ± 5.9 | 20.6 ± 6.9 | 3 |
CNR, contrast-to noise-ratio; HU Hounsfield unit; L, left; R, right; SD, standard deviation.
Data are expressed as mean ± SD.
Interobserver variability, correlation and reliability
The k value for evaluation of subjective image quality was 0.95 for interobserver agreement. Very good correlation was found between CT derived area and implanted prosthesis size with direct correlation in 108/115 patients (94%) In 2/115 patients (2%) CT measurements were oversized while in 5/115 patients (4%) an undersized measure was found. A very good measurements reproducibility for aortic annulus area and diameters was found with minimal intraobserver and interobserver variability (k = 0.95 and 0.94 respectively). The related Pearson correlation and Bland- Altman analysis are shown in Figure 3.
Figure 3.
Measurements reproducibility: Pearson correlation (upper panels) and Bland-Altman analysis (lower panels) between Observer 1 and 2 for assessment of AoA maximum diameter, minimum diameter, and area.
Cardiac ct evaluability and image quality
Coronary arteries image quality evaluation showed mean attenuation values and mean CNR values for LM, LAD, LCX and RCA of 549.7 ± 16.9, 515.3 ± 39.7, 542.0 ± 57.5 and 546.7 ± 48.7 respectively and 17.6 ± 3.3, 15.5 ± 5.7, 13.9 ± 3.6 and 12.8 ± 3.4 respectively. (Table 4a). 1644 coronary segments on a total of 1725 (96%) were considered as evaluable. In 81/1725 (4%) segments the image quality score was poor/not diagnostic because of large calcifications (63 segments) and motion artifacts due to incomplete breath holding/movements (18 segments). No vessels were scored as not evaluable due to impaired signal/image noise ratio. The evaluability of coronary stents was 80% (39/49) with 10 stent segments scored as not evaluable due to significant beam-hardening artifacts. 100% of CABG was considered as evaluable (7 left internal mammary arteries, 9 vein grafts) (Table 4b).
Table 4.
(a) CT attenuation values, image noise, signal-to-noise ratio and contrast to- noise ratio of coronary arteries (b) Coronary arteries and bypass graft image quality score
| (a) Vessel | CT attenuation (HU) | Noise (SD) | SNR | CNR |
| LM | 549.7 ± 16.9 | 28.3 ± 5.5 | 19.8 ± 3.6 | 17.6 ± 3.3 |
| LAD | 515.3 ± 39.7 | 32.0 ± 13.2 | 17.7 ± 6.7 | 15.5 ± 5.7 |
| LCX | 542.0 ± 57.5 | 34.7 ± 7.6 | 15.8 ± 2.1 | 13.9 ± 3.6 |
| RCA | 546.7 ± 48.7 | 38.3 ± 8.5 | 14.5 ± 3.2 | 12.8 ± 3.4 |
| (b) | n | Excellent n (%) |
Good n (%) |
Adequate n (%) |
Poor n (%) |
| Native coronary segments | 1725 | 412 (23) | 1154 (67) | 78 (6) | 81 (4) |
| Stented segments | 49 | 7 (14) | 23 (46) | 10 (20) | 10 (20) |
| CABG | 16 | 12 (75) | 3 (19) | 1 (6) | 0 |
CABG, coronary artery bypass graft.
(b) Data are expressed as mean ± SD. CNR contrast-to noise-ratio; HU, Hounsfield unit; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LM, left main coronary artery; RCA, right coronary artery; SD, standard deviation; SNR, signal-to-noise ratio.
Cardiac ct diagnostic accuracy
Table 5a shows the diagnostic accuracy of pre-TAVI CT for assessment of coronary arteries stenosis more than 50% in comparison with ICA Including only evaluable segments CT showed sensitivity, specificity, negative predictive value, positive predictive value and accuracy of 97, 90, 98, 71 and 91% respectively. When all segments where included in the analysis, CT showed sensitivity, specificity, negative predictive value, positive predictive value and accuracy of 97, 85, 99,62 and 88% respectively. Sensitivity, specificity, negative predictive value, positive predictive value and accuracy of CT including all vessels in a vessel-based and patient based analysis were instead 95, 88, 98, 68 and 89% and 95, 86, 98, 64 and 88% respectively (Table 5b). Lower sensitivity, specificity, negative predictive value, positive predictive value and accuracy were found for stents patency assessment in a segment based analysis (including all stent segments) with 91, 75, 96, 55 and 79% respectively. No CABG were scored as not diagnostic with an accuracy of 100% (Table 6). Diagnostic accuracy of CT in comparison with ICA as standard reference for the detection of coronary arteries stenosis >70% including all segments is showed in Table 7. In a vessel-based and patient based analysis sensitivity, specificity, negative predictive value, positive predictive value and accuracy were 85, 91, 97, 55, 90% and 88, 91, 97, 66 and 91% respectively. The k values for for intraobserver agreement in detection of coronary artery stenosis >50% and ISR with CT were 0.9 and 0.88 while for interobserver agreement 0.85 and 0.85, respectively. Concerning the detection of coronary arteries stenosis >70% the k value was 0.87 and 0.86 for intraobserver agreement and 0.83 and 0.82 for interobserver agreement, respectively. The k value for CT assessment of significant CABG stenosis resulted in 1 for both intra and –inter observer agreements. CT imaging findings of native coronary arteries and bypass grafts in comparison with ICA in TAVI candidates are shown in Figures 4 and 5.
Table 5.
(a) Diagnostic accuracy of pre-TAVI CT vs invasive coronary angiography using a cutoff value >50% of coronary artery stenoses (b) diagnostic accuracy of pre-TAVI CT vs invasive coronary angiography using a cutoff value >50% of coronary artery stenoses (including all vessels)
| (a) Segment-based analysis (excluding not evaluable segments) | |||||||||
| No. of segments | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 1644 | 1194 | 315 | 9 | 126 | 97 (96–98) |
90 (87–92) |
99 (98–99) |
71 (67–75) | 91 (86–93) |
| Segment-based analysis (with not-evaluable segments censored as positive findings) | |||||||||
| No. of vessels | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 1725 | 1194 | 327 | 9 | 195 | 97 (96–98) |
85 (84–87) |
99 (98–99) |
62 (58–66) | 88 (86–89) |
| (b) Vessel-based analysis | |||||||||
| No. of vessels | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 345 | 242 | 68 | 3 | 32 | 95 (93–98) |
88 (84–92) |
98 (97–100) |
68 (58–77) | 89 (86–92) |
| Patient-based analysis | |||||||||
| No. of patients | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 115 | 80 | 22 | 1 | 12 | 95 (91–97) |
86 (80–93) |
98 (96–100) |
64 (48–80) | 88 (82–94) |
FN, false-negative; FP, false-positive; NPV, negative predictive value; PPV, positive predictive value; TN, true-negative; TP, true-positive.
In parentheses data are 95% confidence interval.
Table 6.
Diagnostic accuracy of pre-TAVI CT vs invasive coronary angiography in patients with previous revascularization for assessment of in-stent restenosis (ISR) ≥50% and bypass grafts patency
| Stents: Segment-based Analysis (with not evaluable stents censored as positive) | |||||||||
| No. of stents | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 49 | 28 | 11 | 1 | 9 | 91 (82–100) |
75 (61–89) | 96 (89–100) |
55 (33–76) |
79 (68–90) |
| CABG: Vessel-based analysis (with occluded grafts censored as positive) | |||||||||
| No. of CABG | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 16 | 12 | 4 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
CABG, Coronary Artery Bypass Graft; FN, false-negative; FP, false-positive; NPV, negative predictive value; PPV, positive predictive value; TN, true-negative; TP, true positive.
Table 7.
Diagnostic accuracy of pre-TAVI CT vs invasive coronary angiography using a cutoff value >70% of coronary artery stenoses (including all vessels)
| Vessel-based analysis | |||||||||
| No. of vessels | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 345 | 278 | 27 | 6 | 27 | 85 (81–94) | 91 (87–94) | 97 (96–99) | 55 (43–68) | 90 (87–93) |
| Patient-based analysis | |||||||||
| No. of patients | No of TN findings | No of TP findings | No of FN findings | No of FP findings | Sensitivity | Specificity | NPV | PPV | Accuracy |
| 115 | 89 | 16 | 21 | 8 | 88 (82–88) | 91 (86–97) | 97 (94–100) | 66 (47–85) | 91 (86–96) |
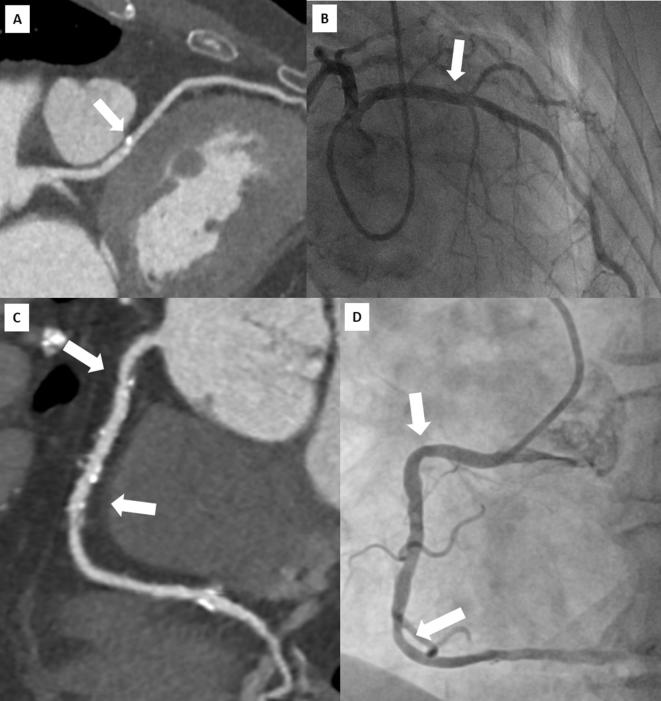
Figure 4.
Head-to-head comparison of CT multiplanar reconstruction (A) and ICA (B) showing a calcified plaque of proximal LAD (arrow) correctly ruled out as not significant at CT examination. Lower panels show a multiplanar reconstruction of a RCA with widespread atheromatous lesions (arrows) defined as “positive” by CT (C) but not confirmed during ICA (D).
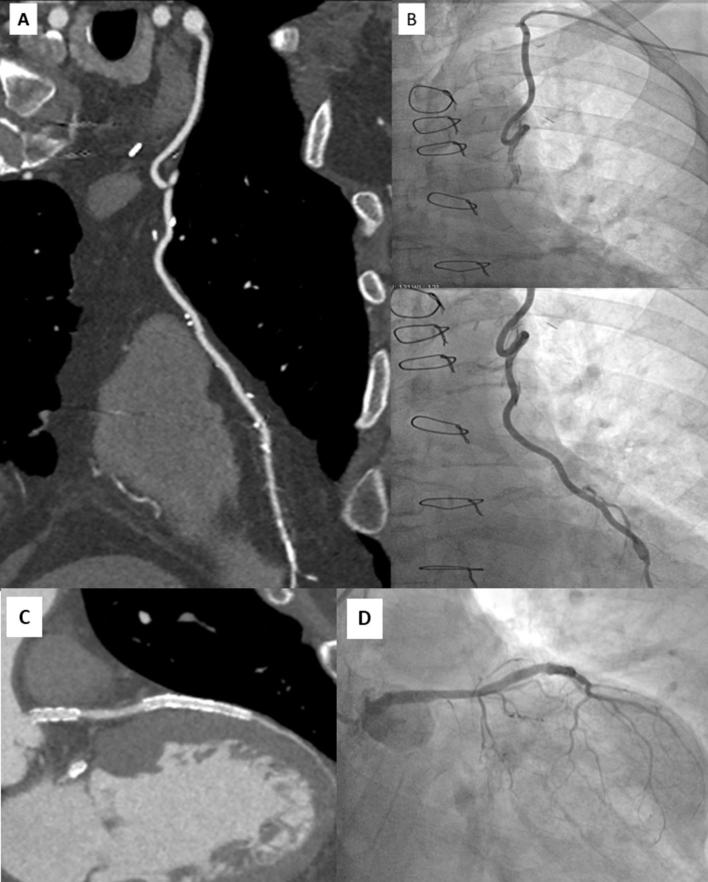
Figure 5.
Head-to-head comparison of CT multiplanar reconstruction showing patency of a bypass graft with LIMA anatomosed to LAD (A) confirmed by ICA (B). (C) Multiplanar CT reconstruction showing the patency of 2 stents implanted in the LM and LAD confirmed at ICA (D). LIMA, left internal mammary artery; LAD, left anterior descending coronary artery; LM, left main; RCA, right coronary artery.
Renal function
No patients presented CIN during the follow-up period. Renal function monitoring results are listed in Table 8. Mean creatinine and eGFR at baseline and after 72 h were 1.19 ± 0.4 mg dl−1, 42.09 ± 4.2 ml/min and 1.2 ± 0.42 mg dl−1, 41.8 ± 3.8 ml/min respectively, with a mean difference of 0.017 ± 0.28 mg dl−1.
Table 8.
Renal function monitoring
| Baseline | 72 h follow-up | Mean difference | p value | |
| Creatinine (mg/dl) | 1.19 ± 0.43 | 1.20 ± 0.42 | 0.01 ± 0.28 | 0.9 |
| eGFR (ml/min) | 42.09 ± 4.2 | 41.8 ± 3.8 | 0.15 ± 1.1 | 0.7 |
Data are expressed as mean ± SD; dL, deciliter; eGFR, estimated Glomerular Filtration Rate; mg, milligrams; min, minutes ml, milliliter.
Radiation dose
Mean CTDIVol and DLP for the whole evaluation of thoracoabdominal aorta and coronary arteries were 36.9 ± 15.1 mGy and 479.1 ± 45.7 mGy/cm respectively with a mean cumulative estimated effective dose of 6.5 ± 2.6 mSv (assuming an average conversion factor k = 0.015 mSv/[mGy.cm]) (Table 9).
Table 9.
Radiation dose
| CTDIVol (mGy) |
DLP (mGy/cm) |
mSv | |
| Mean Dose | 36.9 ± 15.1 | 479.1 ± 45.7 | 6.5 ± 2.6 |
Data are expressed as mean ± SD. CTDIVol, CT dose index Volume, DLP, dose-length product; mGy/cm, MilliGray/centimeter, mSv, MilliSievert.
discussion
Our study demonstrates that a low contrast scan-protocol for CT angiography using third generation CT scanner is safe, feasible and provides accurate informations for pre-procedural TAVI planning determining patient eligibility, access strategy, and prosthesis selection with very good image quality of both aorta and coronary arteries. The use of 50 ml of contrast agent allowed an overall mean attenuation value >500 HU of aortic segments, peripheral accesses and coronary arteries with high reproducibility of aortic annulus measurements and very good reliability for prosthesis sizing (100% procedural success rate). Previous studies with smaller patient population have been published about feasibility of low contrast scan protocol for CT angiography prior to TAVI procedure6, 7,19 with lower amounts of CM in comparison with our study (range 38–40 ml) with evidence however of overall lower CT attenuation values in all aorto-iliac segments. Moreover in these studies no contemporary evaluation of coronary arteries was assessed. Pre-procedural assessment of CAD and patency of CABG or coronary stents is of crucial importance to stratify patient risk and to determine whether myocardial revascularization is indicated before TAVI and previous studies have demonstrated the high CT accuracy to rule out CAD in patients referred to heart valve surgery.20 Furthermore the importance of reducing the volume of CT contrast agents and consequently the risk of contrast-induced nephropathy has been previously reported. Several studies have proved a strong correlation between renal disease and poor outcomes in patients with TAVI showing a graded inverse relationship between creatinine clearance and mortality.21, 22 Although the topic related to the risk of CIN could appear controversial23 an optimized scan protocol to reduce any potential risk before TAVI procedure seems to be mandatory. In this scenario in spite of a total amount of 50 ml of high concentration CM, none of our 115 patients had significant changes in creatinine clearance nor eGFR after CT procedure. Thus, another way to reduce the risk of CIN in TAVI candidates could be to reduce the amount of contrast agents related to unnecessary ICA. To the best of our knowledge this is one of the largest studies to assess the performance of a reduced contrast protocol in pre-TAVI planning including coronary arteries assessment using a third generation CT scanner with wide volume coverage. It has been demonstrated that low coronary attenuation values can lead to stenosis overestimation24 and that coronary CT diagnostic performance may be limited in the elderly TAVI patients because of the age-related high atherosclerotic burden. In particular, in a large study by Rossi et al a lack of CT performance has been demonstrated in patients with heavy calcification suggesting specific contrast saving protocols in this specific population avoiding coronary arteries assessment25 Moreover the high HeartRate frequently observed in patients with severe aortic stenosis or the high prevalence of atrial fibrillation may lead to a significant impairment in CT evaluability and specificity in the assessment of native coronary arteries.26 By using a third generation CT scanner with wide scan coverage along the patient Z-axis and 16 cm detector coverage in our study we were able to perform a scan of the whole heart volume during one heartbeat (0.8 s scan time) reducing the potential artefacts related to HR or movement, even in case of patients with AF (n = 15). Moreover the same technology allowed to perform the examinations with a contrast injection flow rate of 5 ml s−1 according to the SCCT recommendations for CT angiography prior to TAVI27 maintaining an optimal vessel attenuation for both coronary tree and thoraco-abdominal aorta. CT showed high evaluability and diagnostic accuracy for the assessment of native coronary arteries and CABGs, with excellent NPV and overall accuracy. Concerning native coronary segments CT showed an overall evaluability of 96% with only 81/1725 segments scored as not evaluable. In a vessel based analysis including all segments sensitivity, specificity, negative predictive value, positive predictive value and accuracy for stenosis >50% assessment were 95, 88, 98, 68 and 89%. In a patient based analysis NPV and overall accuracy resulted in 98 and 88% respectively. Even in detection of stenosis of more than 70% in a vessel based analysis and patient-based analysis CT showed negative predictive value and accuracy of 97, 90 and 97, 91% respectively, showing a good CT capability to rule out stenosis that should require ICA before TAVI procedure. These findings appear consistent in comparison with previous data regarding non-TAVI patients of a recent published trial.28 Moreover our findings appear consistent with data regarding pre-TAVI patients of a recent study performed with a not significant lower dose of CM (total amount of 19.2 Iodine grams in comparison with a total amount of 20 Iodine grams of our protocol, resulting in a difference of <1 Iodine gram) but in presence of an overall lower specificity and lower PPV in a vessel based model in comparison with our results (68 and 67% respectively).29 As cited in the same study, our results regarding specificity should be related to the use of a last generation iterative reconstruction algorithm (ASIR-V) which was shown to improve the specificity of cardiac CT in calcified vessels.30 Another recent study conducted with a total CM amount of 38.7 ± 8.5 ml of 350 or 370 mgI/ml CM31 demonstrated consistent NPV but lower sensitivity, specificity and accuracy. This could be related to the performance of new generation scanners and particularly to the above described whole heart coverage, that leads to significant reduction in image quality impairment and artefacts related to HR changes and AF as recently demonstrated in a study by our group.32 The CT evaluation of coronary stents demonstrated high NPV (96%) but lower PPV (55%) and accuracy (79%) with an overall evaluability of 80%. Although in presence of a small group of 17 patients with stent in our population these findings appear consistent with CT accuracy for stent patency assessed by large populations studies.33 CT high diagnostic accuracy for assessment of the patency of coronary bypass grafts has been well demonstrated in TAVI patients,34 and this was also confirmed in our study population with overall evaluability of 100 and 100% accuracy. As above stated, pre-procedural assessment of CAD in patient risk stratification to determine myocardial revascularization indication is highly indicated before valve surgery.20 For this reason, in most cases patients scheduled for TAVI procedure routinely undergo a CT examination for pre-procedural planning and ICA to rule out CAD. Given the high sensitivity, NPV and accuracy of CT imaging for the detection of >50% stenosis of native coronaries in our study, the high NPV for the detection of >70% stenosis and given the higher specificity of our data in comparison with previous similar studies28, 29,31 our results suggest that invasive angiographic examinations could be avoided in case of not significant CAD showed during CT examination. Concerning the clinical value of this study, because the high sensitivity and negative predictive value showed by CT, in our population ICA could have been excluded from the pre-procedural diagnostic work-up in those patients with evidence of CAD with less than 50%, consequently reducing further radiation, contrast media administration, hospitalization costs (in our institution about 52 ml of CM and 1200 € for a diagnostic ICA) as well as the potential risks related to invasive procedures. Finally although radiation dose is not the topic of utmost importance in elderly patients undergoing TAVI, this scan protocol resulted in an average total effective dose to the patient of about 6 mSv only.
Limitations
Some limitations of this study must be acknowledged. Only inpatients TAVI candidates with ICA scheduled at least 3 days after CT were included in our study and this could result in an inclusion bias. Second patients with severe renal failure were excluded from the study. Third renal function monitoring has been limited to the first 72 h after CT procedure without long term follow up. Furthermore no cumulative evaluation after ICA procedures has been performed. Fourth our data concerning CT diagnostic performance in vessels with stents and CABG is limited to a small number of patients and this could influence our accuracy results (even if consistent with previous largest trials). Similarly our study population has lower incidence of atrial fibrillation (13%) in comparison with previous studies and this also could influence our study results.
CONCLUSIONS
The use of a low contrast scan-protocol for CT angiography in pre-procedural TAVI planning using third generation CT scanner is safe, feasible and provides accurate informations with very good image quality of both aorta and coronary tree. Moreover the whole-heart coverage scan provides accurate coronary artery assessment with a high negative predictive value and overall accuracy. These findings support the use of CT in the diagnostic workup of patients scheduled for TAVI procedures helping to avoid unnecessary invasive coronary angiographies.
Contributor Information
Andrea D Annoni, Email: andrea.annoni@ccfm.it.
Daniele Andreini, Email: daniele.andreini@ccfm.it.
Gianluca Pontone, Email: gianluca.pontone@ccfm.it.
Maria Elisabetta Mancini, Email: melisabetta.mancini@gmail.com.
Alberto Formenti, Email: alberto.formenti@ccfm.it.
Saima Mushtaq, Email: saima.mushtaq@ccfm.it.
Andrea Baggiano, Email: andrea.baggiano@gmail.com.
Edoardo Conte, Email: edoardo.conte86@gmail.com.
Marco Guglielmo, Email: marco.guglielmo@ccfm.it.
Giuseppe Muscogiuri, Email: g.muscogiuri@gmail.com.
Manuela Muratori, Email: manuela.muratori@ccfm.it.
Laura Fusini, Email: laura.fusini@ccfm.it.
Daniela Trabattoni, Email: daniela.trabattoni@ccfm.it.
Giovanni Teruzzi, Email: giovanni.teruzzi@ccfm.it.
Ana I Coutinho Santos, Email: a.icoutinho.santos@gmail.com.
Marco Agrifoglio, Email: marco.agrifoglio@ccfm.it.
Mauro Pepi, Email: mauro.pepi@ccfm.it.
REFERENCES
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597–607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790–8. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 3.Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012; 6: 366–80. doi: 10.1016/j.jcct.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Hayashida K, Mouillet G, Chevalier B, Meguro K, Watanabe Y, et al. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv 2013; 6: 479–86. doi: 10.1016/j.jcin.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Becker CR, Davidson C, Lameire N, McCullough PA, Stacul F, Tumlin J, et al. High-risk situations and procedures. Am J Cardiol 2006; 98: 37–41. doi: 10.1016/j.amjcard.2006.01.025 [DOI] [PubMed] [Google Scholar]
- 6.Kok M, Turek J, Mihl C, Reinartz SD, Gohmann RF, Nijssen EC, et al. Low contrast media volume in pre-TAVI CT examinations. Eur Radiol 2016; 26: 2426–35. doi: 10.1007/s00330-015-4080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittner DO, Arnold M, Klinghammer L, Schuhbaeck A, Hell MM, Muschiol G, et al. Contrast volume reduction using third generation dual source computed tomography for the evaluation of patients prior to transcatheter aortic valve implantation. Eur Radiol 2016; 26: 4497–504. Epub 2016 Mar 19. doi: 10.1007/s00330-016-4320-8 [DOI] [PubMed] [Google Scholar]
- 8.Dankerl P, Hammon M, Seuss H, Tröbs M, Schuhbaeck A, Hell MM, et al. Computer-aided evaluation of low-dose and low-contrast agent third-generation dual-source CT angiography prior to transcatheter aortic valve implantation (TAVI). Int J Comput Assist Radiol Surg 2017; 12: 795–802. Epub 2016 Sep 7. doi: 10.1007/s11548-016-1470-8 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds MR, Magnuson EA, Lei Y, Wang K, Vilain K, Li H, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A). J Am Coll Cardiol 2012; 60: 2683–92. doi: 10.1016/j.jacc.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 10.Naidu SS, Rao SV, Blankenship J, Cavendish JJ, Farah T, Moussa I, et al. Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv 2012; 80: 456–64. doi: 10.1002/ccd.24311 [DOI] [PubMed] [Google Scholar]
- 11.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK. Contrast media safety committee of European society of urogenital radiology (ESUR). Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol 2011; 21: 2527–41. [DOI] [PubMed] [Google Scholar]
- 12.Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011; 4: 416–29. doi: 10.1016/j.jcmg.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 13.Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012; 6: 366–80. doi: 10.1016/j.jcct.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation 1975; 51(4 Suppl): 5–40. doi: 10.1161/01.CIR.51.4.5 [DOI] [PubMed] [Google Scholar]
- 15.Pflederer T, Rudofsky L, Ropers D, Bachmann S, Marwan M, Daniel WG, et al. Image quality in a low radiation exposure protocol for retrospectively ECG-gated coronary CT angiography. AJR Am J Roentgenol 2009; 192: 1045–50. doi: 10.2214/AJR.08.1025 [DOI] [PubMed] [Google Scholar]
- 16.Kasel AM, Cassese S, Bleiziffer S, Amaki M, Hahn RT, Kastrati A, et al. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging 2013; 6: 249–62Review. doi: 10.1016/j.jcmg.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield GS, Gillam LD, Hahn RT, Kapadia S, Leipsic J, Lerakis S, al HRTet, et al. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2012; 5: 441–55. doi: 10.1016/j.jcmg.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 18.Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010; 257: 158–66. doi: 10.1148/radiol.10100047 [DOI] [PubMed] [Google Scholar]
- 19.Spagnolo P, Giglio M, Di Marco D, Latib A, Besana F, Chieffo A, et al. Feasibility of ultra-low contrast 64-slice computed tomography angiography before transcatheter aortic valve implantation: a real-world experience. Eur Heart J Cardiovasc Imaging 2016; 17: 24–33. doi: 10.1093/ehjci/jev175 [DOI] [PubMed] [Google Scholar]
- 20.Meijboom WB, Mollet NR, Van Mieghem CA, Kluin J, Weustink AC, Pugliese F, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol 2006; 48: 1658–65. doi: 10.1016/j.jacc.2006.06.054 [DOI] [PubMed] [Google Scholar]
- 21.D'Ascenzo F, Moretti C, Salizzoni S, Bollati M, D'Amico M, Ballocca F, et al. 30 days and midterm outcomes of patients undergoing percutaneous replacement of aortic valve according to their renal function: a multicenter study. Int J Cardiol 2013; 167: 1514–8. doi: 10.1016/j.ijcard.2012.04.161 [DOI] [PubMed] [Google Scholar]
- 22.Ferro CJ, Chue CD, de Belder MA, Moat N, Wendler O, Trivedi U, et al. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): an analysis of the UK TAVI registry. Heart 2015; 101: 546–52. doi: 10.1136/heartjnl-2014-307041 [DOI] [PubMed] [Google Scholar]
- 23.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 2013; 267: 106–18. doi: 10.1148/radiol.12121823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cademartiri F, Maffei E, Palumbo AA, Malagò R, La Grutta L, Meiijboom WB, et al. Influence of intra-coronary enhancement on diagnostic accuracy with 64-slice CT coronary angiography. Eur Radiol 2008; 18: 576–83. doi: 10.1007/s00330-007-0773-0 [DOI] [PubMed] [Google Scholar]
- 25.Rossi A, De Cecco CN, Kennon SRO, Zou L, Meinel FG, Toscano W, et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr 2017; 11: 338–46. doi: 10.1016/j.jcct.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 26.Pontone G, Andreini D, Quaglia C, Ballerini G, Nobili E, Pepi M, et al. Accuracy of multidetector spiral computed tomography in detecting significant coronary stenosis in patient populations with differing pre-test probabilities of disease. Clin Radiol 2007; 62: 978–85. doi: 10.1016/j.crad.2007.02.022 [DOI] [PubMed] [Google Scholar]
- 27.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr 2009; 3: 190–204. doi: 10.1016/j.jcct.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Liga R, Vontobel J, Rovai D, Marinelli M, Caselli C, Pietila M, al- et-, et al. Multicentre multi-device hybrid imaging study of coronary artery disease: results from the evaluation of integrated cardiac imaging for the detection and characterization of ischaemic heart disease (EVINCI) hybrid imaging population. Eur Heart J Cardiovasc Imaging 2016; 17: 951–60. doi: 10.1093/ehjci/jew038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris BS, De Cecco CN, Schoepf UJ, Steinberg DH, Bayer RR, Krazinski AW, et al. Dual-source CT imaging to plan transcatheter aortic valve replacement: accuracy for diagnosis of obstructive coronary artery disease. Radiology 2015; 275: 80–8. doi: 10.1148/radiol.14140763 [DOI] [PubMed] [Google Scholar]
- 30.Renker M, Nance JW, Schoepf UJ, O'Brien TX, Zwerner PL, Meyer M, et al. Evaluation of heavily calcified vessels with coronary CT angiography: comparison of iterative and filtered back projection image reconstruction. Radiology 2011; 260: 390–9. doi: 10.1148/radiol.11103574 [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto S, Yamada Y, Hashimoto M, Okamura T, Yamada M, Yashima F, et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol 2017; 27: 1963–70. doi: 10.1007/s00330-016-4547-4 [DOI] [PubMed] [Google Scholar]
- 32.Andreini D, Pontone G, Mushtaq S, Mancini ME, Conte E, Guglielmo M, et al. Image quality and radiation dose of coronary CT angiography performed with whole-heart coverage CT scanner with intra-cycle motion correction algorithm in patients with atrial fibrillation. Eur Radiol 2018; 28: 1383-1392. doi: 10.1007/s00330-017-5131-2 [DOI] [PubMed] [Google Scholar]
- 33.Rief M, Zimmermann E, Stenzel F, Martus P, Stangl K, Greupner J. Computed tomography angiography and myocardial computed tomography perfusion in patients with coronary stents: prospective intraindividual comparison with conventional coronary angiography. J Am Coll Cardiol 2014; 62: 1476–85. [DOI] [PubMed] [Google Scholar]
- 34.Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Ballerini G, Bertella E, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J 2014; 168: 332–9. doi: 10.1016/j.ahj.2014.04.022 [DOI] [PubMed] [Google Scholar]