The kidney plays an important role in the long-term control of blood pressure and is the major organ involved in the regulation of sodium homeostasis.1 The inappropriate sodium retention in hypertension results from enhanced renal sodium transport per se and/or a failure to respond appropriately to signals that decrease renal sodium transport in the face of increased sodium intake. Humans with polygenic essential hypertension have increased renal sodium transport that is not properly regulated by natriuretic and antinatriuretic hormones and humoral factors, including dopamine and angiotensin II (Ang II). Dopamine and Ang II exert their effects via G protein-coupled receptors (GPCRs).1–3
GPCRs constitute by far the largest receptor family in mammals, which are encoded by more than 800 genes in the human genome, and play a vital role in the regulation of most cellular and physiological functions in the body.4 Upon ligand binding, GPCRs regulate and modulate a variety of cell functions by coupling to heterotrimeric G proteins and regulating downstream effectors such as adenylyl cyclases (AC), phospholipases, protein kinases, and ion channels.5 Activation of renal GPCRs, including dopamine and Ang II receptors, leads to either natriuresis (sodium excretion) or anti-natriuresis (sodium retention), keeping a normal sodium balance, resulting in the maintenance of a normal blood pressure.2,3
GPCR kinases (GRKs) constitute a family of seven serine/threonine protein kinases characterized by their ability to specifically recognize and phosphorylate agonist-activated GPCRs.6 GRK-mediated receptor phosphorylation is one of the well-characterized mechanisms for GPCR desensitization. In the process of GPCR desensitization, GRKs phosphorylate agonist-bound receptors, leading to the translocation and binding of arrestins to the receptors and inhibition of subsequent receptor activation by blocking GPCR-G protein coupling. In particular, GRK4 appears to play a vital role in regulating dopamine-mediated natriuresis and renin-angiotensin system (RAS)-mediated anti-natriuresis.7 Increasing number of studies show that GRK4 is associated with hypertension and blood pressure response to antihypertensive medicines and adverse cardiovascular outcomes of antihypertensive treatment.8–12 In this report, we review our evolving understanding of the role of GRK4 in the regulation of dopamine and Ang II receptor function, which advances our understanding of the role of GRK4 in the control of blood pressure and highlights potential and novel strategies for the prevention and treatment of hypertension.
Physiological role of intrarenal dopamine and RAS
Dopamine, via five subtypes of receptors, plays an important role in the control of blood pressure by regulating epithelial sodium transport, vascular smooth muscle (VSM) contractility, inflammation, and production of reactive oxygen species (ROS), and by interacting with the renin-angiotensin and sympathetic nervous systems.1,3,7 Dopamine receptors are classified into D1- and D2-like receptor subtypes: D1-like receptors (D1R and D5R) couple to stimulatory G protein GαS and stimulate AC activity, whereas D2-like receptors (D2R, D3R, and D4R) couple to inhibitory G protein Gαi/Gαo and inhibit AC activity. Disruption of any of the dopamine receptor genes in mice results in hypertension, the pathogenesis of which is specific for each receptor subtype.13
The RAS is classically known as a coordinated hormonal cascade regulating blood pressure as well as electrolyte and fluid homeostasis.1,2 Ang II is a biologically active octapeptide that is considered as the main mediator of classic RAS. Ang II exerts its action through two major receptor subtypes, namely type 1 (AT1R) and type 2 (AT2R). AT1R mediates the vast majority of cardiovascular and renal actions of Ang II, including vasoconstriction, renal tubule sodium reabsorption, ROS generation, and inflammation.1,14,15 In contrast, activation of the AT2R induces vasodilatation, promotes natriuresis, and lowers blood pressure.16
The activity of dopamine receptors and AT1R is regulated by phosphorylation/ dephosphorylation, which is mediated by GRKs and protein phosphatases, respectively. Basal protein phosphatase 2A (PP2A) activity in renal proximal tubules (RPTs) is not different between the normotensive Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs), which have impaired D1R function.17 D1-like receptor agonist treatment of RPT membranes from SHRs failed to increase PP2A activity; an impaired ability to increase PP2A activity would result in continued phosphorylation and desensitization of the D1R.18 However, the GRKs have received, by far, the most attention in the regulation of renal dopamine and Ang II receptors in hypertension.
GPCR kinase family
Classification of GRKs
There are over 800 known GPCRs in the human genome but only seven GRKs have been identified. All GRKs have a similar general structure: a highly conserved central protein kinase domain, inserted in the regulator of G protein signaling homology domain that keeps the ability of the kinase domain to phosphorylate activated GPCRs. The first 20 or so amino acids of GRKs are highly conserved while the carboxy tail region is GRK subtype-specific: prenylated in the GRK1 subfamily; binds to Gβγ and contains a pleckstrin homology domain in the GRK2 subfamily; and has a C-terminal helix/palmitoylation site in the GRK4 subfamily.19 The GRK1 subfamily (opsin kinase family) consists of GRK1 and GRK7; GRK2-like subfamily (β-adrenergic receptor kinase family) consists of GRK2 and GRK3; and the GRK4-like subfamily consists of GRK4, GRK5, and GRK6. GRK1 and GRK7 are found almost exclusively in the retina and modulate opsins. GRK2, GRK3, GRK5, and GRK6 are ubiquitously expressed, while GRK4 is expressed in only a few organs (vide infra).20–22
GRKs and hypertension
GRKs have multiple physiological effects on the regulation of blood pressure. VSM overexpression of GRK2 in transgenic mice attenuates β-adrenergic receptor-induced vasodilation and increases resting blood pressure.23 GRK2 expression and GRK activity are increased in both the lymphocytes and VSMs of patients with essential hypertension and in SHRs.24 GRK2 hemizygous knockout mice have increased nitric oxide bioavailability that protects against Ang II-induced hypertension.25 The transgenic mice with VSM-specific GRK5 overexpression are hypertensive.26 By contrast, GRK3 expression in human lymphocytes has been reported to be inversely correlated with blood pressure, suggesting a protective role for GRK3 in the regulation of blood pressure that is supported by the findings in transgenic mice.27 Overexpression of GRK2, GRK3, and GRK5 in human embryonic kidney (HEK-293) cells desensitizes the D1R.28 Inhibition of GRK6 prevents intestinal D1R desensitization.29 However, renal GRK6 levels are lower in hypertensive subjects and SHRs than their normotensive controls.30
Role of GRK4 in hypertension
GRK4 isoforms
GRK4 has some inherent characteristics. For example, GRK4 has constitutive activity under basal conditions, which may, in part, be due to its ability to bind to inactive GαS and Gα13 subunits.31 It is the only GRK subtype that is capable of phosphorylating unstimulated GPCRs.32 GRK4 is expressed in a limited number of tissues, e.g., artery, bone, cerebellum, heart, kidney, myometrium, small intestines, and testes, unlike GRK2, GRK3, GRK5, and GRK6, which are ubiquitously expressed. Moreover, four splice variants (GRK4α, β, γ and δ) of GRK4 have been identified in humans.21 Alternative splicing generates 4 isoforms of human GRK4 mRNA that differ in the presence or absence of exon 2 and exon 15: GRK4α is the longest isoform and contains all of the 16 exons; GRK4β, which lacks exon 2, has a 32-codon-deletion that encompasses the phosphatidylinositol bisphosphate binding domain near the amino terminus; GRK4γ, which lacks exon 15, has a 46-codon-deletion near the carboxyl terminus; and GRK4δ, lacks both exons 2 and 15.21 The human GRK4 gene locus at 4p16.3 is linked to hypertension.33 Numerous studies show that abnormal GRK4 function has the potential to affect GPCR (such as D1R, D3R, and AT1R)-regulated biological responses in many physiological and pathological conditions such as hypertension,1,3,7,13,20,22,33–38 which makes GRK4 as an attractive candidate for a genetic determinant for essential hypertension.
Distribution of GRK4 in kidney and artery
GRK4 is expressed in the rat renal cortex.34 In both WKY and SHRs, GRK4 is expressed in the subapical membranes of the RPT, thick ascending limb of Henle, and renal artery, with much less expression in the glomerulus. Renal cortical GRK4 expression is increased in SHRs compared with WKY rats, while cardiac GRK4 expression is similar in the two rat strains, indicating that the increased GRK4 expression in hypertension has organ specificity.34 In mice, the renal expression of GRK4 is strain-dependent and influenced by salt intake, e.g., lower on normal but higher on high salt diet in C57BL/6J than in SJL/J mice. C57BL/6 mice are salt-sensitive and have an impaired ability to excrete a NaCl load that is associated with an increase in blood pressure whereas SJL/J mice are salt-resistant.35 All four GRK4 isoforms are expressed abundantly in human RPT cells; GRK4 is localized at the RPT cell surface membrane and cytoplasm, and internalized after stimulation of dopamine receptors.20,36
GRK4 activity is increased in kidneys of hypertensive humans20. Antisense GRK4 oligonucleotides completely blocked the constitutive serine phosphorylation of the D1R and restored the ability of the D1-like receptor agonist, fenoldopam, to stimulate cAMP accumulation in RPT cells from hypertensive subjects, which suggests that the major GRK involved in the phosphorylation of the D1R in hypertension is GRK4 and not the other GRKs.20 However, there are no differences in the expression of the GRK4 isoforms (α/β, γ/δ) in kidneys or cultured RPT cells between hypertensive and normotensive subjects.20 Therefore, we assume that the increased activity of GRK4 in the kidney of hypertensive subjects is not caused by increased renal GRK4 protein expression but rather by constitutively active variants of GRK4.
In Sprague-Dawley rats and C57BL/6J mice, GRK4 is well-expressed in large and small arterial vessels, including the carotid arteries, thoracic aorta, superior mesenteric artery, and renal artery.22 In the aorta, GRK4 is expressed in the tunica media and adventitia. However, removal of the adventitia does not affect the Ang II–mediated vasoconstriction, suggesting that GRK4 in the adventitia does not participate in Ang II–mediated vasoconstriction.22
Regulation of GRK4
As a regulator of GPCRs, GRK4 per se is regulated by transcription factors and signaling molecules. The GRK4 promoter region, containing 1851 bp of the 5’-flanking region and 275 bp of the 5’-untranslated region, is reported to be highly active.39 The GRK4 core promoter resides in the first 1851 bp upstream of its transcription start site,39 suggesting that the complex DNA-protein and protein-protein interaction patterns at this portion may affect the transcriptional and expression capacities of GRK4. The transcription factor c-Myc, binding to the promoter of GRK4, positively regulates GRK4 protein expression in human RPT cells, which connects aberrant Ang II activation to D1R-AC uncoupling.40 The GRK4 subfamily, including GRK4, is potently inhibited by ubiquitous calcium-binding protein calmodulin (CaM), which has little or no effect on members of other GRK subfamilies.41 Sorting nexins (SNXs) are involved in receptor endocytosis and trafficking through the endosomes. SNX5 directly interacts with GRK4 and prevents GRK4 from targeting the phosphorylation sites of the D1R, which is enhanced after D1R activation. In contrast, depletion of SNX5 markedly increases the ability of GRK4 to constitutively phosphorylate D1R in human RPT cells, consistent with the in vivo studies showing that renal SNX5 depletion increases blood pressure and decreases D1R-mediated sodium excretion.42
GRK4 regulation of D1R and AT1R
Studies have provided direct evidence of a crucial role of renal GRK4 in the D1R-mediated control of sodium excretion and blood pressure in genetic hypertension. GRK4 is more effective than GRK2 in attenuating the D1-like agonist-induced desensitization, suggesting a greater role for GRK4 in the D1R homologous desensitization in human RPT cells.43 Increased GRK4 activity causes an impairment of renal D1R function in hypertension. Both basal GRK4 and serine-phosphorylated D1R levels are much higher in renal cortical membranes of SHRs than WKY rats. Silencing of renal cortical GRK4 decreases serine-phosphorylated D1R to a greater extent in SHRs than WKY rats.34 Depletion of renal cortical GRK4 also increases urine flow and sodium excretion and attenuates the increased blood pressure in SHRs, but does not affect the blood pressure in WKY rats.34
In vitro studies also support the regulation of D1R by GRK4. GRK4 constitutively phosphorylates the D1R in the absence of agonist activation, while depletion of GRK4 blunts the D1R desensitization.43,44 Moreover, increased activity of GRK4 because of constitutively active GRK4 variants, not GRK4 protein abundance, causes the decrease in D1R function in RPT cells from hypertensive subjects, which is restored by GRK4 depletion.20 All four GRK4 isoforms are expressed in RPT cells. GRK4α and not the other GRK4 isoforms can phosphorylate D1R in certain HEK-293 (e.g., HEK293T) cells44, while GRK4γ can phosphorylate D1R in Chinese hamster ovary (CHO) cells20 and HEK293 that do not stably express the SV40 large T antigen (J.J. Gildea, P.A. Jose, R.A. Felder, unpublished data, 2015). The phosphorylation of D3R is regulated by both GRK4α and GRK4γ (GRK4γ > GRK4α) isoforms in human RPT cells.36 These results indicate that the regulatory activity of GRK4 isoforms on the desensitization of dopamine receptors is cell-specific. It should also be noted that the effect of human GRK4α variant on blood pressure in mice has not been studied while the effect of human GRK4γ wild-type and variants on blood pressure has been reported in transgenic mice.20,45
Similar to D1Rs, the AT1R is also regulated by GRK4. Our previous studies have shown that AT1R expression and actions are enhanced in both kidney and artery in transgenic mice expressing GRK4 variants (vide infra).22,46 The increased renal GRK4 expression in old rats is also associated with increased renal AT1R expression and function.47 Interestingly, the renal-selective depletion of AT1R in SHRs does not decrease blood pressure because the interruption of the renin-angiotensin negative feedback loop results in increased circulating renin and Ang II.48 However, combined renal-selective silencing of both GRK4 and AT1R decreases blood pressure, as well as plasma renin activity and Ang II levels in both rat strains,49 which can explain, in part, the crucial role of GRK4 in the regulation of sodium excretion and blood pressure.
Regulation of blood pressure by GRK4 variants
Three missense single nucleotide polymorphisms (SNPs) in the coding region of GRK4γ impair D1R function (Table 1). They are: nucleotide 448, CGT to CTT (amino acid 65R>L, rs2960306); nucleotide 679, GCC to GTC (amino acid 142A>V, rs1024323); and nucleotide 1711, GCG to GTG (amino acid 486A>V, rs1801058). GRK4γ SNPs (65R>L, 142A>V, 486A>V) markedly impair D1R-mediated cAMP accumulation that is not due to differences in the quantity of the expression of either D1R or GRK4γ.20 Human GRK4γ (hGRK4γ) 142V transgenic mice have higher blood pressures and greater heart weights than hGRK4γ wild-type transgenic mice.22,45 Infusion of fenoldopam, a D1-like receptor agonist, increases urine flow and sodium excretion in hGRK4γ wild-type mice but not in the hypertensive hGRK4γ 142V transgenic mice.20 Additional studies showed that the higher blood pressure in hGRK4γ 142V transgenic mice is not due to the transgene integration sites, flanking genes, or copy numbers, but due mainly to the effect of hGRK4γ 142V transgene acting via D1R.45 This is confirmed by in vitro studies: in the transfected CHO cells, GRK4γ 142V increases GRK activity and causes D1R phosphorylation, which may explain, in part, the decreased responsiveness of the D1R in hypertensive hGRK4γ 142V transgenic mice.20 There is however, cell specificity of the ability of GRK4γ to regulate D1R function; GRK4γ regulates D1R function in human RPT, CHO cells20, and HEK293 cells (vide supra) while it is GRK4α in HEK293T cells.44 We also found that the function of D3R is also impaired in the hGRK4γ 142V-transfected human RPT cells (J. Yang, V.A.M. Villar, and P.A. Jose, unpublished data, 2015).
Table 1.
GRK4 Variant Transgenic Mice and in vitro Studies
| GRK4 Variants |
Transgenic Mice | Variants Transduced Cell Studies | |||
|---|---|---|---|---|---|
| Mouse Phenotype | Functional Deficit(s) | Receptor Defect(s) | Cell Line | Receptor Defect(s) | |
| R65L | Salt-sensitive hypertension (high salt diet) |
Not determined | Not determined | CHO | Increased renal D1R phosphorylation and impaired renal D1R function, R65L alone or combined with 486V variant20 |
| A142V | Hypertension (normal salt diet) | Decreased urine flow and sodium excretion20 | Decreased renal expression and responsiveness of D1R;20 | CHO | Increased renal D1R phosphorylation; Impaired renal D1R function20 |
| Increased renal AT1R expression and responsiveness46 | Human RPT cells |
Impaired D3R function | |||
| Increased AT1R-mediated vasoconstriction22 | Higher arterial AT1R expression;22 | Vascular smooth muscles cells |
Increased AT1R expression; Decreased AT1R phosphorylation; Decreased AT1R protein degradation; Increased AT1R function22 |
||
| Increased AT1R-mediated blood pressure response to Ang II infusion and AT1R blockade22 | |||||
| A486V | Salt-sensitive hypertension (high salt diet) |
Impaired pressure-natriuresis plot50 | Increased AT1R expression50 | CHO | Increased renal D1R phosphorylation and impaired renal D1R function20 |
The hypertension in hGRK4γ 142V transgenic mice is also associated with increased AT1R expression and function in both kidney and artery.22,46 GRK4γ 142V transgenic mice have increased renal AT1R expression and function, e.g., increased blood pressure response to Ang II infusion and AT1R blockade. By contrast, GRK4γ 142V transgenic mice that are deficient of AT1R have normal blood pressure.46 We have reported that both AT1R expression and AT1R-mediated vasoconstriction are higher in the aorta of hGRK4γ 142V transgenic mice.22 Moreover, infusion of Ang II causes a greater increase in blood pressure while infusion of the AT1R antagonist candesartan causes a greater decrease in blood pressure in hGRK4γ 142V transgenic mice than GRK4 wild-type transgenic mice.46 AT1R mRNA and protein expression and function are higher in hGRK4γ142V than in GRK4γ wild-type cells, but the opposite is true for AT1R phosphorylation and degradation,22 indicating that the regulation of AT1R expression by hGRK4γ occurs at both transcriptional and post-translational levels.
Depending upon the genetic background and sodium intake, hGRK4γ 486V transgenic mice may develop increased blood pressure. hGRK4γ 486V transgenic mice have increased renal AT1R expression and develop hypertension only after an increase in sodium intake, in contrast to GRK4γ 142V transgenic mice which have increased AT1R expression and develop hypertension even on a normal salt diet.46,50 Depending upon the genetic background of the mouse, hGRK4γ wild-type prevents salt-sensitive hypertension, while hGRK4γ 486V converts a salt-resistant phenotype to a salt-sensitive phenotype.50 hGRK4γ 65L transgenic mice, similar to hGRK4γ 486V transgenic mice, have normal blood pressure on a normal salt diet but have increased blood pressure on high salt diet (L.D. Asico and P.A. Jose, unpublished data, 2015). In vitro studies showed that in single (65L or 486V) or double variant GRK4 (65L/486V)-transfected CHO cells, there is an increase in basal D1R phosphorylation and impairment of D1R-mediated cAMP production.20 The mechanism for the increase in blood pressure in GRK4γ 65L or 486V transgenic mice only when salt intake is increased remains unclear.
GRK4 polymorphisms and hypertension
The GRK4 gene polymorphisms have different allele frequencies among different populations. GRK4 486V is more frequent in Asians and less frequent in African-Americans than in other populations (Hispanics and Caucasians).51 The GRK4 locus on human chromosome 4p16.3 is linked to essential hypertension and salt sensitivity.33 The first report in 2002 by Bengra et al found a significant association between GRK4 A486V variant and an Italian population of mildly hypertensive patients.52 Subsequent studies showed that GRK4 gene variants R65L, A142V, and A486V are each associated with essential hypertension in several ethnic groups (Table 2). In Euro-Australians, GRK4 486V is associated with essential hypertension, while the 65L and 142V variants track with elevation in diastolic blood pressure only in male hypertensives.53 In a Chinese Han population, GRK4 A486V is also associated with hypertension in additive, dominant, and recessive model, while GRK4 142V is associated with hypertension in an additive model only.54,55 However, there are reports that do not show the association between GRK4 variants and hypertension.56,57 In a population of African-Americans 18 to 49 years of age, GRK4 A486V variant was found to be negatively associated with hypertension.58 Although the reasons leading to the differences among studies are not known, the negative studies could be the consequence of not taking into account salt sensitivity (particularly for GRK4 R65L and A486V) or assessing the role of GRK4 in conjunction with other single nucleotide polymorphisms of GRK459 and other genes.55,60,61
Table 2.
Association Studies of GRK4 Variants in Hypertensive or Normotensive Subjects
| GRK4 Variants |
Hypertensive Subjects | Normotensive Subjects | ||||
|---|---|---|---|---|---|---|
| Ethnic Group (Year) |
Single or Multi-locus Analyses | Hypertension Phenotype | Ethnic Group (Year) |
Single or Multi-locus Analyses |
Blood Pressure or Sodium Excretion | |
| R65L (rs2960306) |
Ghanaian (2004) | R65L and ACE | not classified60 | Euro- and African-American (2006) | Single-locus | Increased SBP64 |
| European ancestry (2012) | Single-locus | Salt sensitivity62 | ||||
| Euro- and African-American (2006) | Single-locus | Stress-induced UNaV reduction63 | ||||
| A142V (rs1024323) |
Japanese (2006) | Single-locus | low-renin hypertension59 | Japanese (2006) | R65L, A142V, and A486V | Impaired natriuretic response to dopaminergic stimulation59 |
| Japanese (2006) | A142V and CYP11B2 | low-renin hypertension59 | ||||
| Japanese (2006) | R65L, A142V, and A486V | salt-sensitive hypertension59 | ||||
| A486V (rs1801058) |
Italian (2002) | Single-locus | Mild salt-sesitive hypertension52 |
|||
| Euro- Australian (2004) | Single-locus | not classified53 | ||||
| Chinese (2006) | Single-locus | not classified54 | ||||
| Chinese (2006) | Single-locus | not classified55 | ||||
| African- American (2010) | Single-locus | negative association58 | ||||
| African-derived Brazilian (2012) | A486V and NOS3 | not classified66 | ||||
Abbreviations: SBP, systolic blood pressure; UNaV, Urinary sodium excretion.
Salt-sensitive hypertension is associated with GRK4 gene variants. The Italian patients whose hypertension is associated with GRK4 A486V are actually salt-sensitive.52 In a Japanese population, the GRK4 variants (R65L, A142V, and A486V) are more frequent in salt-sensitive than salt-resistant hypertensive patients.59 A genetic model of GRK4 R65L, A142V, and A486V, is 94.4% predictive of salt sensitivity. By contrast, the single-locus model with only GRK4 A142V is 78.4% predictive, while a 2-locus model of GRK4 A142V and aldosterone synthase CYP11B2 is 77.8% predictive of low-renin hypertension.59 The ability to excrete a salt load is inversely related to the number of GRK4 variant alleles (R65L, A142V, and A486V) in hypertensive Japanese.59 GRK4 variants are also associated with salt sensitivity in normotensive subjects.59,62,63 In black normotensive adolescents, the GRK4 65L allele is associated with a reduced urinary sodium excretion in response to stress.63 In young normotensive twins, GRK4 65L was associated with the steepest increase in blood pressure; the GRK4 65L-142V-A486V haplotype had a 1.05 mm Hg steeper increase in systolic blood pressure per year increase in age, relative to those with GRK4 R65, A142, and A486 haplotype.64 Therefore, genetic variations of GRK4 may contribute to variations of blood pressure in normotensive individuals, potentially influencing the development of hypertension.
As indicated above, GRK4 variants interact with other genes in the pathogenesis of hypertension. Multilocus analyses have shown association between GRK4 variants and other gene variants with high blood pressure. In an African population from Ghana, the combination of angiotensin-converting enzyme (ACE) and GRK4 R65L is the best genetic model to predict hypertension (70.5% prediction of hypertension).60 Among Japanese subjects, the best combination that is predictive of hypertension, not classified according to salt sensitivity, is GRK4, ACE, and CYP11B2, with an estimated prediction success of 63%; however, for low-renin hypertension in Japanese, the single best genetic model includes only GRK4 A142V and CYP11B2, with an estimated prediction success of 77.8%.59,65 Normotensive Japanese with GRK4 polymorphisms were reported to have increased serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels.61 A recent study among African-derived Brazilian populations reported that an interaction between GRK4 A486V and endothelial nitric oxide synthase (NOS3) is associated with increased diastolic blood pressure.66
There are currently two meta-analyses on the associations of GRK4 polymorphisms with hypertension risk. Our previous meta-analysis showed that GRK4 486V increases the risk for essential hypertension with an odds ratio (OR) of 1.5 (95% CI:1.2–1.9).7 A more recent meta-analysis showed that GRK4 486V is inversely associated with hypertension among East Asians (OR=0.39, 95% CI: 0.28–0.55), but positively associated with hypertension among Europeans (OR=2.38, 95% CI:1.38–4.10); GRK4 65L was also associated with hypertension among Europeans.11
GRK4 and pharmacogenomics of antihypertensive medicines
Hypertension is a condition involving the interaction between genetics and environment that includes diet and lifestyle, among others. Although it is generally believed that the heritability of blood pressure is about 30–55%, genome wide association studies (GWAS) have identified less than 5% of the genetic factors believed to be involved in the pathogenesis of hypertension.67 Epigenetics, ethnicity, and low frequency of the variants (e.g., SLC12A3 [thiazide-sensitive sodium/chloride cotransporter], SLC12A1 [sodium-potassium 2 chloride cotransporter]), are some reasons.68 Many of the genes identified in GWAS are also found in non-coding regions. Another limitation of GWAS is the absence of some genes in the chips. For example, GRK4 was not linked to hypertension in the GWAS probably because the GRK4 polymorphisms are not consistently present in the Affymetrix and Illumina chips; GRK4 65L, 142V, and 486V are present only in the Illumina Human 1 M beadchip and GRK4 142V is not present in any of the Affymetrix Chips. These limitations may also be found in GWAS for pharmacogenomics of essential hypertension.69
There are several classes of antihypertensive medicines used in clinic and include calcium channel blockers, drugs that target the renin angiotensin system (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitor), diuretics (thiazides and thiazide-like diuretics, loop diuretics, potassium sparing diuretics), and drugs that target the sympathetic nervous system (β-adrenergic receptor blockers, α-adrenergic receptor blockers, direct vasodilators, central α-adrenergic receptor agonists and adrenergic depleters [e.g., reserpine, probably not used any longer]). The treatment of hypertension is currently not based on pharmacogenetics, but rather based on consensus guidelines.70,71
Current evidence shows that the presence or absence of GRK4 gene variants may be important in guiding therapeutic antihypertensive strategies. In the African-American Study of Kidney Disease and Hypertension Study, male (but not female) African-Americans with GRK4 A142 were found to have a faster decrease in blood pressure response to the β1-adrenoceptor blocker metoprolol but were less responsive to metoprolol in the presence GRK4 65L.9 The Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study involving hypertensive African- and Euro-Americans showed that the presence of GRK4 65L, 142V, and 486V variant alleles in Euro-Americans was associated with reduced response to the β1-adrenergic receptor blocker, atenolol.12 Our studies in hypertensive Japanese subjects show that GRK4 142V is associated with greater decrease in both systolic blood pressure and diastolic blood pressure in response to angiotensin receptor blockers (H. Sanada and P.A. Jose, unpublished data, 2015), which also normalize the increased blood pressure of GRK4γ 142V transgenic mice.46 The lowering of blood pressure with a decrease in dietary salt intake among South African blacks was observed in those with no GRK4 variants or one variant GRK4 65L or GRK4 142V allele; the effect of GRK4 486V was not evaluated.8 However, in a small cohort of Japanese hypertensive subjects, those with GRK4 486V had a good antihypertensive response to low-salt diet or diuretics.72 Patients homozygous to 65L and 142V have been reported to need more antihypertensive treatment, especially diuretics.73 These results suggest that the presence of GRK4 variant alleles may be important determinants in the blood pressure response to antihypertensive drugs or dietary intervention and risk for adverse cardiovascular events.
Conclusions and Perspectives
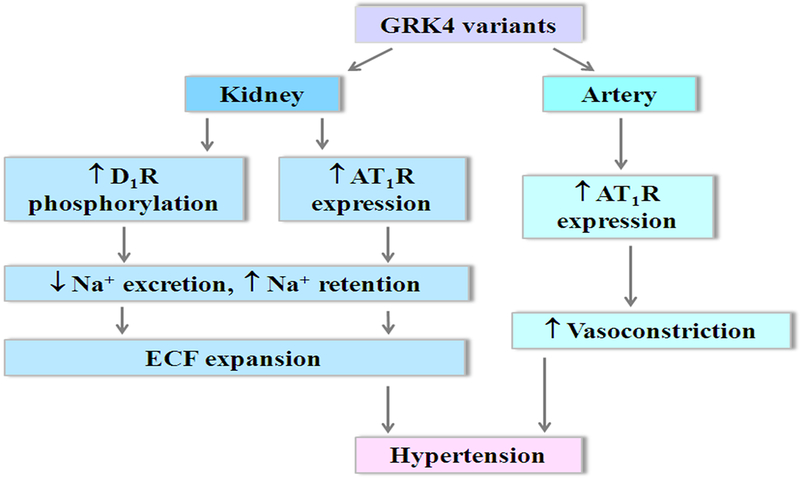
In summary, increasing evidence shows that constitutively active GRK4 variants, specifically the human GRK4γ 65L, 142V, and 486V variants, play a crucial role in regulating the function of the dopamine receptors and AT1R, and are, therefore, involved in the pathogenesis of hypertension (Figure 1). Genetic association has been found between GRK4 gene variants and hypertension. Antihypertensive medicines have different responses in patients with individual GRK4 variants, indicating that GRK4 variants may be important in choosing the initial antihypertensive medication. Increased understanding of GRK4 in the genetics and pharmacogenetics of hypertension may provide therapeutic targets for hypertension in the future.
Figure 1.
Regulation of D1R and AT1R by GRK4 variants. In the kidney, constitutively active GRK4 gene variants cause D1R phosphorylation and desensitization and increase AT1R expression, leading to impaired sodium excretion. In the artery, GRK4 variants increase AT1R expression, resulting in greater vasoconstriction. GRK4-mediated impairment in renal function and increase in vasoconstriction lead to the development of hypertension.
Abbreviations: ECF, extracellular fluid.
Acknowledgments
Sources of Funding
These studies were supported in part by grants from National international technology special grant (2014DFA31070), National Natural Science Foundation of China (81470475, 81270337), and by grants from National Institutes of Health, United States (R37HL023081, R01HL092196, and P01HL074940) and the National Kidney Foundation of Maryland (10013556).
Footnotes
Disclosures
Dr. Jose, who is the Scientific Director of Hypogen, Inc, owns US Patent Number 6,660,474 for G protein–related kinase mutants in essential hypertension. The other authors report no conflicts.
References
- 1.Herrera M, Coffman TM. The kidney and hypertension: novel insights from transgenic models. Curr Opin Nephrol Hypertens. 2012;21:171–178. [DOI] [PubMed] [Google Scholar]
- 2.Shirai A, Yamazaki O, Horita S, Nakamura M, Satoh N, Yamada H, Suzuki M, Kudo A, Kawakami H, Hofmann F, Nishiyama A, Kume H, Enomoto Y, Homma Y, Seki G. Angiotensin II dose-dependently stimulates human renal proximal tubule transport by the nitric oxide/guanosine 3’,5’-cyclic monophosphate pathway. J Am Soc Nephrol. 2014; 25: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep. 2012;14:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. [DOI] [PubMed] [Google Scholar]
- 6.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res. 2011;109:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, Jose PA. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension. 2008;51:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner B, Ramesar R, Steyn K, Levitt N, Lombard C, Charlton K. G-protein-coupled receptor kinase 4 polymorphisms predict blood pressure response to dietary modification in Black patients with mild-to-moderate hypertension. J Hum Hypertens. 2012;26:334–339. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar V, O’Connor DT, Brophy VH, et al. G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. 2009;22:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner F, Malice MP, Wiegert E, McGrath HE, Gildea J, Mitta S, Van Dyck K, De Lepeleire I, Johnson-Levonas AO, Sisk CM, Fernandez R, Greenwalt DM, Beals C, Carey RM, Nunes I. A comparison of the natriuretic and kaliuretic effects of cicletanine and hydrochlorothiazide in prehypertensive and hypertensive humans. J Hypertens. 2012;30:819–827. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Xi B. Pooled analyses of the associations of polymorphisms in the GRK4 and EMILIN1 genes with hypertension risk. Int J Med Sci. 2012;9:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, Beitelshees AL, Turner ST, Chapman AB, Cooper-DeHoff RM, Bailey KR, Boerwinkle E, Pepine CJ, Liggett SB, Johnson JA. G protein receptor kinase 4 polymorphisms: β-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension. 2012;60:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giani JF, Janjulia T, Taylor B, Bernstein EA, Shah K, Shen XZ, McDonough AA, Bernstein KE, Gonzalez-Villalobos RA. Renal generation of angiotensin II and the pathogenesis of hypertension. Curr Hypertens Rep. 2014;16:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen Dinh Cat A, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13:122–128. [DOI] [PubMed] [Google Scholar]
- 16.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 Receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Yu P, Asico LD, Felder RA, Jose PA. Protein phosphatase 2A B56alpha during development in the spontaneously hypertensive rat. Clin Exp Hypertens. 2004;26:243–254. [DOI] [PubMed] [Google Scholar]
- 18.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. [DOI] [PubMed] [Google Scholar]
- 19.Homan KT, Tesmer JJ. Structural insights into G protein-coupled receptor kinase function. Curr Opin Cell Biol. 2014;27:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1996;271:6403–6410. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Fu C, Chen C, Liu L, Ren H, Han Y, Yang J, He D, Zhou L, Yang Z, Zhang L, Jose PA, Zeng C. Role of GRK4 in the regulation of arterial AT1 receptor in hypertension. Hypertension. 2014;63:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. [DOI] [PubMed] [Google Scholar]
- 24.Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, Vasta A, Formisano S, Trimarco B, Iaccarino G. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avendaño MS, Lucas E, Jurado-Pueyo M, Martínez-Revelles S, Vila-Bedmar R, Mayor F Jr, Salaices M, Briones AM, Murga C. Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II-induced hypertension. Hypertension. 2014;63:369–375. [DOI] [PubMed] [Google Scholar]
- 26.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation. 2005; 112:1145–1153. [DOI] [PubMed] [Google Scholar]
- 27.Oliver E, Rovira E, Montó F, Valldecabres C, Julve R, Muedra V, Ruiz N, Barettino D, D’Ocon P. beta-Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. J Hypertens. 2010;28:1281–1289. [DOI] [PubMed] [Google Scholar]
- 28.Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. [DOI] [PubMed] [Google Scholar]
- 29.Fraga S, Luo Y, Jose P, Zandi-Nejad K, Mount DB, Soares-da-Silva P. Dopamine D1-like receptor-mediated inhibition of Cl/HCO3- exchanger activity in rat intestinal epithelial IEC-6 cells is regulated by G protein-coupled receptor kinase 6 (GRK 6). Cell Physiol Biochem. 2006;18:347–360. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Watanabe H, Felder RA, Jose PA. GRK6 in the kidney in human and rat genetic hypertension [abstract]. FASEB J. 2001;15:A774. [Google Scholar]
- 31.Keever LB, Jones JE, Andresen BT. G protein-coupled receptor kinase 4gamma interacts with inactive Galpha(s) and Galpha13. Biochem Biophys Res Commun. 2008;367:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–650. [DOI] [PubMed] [Google Scholar]
- 34.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006; 47:1131–1139. [DOI] [PubMed] [Google Scholar]
- 35.Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660–R1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, Jose PA. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montasser ME, Shimmin LC, Gu D, et al. Variation in genes that regulate blood pressure are associated with glomerular filtration rate in Chinese. PLoS One. 2014;9:e92468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–F304. [DOI] [PubMed] [Google Scholar]
- 39.Hasenkamp S, Telgmann R, Staessen JA, Hagedorn C, Dördelmann C, Bek M, Brand-Herrmann SM, Brand E. Characterization and functional analyses of the human G protein-coupled receptor kinase 4 gene promoter. Hypertension. 2008;52:737–746. [DOI] [PubMed] [Google Scholar]
- 40.Gildea JJ, Tran HT, Van Sciver RE, Bigler Wang D, Carlson JM, Felder RA. A novel role for c-Myc in G protein-coupled receptor kinase 4 (GRK4) transcriptional regulation in human kidney proximal tubule cells. Hypertension. 2013;61:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, DeBlasi A. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta. 2000;1498:112–121. [DOI] [PubMed] [Google Scholar]
- 42.Villar VA, Armando I, Sanada H, Frazer LC, Russo CM, Notario PM, Lee H, Comisky L, Russell HA, Yang Y, Jurgens JA, Jose PA, Jones JE. Novel role of sorting nexin5 in renal D1 dopamine receptor trafficking and function: implications for hypertension. FASEB J. 2013;27:1808–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. [DOI] [PubMed] [Google Scholar]
- 44.Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–769. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4gamma A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–H2092. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Chen S, Asico LD, Escano CS, Villar VA, Lu Q, Coffman TM, Jones JE, Armando I, Felder RA, Jose PA. AT1R dysregulation is crucial in the hypertension of human GRK4 A142V transgenic mice [abstract]. FASEB J. 2009;23:802.7. [Google Scholar]
- 47.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneda M, Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Sasaki M, Katoh T, Watanabe T, Andrews PM, Jose PA, Felder RA. Differential effects of angiotensin II type-1 receptor antisense oligonucleotides on renal function in spontaneously hypertensive rats. Hypertension. 2005;46:58–65. [DOI] [PubMed] [Google Scholar]
- 49.Yatabe J, Sanada H, Midorikawa S, Hashimoto S, Watanabe T, Andrews PM, Armando I, Wang X, Felder RA, Jose PA. Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats. Hypertens Res. 2008;31:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Asico L, Wang X, Escano C, Jose P. Human G protein-coupled receptor kinase type 4γ (GRK4γ) 486V-promoted salt sensitivity in transgenic mice is related with increased AT1 receptor (AT1R) [abstract]. J Am Soc Nephrol. 2007;18:148A. [Google Scholar]
- 51.Lohmueller KE, Wong LJ, Mauney MM, Jiang L, Felder RA, Jose PA, Williams SM. Patterns of genetic variation in the hypertension candidate gene GRK4: ethnic variation and haplotype structure. Ann Hum Genet. 2006;70:27–41. [DOI] [PubMed] [Google Scholar]
- 52.Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–2140. [PubMed] [Google Scholar]
- 53.Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, Morris BJ. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Li B, Zhao W, Liu P, Zhao Q, Chen S, Li H, Gu D. Association study of G protein-coupled receptor kinase 4 gene variants with essential hypertension in northern Han Chinese. Ann Hum Genet. 2006;70:778–783. [DOI] [PubMed] [Google Scholar]
- 55.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. [DOI] [PubMed] [Google Scholar]
- 56.Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, Westerkamp J, Richart T, Thijs L, Li X, Brand-Herrmann SM, Burnier M, Brand E. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–1650. [DOI] [PubMed] [Google Scholar]
- 57.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. [DOI] [PubMed] [Google Scholar]
- 58.Martinez Cantarin MP, Ertel A, Deloach S, Fortina P, Scott K, Burns TL, Falkner B. Variants in genes involved in functional pathways associated with hypertension in African Americans. Clin Transl Sci. 2010;3:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–360. [DOI] [PubMed] [Google Scholar]
- 60.Williams SM, Ritchie MD, Phillips JA 3rd, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38. [DOI] [PubMed] [Google Scholar]
- 61.Yatabe J, Yatabe MS, Yoneda M, Felder RA, Jose PA, Sanada H. Hypertension-related gene polymorphisms of G-protein-coupled receptor kinase 4 are associated with NT-proBNP concentration in normotensive healthy adults. Int J Hypertens. 2012;2012:806810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carey RM, Schoeffel CD, Gildea JJ, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu H, Lu Y, Wang X, Snieder H, Treiber FA, Harshfield GA, Dong Y. The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr Res. 2006;60:440–442. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H, Lu Y, Wang X, Treiber FA, Harshfield GA, Snieder H, Dong Y. The G protein-coupled receptor kinase 4 gene affects blood pressure in young normotensive twins. Am J Hypertens. 2006;19:61–66. [DOI] [PubMed] [Google Scholar]
- 65.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. [DOI] [PubMed] [Google Scholar]
- 66.Kimura L, Angeli CB, Auricchio MT, Fernandes GR, Pereira AC, Vicente JP, Pereira TV, Mingroni-Netto RC. Multilocus family-based association analysis of seven candidate polymorphisms with essential hypertension in an african-derived semi-isolated brazilian population. Int J Hypertens. 2012;2012:859219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabrera CP, Ng FL, Warren HR, Barnes MR, Munroe PB, Caulfield MJ. Exploring hypertension genome-wide association studies findings and impact on pathophysiology, pathways, and pharmacogenetics. Wiley Interdiscip Rev Syst Biol Med. 2015;7:73–90. [DOI] [PubMed] [Google Scholar]
- 68.Franceschini N, Chasman DI, Cooper-DeHoff RM, Arnett DK. Genetics, ancestry, and hypertension: implications for targeted antihypertensive therapies. Curr Hypertens Rep. 2014;16:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Natekar A, Olds RL, Lau MW, Min K, Imoto K, Slavin TP. Elevated blood pressure: Our family’s fault? The genetics of essential hypertension. World J Cardiol. 2014;6:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. [DOI] [PubMed] [Google Scholar]
- 71.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 72.Sanada H, Yatabe J, Yatabe MS, Yokokawa H, Williams S, Bartlett J, Wang Z, Felder R, Jose PA. G Protein-coupled receptor type 4 gene variants and response to antihypertensive medication [Abstract]. Circulation. 2009;120:S1087. [Google Scholar]
- 73.Muskalla AM, Suter PM, Saur M, Nowak A, Hersberger M, Krayenbuehl PA. G-protein receptor kinase 4 polymorphism and response to antihypertensive therapy. Clin Chem. 2014;60:1543–1548. [DOI] [PubMed] [Google Scholar]