Summary
Background
Disease extent in ulcerative colitis is one of the major factors determining prognosis over the long-term. Disease extent is dynamic and a proportion of patients presenting with limited disease progress to more extensive forms of disease over time.
Aim
To perform a systematic review and meta-analysis of epidemiological studies reporting on extension of ulcerative colitis to determine frequency of disease extension in patients with limited ulcerative colitis at diagnosis.
Methods
We performed a systematic literature search to identify studies on disease extension of ulcerative colitis (UC) and predictors of disease progression.
Results
Overall, 41 studies were eligible for systematic review but only 30 for meta-analysis. The overall pooled frequency of UC extension was 22.8% with colonic extension being 17.8% at 5 years and 31% at 10 years. Extension was 17.8% (95% CI 11.2–27.3) from E1 to E3, 27.5% (95% CI 7.6–45.6) from E2 to E3 and 20.8% (95% CI 11.4–26.8) from E1 to E2. Rate of extension was significantly higher in patients younger than 18 years (29.2% (CI 6.4–71.3) compared to older patients (20.2% (CI 13.0–30.1) (P<.0001). Risk of extension was significantly higher in patients from North America (37.8%) than from Europe (19.6%) (P<.0001).
Conclusions
In this meta-analysis, approximately one quarter of patients with limited UC extend over time with most extension occurring during the first 10 years. Rate of extension depends on age at diagnosis and geographic origin. Predicting those at high risk of disease extension from diagnosis could lead to personalised therapeutic strategies.
1 | INTRODUCTION
The extent of the disease in ulcerative colitis (UC) is clinically relevant, as it is one of the major determinants of long-term outcomes.1–3,5–7 Ulcerative colitis can be classified (according to the Montreal classification) into three different sub-groups based on the extent of colorectal inflammation: disease limited to the rectum (E1), involvement up to the splenic flexure (E2), or extension beyond the splenic flexure (E3).4 Disease extent in UC is dynamic, as 27%–54% of patients who are initially diagnosed with proctitis (E1) and/or left-sided colitis (E2) will progress to develop more extensive disease (extensive colitis or pancolitis).1 The natural history of the disease depends on the original anatomic location. Patients with an initial diagnosis of pancolitis have more frequent complications and extraintestinal manifestations (EIMs), need more immunosuppressive and surgical therapy, and have greater cancer risk.2,3,5–8 Distal UC is associated with fewer complications, EIMs and cancer.9 In the past, ulcerative proctitis and UC were discussed as two independent diseases. However, long-term epidemiological studies have revealed that proctitis often extends proximally and can progress to total colitis.10
Proximal disease extension appears to carry a poor prognosis, not only because it implies a higher disease burden for the individual patient, with higher therapeutic requirements, but also because it is associated with a more severe course. This was originally suggested in population-based inception cohorts, where disease extension was associated with a higher rate of colectomy.1 Patients with proximal extension following a period of stable proctitis or left-sided disease had (after extension) higher colectomy rates, higher need for biologics, more active disease, and increased hospitalisations than controls who started off with extensive colitis.11
Few clinical or pathological factors which predict likelihood of disease extension have emerged from prior studies. Young age at diagnosis, extra-intestinal manifestations, refractory disease and nonsmoking have all been proposed as risk factors, but these findings have been inconsistent.11–22
We therefore performed a systematic review and meta-analysis to identify the rates of extension in patients diagnosed with proctitis or left-sided UC and to examine several risk factors which may be associated with disease extension.
2 | METHODS
2.1 | Literature search
This study was conducted according to the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.23
2.1.1 | Search strategy and study selection
A comprehensive search strategy was designed and executed in PubMed/MEDLINE, Embase, and Scopus to identify all epidemiological studies reporting on extension of ulcerative colitis. The search query employed both an exhaustive list of keywords and index terminology whenever possible. Animal studies were excluded as recommended in the Cochrane Handbook of Systematic Reviews of Interventions.24 No date or language filters were employed in the search, although subsequently all articles not written in English, French, Italian, Greek, Spanish, Catalan or Portuguese were excluded. The full search strategy for each database is reported in Table S1.
All studies identified by the electronic searches were independently screened by two reviewers (GR and KK). In the case of disagreement between the two reviewers, a third author (JT) was consulted. If the study title and/or abstract clearly indicated that the study did not meet the pre-defined selection criteria, it was excluded from further analysis. The remaining results were assessed for inclusion based on the full text of the article. Reviewers sought to identify epidemiological studies, including cohort, longitudinal, case– control and other observational studies reporting on extension of ulcerative colitis. Studies which did not include a baseline endoscopic assessment were excluded. Figure 1 is a flow chart outlining the study-selection process.
FIGURE 1.
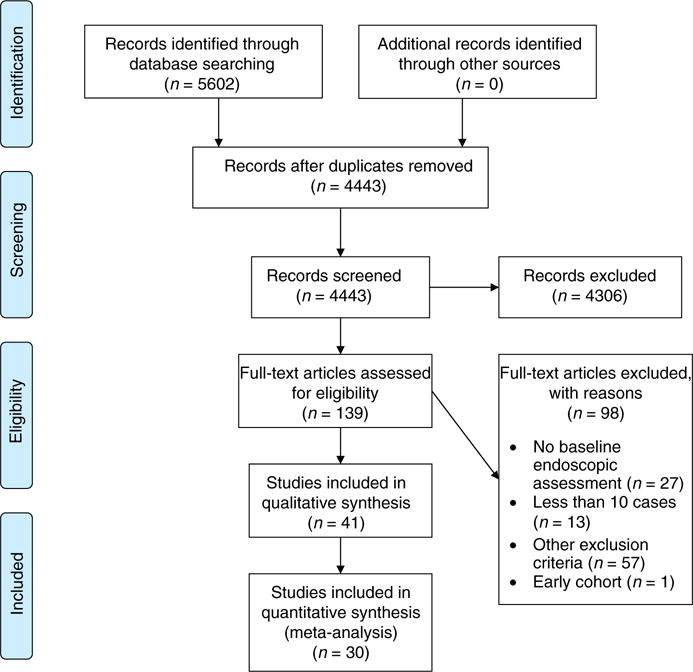
Prisma flow chart illustrating the selection of the included studies. 139 full-text articles were assessed for eligibility, of which 30 studies were included in the quantitative synthesis (meta-analysis)
The following data were extracted from those studies which met the eligibility criteria: general study information including the name of the first author, year of publication, full title, and outcomes of interest as specified below.
2.2 | Inclusion and exclusion criteria
Participants of all ages previously diagnosed with UC using standard clinical, endoscopic, radiological and histologic criteria were considered eligible for inclusion in this review. Due to the expected heterogeneity in diagnostic criteria and assessment of disease activity, any study using a commonly accepted method to diagnose or assess UC was considered for inclusion in this review. We excluded studies in which UC extension was not reported as well as studies with no baseline endoscopic assessment, less than 10 cases or with insufficient information on patients. Moreover, studies showing only preliminary data were excluded.
2.3 | Outcomes of interest
The primary outcome of our meta-analysis was the overall extension rate. Secondary outcomes were extension of disease defined as extension of E1 to E2, E2 to E3 or E1 to E3, the cumulative extension at 5 and 10 years, and the clinical factors that were associated with the primary outcome. Geographic location of the studies was also extracted. We also documented study design, patient population when reported (paediatric vs adult), accrual period, and follow-up length. With regard to risks factors of extension, we extracted the following information: therapy pre-extension (local therapy with 5-ASA enemas/suppositories, or steroids enemas, systematic 5-ASA, azathioprine, methotrexate, anti-TNFa, other biological, AZA+anti-TNFa, methotrexate+anti-TNFa, other immunosuppressant, steroids); EIMs including skin, muskuloskeletal, eyes, or primary sclerosing cholangitis (PSC), tobacco usage and age at diagnosis.
2.4 | Study quality
The quality of nonrandomised studies was assessed using the New-castle-Ottawa scale, a tool that allows for quality appraisal of nonrandomised studies in meta-analyses.25 Detailed results from the quality assessment are provided in Table S2.
2.5 | Statistical analysis
Data from studies were pooled if the studies provided sufficient information for meta-analysis. Comprehensive Meta-Analysis version 2.0 software (Biostat, Inc. Englewood, NJ, USA) was used to calculate pooled incidence of UC extension and perform subgroup analysis of different geographic regions, age categories and by original disease location. Random effect modelling was conducted. Chi-squared tests were used to compare frequencies of extension in subgroup analyses. We tested for heterogeneity using the chi-squared test and the I2 test. Publication bias was assessed with Egger’s test. A two-tailed P<.10 was considered statistically significant.
3 | RESULTS
3.1 | Literature search
Our search identified 5602 citations in PubMed/MEDLINE, Embase and Scopus (Figure 1). After exclusion of duplicates, 4443 records were screened. After reviewing the title and abstract and if necessary the full publications, 41 relevant studies were retrieved for full review1,8,12–17,21,26–40,42–58 out of 139 full-text articles were assessed for eligibility. Studies with no baseline endoscopic assessment, less than 13 cases or with insufficient information on patients were excluded. One study reporting preliminary data from an early cohort of patients was excluded.41 We conducted a systematic review on 41 studies. Eleven of these studies lacked sufficient information for inclusion in the meta-analysis. A final cohort of 30 unique studies were used for the meta-analysis (Figure 1).8,12,13,15,17,21,26–30,32–35,39,42–45,47,49–52,54–58
3.2 | Characteristics of the included studies
Characteristics of the 41 studies fulfilling inclusion criteria are detailed in Table 1. Twelve of the 41 studies were abstracts28,32,34–36,39,40,42,46,50,52,54 and 27 were retrospective studies. Twenty-four studies were from Europe1,6,8,13,15,21,27,30,31,33–36,39,42,43,46,47,49–53,56, seven from North America,17,32,36,44,48,55,58 seven from Asia12,16,29,37,38,54,57 and one from Africa.28 Accrual period for these studies, reported in 38 studies1,8,12–17,21,26–40,42,45–54,56–58 of 41 ranged from 1953 to 2016. Eighteen studies detailed patient gender with more than half of cases being male among all the 7 studies.
TABLE 1.
Characteristics of the 41 studies fulfilling inclusion criteria
| Author | Year | Designa | Study region |
Number of UC cases |
Number of males |
Age of partecipants (mean) |
Age at diagnosis (years) |
% of UC patients with extension overall |
Therapy pre- extensionb |
Number of Smokers |
EIMs pre- extensionc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ajana28 | 2012 | R | Morocco | 300 | 83 | 30 | 2,3,+ | ||||
| Alkim29 | 2011 | R | Turkey | 193 | 102 | 43.8 | 14 | 0,1,2,+ | |||
| Aloi30 | 2013 | R | Italy | 110 | 42 | 10.2 | 29 | 1,2,3,4,5,+ | 0,1,2,3,4,5 | ||
| Ayres27 | 1996 | R | GB | 145 | 72 | 28.3 | 37 | ||||
| Bareiro-deAcosta31 | 2010 | R | Portugal/Spain | 1549 | 35 | 35 | 1,2,3,4,5,+ | ||||
| Belkin32 | 2013 | R | USA | 385 | 27 | 27 | 10 | ||||
| Bresci33 | 1997 | R | Italy | 112 | 15 | 0,1,2,3,4 | |||||
| Capello34 | 2011 | R | Italy | 204 | 121 | 38.9 | 17 | ||||
| Charpentier35 | 2012 | R | France | 561 | 69 | 69 | 3 | ||||
| Chatzicostas14 | 2006 | R | Greece | 256 | 51 | ||||||
| Childers36 | 2011 | R | USA | 170 | 0,1,2,3,5,11,+ | ||||||
| Chow37 | 2009 | R | China | 172 | 48.4 | 40.4 | 1,2,3,5,11,+ | 26 | |||
| Chowdhur38 | 2014 | R | Bangladesh | 164 | |||||||
| Cuomo39 | 2015 | R | Italy | 156 | 81 | 22 | 1.2 | ||||
| Farmer17 | 1993 | R | USA | 1412 | 32.2 | 59 | 0,1,2,3,4,5 | ||||
| Garcia-Planella40 | 2009 | P | 100 | 35.5 | 11,+ | ||||||
| Gower-Rousseau42 | 2014 | P | France | 159 | 44 | 14.5 | 14.5 | 50 | |||
| Henriksen13 | 2006 | R | Norway | 518 | 235 | 37 | 37 | 17 | 0,1,2,3,4,11,+ | ||
| Halfvarson43 | 2007 | R | Sweden/Denmark | 158 | 27 | 23.5 | 22 | ||||
| Hyams44 | 1996 | R | USA | 171 | 94 | 11.2 | 11.2 | 3 | |||
| Kalkan45 | 2015 | R | Turkey | 612 | 37.9 | 37.9 | 9 | 1,2,3,5, 11,+ | 70 | ||
| Katsanos46 | 2013 | R | Greece | 443 | |||||||
| Kim12 | 2014 | R | Korea | 457 | 228 | 38.1 | 38.1 | 28 | 0,1,2,11,+ | 0,1,2,,3,4,5 | |
| Lakatos47 | 2011 | R | Hungary | 220 | 125 | 40.5 | 40.5 | 12 | 136 | 0,1,2,3,4,5 | |
| Malaty48 | 20113 | R | USA | 115 | 10.6 | 10.6 | 0,1,2,3,4,5,11,+ | 0,1,2,5 | |||
| Malmborg49 | 2015 | R | Sweden | 74 | 163 | 22 | |||||
| Manetti50 | 2015 | R | Italy | 1772 | 1011 | 45 | 45 | 20 | |||
| Margagnocni51 | 2014 | R | Italy | 1387 | 454 | 38 | 38 | 17 | |||
| Mazza52 | 2011 | Italy | 204 | 121 | 38.9 | 18 | |||||
| Meucci15 | 2000 | R | Italy | 341 | 202 | 38.5 | 27 | 164 | |||
| Moum21 | 1999 | P | Norway | 496 | 37 | 14 | |||||
| Park16 | 2014 | R | Korea | 240 | 132 | 41 | 41 | ||||
| Ritchie8 | 1978 | R | St mark | 269 | 162 | 22 | |||||
| Safroneeva26 | 2014 | R | Switzerland | 918 | 502 | 40.9 | 16 | 2,3,4,5,11 | |||
| Solberg1 | 2009 | R | Norway | 357 | 179 | ||||||
| Stewenius52 | 1996 | R | Sweden | 354 | 209 | 37.2 | 37.2 | ||||
| Takeuchi53 | 2011 | R | Japan | 53 | 15 | 33 | 26 | ||||
| Tsang54 | 2012 | R | Canada | 54 | 23 | 9.36 | 10.6 | 67 | |||
| Vester-Andersen55 | 2014 | P | Denmark | 300 | 151 | 37.3 | 37.3 | 28 | |||
| Watermn58 | 2015 | R | Canada | 601 | 283 | 27 | 28.9 | 2,3,5,+ | 83 | ||
| Anzai57 | 2016 | R | Japan | 66 | 36 | 34.9 | 51.5 | + | 15 |
Design (P=prospective; R=retropective).
Therapy pre-extension (0=nothing, 1=Local therapy only [5-ASA enemas/suppositories, or CS=ciclosporin enemas], 2=5-ASA systematic, 3=Azathioprine, 4=Methotrexate, 5=anti-TNFa, 6=other biological, 7=AZA+anti-TNF. 9=methotrexate+anti-TNF, 10=other immunosuppressant, 11=other therapy, +=steroids).
EIMs, extraintestinal manifestations pre extension (0=none, 1=skin, 2=muskuloskeletal, 3=eyes, 4=PSC, 5=other).
Ulcerative colitis overall rate of extension was reported in 30 studies1,12,13,15,17,21,26–30,32–35,39,42–45,47,49–52,54–58 with information on rate of extension from proctitis (E1) to left side colitis (E2) in 11 studies1,8,16,26,42,43,46,48,53,56,57 from left side colitis (E2) to pancolitis (E3) in 13 studies1,8,13,16,17,26,40,42,43,48,53,56,57 and from proctitis (E1) to pancolitis (E3) in 10 studies1,16,17,26,42,46,48,53,56,57 Information on extension over time (at 5 and 10 years) was reported in 11 studies,1,5,14,18,27,37,51,53,54,57,59 of which 35,18,59 were not included in the meta-analysis because of insufficient information.
Treatments prior to extension were reported in 15 studies12,13,26,28–31,36,37,39,42,45,48,57,58 with use of steroids in 13 studies.12,13,28–31,36,37,42,45,48,57,58 EIMs were reported in seven studies12,17,30,33,42,47,48 and tobacco usage in 12 studies.12,14,15,27,32,35,37,45,47,48,57,58 Age at diagnosis was reported in 20 studies12,13,16,31,35,37,42–45,47,48,50,51,53–58 with a median age of 37.1 years (10.6–69).
3.3 | Meta-analysis
We performed a meta-analysis on a final cohort of 30 unique studies.1,12,13,15,17,21,26–30,32–35,39,42–45,47,49–52,54–58 Meta-analyses were performed for overall rate of extension, E1/E2 to E2/E3 rate of extension, and how extension varies based on age at diagnosis and geographic area was examined. Too few studies reported on other risk factors such as tobacco usage, treatment prior to extension and EIMs to perform any significant analyses on these risk factors.
3.3.1 | Rates of extension
Overall rate of extension was reported in 31 studies including one early cohort of patients that was excluded from the meta-analysis.42 The overall pooled frequency of UC extension was 22.8% (95% CI 17.4–29.3; I(2)=97.8%; chi-squared test P<.001). When we assessed extension over time, the pooled proportion for proximal extension was 17.8% (95% CI 12.3–25.1; I(2)= 92.9%; chi-squared test P<.001) at 5 years and 31.0% (95% CI 23.5–39.7; I(2)=94.9%, chi-squared test P<.001) at 10 years (Figure 2). Sub-analyses looking at extension from E1 to E2, E2 to E3 and E1 to E3 were performed including studies for which data was provided on number of total proctitis or left-sided colitis patients, and how many progressed to left-sided colitis or pancolitis (Figure 3). Rate of extension was 17.8% (95% CI 11.4–26.8; I(2)=86.2%, chi-squared test P<.001) from E1 to E2, 17.8% (95% CI 11.2–27.3; I(2)=90.3%; chi-squared test P<.001) from E1 to E3 and 20.8% (95% CI 7.6–45.6; I(2)=97.3%; chi-squared test P<.001) from E2 to E3. When stratifying by study type, the rate of extension in prospective studies was 22.9% (95% CI 14.0–41.7) and the rate of extension in retrospective studies was 25.9% (95% CI 16.5–29.6), which was not a statistically significant difference (P=.069).
FIGURE 2.

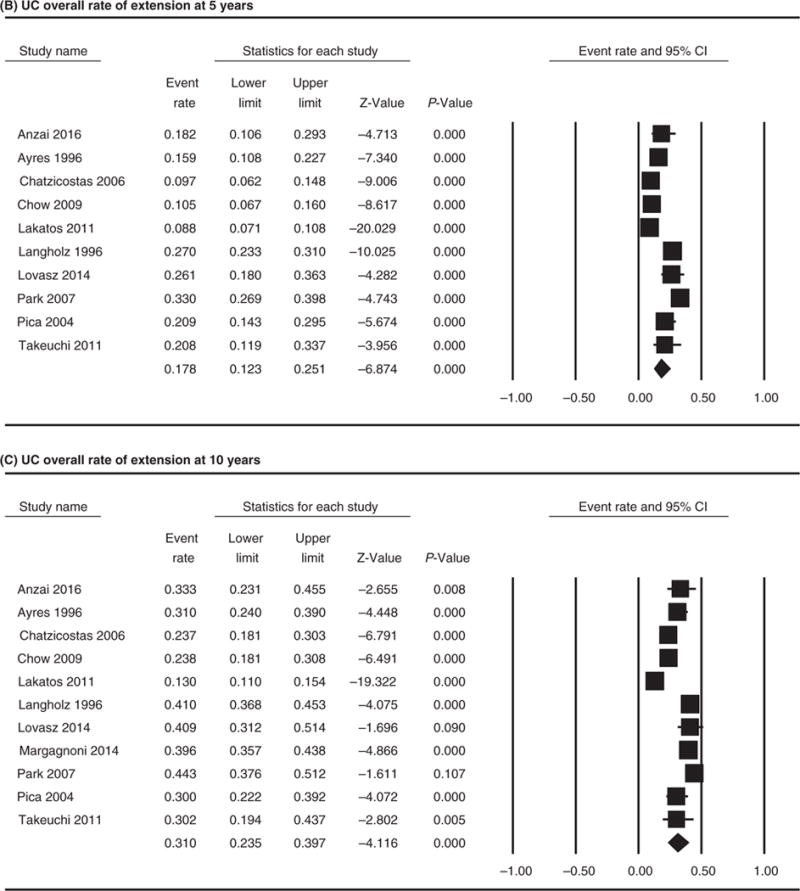
Forest plot of the included studies comparing (A) UC overall rate of extension. Extension over the time: (B) UC overall rate of extension at 5 years and (C) UC overall rate of extension at 10 years
FIGURE 3.

Forest plot of the included studies comparing rate of extension from E1 (proctitis) to E2 (left side colitis) (A), E1 to E3 (extensive colitis) (B) and E2 to E3 (C)
3.3.2 | Age at diagnosis
We performed a sub-analysis by age at diagnosis for the 13 studies where this information was available based on overall extension data (Figure 4). Studies were analysed by dichotomising and comparing the three studies which contained patients younger than 18 years old and the 11 studies including patients older than 18 years.12,13,35,43,45,47,50,51,54,57,58 The rate of extension was 20.2% (95% CI 13.0–30.1; I(2)=97.4%; chi-squared test P<.001) for patients older than 18 years as compared to 29.2% (95% CI 6.4–71.3; I(2)=96.9%; chi-squared test P<.001) for patients younger than 18 years (P<.0001). Chi-squared test comparing the rate of extension in younger patients vs older showed significant difference (P<.0001).
FIGURE 4.
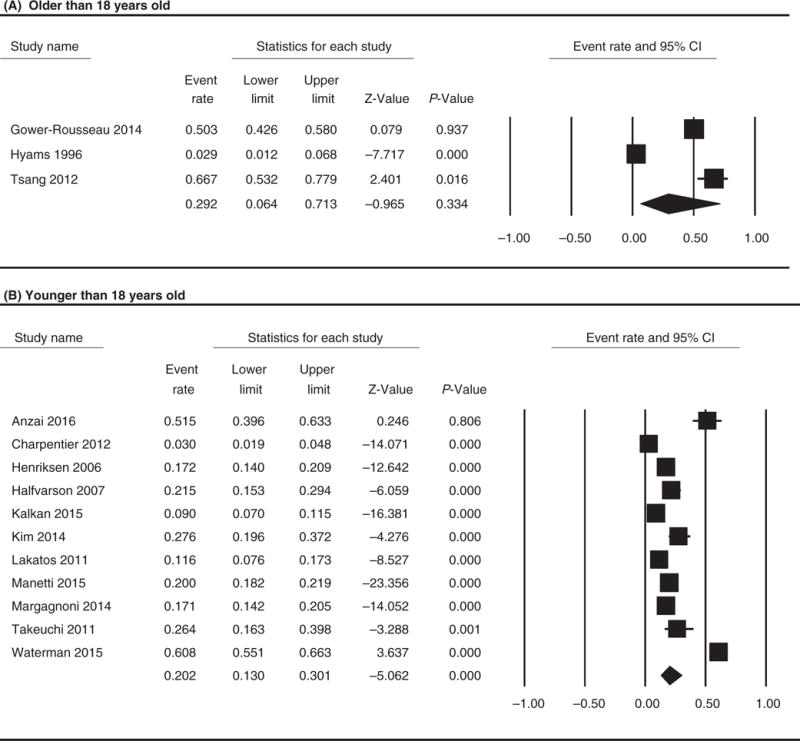
Forest plot of the included studies comparing overall rate of extension in patient older (A) vs younger than 18 years old (B). Chi-squared test comparing the rate of extension in younger patients vs older showed significant difference (P<.0001)
3.3.3 | Geographic region
Rate of extension based on geographic region was 19.6% (95% CI 16.1–23.7; I(2)=92.5%; chi-squared test P<.001), in the European group, 37.8% (95% CI 21.8–57.0; I(2)=97.9%; chi-squared test P<.001) in the North America group and 23.8% (95% CI 13.5–38.5; I (2)=95.2%; chi-squared test P<.001) in the rest of the world (Figure 5). The difference in rate of extension between North America (37.8%) and Europe (19.6%) was statistically significant (P<.0001) as well as between North America and the rest of the world (P<.0001) and Europe and the rest of World (P=.005).
FIGURE 5.

Forest plot of the included studies comparing overall rate of extension based on geographic area. A, Europe; B, North America; C, Rest of the world. The difference in rate of extension between North America (37.8%) and Europe (19.6%) was statistically significant (P<.0001) as well as between North America and the rest of the world (P<.0001) and Europe and the rest of World (P=.005)
3.3.4 | Study quality and publication bias
The chi-squared test for heterogeneity revealed a value of 27.0, and the I2 test result was 97.7%, indicating significant variability in effect estimates that is likely due to heterogeneity rather than chance. Egger’s test showed no evidence of publication bias (Egger’s t-value=1.99, P=.057).
4 | DISCUSSION
We performed a systematic review and meta-analysis of the available literature to determine the rate of extension from limited colitis (proctitis or left-sided colitis) to more extensive disease and to assess the impact of known risk factors. We found that the overall rate of extension was 22.8% accounting for extension from E1 to E2 or E3, and E2 to E3. The pooled proportion for proximal extension was 17.8% at 5 years and 31% at 10 years. Rates of extension were higher in younger patients and in patients from North America compared to Europe and the rest of the world.
Previous estimates of disease extension have varied widely. In the IBSEN study1 and others, one-fifth to one-third of patients with proctitis or left-sided colitis showed disease extension to the proximal colon.14,15,20,51 However, other studies have suggested much higher extension rates. Fumery et al.60 in their review on the natural history of paediatric-onset ulcerative colitis, that included 26 population-based studies, found that paediatric-onset UC is characterised by a high rate of disease extension with most patients experiencing disease extension and about two-thirds of patients having pancolitis at the end of follow-up. Farmer et al.17 reported that 53% of patients with UC had extension and a Danish inception cohort of 1161 UC patients demonstrated 53% of those with proctosigmoiditis had progression after 25 years.5 The range of prior findings is likely due to differences in study design (retrospective vs prospective), varying durations of follow-up, ages included, treatments received and potentially environmental differences between different countries and regions. In addition, the way in which disease extent was ascertained varied, for example, some studies (particularly older ones) utilising barium enema or flexible sigmoidoscopy findings to define extension. Last, changes in disease management over time may also affect the cumulative rate of disease extension.
Our meta-analysis has shown that disease extension may occur any time after initial diagnosis with an increasing probability after the first decade of follow-up (31.1%) as reported by previous groups.16,27 Moreover, we found that initial disease location does not impact the risk of extension. Patients with initial proctitis or left-sided colitis are at the same risk to extend to pancolitis. Several studies have demonstrated that patients with proctitis are at greater risk to extend to pancolitis,15 although this has not been a consistent finding.16
Our study confirmed that young age at diagnosis is a risk factor to predict extension in a patient with limited colitis. Few studies have investigated young age as a risk factor of extension over the time, and data are inconsistent.5 Hochart et al.61 reported a pooled proportion for colonic extension of 10% at 1 year, 45% at 5 years and 52% at 10 years in paediatric proctitis patients. The high likelihood of colonic extension suggests paediatric-onset ulcerative proctitis is not a minor, self-limited disease. Other studies have reported high rates of colonic extension in the paediatric population, ranging between 38% and 65%.5,12,29,42 For example, in an incidence cohort of 113 paediatric UC patients who were followed up for at least 2 years, disease extension was observed in 49% of patients.42 The risk of extension seems highest within the first 5 years of follow-up, suggesting a role for monitoring paediatric patients closely after their diagnosis.
North American studies in our meta-analysis reported the highest rates of extension when compared to European and studies from other parts of the world. This finding may be due to a number of reasons. This may represent a real difference in disease behaviour based on geography which could be due to variation in environmental factors pre-disposing to UC extension. Alternatively, the time it takes to diagnose UC can vary from country to country and region to region. There may be a longer delay in diagnosis in North American countries compared to Europe for example. If the diagnosis is significantly delayed and therefore treatment as well, this may predispose patients to have progression of their UC. Geographic differences might also suffer from bias. Included studies follow patients for different periods of time, and some studies are following younger vs older patients whereas others are restricted to adults. Furthermore, no adjustments are performed for different treatment patterns in the different countries.
There are several strengths and limitations of this meta-analysis. Our extensive literature search allowed us to include 1772 UC patients for study. We also included both paediatric and adult patients and confirmed young age at diagnosis as a risk factor of extension. One of the major limitations of our study is that many included studies did not provide sufficient data on previously reported risk factors such as EIMs, pre-extension medications, smoking, severity of the disease or previously suggested risk factors such as delay in diagnosis of more than 6 months, a family history of inflammatory bowel disease, continuous disease activation within 6 months of the initial diagnosis, frequent relapses, severe bleeding, refractoriness to therapy, toxic colitis and inflammation of the appendiceal orifice.15–22 Of note, 12 of 30 studies included were in abstract form so information was limited. Moreover, there are many inherent as well as technical difficulties in studies investigating factors related to UC proximal extension, as the methods used to determine disease extent over time have changed.62,63
In summary, the extent of colonic involvement in UC is an important clinical feature, because it serves as an indicator of the severity and activity of the disease, the type of treatment needed, as well as the future risk of high grade dysplasia and colorectal cancer.3–9 In this meta-analysis we found that 22.8% of patients with limited colitis (E1 or E2) are at risk to progress to more extensive disease (E2 or E3), most frequently during the first 10 years after diagnosis. There also appears to be a higher risk of extension in patients diagnosed at a younger age and in North American countries. This finding may have implications for clinical care and patient monitoring although there remain several issues for clarification. Larger prospective studies are needed to better determine predictors of disease extension, including clinical and molecular predictors, in patients with limited colitis at diagnosis.
Supplementary Material
Acknowledgments
Funding information: None.
Footnotes
Declaration of personal interests: None.
As part of AP&T’s peer-review process, a technical check of this meta-analysis was performed by Dr Y. Yuan. The Handling Editor for this article was Professor Ashwin Ananthakrishnan, and it was accepted for publication after full peer-review.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
AUTHORSHIP
Guarantor of the article: Giulia Roda.
Author contributions: GR: study concept and design, literature search, data abstraction, data analysis and manuscript writing. NN: data analysis, manuscript writing. RP: literature search and preparation of results for screenig. KK: study concept and design, data abstraction and manuscript writing AA: data abstraction JT: data abstraction UR and JB: manuscript writing JFC: study design and concept, and manuscript writing. All authors have approved the final version of this manuscript.
LINKED CONTENT
This article is linked to Lamba et al paper. To view this article visit https://doi.org/10.1111/apt.14129.
References
- 1.Solberg IC, Lygren I, Jahnsen J, et al. Group IS Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44:431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick CG, Zack M, Holmberg L, Adami HO. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology. 1992;103:954–960. doi: 10.1016/0016-5085(92)90029-x. [DOI] [PubMed] [Google Scholar]
- 4.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 6.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Survival and cause-specific mortality in ulcerativecolitis: follow-up of a population-based cohort in Copenhagen County. Gastroenterology. 2003;125:1576–1582. doi: 10.1053/j.gastro.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Jess T, Loftus EV, Jr, Harmsen WS, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut. 2006;55:1248–1254. doi: 10.1136/gut.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie JK, Powell-Tuck J, Lennard-Jones JE. Clinical outcome of the first ten years of ulcerative colitis and proctitis. Lancet. 1978;1:1140–1143. doi: 10.1016/s0140-6736(78)90312-4. [DOI] [PubMed] [Google Scholar]
- 9.Katz J. The course of inflammatory bowel disease. Med Clin North Am. 1994;78:1275–1280. doi: 10.1016/s0025-7125(16)30100-6. [DOI] [PubMed] [Google Scholar]
- 10.Binder V. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol. 2004;18:463–479. doi: 10.1016/j.bpg.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356–1363. doi: 10.1002/ibd.22839. [DOI] [PubMed] [Google Scholar]
- 12.Kim B, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Proximal disease extension and related predicting factors in ulcerative proctitis. J Crohns Colitis. 2014;8:S128. doi: 10.3109/00365521.2013.867360. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen M, Jahnsen J, Lygren I, et al. Ulcerative colitis and clinical course: result of a 5-year population-based follow- up study (The IBSEN Study) Inflamm Bowel Dis. 2006;12:543–550. doi: 10.1097/01.MIB.0000225339.91484.fc. [DOI] [PubMed] [Google Scholar]
- 14.Chatzicostas C, Roussomoustakaki M, Potamianos S, et al. Factors associated with disease evolution in Greek patients with inflammatory bowel disease. BMC Gastroenterol. 2006;6:21. doi: 10.1186/1471-230X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meucci G, Vecchi M, Astegiano M, et al. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII) Am J Gas-troenterol. 2000;95:469–473. doi: 10.1111/j.1572-0241.2000.t01-1-01770.x. [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007;13:278–283. doi: 10.1002/ibd.20015. [DOI] [PubMed] [Google Scholar]
- 17.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A longterm follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 18.Langholz E, Munkholm P, Davidsen M, Nielsen OH, Binder V. Changes in extent of ulcerative colitis: a study on the course and prognostic factors. Scand J Gastroenterol. 1996;31:260–266. doi: 10.3109/00365529609004876. [DOI] [PubMed] [Google Scholar]
- 19.Eleftheriadis N, Lambrecht G, D’Haens G, et al. Maintenance therapy for ulcerative colitis has no impact on changes in the extent of ulcerative colitis. J Crohns Colitis. 2007;1:21–27. doi: 10.1016/j.crohns.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Karoui S, Kallel L, Dahmani Z, Boubaker J, Filali A. Frequency of proximal colonic extension of distal ulcerative colitis. Tunis Med. 2007;85:669–672. [PubMed] [Google Scholar]
- 21.Moum B. Medical treatment: does it influence the natural course of inflammatory bowel disease? Eur J Intern Med. 2000;11:197–203. doi: 10.1016/s0953-6205(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 22.Fdez-Morera JL, Rodrigo L, Lopez-Vazquez A, et al. MHC class I chain-related gene A transmembrane polymorphism modulates the extension of ulcerative colitis. Hum Immunol. 2003;64:816–822. doi: 10.1016/s0198-8859(03)00121-6. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, editor. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008. [Google Scholar]
- 25.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. 2011 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 17, 2013.
- 26.Safroneeva E, Vavricka S, Fournier N, et al. Systematic analysis of factors associated with progression and regression of ulcerative colitis in the SWISS IBD cohort study. Gastroenterology. 2014;1:S-441. [Google Scholar]
- 27.Ayres RC, Gillen CD, Walmsley RS, Allan RN. Progression of ulcerative proctosigmoiditis: incidence and factors influencing progression. Eur J Gastroenterol Hepatol. 1996;8:555–558. doi: 10.1097/00042737-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Ajana FZ, Bousseaden A, Essamri W, Benlbaghdadi I, Essaid A. Distal colitis: evolution and prognosis during 10 years. J Crohns Colitis. 2012;6:S127. [Google Scholar]
- 29.Alkim C, Alkim H, Dagli U, Parlak E, Ulker A, Sahın B. Extension of ulcerative colitis. Turk J Gastroenterol. 2011;22:382–387. doi: 10.4318/tjg.2011.0241. [DOI] [PubMed] [Google Scholar]
- 30.Aloi M, D’Arcangelo G, Pofi F, et al. Presenting features and disease course of pediatric ulcerative colitis. J Crohns Colitis. 2013;7:e509–e515. doi: 10.1016/j.crohns.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 31.deBarreiro-Acosta M, Magro F, Carpio D, et al. Ulcerative colitis in northern Portugal and Galicia in Spain. Inflamm Bowel Dis. 2010;16:1227–1238. doi: 10.1002/ibd.21170. [DOI] [PubMed] [Google Scholar]
- 32.Belkin E, Cherng N, Pellish R. Progression of ulcerative proctitis to proximal disease. Inflamm Bowel Dis. 2013;19:S48–S49. [Google Scholar]
- 33.Bresci G, Parisi G, Gambardella L, et al. Evaluation of clinical patterns in ulcerative colitis: a long-term follow-up. Int J Clin Pharmacol Res. 1997;17:17–22. [PubMed] [Google Scholar]
- 34.Cappello M, Peralta S, Mazza M, et al. Disease extension is the major factor affecting the disease course of ulcerative colitis (UC) J Crohns Colitis. 2011;5:S38–S39. [Google Scholar]
- 35.Charpentier C, Salleron J, Savoye G, et al. Natural history of ulcerative colitis in the elderly: a population-based study. Gastroenterology. 2012;1:S25. [Google Scholar]
- 36.Childers RE, Baron P, Kahare B, et al. Predictors of progression to pancolitis in ulcerative colitis. Gastroenterology. 2011;1:S426. [Google Scholar]
- 37.Chow DKL, Leong RW, Tsoi KK, et al. Long-term follow-up of ulcerative colitis in the Chinese population. Am J Gastroenterol. 2009;104:647–654. doi: 10.1038/ajg.2008.74. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury MS, Khan MR, Mahmuduzzaman M, et al. Disease extent and local complication of ulcerative colitis in Bangladeshi population. Mymensingh Med J. 2014;23:720–723. [PubMed] [Google Scholar]
- 39.Cuomo A, D’Auria MV, Miranda A, et al. Combined mesalazine MMX plus rectal mesalazine in the treatment of mild to moderately active ulcerative proctitis. Dig Liver Dis. 2015;47:e139. doi: 10.1159/000485614. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Planella E, Manosa M, van Domselaar M, et al. Long-term outcome of ulcerative colitis in patients responding to a first course of steroids. Gastroenterology. 2009;1:A357. doi: 10.1016/j.dld.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104:2080–2088. doi: 10.1038/ajg.2009.177. [DOI] [PubMed] [Google Scholar]
- 42.Gower-Rousseau C, Sarter H, Turck D, et al. Long-term outcome of paediatric-onset ulcerative colitis: early years are shaping the future. J Crohns Colitis. 2014;8:S32–S33. [Google Scholar]
- 43.Halfvarson J, et al. Longitudinal concordance for clinical characteristics in a Swedish-Danish twin population with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1536–1544. doi: 10.1002/ibd.20242. [DOI] [PubMed] [Google Scholar]
- 44.Hyams JS, Jess T, Bodin L, et al. Clinical outcome of ulcerative colitis in children. J Pediatr. 1996;129:81–88. doi: 10.1016/s0022-3476(96)70193-2. [DOI] [PubMed] [Google Scholar]
- 45.Kalkan IH, Dagli U, Kekilli M, et al. Clinical course and predictors of total colectomy in ulcerative colitis: a referral center experience from Turkey. Turk J Gastroenterol. 2015;26:25–30. doi: 10.5152/tjg.2015.5071. [DOI] [PubMed] [Google Scholar]
- 46.Katsanos K, Tsianos V, Vasileiou T, et al. Endoscopic extension of inflammation progresses more frequently in ulcerative colitis compared to Crohn’s disease patients. J Crohns Colitis. 2013;7:S109. [Google Scholar]
- 47.Lakatos L, David G, Erdelyi Z, et al. Incidence and early disease course of ulcerative colitis in western Hungary between 2002–2006. Gastroenterology. 2010;1:S202. [Google Scholar]
- 48.Mataly HM, Abraham BP, Mehta S, et al. The natural history of ulcerative colitis in a pediatric population: a follow-up population-based cohort study. Clin Exp Gastroenterol. 2013;6:77–83. doi: 10.2147/CEG.S40259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malmborg P, Grahnquist L, Idestrom M, et al. Presentation and progression of childhood-onset inflammatory bowel disease in northern Stockholm county. Inflamm Bowel Dis. 2015;21:1098–1108. doi: 10.1097/MIB.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 50.Manetti N, Bagnoli S, Rogai F, et al. Disease course and colectomy rate in ulcerative colitis: a follow-up cohort study of a tertiary referral center in Tuscany. Digest Liver Dis. 2015;47:e135. doi: 10.1097/MIB.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 51.Margagnoni G, Fasci Spurio F, Feigusch L, et al. Long term course of ulcerative colitis in the prebiologic era. A retrospective study in a tertiary referral center. Minerva Gastroenterol Dietol. 2014;60:275–283. [PubMed] [Google Scholar]
- 52.Mazza M, Cappello M, Almasio PL, et al. Disease extention is the major prognostic factor of clinical course in patients with ulcerative colitis. Digest Liver Dis. 2011;43:S189. [Google Scholar]
- 53.Stewenius J, Adnerhill I, Ekelund GR, et al. Risk of relapse in new cases of ulcerative colitis and indeterminate colitis. Dis Colon Rectum. 1996;39:1019–1025. doi: 10.1007/BF02054693. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi Y, Ohishi C, Arai K, et al. The natural course of ulcerative proctitis in Japan: disease extension and related factors. Gastroenterology. 2011;1:S791. [Google Scholar]
- 55.Tsang J, Sikora S, Spady D, et al. Histopathological changes in anatomical distribution of inflammatory bowel disease in children: a retrospective cohort study. BMC Pediatr. 2012;12:162. doi: 10.1186/1471-2431-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vester-Andersen MK, Prosberg MV, Jess T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705–714. doi: 10.1038/ajg.2014.45. [DOI] [PubMed] [Google Scholar]
- 57.Anzai H, Hata K, Kishikawa J, et al. Clinical pattern and progression of ulcerative proctitis in the Japanese population: a retrospective study of incidence and risk factors influencing p regression. Colorectal Dis. 2016;18:O97–O102. doi: 10.1111/codi.13237. [DOI] [PubMed] [Google Scholar]
- 58.Waterman M, Knight J, Dinani A, et al. Predictors of outcome in ulcerative colitis. Inflamm Bowel Dis. 2015;21:2097–2105. doi: 10.1097/MIB.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pica R, Paoluzi OA, Iacopini F, et al. Oral mesalazine (5-ASA) treatment may protect against proximal extension of mucosal inflammation in ulcerative proctitis. Inflamm Bowel Dis. 2004;10:731–736. doi: 10.1097/00054725-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Fumery M, Duricova D, Gower-Rousseau C, Annese V, Peyrin-Biroulet L, Lakatos PL. Review article: the natural history of paediatric-onset ulcerative colitis in population-based studies. Aliment Pharmacol Ther. 2016;43:346–355. doi: 10.1111/apt.13478. [DOI] [PubMed] [Google Scholar]
- 61.Hochart A, Gower-Rousseau C, Sarter H, et al. Ulcerative proctitis is a frequent location of paediatric-onset UC and not a minor disease: a population-based study. Gut. 2016 doi: 10.1136/gutjnl-2016-311970. [DOI] [PubMed] [Google Scholar]
- 62.Leidenius M, Kellokumpu I, Linden H, Taskinen E. The true extent of ulcerative colitis? A radiological, endoscopic and histological study. Apmis. 1994;102:950–955. doi: 10.1111/j.1699-0463.1994.tb05257.x. [DOI] [PubMed] [Google Scholar]
- 63.Niv Y, Bat L, Ron E, Theodor E. Change in the extent of colonic involvement in ulcerative colitis: a colonoscopic study. Am J Gastroenterol. 1987;82:1046–1051. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


