Abstract
The UK screening programme began in 2009, and has now been expanded around the UK. Long-term follow-up of the original cohorts continues to demonstrate significant benefits for abdominal aortic aneurysm (AAA)-related and all-cause mortality, and results from the first 5 years of the formal screening programme have demonstrated similar success. Ultrasound scanning is an effective and safe screening tool for the detection of AAA, although a variety of measurement protocols are employed internationally. Key challenges for the future of the programme relate to declining incidence of screen detected aneurysms. Recent publications have demonstrated a UK incidence of only 1.34%, compared to 4.9–7.2% of men invited for screening in the original trials. Work into increasing engagement amongst the target group, and expanding screening to siblings and women is underway to address this issue. This review describes the evidence behind the screening programme, its justification in addressing AAA as a significant health problem and discusses some of the potential developments in the future.
Introduction to the UK national screening programme
First introduced in selected UK regions in 2009, the national abdominal aortic aneurysm screening programme (NAAASP) is now offered to all men aged 65 years, registered with a general practitioner.1 The original UK-based multicentre aneurysm screening study (MASS) demonstrated the effectiveness of one-time ultrasound screening in men aged 65–74 years, with an associated relative risk reduction on abdominal aortic aneurysm (AAA)-related mortality at 7 and 10 years.2, 3 A 2007 Cochrane review collating results from the four UK, Australian and Danish randomised controlled studies confirmed the benefits of a screening programme in men.4
Background and Evidence
The evidence supporting AAA screening in men originates from four randomised controlled trials performed between 1991 and 2004.
The first randomised controlled trial, the Chichester study, identified 15,775 men and women registered with a general practitioner from a single area (Chichester, UK), randomising them to aortic screening or standard care from 1988–1991.2,5,6 Aortas between 3–4.4 cm were re-imaged annually, and those 4.5–5.9 cm every 3 months. Criteria for surgery included AAA ≥6 cm, symptomatic AAA (pain) and growth of ≥1 cm year–1. The MASS trial3 randomised 67,770 men aged 65–74 from Oxford, Portsmouth, Winchester and Southampton (UK) between 1997–1999. Threshold for surgery was lower (5.5 cm).7 The Western Australia study invited 41,000 men aged 65 to 79 years for screening, identified from the electoral roll (Perth).8 Unlike the UK trials, ongoing management was at the discretion of patients’ general practitioners.
The Viborg trial recruited 12,628 men (aged 65–73 years) between 1994 and 1998 using residential records for a single county (Viborg, Denmark).9 Patients with an aortic measurement of ≥3 cm were offered annual screening, and those ≥5 cm were offered surgical repair. Mean follow-up was 5.1 years, and like the MASS study, data for both screening and subsequent intervention was gathered to estimate cost- effectiveness.10, 11
All four trials analysed results on an intention-to-treat basis; however, the Viborg trial only gathered data for in hospital deaths. A recent pooled analyses confirmed significant reductions in AAA-related mortality and incidence of rupture.12 This did not only relate to events around the time of screening; benefits were seen up to 15-year follow-up, with a reduction in AAA-related mortality and rupture rates
Based on extrapolation of results from these first screening trials, a detection rate of at least 4.5% in men was anticipated.13 However published data from the first 5-year results of the NAAASP found a fall in UK incidence to 1.34%,14 thought to reflect reduced rates of smoking and improved medical therapy.
AAA as an important health problem
AAA is defined as a full thickness dilatation of the abdominal aortic diameter of ≥×1.5, measured in the anteroposterior plane. In men, this is taken to mean 3 cm or greater. Around 85% of aortic aneurysms occur within the infra-renal segment of the abdominal aorta.15 The most common risk factors for AAA include smoking, hypertension, hypercholesterolemia, increasing age and family history, in common with other cardiovascular disease. Other risks, such as connective tissue disorders are much less common, and associated with AAA in younger patients.16
Risk of AAA begins to rise around the age of 50 in men in whom it is significantly more common (ratio of approximately 4:1), and later in women. AAA is usually asymptomatic until it ruptures, although pain in the abdomen or lower back can represent a rapidly enlarging or mycotic aneurysm, which should be considered for emergency repair. Aneurysm-related and all-cause mortality following ruptured AAA remains high (up to 80%),17 a combination of pre-hospital death and failure to survive to discharge. Recent published analysis calculated a pooled risk of rupture of 3.5% for AAA 5.5–6 cm, 4.1% for 6.1–7 cm and 6.3% for AAA ≥7 cm,18 with risk accumulating over time. This has decreased over time; previously AAA ≥6 cm carried a rupture risk of 14.1% in men and 22.3% in women,suggesting changes in patient behaviour could contribute to a reduction in AAA-related mortality.19 The average risk of rupture in women with AAA of between 5–5.9 cm is up to four times as high as in men, hence ongoing debate and suggestions that repair should be considered once diameter reaches 5 cm in women.
Although the screening programme in the UK is active, many men are still referred with an incidental finding of AAA following investigations for pathology such as prostate cancer. The National Vascular Registry (the vascular services quality improvement programme that collates and publishes outcomes for all major vascular procedures) suggests over half of patients are referred to a vascular surgeon over the age of 65 via channels other than the screening programme.20
Suitable screening test
Ultrasound imaging can reliably visualise the aorta in 99% of individuals,21 and has been validated against reconstructed three-dimensional CT imaging of the aorta with the benefits of being non-invasive, non-ionising and not requiring nephrotoxic contrast use.22, 23 Ultrasound was utilised in all of the major AAA screening trials, and supported the significant body of literature concluding that AAA screening using ultrasound was time-efficient, inexpensive and accurate.21,24–29
The method at which the aorta is measured is still under considerable debate. The three most widely recognised techniques for measuring the aorta with ultrasound are inner-to-inner (ITI), outer-to-outer (OTO) and leading edge-to-leading edge (LELE).30 The UK NAAASP employs the ITI method used in the MASS trial. Hartshorne et al showed ITI gave less inter and intra observer variability compared to OTO when performed by screening technician (reproducibility coefficient 0.3 vs 0.42 cm) (Figure 1).31
Figure 1.
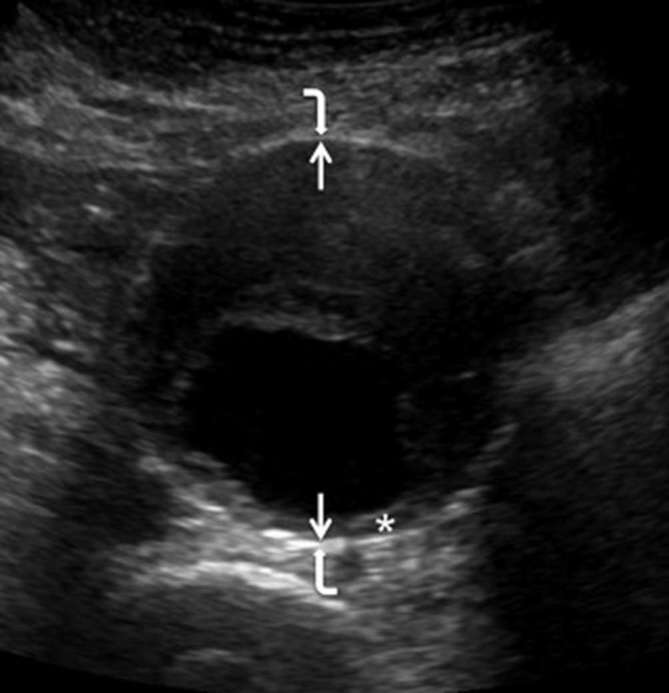
A transverse image of an AAA from Hartshorne et al demonstrating the inner to inner measurement used in the NAAASP.31 The aortic diameter is measured in the anteroposterior plane. The straight arrows indicate the position of the inner anterior and inner posterior wall. The angle arrows indicate the position of the outer anterior and outer posterior wall. The asterisk indicates an area of mural thrombus on the posterior wall. The authors have shown this to demonstrate the importance of not placing the posterior inner wall calliper on the inner border of the thrombus. AAA, abdominal aortic aneurysm; NAAASP, national abdominal aortic aneurysm screening programme.
The threshold for diagnosis of AAA is currently set at an infra-renal aortic diameter of 30 mm. The threshold for referral to a vascular surgeon for consideration of treatment is based on the outcomes of the UK small aneurysms trial, which clearly demonstrated a shift toward benefit of surgical intervention at 55 mm.32 Data has also failed to demonstrate longer-term mean survival benefits of early surgery for patients with AAA smaller than 5.5 cm.33
Surveillance intervals are set at arbitrary timeframes based on less robust evidence, with the assumption that larger AAA should be surveyed more frequently. The NAAASP surveillance programme has recently reviewed its protocols and is set to change, based on data from the RESCAN study showing a mean rate of growth of 1.28–2.44 mm year–1 for AAA between 30–44 mm, and 3.61 mm year–1 for AAA of 50 mm. It is therefore certain that screening intervals within NAAASP will increase to optimise the current service.34
Further investigation and patient pathway
The UK NAAASP standard operating procedure dictates that on detection of a threshold-sized aneurysm (ITI AP diameter of 5.5 cm or greater on ultrasound) referral to a vascular surgeon should be made. All referrals from the screening programme should be assessed in a vascular outpatients' department within 2 weeks. Aneurysms of over 7 cm on screening should be seen in the next available outpatient clinic.35
Confirmatory diagnostic re-imaging should be undertaken at this stage. This takes the form of CT angiogram (CTA). Reconstruction of CT images allows assessment of aortic and aneurysm morphology.36 MRI/MRA confers the same anatomical information as CTA. Benefits of MRA include negating the need for ionising radiation exposure and iodine-based intravenous contrast. MRA is more susceptible to image degradation secondary to movement artefact due to the need for the patient to lie still for a prolonged period during scanning.36
Digital subtraction angiography has now been superseded by CTA/MRA in the initial assessment of AAA. However it does still play a crucial role in the dynamic assessment of endovascular aneurysm repair (EVAR) suspected to be suffering from endoleak.37
All of these combined with global assessment of the patient suitability for surgical intervention including anaesthetic assessment by a specialist vascular anaesthetist and discussion at an AAA multidisciplinary team meeting will influence choice of intervention discussed with patients.38 If surgical or endovascular intervention is to be undertaken this should be completed within 8 weeks of referral from the screening programme.35
Generally accepted treatment
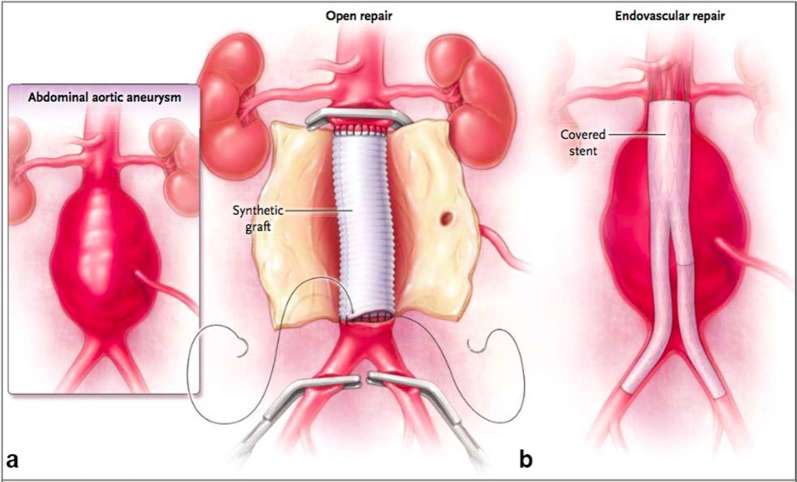
Broadly speaking there are two surgical options for AAA repair (Figure 2). Open surgery requires laparotomy and exposure of the aneurysm, proximal and distal control using arterial clamps, and sewing in a synthetic graft to healthy artery to completely replace the diseased segment. EVAR describes endovascular surgery, in which a fabric covered component stent graft is deployed within the aneurysmal component of the aorta to create an impermeable seal proximally and distally in unaffected areas of the vessel (most commonly a non-diseased infra-renal section of the aorta proximally, and the common iliac arteries distally). Access is obtained via the common femoral arteries in the vast majority of cases.
Figure 2.
Techniques used for repair of infra-renal AAA.16 (a) Open repair. Once inflow and outflow are controlled the aneurysm sac is opened and a synthetic graft is sutured in place onto normal aorta. (b) EVAR. A covered stent graft is inserted into the AAA via the common femoral arteries and is anchored to the normal aorta above the aneurysm and in the iliac arteries below, either by radial force from the spring mounted fabric, or supra-renal fixating hooks. Newer generation stent grafts have also been introduced that use aortic sealing technology. AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair.
Treatment outcomes for patients followed-up from the first 5 years of the screening programme showed a mortality rate of only 0.8% following elective surgery.39 The most recent UK NVR data for all patients undergoing elective AAA repair, has shown a mean in hospital mortality of 3% for open repair and 0.4% for EVAR.20 However, surgical risk is rarely decided by the surgeon alone, and is made on a case by case basis. Quality improvement initiatives run by the Vascular Society of Great Britain have led to the development of several recommendations with respect to the patient pathway including: all elective patients should undergo review in a multidisciplinary setting including a surgeon and radiologist to ascertain suitability for EVAR, all patients should undergo standard pre-operative assessment such as Cardiopulmonary Exercise testing, and review by an experienced anaesthetist.
Cost Efficiency
The initial cost-effectiveness analysis from the Viborg trial demonstrated significant benefits in terms of prevention of emergency hospital admission.40 The calculations and assumptions made in the original cost assessments were based on prevalence far higher than those found in current practice. The most recent analysis performed by the Swedish group in Uppsala used data from the MASS follow- up and their own Swedish database to calculate cost efficiency.41 They concluded that at current AAA prevalence the relative risk reduction of screening is 42% at 13 years. The absolute risk reduction for death from AAA was 15.1/10,000 invited men. That is, 530 patients required screening to prevent one death from AAA, resulting in a 4.8 life year gain. The incremental cost efficiency ratio of Euro per quality-adjusted life year and Euro per life year were €14,706 and €11,558 respectively, confirming that the programme will remain cost-efficient down to a prevalence of 0.5%. Taking into account the cost associated with incidental identification and treatment of AAA in a non-screened control group, incidental detection would need to increase by 100% to affect the cost benefit of screening.
Comparison with international screening programmes
AAA programmes have been established in the UK, Sweden and the USA with fairly consistent design. Each programme is based on the same available evidence, and in the current climate, has had to undergo significant scrutiny to ensure it is both beneficial to the population and cost-effective for the initiating health programme. The largest of all the screening programmes is the UKNAASSP,1 whose design follows that of the MASS trial.32 The UK and Sweden initially invite men in their 65th year for a one-off screening ultrasound, whereas the USA programme invites men aged 65–75 years with a smoking history.42, 43 Incidentally, the USA programme also invites women with a significant family history of AAA.
All programmes use ultrasound to screen for AAA with a diameter of ≥30 mm as cut-off; however, the method of measurement differs between programmes. The UK uses the (ITI method, the Swedish programme uses the LELE method, and the USA does not stipulate which measurement is used. To account for possible discrepancies, the Swedish programme includes patients with sub-aneurysmal aortas (25–29 mm) into a delayed (5-year) surveillance. Surveillance intervals vary between programmes and are based on aortic measurements (Table 1).
Table 1.
Features of current AAA screening programmes worldwide
| Programme | Patients | Method | Threshold for inclusion into surveillance | Surveillance Schedule | Referral to surgeon |
| UK NAAASP | Men 65th year |
Ultrasound ITI |
30 mm | 30–44 mm yearly 45–54 mm 3 months |
55 mm |
| Sweden | Men 65th year |
Ultrasound LELE |
30 mm (25–29 mm rescreened at 5 years) |
25–29 mm 5 years 30–39 mm 2 years 40–44 mm 1 year 45–50 mm 6 months 50–54 mm 3 months |
55 mm |
| USA | Men 65–75 years Women with significant family history |
Ultrasound Not specified |
30 mm | 29–29 mm 5 years 30–34 mm 3 years 35–44 mm 12 months 45–54 6 months |
50 mm |
ITI, inner-to-inner measurement; LELE, leading edge-to-leading dge; NAAASP, national abdominal aortic aneurysm screening programme.
Challenges and controversies
The most significant challenge to the AAA screening programmes is the declining incidence of AAA in western populations. Prevalence of AAA in the seminal trials was between 4.9–7.2%.44, 45 However, prevalence of AAA detection in NAAASP is currently only 1.34%,39 in-line with other evidence demonstrating a reduction in AAA prevalence in western countries.46–49 Due to this, various strategies are being investigated to improve uptake and AAA detection.
Currently, women are not invited to AAA screening in the UK, due in part to a lack of evidence for a benefit in aneurysm-specific mortality or cost-effectiveness.50 This is changing, with greater interest in studies aiming to investigate screening in women, such as the SWAN study (screening women for abdominal aortic aneurysms).51 AAA prevalence is lower in women, with a tendency for the aorta to become aneurysmal in older age, but with an increased rupture rate and worse reported outcomes in both the elective and emergency setting.50, 52 Economic analysis in men has concluded AAA screening would be beneficial at levels of prevalence that are similar to that currently reported in women, therefore rightly, inclusion of women in screening is being debated and may well become cost- effective in future analysis.53 The debate extends to follow-up of women with sub-aneurysmal aortas, in whom an equally high proportion will go on to reach threshold for surgery.54
Current uptake in screening is approximately 80% in the NAAASP, with regular review to improve access and coverage. Turn down rates during the Chichester trial were higher in the older age group (33.8% for subjects aged 76–80 years vs 19.5% for those aged 65 years). Aneurysm screening targets a potentially challenging group i.e. patients at risk of AAA as well as other concurrent cardiorespiratory conditions, associated historically with lower socio-economic status and poorer engagement with medical services.55 Targeting patients with pre-existing high-risk conditions such as coronary disease and peripheral arterial disease, using combination echocardiogram or peripheral arterial imaging is one way to increase participation, although evidence is limited. Automatically inviting siblings of patients diagnosed with AAA has been trialled in Sweden, with successful detection in 14% of brothers and 6% of sisters. Sibling age ranged from 40 to 80 years, with the associated risk of identifying AAA due to connective tissue disorders as well as atherosclerotic disease.56
Risk assessment for potential harms of screening and over diagnosis is mandatory for any screening programme. As the majority of AAA are asymptomatic, newly diagnosed men are informed of a potentially life-threatening condition requiring life-long imaging and possible surgery with an appreciable risk of death. It has been suggested that the greatest psychological impact is on patients identified as having an AAA, who go on to receive conservative management, as this group report lower quality of life measurements than patients who undergo surgery at the time of screening.33, 57 Screening itself has only been linked to transient changes in anxiety. At present, as screening has been shown to demonstrate significant benefit, the associated effects on measured quality of life have been accepted. There are continuous efforts to review patient information to allow informed decision making prior to attending screening.
Variation in the ultrasound measurement protocol used by each programme represents the different approaches to sub-threshold aortas. The ITI technique used in the NAAASP provides measurements that can be as much as 4 mm smaller than the OTO measurement, potentially underestimating the aortic diameter.58 There is ongoing debate regarding management of those patients with an initial “sub-aneurysmal” measurement, i.e. ITI diameter 26–29 mm. Under the current protocol these patients are discharged from screening. The MASS trial reported an increased late rupture rate in the one-off screening group, thought to be due to these “sub-aneurysmal” aortas continuing to grow during long- term.44 The natural history of these smaller but abnormal aortas is of significant interest, as evidence suggests risk of ongoing expansion and rupture.59–61 To counter this, the Swedish screening programme currently invites this cohort for a further scan at 5 years, a strategy under review by the NAAASP.
Future Directions
A strong evidence base and the success of current national screening models make it seem inevitable that some form of AAA screening will become available in all regions where AAA is a significant health risk. Variations in models of healthcare provision and other more significant public health priorities will dictate pace of uptake. One-time screening in men aged 65 years continues to demonstrate overall benefit in comparison with other screening programmes, and avenues to ensure maximum benefit across all at-risk populations continue to be the subject of ongoing research. As with all screening, targeting of resources towards highest risk groups remains a significant challenge.
Contributor Information
Ruth A Benson, Email: ruth.benson@gmail.com.
Lewis Meecham, Email: Lewis.Meecham@heartofengland.nhs.uk.
Owain Fisher, Email: owain.fisher@uhcw.nhs.uk.
Ian M Loftus, Email: ian.loftus@stgeorges.nhs.uk.
REFERENCES
- 1.Davis M, Harris M, Earnshaw JJ. Implementation of the national health service abdominal aortic aneurysm screening program in England. J Vasc Surg 2013; 57: 1440–5. doi: 10.1016/j.jvs.2012.10.114 [DOI] [PubMed] [Google Scholar]
- 2.Thompson SG, Ashton HA, Gao L, Scott RA, Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised multicentre aneurysm screening study. BMJ 2009; 338: b2307. doi: 10.1136/bmj.b2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. . The multicentre aneurysm screening study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360: 1531–9. [DOI] [PubMed] [Google Scholar]
- 4.Cosford PA, Leng GC, Thomas J, Cochrane Vascular Group. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007; 2: CD002945. doi: 10.1002/14651858.CD002945.pub2 [DOI] [PubMed] [Google Scholar]
- 5.Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 2007; 94: 696–701. doi: 10.1002/bjs.5780 [DOI] [PubMed] [Google Scholar]
- 6.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 1995; 82: 1066–70. [DOI] [PubMed] [Google Scholar]
- 7.Kim LG, P Scott RA, Ashton HA, Thompson SG, Multicentre Aneurysm Screening Study Group. A sustained mortality benefit from screening for abdominal aortic aneurysm. Ann Intern Med 2007; 146: 699–706. doi: 10.7326/0003-4819-146-10-200705150-00003 [DOI] [PubMed] [Google Scholar]
- 8.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. . Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329: 1259–0. doi: 10.1136/bmj.329.7477.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330: 750. doi: 10.1136/bmj.38369.620162.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindholt JS, Juul S, Fasting H, Henneberg EW. Preliminary ten year results from a randomised single centre mass screening trial for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2006; 32: 608–14. doi: 10.1016/j.ejvs.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 11.Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97: 826–34. doi: 10.1002/bjs.7001 [DOI] [PubMed] [Google Scholar]
- 12.Takagi H, Goto SN, Matsui M, Manabe H, Umemoto T. A further meta-analysis of population-based screening for abdominal aortic aneurysm. J Vasc Surg 2010; 52: 1103–8. doi: 10.1016/j.jvs.2010.02.283 [DOI] [PubMed] [Google Scholar]
- 13.Chichester Aneurysm Screening Group, Viborg Aneurysm Screening Study, Western Australian Abdominal Aortic Aneurysm Program, Multicentre Aneurysm Screening Study. A comparative study of the prevalence of abdominal aortic aneurysms in the United Kingdom, Denmark, and Australia. J Med Screen 2001; 8: 46–50. doi: 10.1136/jms.8.1.46 [DOI] [PubMed] [Google Scholar]
- 14.Benson RA, Poole R, Murray S, Moxey P, Loftus IM. Screening results from a large United Kingdom abdominal aortic aneurysm screening center in the context of optimizing United Kingdom national abdominal aortic aneurysm screening programme protocols. J Vasc Surg 2016; 63: 301–4. doi: 10.1016/j.jvs.2015.08.091 [DOI] [PubMed] [Google Scholar]
- 15.Jongkind V, Yeung KK, Akkersdijk GJ, Heidsieck D, Reitsma JB, Tangelder GJ, et al. . Juxtarenal aortic aneurysm repair. J Vasc Surg 2010; 52: 760–7. doi: 10.1016/j.jvs.2010.01.049 [DOI] [PubMed] [Google Scholar]
- 16.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 2014; 371: 2101–8. doi: 10.1056/NEJMcp1401430 [DOI] [PubMed] [Google Scholar]
- 17.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg 2013; 100: 1405–13. doi: 10.1002/bjs.9235 [DOI] [PubMed] [Google Scholar]
- 18.Parkinson F, Ferguson S, Lewis P, Williams IM, Twine CP, South East Wales Vascular Network. Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg 2015; 61: 1606–12. doi: 10.1016/j.jvs.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 19.Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: the impact of size, gender, and expansion rate. J Vasc Surg 2003; 37: 280–4. doi: 10.1067/mva.2003.119 [DOI] [PubMed] [Google Scholar]
- 20. Royal Society of Chemistry. National vascular registry 2016 annual report; 2016. pp 1–83. http://xlink.rsc.org/?DOI=c1dt90165f. [Google Scholar]
- 21.Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999; 17: 472–5. doi: 10.1053/ejvs.1999.0835 [DOI] [PubMed] [Google Scholar]
- 22.Meyenburg M, Lang R, Saling E, Wegner R. Does pulsed-Doppler ultrasound have mutagenic effects? Application of the Ames mutagenicity assay to test pulsed-Doppler equipment. Echocardiography 1990; 7: 657–60. doi: 10.1111/j.1540-8175.1990.tb00417.x [DOI] [PubMed] [Google Scholar]
- 23.Sprouse LR, Meier GH, Parent FN, DeMasi RJ, Glickman MH, Barber GA. Is ultrasound more accurate than axial computed tomography for determination of maximal abdominal aortic aneurysm diameter? Eur J Vasc Endovasc Surg 2004; 28: 28–35. doi: 10.1016/j.ejvs.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 24.Smith FC, Grimshaw GM, Paterson IS, Shearman CP, Hamer JD. Ultrasonographic screening for abdominal aortic aneurysm in an urban community. Br J Surg 1993; 80: 1406–9. doi: 10.1002/bjs.1800801117 [DOI] [PubMed] [Google Scholar]
- 25.O’Kelly TJ, Heather BP. General practice-based population screening for abdominal aortic aneurysms: a pilot study. Br J Surg 1989; 76: 479–80. doi: 10.1002/bjs.1800760517 [DOI] [PubMed] [Google Scholar]
- 26.Morris GE, Hubbard CS, Quick CR. An abdominal aortic aneurysm screening programme for all males over the age of 50 years. Eur J Vasc Surg 1994; 8: 156–60. doi: 10.1016/S0950-821X(05)80451-7 [DOI] [PubMed] [Google Scholar]
- 27.Lucarotti M, Shaw E, Poskitt K, Heather B. The gloucestershire aneurysm screening programme: the first 2 years’ experience. Eur J Vasc Surg 1993; 7: 397–401. doi: 10.1016/S0950-821X(05)80256-7 [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson H, Bergqvist D, Ekberg O, Janzon L. A population based screening of abdominal aortic aneurysms (AAA). Eur J Vasc Surg 1991; 5: 53–7. doi: 10.1016/S0950-821X(05)80927-2 [DOI] [PubMed] [Google Scholar]
- 29.Collin J, Araujo L, Walton J, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet 1988; 2: 613–5. doi: 10.1016/S0140-6736(88)90649-6 [DOI] [PubMed] [Google Scholar]
- 30.Gürtelschmid M, Björck M, Wanhainen A. Comparison of three ultrasound methods of measuring the diameter of the abdominal aorta. Br J Surg 2014; 101: 633–6. doi: 10.1002/bjs.9463 [DOI] [PubMed] [Google Scholar]
- 31.Hartshorne TC, McCollum CN, Earnshaw JJ, Morris J, Nasim A. Ultrasound measurement of aortic diameter in a national screening programme. Eur J Vasc Endovasc Surg 2011; 42: 195–9. doi: 10.1016/j.ejvs.2011.02.030 [DOI] [PubMed] [Google Scholar]
- 32.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. . The multicentre aneurysm screening study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360: 1531–9. [DOI] [PubMed] [Google Scholar]
- 33.The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet 1998; 352: 1649–55. [PubMed] [Google Scholar]
- 34.Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG, RESCAN Collaborators. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 2013; 309: 1720–1. doi: 10.1016/j.jvs.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 35.Public Health England. NHS abdominal aortic aneurysm (AAA) screening programme essential elements in providing an AAA screening and surveillance programme. 2017. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/598365/AAA_Screening_Standard_Operating_Procedures_March_2017.pdf.
- 36.Hong H, Yang Y, Liu B, Cai W, Yunan Y, Bo L. Imaging of abdominal aortic aneurysm: the present and the future. Curr Vasc Pharmacol 2010; 8: 808–19. doi: 10.2174/157016110793563898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang SG, Woo K, Moos JM, Han S, Lew WK, Chao A, et al. . A prospective study of carbon dioxide digital subtraction versus standard contrast arteriography in the detection of endoleaks in endovascular abdominal aortic aneurysm repairs. Ann Vasc Surg 2013; 27: 38–44. doi: 10.1016/j.avsg.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 38. Vascular society of Great Britain and Ireland. Abdominal aortic aneurysm quality improvement program (AAAQIP) AAA preoperative care bundle. Version 5; 2011. [Google Scholar]
- 39.Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br J Surg 2016; 103: 1125–31. doi: 10.1002/bjs.10173 [DOI] [PubMed] [Google Scholar]
- 40.Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. Results from a randomised population screening trial. Eur J Vasc Endovasc Surg 2002; 23: 55–60. doi: 10.1053/ejvs.2001.1534 [DOI] [PubMed] [Google Scholar]
- 41.Svensjö S, Mani K, Björck M, Lundkvist J, Wanhainen A. Screening for abdominal aortic aneurysm in 65-year-old men remains cost-effective with contemporary epidemiology and management. Eur J Vasc Endovasc Surg 2014; 47: 357–65. doi: 10.1016/j.ejvs.2013.12.023 [DOI] [PubMed] [Google Scholar]
- 42.LeFevre ML, U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161: 281–90. doi: 10.7326/M14-1204 [DOI] [PubMed] [Google Scholar]
- 43.Chun KC, Teng KY, Van Spyk EN, Carson JG, Lee ES. Outcomes of an abdominal aortic aneurysm screening program. J Vasc Surg 2013; 57: 376–81. doi: 10.1016/j.jvs.2012.08.038 [DOI] [PubMed] [Google Scholar]
- 44.Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA, Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the multicentre aneurysm screening study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012; 99: 1649–56. doi: 10.1002/bjs.8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaul KA, Lawrence-Brown M, Dickinson JA, Norman PE. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms. JAMA Intern Med 2016; 176: 1761. doi: 10.1001/jamainternmed.2016.6633 [DOI] [PubMed] [Google Scholar]
- 46.Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011; 124: 1118–23. doi: 10.1161/CIRCULATIONAHA.111.030379 [DOI] [PubMed] [Google Scholar]
- 47.Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43: 161–6. doi: 10.1016/j.ejvs.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 48.Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98: 645–51. doi: 10.1002/bjs.7461 [DOI] [PubMed] [Google Scholar]
- 49.Norman PE, Spilsbury K, Semmens JB. Falling rates of hospitalization and mortality from abdominal aortic aneurysms in Australia. J Vasc Surg 2011; 53: 274–7. doi: 10.1016/j.jvs.2010.08.087 [DOI] [PubMed] [Google Scholar]
- 50.Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89: 283–5. doi: 10.1046/j.0007-1323.2001.02014.x [DOI] [PubMed] [Google Scholar]
- 51.National Institute for Health Research. SWAN: screening women for abdominal aortic aneurysms. 2015. Available from: http://www.nets.nihr.ac.uk/projects/hta/1417901.
- 52.Derubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, Faries PL, et al. . Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46: 630–5. doi: 10.1016/j.jvs.2007.06.024 [DOI] [PubMed] [Google Scholar]
- 53.Wanhainen A, Lundkvist J, Bergqvist D, Björck M. Cost-effectiveness of screening women for abdominal aortic aneurysm. J Vasc Surg 2006; 43: 908–14. doi: 10.1016/j.jvs.2005.12.064 [DOI] [PubMed] [Google Scholar]
- 54.Söderberg P, Wanhainen A, Svensjö S. Five year natural history of screening detected sub-aneurysms and abdominal aortic aneurysms in 70 year old women and systematic review of repair rate in women. Eur J Vasc Endovasc Surg 2017; 53: 802–9. doi: 10.1016/j.ejvs.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 55.Crilly MA, Mundie A, Bachoo P, Nimmo F. Influence of rurality, deprivation and distance from clinic on uptake in men invited for abdominal aortic aneurysm screening. Br J Surg 2015; 102: 916–23. doi: 10.1002/bjs.9803 [DOI] [PubMed] [Google Scholar]
- 56.Linné A, Forsberg J, Leander K, Hultgren R. Screening of siblings to patients with abdominal aortic aneurysms in Sweden. Scand Cardiovasc J 2017; 51: 167–71. doi: 10.1080/14017431.2017.1303189 [DOI] [PubMed] [Google Scholar]
- 57.Lindholt JS, Vammen S, Fasting H, Henneberg EW. Psychological consequences of screening for abdominal aortic aneurysm and conservative treatment of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2000; 20: 79–83. doi: 10.1053/ejvs.1999.1087 [DOI] [PubMed] [Google Scholar]
- 58.Meecham L, Evans R, Buxton P, Allingham K, Hughes M, Rajagopalan S, et al. . Abdominal aortic aneurysm diameters: a study on the discrepancy between inner to inner and outer to outer measurements. Eur J Vasc Endovasc Surg 2015; 49: 28–32. doi: 10.1016/j.ejvs.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 59.Wild JB, Stather PW, Biancari F, Choke EC, Earnshaw JJ, Grant SW, et al. . A multicentre observational study of the outcomes of screening detected sub-aneurysmal aortic dilatation. Eur J Vasc Endovasc Surg 2013; 45: 128–34. doi: 10.1016/j.ejvs.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 60.Hafez H, Druce PS, Ashton HA. Abdominal aortic aneurysm development in men following a “normal” aortic ultrasound scan. Eur J Vasc Endovasc Surg 2008; 36: 553–8. doi: 10.1016/j.ejvs.2008.06.032 [DOI] [PubMed] [Google Scholar]
- 61.Darwood R, Earnshaw JJ, Turton G, Shaw E, Whyman M, Poskitt K, et al. . Twenty-year review of abdominal aortic aneurysm screening in men in the county of Gloucestershire, United Kingdom. J Vasc Surg 2012; 56: 8–13. doi: 10.1016/j.jvs.2011.12.069 [DOI] [PubMed] [Google Scholar]