Abstract
Background
A low-carbohydrate diet may improve cancer survival, but relevant clinical evidence remains limited.
Methods
We followed 1542 stages I to III colorectal cancer (CRC) patients who completed a validated food frequency questionnaire between 6 months and 4 years after diagnosis. We calculated overall, animal-, and plant-rich, low-carbohydrate diet scores and examined their associations with CRC-specific and overall mortality using Cox proportional hazards regression after adjusting for potential predictors for cancer survival. We also assessed the intake and changes of macronutrients after diagnosis. Statistical tests were two-sided.
Results
Although no association was found for overall and animal-rich low-carbohydrate diet score, plant-rich, low-carbohydrate diet, which emphasizes plant sources of fat and protein with moderate consumption of animal products, was associated with lower CRC-specific mortality (hazard ratio [HR] comparing extreme quartiles = 0.37, 95% confidence interval [CI] = 0.25 to 0.57, Ptrend < .001). Carbohydrate intake was associated with higher CRC-specific mortality, and this association was restricted to carbohydrate consumed from refined starches and sugars (HR per one-SD increment = 1.36, 95% CI = 1.14 to 1.62, Ptrend < .001). In contrast, replacing carbohydrate with plant fat and protein was associated with lower CRC-specific mortality, with the HR per one-SD increment of 0.81 (95% CI = 0.69 to 0.95, Ptrend = .01) for plant fat and 0.77 (95% CI = 0.62 to 0.95, Ptrend = .02) for plant protein. Similar results were obtained for overall mortality and when changes in macronutrient intake after diagnosis were assessed.
Conclusion
Plant-rich, low-carbohydrate diet score was associated with lower mortality in patients with nonmetastatic CRC. Substituting plant fat and protein for carbohydrate, particularly that from refined starches and sugars, may improve patients’ survival.
Despite advances in early detection and treatment, colorectal cancer (CRC) remains the third leading cause of cancer death in the United States (1). The relative survival rate of CRC is 65% at 5 years after diagnosis, and decreases to 58% at 10 years after diagnosis (2). Currently available treatment options, including surgery and radio-, chemo-, and immunotherapy, are highly selective and only effective in patients with certain characteristics. Moreover, cancer recurrence is common among CRC survivors; approximately one-half of patients treated with surgery will experience a recurrence within the first 3 years after surgery (3). Also, treatment-related side effects are common and impair quality of life. Therefore, developing effective adjuvant therapies is a high priority to improve patients’ long-term survival outcomes.
Enhanced glucose uptake is a key feature of cancer metabolism, leading to secretion of insulin and subsequent elevation of insulin-like growth factor 1 (IGF1) that activates the phosphoinositide 3-kinase-Akt-mammalian/mechanistic target of rapamycin pathway, a major metabolic signaling pathway that drives anabolic metabolism and tumorigenesis (4). Both hyperglycemia and hyperinsulinemia have been linked to poor prognosis of CRC (5–8). These data together support a central role of glucose metabolism in carcinogenesis and lead to substantial interest in a low-carbohydrate diet as an effective dietary approach to facilitate cancer treatment.
Despite the mechanistic data, however, human evidence remains sparse. So far, only one prospective study has examined the prognostic influence of carbohydrate intake on CRC and found that stage III colon cancer patients with higher intake of carbohydrate and higher glycemic load had worse survival (9).
To address this knowledge gap, we examined low-carbohydrate diet and macronutrient intake in relation to survival among stage I to III CRC patients documented in two large prospective cohorts: the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). Given the increasing recognition for the importance of food sources of macronutrients (10–12), we considered animal and plant food sources separately in the analysis.
Methods
Study Population
Details about the NHS/HPFS cohorts have been described elsewhere (13,14). In brief, the NHS enrolled 121 700 US registered female nurses ages 30–55 years in 1976, and the HPFS enrolled 51 529 US male health professionals ages 40–75 years in 1986. Participants completed a mailed questionnaire regarding their lifestyle and medical history at baseline and every 2 years thereafter. Diet was assessed by a validated food frequency questionnaire (FFQ) in 1980, 1984, 1986, and every 4 years thereafter in the NHS, and every 4 years since 1986 in the HPFS. The follow-up rate in the two cohorts has been higher than 90% for each questionnaire cycle. This study was approved by the Institutional Review Board at the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
Ascertainment of CRC Cases
On each biennial questionnaire, participants were asked whether they had been diagnosed with CRC in the past 2 years. For those who reported CRC, we asked for their permission to acquire medical records and pathologic reports. Study physicians, blinded to exposure data, reviewed all medical records to confirm the diagnosis and record the date of diagnosis and tumor stage, histology, and location (15). In this analysis, we included participants who were diagnosed with stage I to III CRC throughout follow-up and completed the FFQ after diagnosis (N = 919 in the NHS and 623 in the HPFS) (Supplementary Figure 1, available online). These participants did not differ from the overall patient sample of the two cohorts (Supplementary Table 1, available online).
Death Ascertainment
Most of the deaths were identified through family members or the postal system in response to the follow-up questionnaires. We also searched the names of persistent nonresponders in the National Death Index. The cause of death was assigned by study physicians blinded to exposure data. More than 96% of deaths have been identified using these methods (16).
Dietary Assessment
In each FFQ, participants were asked how often, on average, they consumed each food of a standard portion size during the previous year. We calculated the average daily intake for each nutrient by multiplying the reported frequency of consumption of each item by its nutrient content and then summing across from all foods. Total carbohydrate, fat, and protein intake was expressed as percentage of total energy consumption. To account for food sources, we also calculated fat and protein intake from animal and plant sources separately and assessed carbohydrate intake from fruits, vegetables, and whole grains, and from refined starches and sugars separately. Major food sources of these carbohydrate categories are provided in Supplementary Table 2 (available online). The validity of FFQ for macronutrient assessment has been documented previously (Supplementary Methods, available online).
Overall, animal- and plant-rich, low-carbohydrate diet scores were developed previously (17). Briefly, the percentages of energy intake from carbohydrate, protein, and fat were divided into 11 categories with equal sample sizes. The carbohydrate categories were ranked from 10 (lowest intake) to 0 (highest intake), whereas protein and fat categories were ranked from 0 (lowest intake) to 10 (highest intake). Ranks were added to create the overall low-carbohydrate score with a maximum value of 30, which represented the highest intake of protein and fat and the lowest intake of carbohydrate. In addition, we created a plant-rich and animal-rich, low-carbohydrate score based on the intake of carbohydrate, plant/animal protein, and plant/animal fat (instead of total protein and fat). To facilitate the understanding about the three low-carbohydrate scores, we presented in Supplementary Table 3 (available online) the mean intake of macronutrients and major food groups among participants with extreme scores (≤5 and ≥25).
For this study, diet assessed on the first FFQ at least 6 months but no more than 4 years after CRC diagnosis (median, 2.1 years) was used for postdiagnostic intake to avoid assessment during the period of active treatment. We also calculated the change in dietary intake by subtracting from the postdiagnostic intake the intake reported on the last FFQ before CRC diagnosis (prediagnostic intake).
Statistical Analysis
We calculated person-time of follow-up from the return date of the FFQ that was used for postdiagnostic assessment to death or the end of the study period (June 1, 2014 for the NHS, January 31, 2014 for the HPFS), whichever came first. Cause-specific Cox proportional hazards regression model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for CRC-specific mortality and all-cause mortality. Because participants can only contribute person-time until completion of their postdiagnostic FFQ, we used the time since diagnosis as the time scale to account for left truncation due to delayed entry (18).
We performed the inverse probability weighting in all survival analyses to minimize any bias resulting from the availability of postdiagnostic questionnaire data (Supplementary Methods, available online) (19). To control for confounding, we adjusted for prediagnostic exposure and other potential predictors for cancer survival (see Table 1 and footnote of Table 2). Details about covariate assessments are provided in the Supplementary Methods (available online). We tested the proportional hazards assumption by including the interaction term between low-carbohydrate diet score and follow-up time in the model and did not find statistical evidence for violation of this assumption.
Table 1.
Baseline characteristics of CRC patients at diagnosis according to quartiles of overall and animal- and plant-rich, low-carbohydrate diet scores (n = 1542)*
| Overall low-carbohydrate diet |
Animal-rich, low-carbohydrate diet |
Plant-rich, low-carbohydrate diet |
||||
|---|---|---|---|---|---|---|
| Characteristic | Quartile 1 (n = 396) | Quartile 4 (n = 372) | Quartile 1 (n = 383) | Quartile 4 (n = 399) | Quartile 1 (n = 396) | Quartile 4 (n = 352) |
| Female, % | 56 | 58 | 61 | 61 | 59 | 64 |
| Age, y | 68.9 (9.5) | 67.1 (9.3) | 67.6 (9.6) | 67.4 (9.3) | 70.1 (9.3) | 66.7 (9.2) |
| Years from diagnosis to postdiagnostic dietary assessment | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 2.2 (1.0) | 2.2 (1.0) | 2.1 (1.0) |
| BMI, kg/m2 | 25.3 (4.6) | 27.2 (4.6) | 25.5 (4.4) | 27.4 (4.7) | 26.3 (5.0) | 26.3 (4.4) |
| Physical activity, MET-h/wk | 20.1 (23.3) | 16.7 (22.6) | 21.0 (24.8) | 15.9 (22.6) | 17.8 (23.6) | 18.6 (20.7) |
| Pack-years of smoking | 14.0 (19.8) | 17.8 (22.9) | 13.3 (19.7) | 18.8 (24.0) | 17.1 (22.5) | 16.2 (22.5) |
| Current smokers, % | 4 | 7 | 4 | 8 | 5 | 6 |
| Regular use of aspirin, %† | 38 | 33 | 37 | 32 | 34 | 36 |
| Dietary consumption | ||||||
| Macronutrient, % of energy | ||||||
| Total fat | 24 (5) | 37 (5) | 25 (6) | 35 (5) | 26 (6) | 36 (6) |
| Animal fat | 10 (4) | 19 (5) | 9 (3) | 20 (4) | 14 (5) | 14 (5) |
| Plant fat | 14 (4) | 18 (6) | 16 (5) | 15 (5) | 11 (3) | 22 (5) |
| Saturated fat | 8 (2) | 12 (3) | 8 (2) | 12 (3) | 9 (3) | 11 (2) |
| Polyunsaturated fat | 5 (1) | 7 (2) | 6 (2) | 6 (2) | 5 (1) | 8 (2) |
| Monounsaturated fat | 9 (2) | 14 (3) | 10 (3) | 13 (2) | 9 (2) | 14 (3) |
| Trans fat | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Total protein | 15 (2) | 19 (3) | 15 (2) | 20 (3) | 17 (4) | 17 (3) |
| Animal protein | 9 (2) | 14 (3) | 9 (2) | 15 (3) | 12 (4) | 11 (3) |
| Plant protein | 6 (2) | 5 (1) | 6 (2) | 5 (1) | 5 (1) | 6 (1) |
| Total carbohydrate | 61 (6) | 43 (5) | 61 (7) | 44 (6) | 57 (8) | 47 (7) |
| Carbohydrate from fruits/vegetables/whole grains | 25 (9) | 18 (6) | 25 (9) | 18 (6) | 24 (8) | 20 (7) |
| Carbohydrate from refined starches/sugars | 36 (9) | 25 (6) | 36 (9) | 26 (6) | 33 (9) | 27 (7) |
| Glycemic load | 147 (21) | 98 (17) | 145 (23) | 101 (18) | 134 (26) | 106 (21) |
| Alcohol, g/d | 7.0 (12.3) | 7.5 (10.7) | 6.0 (11.2) | 8.1 (11.0) | 6.6 (11.4) | 8.8 (13.1) |
| Fiber, g/d | 24.5 (8.5) | 18.7 (5.3) | 25.1 (8.1) | 18.0 (4.9) | 20.8 (7.3) | 21.3 (5.8) |
| Marine omega-3 fatty acid, g/d | 0.3 (0.3) | 0.3 (0.4) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| Folate, μg/d | 724 (380) | 635 (328) | 727 (380) | 626 (333) | 701 (360) | 654 (338) |
| Calcium, mg/d | 1163 (577) | 1091 (502) | 1151 (570) | 1130 (524) | 1249 (555) | 1070 (503) |
| Vitamin D, IU/d | 509 (354) | 562 (461) | 511 (367) | 543 (432) | 572 (347) | 512 (432) |
| Red/processed meat, serving/wk | 2.9 (2.5) | 7.1 (4.2) | 2.8 (2.4) | 7.1 (4.1) | 4.0 (2.9) | 4.8 (3.3) |
| Poultry, serving/wk | 2.0 (1.7) | 3.2 (2.4) | 1.9 (1.6) | 3.2 (2.5) | 2.4 (1.9) | 2.3 (1.8) |
| Fish, serving/wk | 1.3 (1.2) | 1.8 (1.5) | 1.3 (1.2) | 1.7 (1.6) | 1.5 (1.6) | 1.6 (1.3) |
| Cancer subsite, % | ||||||
| Proximal colon | 40 | 42 | 42 | 42 | 43 | 42 |
| Distal colon | 31 | 31 | 29 | 30 | 33 | 30 |
| Rectum | 23 | 22 | 24 | 24 | 19 | 23 |
| Unspecified | 6 | 5 | 5 | 4 | 5 | 5 |
| Stage, % | ||||||
| I | 33 | 35 | 35 | 37 | 35 | 37 |
| II | 31 | 29 | 30 | 30 | 30 | 30 |
| III | 22 | 22 | 22 | 22 | 23 | 21 |
| Unspecified | 14 | 14 | 13 | 11 | 12 | 12 |
*Quartiles are created in women and men separately. Means (SD) are calculated for continuous variables. All variables are age-adjusted except for age itself. The overall study sample was used as the standard population. BMI = body mass index; CRC = colorectal cancer; MET = metabolic equivalent.
†Regular users are defined as those taking two or more standard (325 mg) tablets of aspirin per week.
Table 2.
Postdiagnostic, low-carbohydrate diet score and all-cause and CRC-specific mortality among CRC patients in the NHS and HPFS cohorts
| Outcome | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend |
|---|---|---|---|---|---|
| CRC-specific mortality | |||||
| Overall low-carbohydrate diet | |||||
| Median (IQR) | 5 (3–7) | 11 (10–13) | 17 (16–18) | 23 (21–25) | |
| No. of events (n = 185) | 47 | 52 | 40 | 46 | |
| No. of person-years (n = 14 420) | 4368 | 4115 | 4066 | 3850 | |
| Model 1: HR (95% CI)* | 1 (referent) | 1.18 (0.86 to 1.62) | 1.00 (0.71–1.41) | 1.21 (0.87–1.68) | .55 |
| Model 2: HR (95% CI)† | 1 (referent) | 1.10 (0.79 to 1.55) | 0.91 (0.62–1.32) | 0.95 (0.65–1.41) | .45 |
| Animal-rich, low-carbohydrate diet | |||||
| Median (IQR) | 5 (2–7) | 12 (10–13) | 17 (16–19) | 24 (22–26) | |
| No. of events (n = 185) | 44 | 49 | 37 | 55 | |
| No. of person-years (n = 14 420) | 4518 | 4215 | 3703 | 3964 | |
| Model 1: HR (95% CI)* | 1 (referent) | 1.24 (0.89 to 1.72) | 1.03 (0.72 to 1.48) | 1.50 (1.09 to 2.08) | .04 |
| Model 2: HR (95% CI)† | 1 (referent) | 1.07 (0.75 to 1.51) | 0.89 (0.60 to 1.32) | 1.15 (0.78 to 1.70) | .61 |
| Plant-rich, low-carbohydrate diet | |||||
| Median (IQR) | 8 (6–9) | 13 (12–14) | 16 (15–17) | 21 (20–23) | |
| No. of events (n = 185) | 61 | 50 | 47 | 27 | |
| No. of person-years (n = 14 420) | 3585 | 4236 | 4525 | 4053 | |
| Model 1: HR (95% CI)* | 1 (referent) | 0.68 (0.50 to 0.92) | 0.60 (0.44 to 0.81) | 0.42 (0.29 to 0.61) | <.001 |
| Model 2: HR (95% CI)† | 1 (referent) | 0.66 (0.48 to 0.91) | 0.57 (0.40 to 0.80) | 0.37 (0.25 to 0.57) | <.001 |
| All-cause mortality | |||||
| Overall low-carbohydrate diet | |||||
| No. of events (n = 817) | 227 | 194 | 195 | 201 | |
| Model 1: HR (95% CI)* | 1 (referent) | 0.93 (0.79 to 1.08) | 0.92 (0.79 to 1.08) | 1.13 (0.97 to 1.32) | .11 |
| Model 2: HR (95% CI)† | 1 (referent) | 0.93 (0.79 to 1.10) | 0.89 (0.75 to 1.06) | 1.04 (0.87 to 1.25) | .68 |
| Animal-rich, low-carbohydrate diet | |||||
| No. of events (n = 817) | 204 | 213 | 174 | 226 | |
| Model 1: HR (95% CI)* | 1 (referent) | 0.99 (0.85 to 1.16) | 0.94 (0.79 to 1.11) | 1.32 (1.13 to 1.54) | <.001 |
| Model 2: HR (95% CI)† | 1 (referent) | 0.94 (0.80 to 1.12) | 0.91 (0.75 to 1.10) | 1.21 (1.00 to 1.47) | .02 |
| Plant-rich, low-carbohydrate diet | |||||
| No. of events (n = 817) | 232 | 218 | 210 | 157 | |
| Model 1: HR (95% CI)* | 1 (referent) | 0.92 (0.79 to 1.07) | 0.85 (0.72 to 0.99) | 0.71 (0.60 to 0.84) | <.001 |
| Model 2: HR (95% CI)† | 1 (referent) | 0.90 (0.77 to 1.06) | 0.84 (0.71 to 0.99) | 0.70 (0.58 to 0.84) | <.001 |
*Cox proportional hazards regression model stratified by sex and adjusted for age at diagnosis (continuous) and cancer stage (I, II, III, and unspecified). BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; IQR = interquartile range; MET = metabolic equivalent; NHS = Nurses’ Health Study.
†Further adjusted for year of diagnosis (continuous), subsite (proximal colon, distal colon, rectum, and unspecified), prediagnostic, low-carbohydrate diet score (continuous), postdiagnostic alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-h/wk; men: <7, 7–14.9, 15–24.9, ≥25 MET-h/wk), regular use of aspirin (yes or no), total energy intake, and total fiber intake (in quartiles).
For individual macronutrient analysis, we used a nutrient density model with adjustment for total energy and an alternative macronutrient so that the regression coefficient reflected the isocaloric replacement effect (20). For example, for fat intake, we adjusted for protein and thus the coefficient reflects the substitution effect of an equal amount of energy from fat for carbohydrate. Mutual adjustment was performed when macronutrient from animal and plant food sources was examined separately. We calculated the HR per one-SD increment in both the postdiagnostic intakes and the pre-to-postdiagnosis changes. Ptrend was calculated using the continuous exposure.
We first performed the analysis in the NHS and HPFS separately, and because no appreciable difference was detected by cohort (Supplementary Table 4, available online), we conducted the pooled analysis using the sex-stratified Cox regression. As a secondary analysis, we also stratified by the potential confounding lifestyle and clinicopathological factors, including age at diagnosis, year of diagnosis, smoking, alcohol consumption, body mass index (BMI), physical activity, regular aspirin use, total carbohydrate intake, dietary glycemic load, and tumor stage and subsite. Likelihood ratio test was used to calculate the Pinteraction. We used SAS 9.4 for all analyses (SAS Institute, Cary, NC). All statistical tests were two-sided.
Results
Basic Characteristics of Participants
Among 1542 eligible patients diagnosed with stage I to III CRC, we documented 817 deaths, of which 185 were classified as CRC-specific deaths over a median of 9 years of follow-up. Other major causes of death included cardiovascular diseases (n = 178) and cancers other than CRC (n = 125). Participants with a higher overall low-carbohydrate diet score had a higher BMI and were less physically active and more likely to smoke (Table 1). They also consumed more fat and protein, particularly from red/processed meat, but less fiber and folate. Similar patterns were observed according to the animal-rich, low-carbohydrate diet score. For the plant-rich, low-carbohydrate diet, patients with a higher score were younger and more likely to be females and physically active, whereas the red and white meat intake was similar among patients in the extreme quartiles. In a subset of patients with chemotherapy data (n = 343), the low-carbohydrate diet scores and macronutrient intakes were similar among those who received chemotherapy and those who did not (Supplementary Table 5, available online).
Low-Carbohydrate Diet Scores After Diagnosis and Survival
We did not find any association between the overall low-carbohydrate diet score and mortality (Table 2). In contrast, higher plant-rich, low-carbohydrate diet score was strongly associated with better survival, with the HRs comparing extreme quartiles of 0.37 (95% CI = 0.25 to 0.57, Ptrend < .001) for CRC-specific mortality and 0.70 (95% CI = 0.58 to 0.84, Ptrend < .001) for all-cause mortality. For animal-rich, low-carbohydrate score, participants in the highest quartile had higher all-cause mortality than those in the lowest quartile (HR = 1.21, 95% CI = 1.00 to 1.47, Ptrend = .02), whereas no association was found for CRC-specific mortality (HR comparing extreme quartiles = 1.15, 95% CI = 0.78 to 1.70, Ptrend = .61).
In the sensitivity analysis, we further adjusted for other dietary factors that may influence CRC prognosis, including marine omega-3 fatty acid, vitamin D, folate, and calcium intake. The results remained essentially unchanged. Similar findings were obtained when additionally adjusting for a Western dietary pattern. We also did not observe any material change when the 196 patients without stage information were excluded. Furthermore, to assess the possibility of reverse causation due to occult recurrences or other undiagnosed major illnesses that could influence dietary intake, we excluded the first year of follow-up and observed similar results. Finally, to examine the impact of competing risk, we used the subdistribution hazard method (21) and observed similar associations. All the results of the sensitivity analyses are summarized in Supplementary Table 6 (available online).
Macronutrient Intake After Diagnosis and Survival
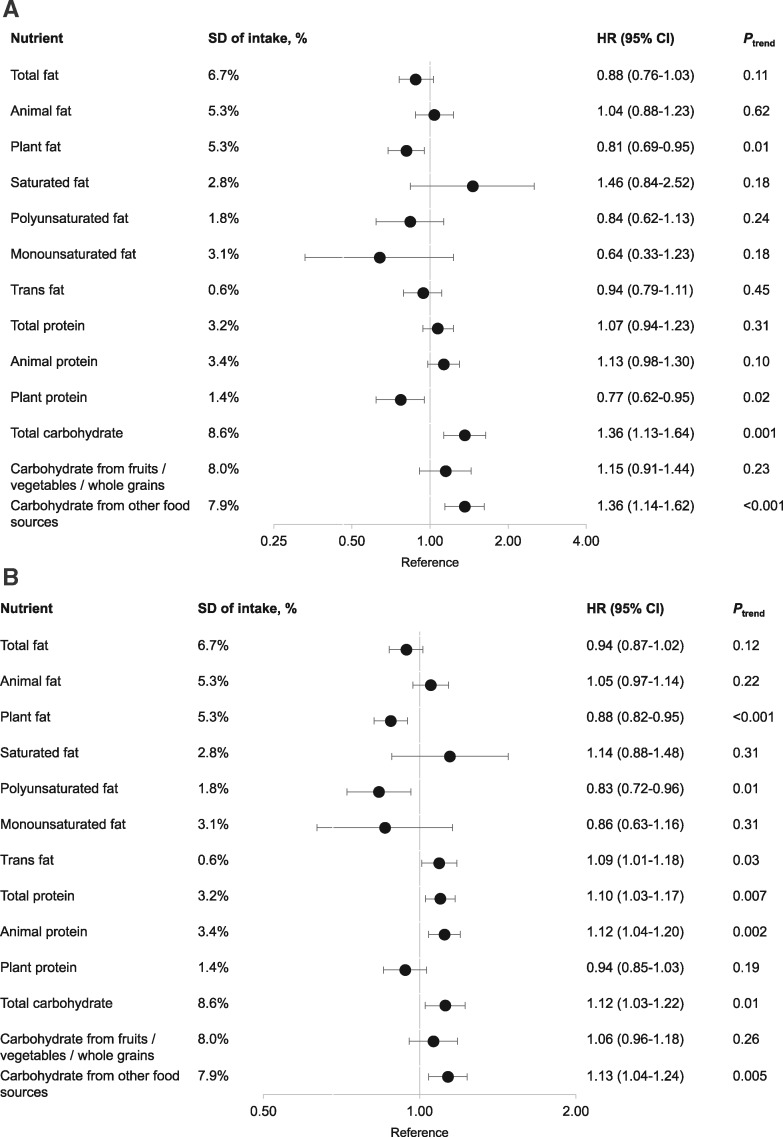
We then examined the association between mortality and each macronutrient separately (Figure 1 , detailed data in Supplementary Tables 7 and 8, available online). For CRC-specific mortality, substituting plant fat and protein for carbohydrate was associated with lower risk, with the HR per one-SD increment of 0.81 (95% CI = 0.69 to 0.95, Ptrend = .01) for plant fat and 0.77 (95% CI = 0.62 to 0.95, Ptrend = .02) for plant protein. In contrast, substituting carbohydrate for fat was associated with higher CRC mortality (HR = 1.36, 95% CI = 1.13 to 1.64, Ptrend = .001), and this association was restricted to carbohydrate consumed from refined starches and sugars (HR = 1.36, 95% CI = 1.14 to 1.62, Ptrend < .001). No association was found for other macronutrients.
Figure 1.
Association of postdiagnostic macronutrient intake and colorectal cancer (CRC)-specific mortality (A) and all-cause mortality (B) among CRC patients in the Nurses’ Health Study and the Health Professionals Follow-up Study cohorts. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CI) per one-SD increment in intake, expressed as percentage of total energy intake, are presented on the axis with log(2) scale. The HRs were derived from the Cox proportional hazards regression model stratified by sex and adjusted for age at diagnosis (continuous), cancer stage (I, II, III, and unspecified), year of diagnosis (continuous), subsite (proximal colon, distal colon, rectum, and unspecified), prediagnostic intake of the nutrient under analysis (continuous), postdiagnosis intake of total calories (continuous), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-h/wk; men: <7, 7–14.9, 15–24.9, ≥25 MET-h/wk), regular use of aspirin (yes or no), and total fiber intake (in quartiles). For the analysis of fat and carbohydrate, we further adjusted for postdiagnostic protein intake (continuous); for the analysis of protein, we further adjusted for postdiagnostic total fat intake. Animal and plant fat were adjusted for each other; animal and plant protein were adjusted for each other; carbohydrates from fruits, vegetables, whole grains, and other food sources were adjusted for each other.
Similar associations were observed for all-cause mortality. In addition, we found a statistically significant positive association with trans fat, and total and animal protein; and an inverse association with polyunsaturated fat.
Change in Macronutrient Intake After Diagnosis with Survival
Pre- and postdiagnosis macronutrient intakes were modestly correlated (Spearman correlation coefficient ranged from 0.39 to 0.59; Supplementary Table 9, available online). We assessed whether change in macronutrient intake after diagnosis was associated with mortality. As shown in Table 3, increased intake of plant fat and protein after diagnosis was associated with lower CRC-specific mortality (for plant fat: HR per one SD = 0.78, 95% CI = 0.65 to 0.93, Ptrend = .005; for plant protein: HR = 0.76, 95% CI = 0.63 to 0.92, Ptrend = .004), whereas increased intake of carbohydrate was associated with higher mortality (HR = 1.22, 95% CI = 1.02 to 1.47, Ptrend = .03). Similar findings were observed for all-cause mortality.
Table 3.
Change in macronutrient intake after diagnosis (per one SD) and CRC-specific mortality and all-cause mortality among CRC patients in the NHS and HPFS cohorts
| Macronutrient | SD of change in intake after diagnosis, % of energy | HR (95% CI) for CRC-specific mortality* | HR (95% CI) for all-cause mortality* |
|---|---|---|---|
| Total fat | 6.5 | 0.92 (0.79 to 1.08) | 0.95 (0.88 to 1.03) |
| Animal fat | 5.4 | 1.18 (0.98 to 1.43) | 1.13 (1.04 to 1.24) |
| Plant fat | 5.6 | 0.78 (0.65 to 0.93) | 0.86 (0.79 to 0.93) |
| Saturated fat | 2.7 | 1.11 (0.91 to 1.34) | 1.05 (0.96 to 1.15) |
| Polyunsaturated fat | 1.9 | 0.85 (0.71 to 1.01) | 0.89 (0.81 to 0.96) |
| Monounsaturated fat | 3.3 | 0.88 (0.71 to 1.09) | 0.92 (0.84 to 1.01) |
| Trans fat | 0.6 | 0.92 (0.80 to 1.07) | 1.07 (1.00 to 1.15) |
| Total protein | 3.3 | 1.08 (0.93 to 1.25) | 1.08 (1.01 to 1.16) |
| Animal protein | 3.4 | 1.18 (1.01 to 1.37) | 1.11 (1.03 to 1.19) |
| Plant protein | 1.2 | 0.76 (0.63 to 0.92) | 0.91 (0.84 to 0.99) |
| Total carbohydrate | 8.2 | 1.22 (1.02 to 1.47) | 1.06 (0.97 to 1.16) |
| Carbohydrate from fruits/vegetables/whole grains | 6.7 | 1.18 (0.97 to 1.43) | 1.06 (0.97 to 1.17) |
| Carbohydrate from refined starches/sugars | 7.7 | 1.26 (1.05 to 1.52) | 1.06 (0.97 to 1.16) |
*Cox proportional hazards regression model stratified by sex and adjusted for age at diagnosis (continuous), cancer stage (I, II, III, and unspecified), year of diagnosis (continuous), subsite (proximal colon, distal colon, rectum, and unspecified), prediagnostic intake of the nutrient under analysis (continuous), postdiagnosis intake of total calories (continuous), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-h/wk; men: <7, 7–14.9, 15–24.9, ≥25 MET-h/wk), regular use of aspirin (yes or no), and total fiber intake (in quartiles). For the analysis of fat and carbohydrate, we further adjusted for the change in protein intake (continuous) after diagnosis; for the analysis of protein, we further adjusted for change in total fat intake. Animal and plant fat were adjusted for each other; animal and plant protein were adjusted for each other; carbohydrate from fruits/vegetables/whole grains and from refined starches/sugars was adjust for each other. BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; MET = metabolic equivalent; NHS = Nurses’ Health Study.
Plant-Rich, Low-Carbohydrate Diet Score and Survival Within Subgroups
In the stratified analysis according to clinical and lifestyle factors (Supplementary Table 10, available online), we found a statistically significant interaction between plant-rich, low-carbohydrate diet score and alcohol consumption (Pinteraction = .007); the HRs of CRC mortality comparing extreme quartiles of the diet score were 0.29 (95% CI = 0.16 to 0.53, Ptrend < .001) and 1.01 (95% CI = 0.39 to 2.59, Ptrend = .76) among patients consuming less than and at least 7 g/d of alcohol, respectively. No interaction was detected with other variables, including cancer stage and subsite.
Discussion
In this prospective study of 1542 stage I-III CRC patients, we found that a higher plant-rich, low-carbohydrate diet score was associated with lower mortality. Higher consumption of carbohydrate, primarily from refined grains and sugars, was associated with worse survival, whereas substituting plant fat and protein for carbohydrate was associated with better survival. Patients who increased their intake of plant fat and protein after diagnosis had a better survival, whereas those who increased their carbohydrate intake had a worse survival.
To our knowledge, only one study has examined the prognostic association of carbohydrate intake in stage III colon cancer patients (9). Consistent with our findings, higher carbohydrate intake was associated with worse survival, and this association appeared to be driven by foods with high glycemic index. Mechanistically, high dietary glycemic load and carbohydrate intake may result in increased blood glucose concentrations that in turn stimulate insulin production (22,23). Insulin can directly promote cell proliferation and inhibit apoptosis and also indirectly enhance carcinogenesis through bioactive IGF1 by decreasing levels of IGF binding proteins (24). Higher levels of circulating C-peptide, a marker of longer-term insulin production, have been linked to increased mortality in nonmetastatic CRC patients (5). Moreover, major predictors of hyperinsulinemia, including obesity (25), sedentary lifestyle (26), and Western dietary pattern (27), have all been associated with an increased risk of cancer recurrence and death among CRC patients. Recently, we reported that higher intake of fiber and whole grains, which have insulin-sensitizing properties, was associated with better survival after CRC diagnosis (28). These findings together support the hypothesis that high intake of carbohydrate, primarily from refined starches and sugars, may promote cancer cell proliferation and inhibit apoptosis of micrometastases by increasing glucose availability and insulin production.
In addition to carbohydrate, we also performed a comprehensive analysis of macronutrient composition by studying the low-carbohydrate diet scores and also isocaloric substitution of individual macronutrients. Particularly, to facilitate clinical translation, we considered different food sources of macronutrients. Although the overall low-carbohydrate diet score and intake of total fat and protein did not show any association with CRC-specific mortality, reducing carbohydrate intake in a plant-rich, diet with moderate consumption of animal products (as shown in Table 1) was associated with substantially improved survival. These findings are in line with other prospective studies that found a beneficial association between plant-rich, rather than overall, low-carbohydrate diet and lower risk of death (12), type 2 diabetes (17), and cardiovascular disease (11). These data indicate that, besides the composition and amount of intake, food source is another critical predictor for the health effect of macronutrients and that other components in foods (eg, nitrates and nitrites in processed meats), in addition to macronutrients per se, may have a role.
Mechanistically, a high-protein, low-carbohydrate diet enriched with animal foods has been shown to decrease cancer-protective metabolites found in stool and increase cancer-promoting metabolites (29). In contrast, food sources of plant fats (eg, oils and nuts) may reduce circulating insulin and markers of inflammation and glycemic control (30,31), all of which may affect CRC progression (5,32,33). Moreover, unlike animal protein, plant protein has not been associated with increased levels of IGF1 (34,35), an important promoter of tumor growth (36), and has been suggested to improve insulin sensitivity (37). Therefore, given that cancer patients are at particularly high risk of developing insulin resistance and hyperglycemia (due to either preexisting metabolic syndrome or cancer-induced metabolic reprograming) (38), it is possible that replacing carbohydrate, especially that consumed from refined food sources, with plant fat and protein may improve glycemic control and mitigate cancer-related metabolic disturbances, leading to a better survival after CRC diagnosis. On the other hand, for participants adhering to a general or animal-rich, low-carbohydrate diet, the increased consumption of animal products may offset any benefit conferred by lowered intake of carbohydrate.
Our study has several strengths, including the prospective design, long-term follow-up, and detailed collection of pre- and postdiagnostic data that allows for adjustment for various potential confounding factors and characterization of the associations of postdiagnosis intake of different macronutrients according to food sources and their pre- to postdiagnosis changes with survival outcomes.
Some limitations are worth noting. First, the low-carbohydrate diet scores were not designed to mimic any particular versions of low-carbohydrate diets available in the literature. Therefore, our findings do not directly translate to the assessment of benefit or risk associated with the popular versions of the diet (12). Second, detailed treatment data were largely unavailable in the cohorts. However, among a subset of patients with chemotherapy data, we did not find any difference in macronutrient intake according to the use of chemotherapy. Moreover, adjuvant treatment was largely standardized during the time period of the study and primarily correlated with disease stage. Thus, our ability to control for stage minimized any potential confounding by treatment. In addition, our stratified analysis showed a similar association between higher plant-rich, low-carbohydrate diet score and better survival for patients at different stages and for those who were diagnosed before and after 2004, when the routine use of adjuvant chemotherapy (eg, FOLFOX and FOLFIRI) was standardized in clinical practice (Supplementary Table 10, available online). Third, given the observational design, we cannot exclude the possibility of residual confounding, although our results appeared to be robust to adjustment for various major risk factors of mortality. Also, because all participants were health professionals, any residual cofounding may have likely been minimized.
In conclusion, among patients with nonmetastatic CRC, consumption of a plant-rich, low-carbohydrate diet was associated with a lower risk of CRC-specific and all-cause mortality. Substituting plant fat and protein for carbohydrate, particularly that from refined starches and sugars, may improve patients’ survival.
Supplementary Material
Funding
This work was supported by the American Cancer Society (grant no. MRSG-17–220–01 – NEC to MS); the 2017 AACR-AstraZeneca Fellowship in Immuno-oncology Research (grant no. 17–40–12-SONG to MS); the US National Institutes of Health (NIH) grants (K99 CA215314 and R00 CA215314 to MS; U01 CA167552 to L. A. Mucci and W. C. Willett; P01 CA87969 to M. J. Stampfer; UM1 CA186107 to M. J. Stampfer; P01 CA55075 to W. C. Willett; UM1 CA167552 to W. C. Willett; P50 CA127003 to CSF; K24 DK098311, R01 CA137178, R01 CA202704, and R01 CA176272 to ATC; R01 CA151993 and R35 CA197735 to SO); and by grants from the American Institute for Cancer Research (KW), the Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. Dr Chan is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Notes
Affiliations of authors: Department of Epidemiology (MS, SO, ELG) and Department of Nutrition (MS, KW, MW, ELG), Harvard T.H. Chan School of Public Health, Boston, MA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA (MS, ATC); Division of Gastroenterology, Massachusetts General Hospital, Boston, MA (MS, ATC); Department of Oncologic Pathology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA (JAM, SO); Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA (OY); Department of Pathology, Massachusetts General Hospital, Boston, MA (OY); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA (MW); Channing Division of Network Medicine, Department of Medicine (MW, ELG, ATC) and Program in MPE Molecular Pathological Epidemiology, Department of Pathology (SO), Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA; Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, MA (SO, ATC); Yale Cancer Center, New Haven, CT (CSF); Department of Medicine, Yale School of Medicine, New Haven, CT (CSF); Smilow Cancer Hospital, New Haven, CT (CSF); Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, MA (ATC).
We thank the participants and staff of the NHS and the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. Thethors assume full responsibility for analyses and interpretation of these data.
Andrew T. Chan previously served as a consultant for Bayer Pharma AG, Pfizer Inc, and Janssen for work unrelated to the topic of this manuscript. This study was not funded by Bayer Pharma AG, Janssen, or Pfizer Inc. No other conflict of interest exists.
Author contributions: study concept and design: MS, KW, JAM, OY, ELG, ATC; acquisition of data: MS, KW, JAM, SO, CSF, ELG, ATC; analysis and interpretation of data: MS, JAM, MW, CSF, ELG, ATC; drafting of the manuscript: MS; critical revision of the manuscript for important intellectual content: MS, KW, JAM, OY, MW, SO, CSF, ELG, ATC; statistical analysis: MS, MW; obtained funding: KW, SO, CSF, ELG, ATC; administrative, technical, or material support: SO, CSF, ELG, ATC; study supervision: ELG, ATC.
References
- 1. Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;673:177–193. [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;664:271–289. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, DeSantis C, Virgo K et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;624:220–241. [DOI] [PubMed] [Google Scholar]
- 4. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;44:257–262. [DOI] [PubMed] [Google Scholar]
- 5. Wolpin BM, Meyerhardt JA, Chan AT et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;272:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng F, Hu D, Lin X et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Int J Cancer. 2016;13912:2705–2713. [DOI] [PubMed] [Google Scholar]
- 7. Yang IP, Tsai HL, Huang CW et al. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016;714:18837–18850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson RS, Amdur RL, White JC et al. Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg. 2012;2141:68–80. [DOI] [PubMed] [Google Scholar]
- 9. Meyerhardt JA, Sato K, Niedzwiecki D et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst. 2012;10422:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song M, Fung TT, Hu FB et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;17610:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halton TL, Willett WC, Liu S et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;35519:1991–2002. [DOI] [PubMed] [Google Scholar]
- 12. Fung TT, van Dam RM, Hankinson SE et al. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;1535:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rimm EB, Giovannucci EL, Willett WC et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;3388765:464–468. [DOI] [PubMed] [Google Scholar]
- 14. Colditz GA, Manson JE, Hankinson SE. The nurses’ health study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;61:49–62. [DOI] [PubMed] [Google Scholar]
- 15. Edge SB, Byrd DR, Compton CC et al. AJCC cancer staging manual. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti III A, eds. American Joint Committee on Cancer, (ed). 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 16. Stampfer MJ, Willett WC, Speizer FE et al. Test of the National Death Index. Am J Epidemiol. 1984;1195:837–839. [DOI] [PubMed] [Google Scholar]
- 17. Halton TL, Liu S, Manson JE et al. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008;872:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cain KC, Harlow SD, Little RJ et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol. 2011;1739:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;223:278–295. [DOI] [PubMed] [Google Scholar]
- 20. Willett WC. Implications of total energy intake for epidemiologic analyses. In: Willett WC, ed. Nutritional Epidemiology. 3rd ed New York, NY: Oxford University Press; 2013. [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94446:496–509. [Google Scholar]
- 22. Solomon TP, Haus JM, Kelly KR et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;926:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Runchey SS, Pollak MN, Valsta LM et al. Glycemic load effect on fasting and post-prandial serum glucose, insulin, IGF-1 and IGFBP-3 in a randomized, controlled feeding study. Eur J Clin Nutr. 2012;6610:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;863:s836–s842. [DOI] [PubMed] [Google Scholar]
- 25. Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA et al. Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. J Clin Oncol. 2016;34:3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;257:1293–1311. [DOI] [PubMed] [Google Scholar]
- 27. Meyerhardt JA, Niedzwiecki D, Hollis D et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;2987:754–764. [DOI] [PubMed] [Google Scholar]
- 28. Song M, Wu K, Meyerhardt JA et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018;41:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russell WR, Gratz SW, Duncan SH et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;935:1062–1072. [DOI] [PubMed] [Google Scholar]
- 30. Viguiliouk E, Kendall CW, Blanco Mejia S et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;97:e103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez-Gonzalez MA, Salas-Salvado J, Estruch R et al. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis. 2015;581:50–60. [DOI] [PubMed] [Google Scholar]
- 32. Volkova E, Willis JA, Wells JE et al. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;1041:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheridan J, Wang LM, Tosetto M et al. Nuclear oxidative damage correlates with poor survival in colorectal cancer. Br J Cancer. 2009;1002:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen NE, Appleby PN, Davey GK et al. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002;1111:1441–1448. [PubMed] [Google Scholar]
- 35. Holmes MD, Pollak MN, Willett WC et al. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;119:852–861. [PubMed] [Google Scholar]
- 36. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;812:915–928. [DOI] [PubMed] [Google Scholar]
- 37. Tremblay F, Lavigne C, Jacques H et al. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. [DOI] [PubMed] [Google Scholar]
- 38. Seyfried TN, Flores RE, Poff AM et al. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;353:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.