Abstract
Children’s exposures to chemical and non-chemical stressors from their everyday environment affects their overall health and well-being. American-Indian/Alaska-Native (AI/AN) children may have a disproportionate burden of stressors from their built and natural environments when compared to children from other races/ethnicities. Our objectives were to identify chemical and non-chemical stressors from AI/AN children’s built and natural environments and evaluate their linkages with health and well-being outcomes from the peer reviewed literature. Library databases (e.g. PubMed) were searched to identify studies focused on these stressors. References were excluded if they: did not discuss AI/AN children or they were not the primary cohort; discussed tribes outside the United States (U.S.); were reviews or intervention studies; or did not discuss stressors from the built/natural environments. Out of 2539 references, 35 remained. Sample populations were predominantly (70%) in New York (NY) and Alaska (AK); 14 studies reported on the same cohort. Studies with matching stressors and outcomes were few, ruling out a quantitative review. Respiratory and developmental outcomes were the main outcomes evaluated. Primary non-chemical stressors were residential proximity to polluted landscapes, lack of indoor plumbing, and indoor use of wood for heating or cooking. The main chemical stressors were volatile organic compounds (VOCs), particulate matter (PM2.5), polychlorinated biphenyls (PCBs), p,p′-DDE, hexachlorobenzene (HCB), lead, and mercury. Our qualitative review was suggestive of a potential increase in respiratory illness from indoor wood use or no plumbing, which can be used as a guide to promote healthy environments for AI/AN children. We identified limited studies (<40), demonstrating this population as understudied. Future studies need to consider: sample populations from other tribes in the U.S., stressors outside the household, other elements of the natural environment, and an evaluation of stressors from AI/AN children’s total environment (built, natural, and social).
Keywords: review, children, American Indian/Alaska Native, built environment, natural environment, stressors
Introduction
When compared to adults, children are more vulnerable to exposures from environmental contaminants found in their everyday environments. This vulnerability can be due to age-specific factors such as differences in physiology, developmental stages, surface-to-volume ratio, lifestage-specific activities and behaviors (e.g. object/surface-to-hand-to-mouth) (1), (2), (3), (4). Because of the way children interact with their environment, they can be exposed to the same chemical through multiple exposure routes (2), (4).
Exposure to stressors during critical stages of development may lead to growth abnormalities, structural impairments, functional deficits, and altered survival (2), (5). Stressors are defined as any physical, chemical, social, or biological entity that can induce change in health and well-being (6), (7). For assessing childhood exposures, early lifestage groupings are narrow when rapid development occurs (i.e. birth to <1 month, 1 to <3 months, 3 to <6 months, 6 to <12 months, 1 to <2 years, 2 to <3 years) and broader in later childhood when the rate of development slows (i.e. 3 to <6 years, 6 to <11 years, 11 to <16 years, 16 to <21 years) (8).
Stressors between children’s everyday environments (built, natural, and social) may contribute to differences in children’s exposures, thereby impacting their health and well-being. The built environment represents man-made surroundings such as land use, transportation, buildings, and infrastructure. The natural environment represents naturally-occurring surroundings, living and non-living, such as the atmosphere, water bodies, forests, and mountains. The social environment may include factors related to social interactions, the economy, the community, school, safety, parental level of education, number of people in home, and access to resources (6).
Children from some communities, such as those from American-Indian/Alaska-Native (AI/AN) tribes, are disproportionately burdened with adverse health and well-being outcomes compared to other populations in the U.S. (9). According to the U.S. Department of Health and Human Services’ Indian Health Service (IHS) (9), the leading causes of post-neonatal mortality among AI/ANs (2007-2009) were sudden infant death syndrome (SIDS); congenital malformations, deformations, and chromosomal abnormalities; and unintentional injuries. The AI/AN mortality rate for SIDS, for example, was twice as high when compared to all races in the U.S. (9). For AI/AN children aged between 1 and 4 years, the leading causes of mortality were unintentional injuries (rate was 4 times greater than the rate among all races in the U.S.), homicide, and congenital anomalies (9). For children between 5 and 14 years, the leading causes of mortality were unintentional injuries, suicide, and malignant neoplasms (9). And, for children between 1 and 14 years, the primary contributor to their hospitalizations was respiratory diseases (9).
AI/ANs encounter a multitude of stressors from their built, natural, and social environments related to hazards around their communities (10). Previous reviews of the peer reviewed literature have focused on stressors from AI/AN children’s social environment. These studies identified societal, cultural, community, school, and family factors (11); cultural implications when AI children are placed away from their communities (12); adolescent socialization (13); and the benefits of breastfeeding (14). We could not find a published review of chemical and non-chemical stressors from AI/AN children’s built and natural environments.
To that end, we aimed to review the published literature to: identify and characterize chemical and non-chemical stressors from AI/AN children’s built and natural environments and assess relationships between these stressors and any health and well-being outcomes. This review highlights how disparities in chemical and non-chemical stressors affecting AI/AN children need to be considered when examining their health and well-being.
Methods
This review was guided by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (15).
Eligibility criteria
Studies were eligible if the title or abstract included AI/AN children in the study sample, presented findings of stressors (chemical or non-chemical that could impact changes in health and well-being) from the built or natural environments, were published in English, and published by January 8, 2018.
Information sources
Three library databases (ProQuest’s Environmental Science Collection, PubMed, and Web of Science) were searched with key words and search strings focused on AI/AN children (e.g. Alaska Native AND child). Bibliographies of relevant studies were also reviewed to locate additional relevant articles.
Search
The key word/search string strategy was similar across the three databases (one database’s key word/search string is listed in Figure 1). The first component of the search string targeted AI/AN background (i.e. “native american” OR “american indian” OR “alaska native” OR “alaskan native”). The second component targeted children (e.g. prenatal OR child* OR adolescent). The only difference across the databases for the key words was the term fetal. In PubMed, the term fetal was lengthened because it would have only used the first few hundred variations with a shortened term (i.e. fet*).
Fig 1.
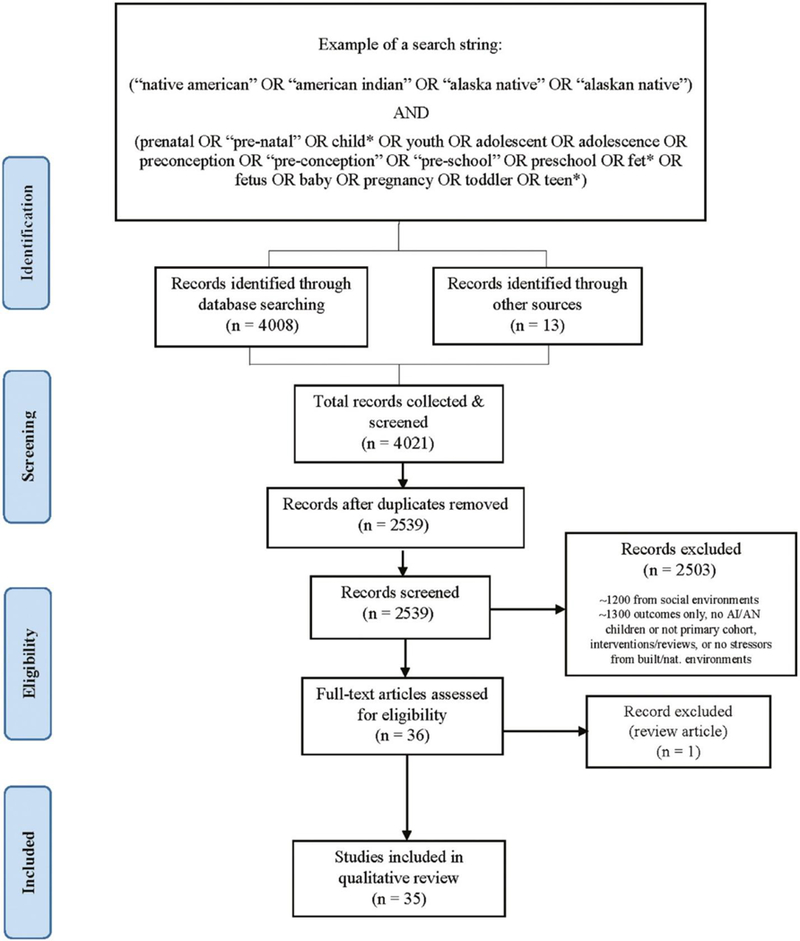
Review study selection (reporting of items adapted from Moher et al. [15]).
These key words/search strings were searched in the title and abstract fields (ProQuest and PubMed). In Web of Science, however, there was no available field to search only abstracts so the ‘Topic’ field was selected; the ‘Abstract of Published Item’ field was bibliographic-only data for a published paper. Results from these searches were limited to a specific end date of January 8, 2018, English language articles, and scholarly journals.
Study selection
Titles and abstracts were screened (n=4021) and duplicate articles removed (n=1482) (Figure 1). References were then excluded (n=2503) if they: (1) did not discuss AI/AN children or AI/AN children were not the primary cohort of interest (n=431); (2) discussed tribes outside the U.S. (n=134); (3) described reviews or interventions (n=283); or (4) did not provide findings about chemical or non-chemical stressors from the built or natural environments (n=1655). Full-text reviews were conducted for the remaining articles (n=36).
Synthesis of results
Results were reported for the occurrence of chemical and non-chemical stressors in the study sample by built and/or natural environments, consideration of other factors in the analysis, and the impact of stressor(s) on the outcome(s) described. Results were synthesized to compare studies with matching methodology for similar stressors and outcomes.
Results
Study selection
From the three library databases, 2539 references were screened, resulting in the inclusion of 35 studies (Figure 1). These 35 studies were published between 1986 and 2016. By journal, the greatest number of studies for stressors identified from the built environment were published in Pediatric Infectious Disease (n=3), while for the natural environment, it was Environmental Health Perspectives (n=3). Studies were most frequently excluded for not reporting findings on stressors from the built or natural environments.
Study characteristics
From our 35 studies, sample populations were mainly from rural or isolated settings. Figure 2 illustrates the breadth of federally- and state-recognized tribal entities (reservations and off-reservation trust lands) (red shading) (16), (17) along with the number of studies that we identified by sample population location (U.S. state) (yellow shading). Seventy percent of the sample populations were in only two states: New York (NY) (n=14) and Alaska (AK) (n=11). The few remaining sample populations were from western U.S. states. Population sample sizes ranged from 22 (18) to 10,360 (19) (Tables 1 and 2).
Fig 2.
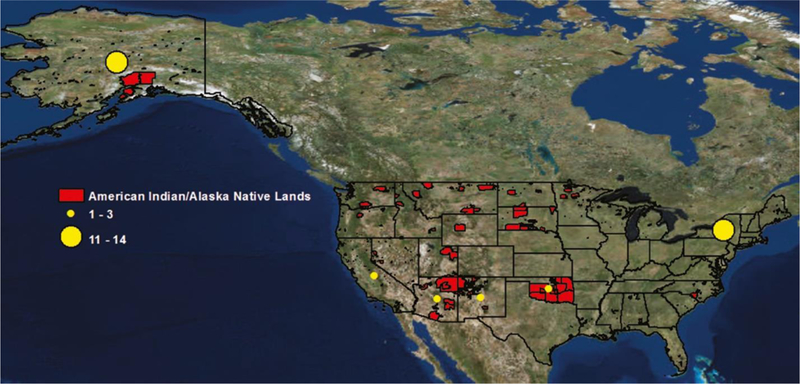
Number of studies by location (U.S. state) of sample populations.
Table 1.
Study characteristics for non-chemical stressors and health outcomes.
| Study | Health outcome(s) |
Lifestage group1 |
U.S. state / observation period |
Sample size/design |
Data source(s) | |
|---|---|---|---|---|---|---|
| Lack of piped/running water | ||||||
| Bruden et al. 2015 | LRTI RSV | <12 mos | AK 1995-2012 | N=49 villages village-level analysis | ACS, Census, med. records | |
| Bulkow et al. 2012 | hMPV hPIV LRTI RSV | <12 mos 1-<2 yrs 2-<3 yrs | AK 2006-2007 | n=128 cases n=186 controls case-control | Med. records, questionnaires | |
| Gessner et al. 2008 | LRTI | <12 mos 1-<2 yrs | AK 1998-2003 | N=108 villages community-level analysis | Census, med. assistance/water service records, provider billing | |
| Hennesy et al. 2008 | PNA and flu RSV | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 11-<16 yrs 16-21 yrs | AK 2000-2004 | N=128 villages, 12,480 homes in 6 regions village-level analysis | Med. records, outbreak investigation, sanitation inventory, surveillance | |
| Morris et al. 1990 | LRTI (PNA, bronchiolitis) | <12 mos 1-<2 yrs | AZ 1988 | n=58 cases n=58 controls case-control | Interviews, med. records | |
| Reisman et al. 2014 | Pneumococcal colonization of nasopharynx | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 11-<16 yrs 16-21 yrs | AK 2008-2011 | N=6,080 cross-sectional | Interviews, med. records, nasopharyngeal swabs for Streptococcus pneumoniae | |
| Wenger et al. 2010 | IPD | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs | AK 2001-2007 | N=50 villages village/city-level analysis | CDC’s Arctic Investigations Program, sanitation inventory | |
| Gilbreath et al. 2006a | Adverse birth outcomes | <12 mos | AK 1997-2001 | N=10,073 from 197 villages retrospective cohort |
Birth certificates, open dumpsite hazard rankings | |
| Gilbreath et al. 2006b | - | <12 mos | AK 1997-2001 | N=10,360 from 197 villages retrospective cohort |
Birth certificates, open dumpsite hazard rankings | |
| Use of wood for heating or cooking | ||||||
| Bruden et al. 2015 | LRTI RSV | <12 mos | AK 1994-2012 | N=49 villages village-level analysis | ACS, Census, med. records | |
| Bulkow et al. 2012 | LRTI | <12 mos 1-<2 yrs 2-<3 yrs | AK 2006-2007 | n=128 cases n=186 controls case-control | Med. records, questionnaires | |
| Morris et al. 1990 | LRTI (PNA, bronchiolitis) | <12 mos 1-<2 yrs | AZ 1988 | n=58 cases n=58 controls case-control | Interviews, med. records | |
| Ware et al. 2014 | Asthma LRTI | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs 16-21 yrs | AK 2011-2012 | N=475 in 241 households cross-sectional | Questionnaires | |
| Robin et al. 1996 | ALRI | <12 mos 1-<2 yrs | AZ 1992-1993 | n=45 cases n=45 controls case-control | Interviews, med. records | |
| Singleton et al. 2016 | Severe/chronic lung disease | 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs | AK 2012-2015 | N=63 households analyses between indoor air pollutants and respiratory symptoms/diagnoses | Air monitoring, interviews | |
| Mold | ||||||
| Bulkow et al. 2012 | LRTI | <12 mos 1-<2 yrs 2-<3 yrs | AK 2006-2007 | n=128 cases n=186 controls case-control | Med. records, questionnaires | |
| Surdu et al. 2006 | Asthma | 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs | NY - | n=25 cases n=25 controls case-control | Air/dust samples, interviews, med. records | |
| Ware et al. 2014 | Respiratory disease | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs 16-21 yrs | AK 2011-2012 | N=475 in 241 households cross-sectional | Questionnaires | |
| Petersen et al. 2003 | CRD | <12 mos >12 mos | AK - | N=22 community members (e.g., parents) qualitative | Interviews | |
| Farm operations | ||||||
| Goldcamp et al. 2006 (J Agric Saf Health) | Non-fatal injury | 6-<11 yrs 11-<16 yrs 16-21 yrs |
Nationwide 2000 | N=7,381 AI youth living on racial minority-operated farms | USDA survey for NIOSH, Census of Agriculture | |
| Goldcamp et al. 2006 (J Agromedicine) | Non-fatal injury | 6-<11 yrs 11-<16 yrs 16-21 yrs |
Nationwide 2000 | N=7,381 youth living on 9,556 AI-operated farms | USDA survey for NIOSH, Census of Agriculture | |
| Dirt floor | ||||||
| Morris et al. 1990 | LRTI (PNA, bronchiolitis) | <12 mos 1-<2 yrs | AZ 1988 | n=58 cases n=58 controls case-control | Interviews, med. records | |
| Garage attached to home | ||||||
| Surdu et al. 2006 | Asthma | 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs | NY - | n=25 cases n=25 controls case-control | Air/dust samples, interviews, med. records | |
| House built before 1985 | ||||||
| Ware et al. 2014 | Respiratory disease | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs 16-21 yrs | AK 2011-2012 | N=475 in 241 households cross-sectional | Questionnaires | |
| Steam baths/housing sand dust/poor sanitation | ||||||
| Petersen et al. 2003 | CRD | <12 mos >12 mos | AK - | N=22 community members (e.g., parents) qualitative | Interviews | |
U.S. Environmental Protection Agency. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Risk Assessment Forum. November 2005. EPA/630/P-03/003F. ACS - American Community Survey; AI - American Indian; AK - Alaska; ALRI - acute lower respiratory illness; AZ - Arizona; CA - California; CDC - Centers for Disease Control and Prevention; CRD - chronic respiratory disease; hMPV - human metapneumovirus; hPIV - human parainfluenza virus; IPD - invasive pneumococcal disease; LRTI -lower respiratory tract infection; NIOSH - National Institute for Occupational Safety and Health; NY- New York; PNA - pneumonia; RSV -respiratory syncytial virus; USDA - U.S. Department of Agriculture.
Table 2.
Study characteristics for chemical stressors and health outcomes.
| Study | Chemical(s) of interest |
Health outcome(s) |
Lifestage group1 |
U.S. state/ observation period |
Sample size/design |
Data source(s) |
|---|---|---|---|---|---|---|
| Youth (cohort from same territory) | ||||||
| Schell et al. 2004 | p,p’-DDE, HCB, mirex, PCBs, lead | Thyroid function | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY - | N=115 - | Blood samples |
| Schell et al. 2008 | p,p’-DDE, HCB, PCBs, lead, mercury | Thyroid hormone levels | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY 1995-2000 | N=232 - | Blood samples, interviews |
| Schell et al. 2009 | p,p’-DDE, HCB, PCBs | Thyroid function | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY 1995-2000 | N=115 - | Blood samples, interviews, questionnaires |
| Newman et al. 2006 | PCBs | Cognitive function | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY - | N=271 - | Blood samples, cognitive tests, interviews |
| Newman et al. 2009 | PCBs | Cognitive function | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY - | N=271 - | Blood samples, cognitive tests, interviews |
| Newman et al. 2014 | PCBs | ADHD | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY - | N=271 cross-sectional | Behavioral ratings, blood samples |
| Denham et al. 2005 | p,p’-DDE, HCB, PCBs, mirex, lead, mercury | Timing of menarche | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY - | N=138 cross-sectional | Blood samples, interviews |
| Ernst et al. 1986 | Fluoride from aluminum smelter | Lung function | 11-<16 yrs 16-21 yrs | NY 1981 | N=253 children analyses btw. community air pollution and individual lung function | Air and urine sampling, interviews, lung function tests, plant fluoride content |
| Gallo et al. 2011 | p,p’-DDE, HCB, PCBs | - | 16-21 yrs | NY - | N=152 - | Blood samples, questionnaires |
| Schell et al. 2003 | p,p’-DDE, HCB, mirex, PCBs, lead, mercury | - | 6-<11 yrs 11-<16 yrs 16-21 yrs | NY 1996-2000 | N=271 - | Blood samples, interviews |
| Youth (other AI cohort) | ||||||
| Malcoe et al. 2002 | Lead from a former uranium mining region | - | 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs | OK - | n=95 AI n=129 White n=26 cases n=198 controls case-control | Blood, dust, paint, soil, water samples; interviews |
| Mothers/infants (cohort from same territory) | ||||||
| Fitzgerald et al. 1998 | PCBs | - | <12 mos >12 mos | NY 1986-1992 | n=97 AI (cases) who gave birth 1969-1992, n=154 White (controls) in counties relatively free of PCB contamination who gave birth during same period cross-sectional | Breast milk samples, interviews |
| Fitzgerald et al. 2004 | PCBs | - | <12 mos | NY 1992-1995 | N=111 pregnant AI women - |
Air, blood, food, soil samples; interviews |
| Hong et al. 1994 | PCBs | - | <12 mos | NY 1988-1990 | n=20 AI (cases), n=30 controls from WIC clinics in 2 NY counties who gave birth 1988-1990 - | Breast milk samples, interviews |
| Fitzgerald et al. 2001 | p,p’-DDE, HCB, mirex | - | <12 mos >12 mos | NY 1986-1992 | n=97 AI (cases), n=154 White (controls) from other rural areas in NY - | Breast milk samples, interviews |
| Mothers/infants (Other AI/AN cohorts) | ||||||
| Gilbreath et al. 2006 (Int J Circumpolar Health) | Potential exposure to hazardous waste and waste disposal methods | Fetal/neonatal deaths, congenital anomalies | <12 mos | AK 1997-2001 |
N=10,360 from 197 villages retrospective cohort |
Birth certificates, open dumpsite hazard rankings |
| Gilbreath et al. 2006 (Am J Epidemiol) | Potential exposure to hazardous waste and waste disposal methods | Adverse birth outcomes | <12 mos | AK 1997-2001 |
N=10,073 from 197 villages retrospective cohort |
Birth certificates, open dumpsite hazard rankings |
| Orr et al. 2002 | COIs, inorganic compounds, nitrates/ nitrites, pesticides, VOCs | Birth defect | <12 mos | CA 1983-1988 | n=431 cases n=392 controls case-control | Birth defects program, residence |
| Shields et al. 1992 | Radiation from a former uranium mining region | Adverse birth outcomes | <12 mos | NM 1964-1981 | N=266 pairs of cases/controls case-control | Interviews, med. records, NIOSH |
| Monheit et al. 2008 | Fluridone (herbicide) | - | <12 mos >12 mos | CA 2005 | N=60 environmental samples human health hazard assessment | Aquatic tule vegetation, sediment, water samples |
| Xue et al. 2014 | PCBs | - | 11-<16 yrs 16-21 yrs | Nationwide 2001-2004 blood PCB levels; 1999-2006 dietary consumption | 3.9% of Asian/Pacific Islander, Native American, or multiracial (A/P/N/M) participants among 12-≤30 yrs of study sample for blood PCB levels, n=321 for A/P/N/M 12-20 years for consumption data - | NHANES, NYC Asian Market Survey, U.S. EPA’s Food Consumption Intake Database |
| Outdoor air pollution | ||||||
| Surdu et al. 2006 | Burn-barrel near home | Asthma | 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs |
NY - | n=25 cases n=25 controls case-control | Air/dust samples, interviews, med. records |
| Ware et al. 2014 | Outdoor smoke | Respiratory disease | <12 mos 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs 16-21 yrs | AK 2011-2012 | N=475 in 241 households cross-sectional | Questionnaires |
| Indoor woodstove use for primary heat source | ||||||
| Singleton et al. 2016 | VOCs, CO2, PM2.5 | Severe/chronic lung disease | 1-<2 yrs 2-<3 yrs 3-<6 yrs 6-<11 yrs 11-<16 yrs | AK 2012-2015 | N=63 households analyses between indoor air pollutants and respiratory symptoms/diagnoses | Air monitoring, interviews |
U.S. Environmental Protection Agency. 2005. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Risk Assessment Forum. EPA/630/P-03/003F. p,p’-DDE - dichlorodiphenyldichloroethylene; AI/AN - American Indian/Alaska Native; ACS - American Community Survey; ADHD - attention deficit hyperactivity disorder; CA - California; COI - cytochrome oxidase inhibitor; HCB - hexachlorobenzene; NHANES - National Health and Nutrition Examination Survey; NIOSH - National Institute for Occupational Safety and Health; NM - New Mexico; NY - New York; OK - Oklahoma; PCB - polychlorinated biphenyl; U.S. EPA - U.S. Environmental Protection Agency; VOC - volatile organic compound; WIC - Women, Infants, and Children.
AI/ANs were the only sample population in all studies except four (32), (44), (49), (52) (Tables 1 and 2). Goldcamp et al. (32) targeted injuries among household youth on minority-operated farms, which were also comprised of Asian, Black, and ‘Other’ operators. Malcoe et al. (44) examined lead exposures around a former mining region among Caucasian children in Oklahoma. Orr et al.’s (49) study cohort included Black/African-American, Hispanic/Latino, and Asian/Pacific-Islander children of women living around hazardous waste sites in California. And, Xue et al.’s (52) cohort was composed of Mexican-American, Caucasian, Black, Other Hispanic, and “Asian, Pacific-Islander, Native-American or multiracial” participants from the U.S.’s National Health and Nutrition Examination Survey (NHANES) for an analysis of blood polychlorinated biphenyl (PCB) concentrations. Village/community sample sizes ranged from 49 (20) to 197 (19). Among studies with information about their design, seven were case-control, six were done at the village/community-level, five were cross-sectional, and one was qualitative.
Overall, 16 studies reported stressors from the built environment, two studies reported stressors from the natural environment, and 22 studies reported stressors from both the built and natural environments (Figure 3A). Ten non-chemical stressors were identified from the built and natural environments (Figure 3A). We identified mold, a biological entity, as a non-chemical stressor in four studies; none of these identified any chemical stressors from mold. Several chemical stressors were identified, including volatile organic compounds (VOCs), particulate matter (PM2.5), carbon dioxide (CO2), PCBs, p,p-DDE, hexachlorobenzene (HCB), lead, mercury, radiation, fluoride, and fluridone.
Fig 3.
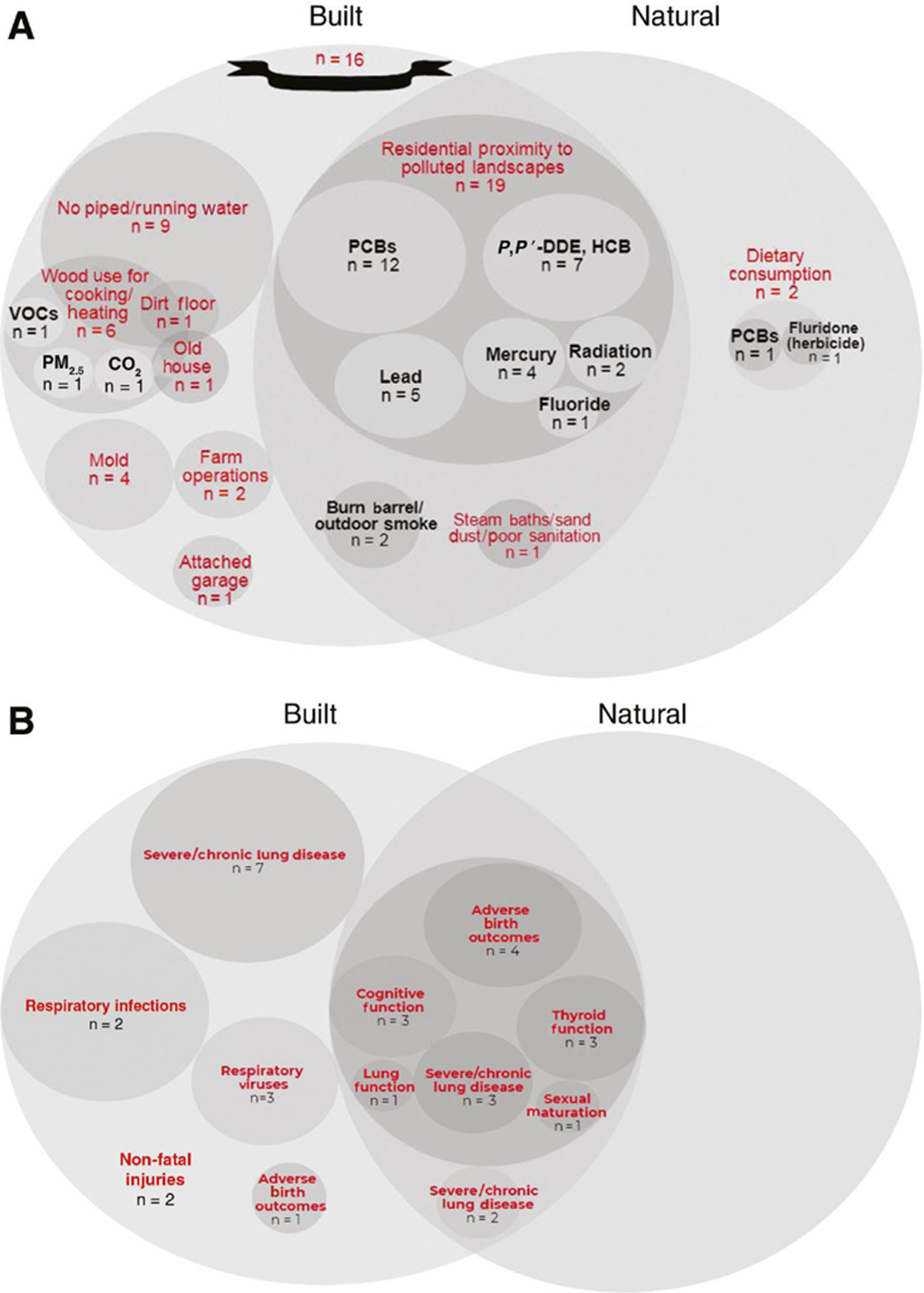
Identification of chemical and non-chemical stressors and health outcomes by environment. (A)Organization of chemical (black) and non-chemical (red) stressors into the built and natural environments as described by Tulve et al. (6). (B) Health outcomes identified from studies linked to the built and natural environments.(B) Few studies with matching stressors and outcomes were identified (Tables 1 and 2), ruling out a quantitative review. Instead, a qualitative review was performed for identified stressors and outcomes (Tables 3 and 4). Respiratory illness (asthma, respiratory infections, respiratory viruses) and developmental outcomes (e.g. adverse birth outcomes, cognitive/thyroid function) were the main outcomes evaluated in these studies (Figure 3B).
Built environment
Sixteen studies described chemical and non-chemical stressors from the built environment during childhood (Figure 1, Table 1). In 12 studies, respiratory illness was the outcome of interest; medical records were the data source for eight studies (Table 1). Stressors were derived around the household, affecting water quality (six studies), air quality (two studies), both (six studies) or due to farm operations (two studies). There were three studies that sampled inside households: Singleton et al. (30) sampled indoor air for particulate matter, carbon dioxide, and volatile organic compounds; Robin et al. (29) sampled respirable particles in indoor air; and Surdu et al. (31) measured mite and cat allergen concentrations in indoor dust.
Household lack of plumbing/running water
Nine studies identified the same non-chemical stressor in the home: lack of plumbing/running water. In seven of the nine studies, an increased risk of respiratory illness was associated with a lack of plumbing/running water (Table 3). Five of the seven studies found an increased risk of lower respiratory tract infections (LRTIs) [(20): relative risk=1.25, 95% confidence interval (CI) 1.05-1.26; (23)]: greatest rate ratio among those younger than 1 year=6.57, 95% CI 5.58-7.72; decreased LRTIs from having plumbing/running water [(21): odds ratio (OR)=0.29, 95% CI 0.14-0.58; (22): outpatient LRTI beta (β) estimate from regression analyses=−0.53, p<0.001; (24): OR=0.5, p=0.061]. Two of the seven studies observed an increased incidence of invasive pneumococcal disease [(26): 391 cases/100,000 children/year in a region with low proportion of households with piped water vs. 147 cases in a high-water service region, p=0.008 or increased pneumococcal colonization (precursor for invasive disease) of the nasopharynx [(25): greatest risk among those aged less than 5 years, OR=1.42]].
Table 3.
Study findings for non-chemical stressors and health outcomes from the built environment.
| Study | Variable of interest |
Health outcome(s) |
Effect estimate/ measure of association/ summary measure |
Other variables considered |
Association(s) between stressor and health outcome(s) |
|---|---|---|---|---|---|
| Lack of piped/running water | |||||
| Bruden et al. 2015 | Lack of plumbed water | LRTI, RSV | ADJUSTED LRTI RR 1.25 (95% CI 1.05-1.26) RSV RR 1.45 (1.19-1.78) UNADJUSTED LRTI RR 1.65 (1.49-1.83) RSV RR 1.85 (1.57-2.17) | Coastal community, community size, healthcare access, household crowding, period, poverty, wood heating | Greater hospitalizations for LRTIs/RSV in areas with higher proportion of households that lack plumbed water |
| Bulkow et al. 2012 | Sinks in 2 or more rooms | hMPV, hPIV, LRTI, RSV | ADJUSTED LRTI OR 0.29 (95% CI 0.14-0.58) UNADJUSTED hMPV OR 0.58 (P=0.350) hPIV OR 0.10 (P=0.030) LRTI OR 0.41 (95% CI 0.23-0.73) RSV OR 0.30 (P=0.081) | Bottle fed, medically high-risk, regularly vomiting after feeding, wood stove in house | Decreased risk of hospitalizations for hMPV, hPIV, LRTIs, and RSV for households with sinks in 2 or more rooms |
| Gessner et al. 2008 | Modern water service (in-home piped water/septic system, water delivered by truck) | LRTI | ADJUSTED Outpatient LRTI β −0.53 (P<0.001) inpatient LRTI β −0.15 (P=0.09) | Adult education, Alaska Native resident, young children in household, cigarette use, employment, household crowding, poverty, wood stove use | Higher incidence of LRTIs among households lacking modern water service |
| Hennessy et al. 2008 | Low in-home water service by region | Pneumonia and influenza, RSV | ADJUSTED Pneumonia and influenza <1 yr rate ratio 6.57 (95% CI 5.58-7.72) pneumonia and influenza 1-4 yrs rate ratio 2.96 (2.51-3.50) pneumonia and influenza 5-19 yrs rate ratio 1.80 (1.39-2.33) RSV <5 yrs rate ratio 3.4 (3.0-3.8) | Household crowding | Higher hospitalization rates for pneumonia and influenza and RSV in regions with lower proportion of home water service |
| Morris et al. 1990 | Presence of running water | LRTI (bronchiolitis or pneumonia) | UNADJUSTED OR=0.5 (P=0.061) | Asthma history, respiratory illness exposure, wood-burning stove | Children in households with running water tended to have fewer LRTIs |
| Reisman et al. 2014 | Lack of in-home running water | Pneumococcal colonization of nasopharynx | ADJUSTED <10 yrs OR 1.35 (95% CI 1.08-1.69) <5 yrs OR 1.42 (no P-value) UNADJUSTED <10 yrs OR 1.25 (P=0.001) 10-17 yrs OR 1.09 (P=0.26) | Antibiotic use, household crowding, no. of young children, otitus media, pneumonia, respiratory infection, strep throat, village | Increased prevalence of pneumococcal colonization significantly associated with lack of in-home running water among children <10 years |
| Wenger et al. 2010 | Lack of in-home piped water | IPD | UNADJUSTED IPD rate for low water service (<10% of households served in region): 391 cases/100,000 children/yr (P=0.008); IPD rate for midlevel water service (10-80%): 263 cases/100,000/yr; IPD rate for high water service (80%+): 147 cases/100,000/yr ADJUSTED IPR rate and water service (P<0.02) | Household crowding, poverty, wood for heating | Higher IPD rates associated with lack of in-home piped water |
| Gilbreath et al. 2006 (Am J Epidemiol) | Some or no piped water to households in village | Adverse birth outcomes | UNADJUSTED low birth weight: some households plumbed OR 1.35 (95% CI 1.06-1.72) no households plumbed OR 1.32 (1.00-1.74) vs. all households plumbed; preterm birth: some households plumbed OR 1.27 (1.07-1.51) no households plumbed OR 1.41 (1.17-1.71); intrauterine growth retardation: some households plumbed OR 0.92 (0.58-1.5) no households plumbed OR 1.12 (0.71-2.0) | Birth weight, gender, healthcare options, interpregnancy interval, maternal age/education, missing values, parity, prenatal care, race, tobacco/alcohol use, year of birth | Among mothers living near open dumpsites, a significantly higher risk of low birth weight, and preterm birth was associated with maternal residence in villages with some households receiving piped water compared to villages with all households receiving piped water. |
| Gilbreath et al. 2006 (Int J Circumpolar Health) | Some or no piped water to households in village | - | UNADJUSTED Villages with high hazard dumpsite rankings: no households plumbed 36%, some households plumbed 39%, all households plumbed 25% vs. villages with low hazard dumpsite rankings: no households plumbed 30%, some households plumbed 57%, all households plumbed 13% | Birth weight, gender, healthcare options, interpregnancy interval, maternal age/education, missing values, parity, prenatal care, race, tobacco/alcohol use, year of birth | Mothers from villages containing high hazard open dumpsite rankings were more likely to be in villages with some or no households with piped water compared to mothers from villages with low hazard ranked dumpsites. |
| Use of wood for heating or cooking | |||||
| Bruden et al. 2015 | Use of wood for heating | LRTI, RSV | UNADJUSTED LRTI RR 1.00 (95% CI 0.95-1.07) RSV RR 1.02 (0.93-1.15) | Coastal community, community size, healthcare access, household crowding, period, poverty, lack plumbing | Hospitalizations for LRTIs and RSV not significantly associated with households using wood for heating |
| Bulkow et al. 2012 | Woodstove for heating and/or cooking in house | hMPV, hPIV, LRTI, RSV | ADJUSTED LRTI OR 2.21 (95% CI 1.20-4.10) unknown viral pathogen OR 6.23 (P=0.01) UNADJUSTED hMPV OR 1.61 (P=0.351) hPIV OR 1.43 (P=0.624) RSV OR 1.22 (P=0.686) unknown viral pathogen OR 3.43 (P=0.01) | Bottle fed, medically high-risk, regularly vomiting after feeding, 2 or more rooms with sinks in house | Increased risk of hospitalizations for LRTIs associated with woodstove use in homes |
| Morris et al. 1990 | Wood-burning stove for heat | LRTI (bronchiolitis or pneumonia) | ADJUSTED OR 4.85 (95% CI 1.69-12.91) UNADJUSTED OR 4.2 (P=0.001) | Asthma history, respiratory illness exposure, running water | Higher risk of LRTIs associated with children living in homes with a wood-burning stove |
| Ware et al. 2014 | Woodstove for heating | Bronchitis, cold, flu, middle-ear infection, pneumonia, throat infection | UNADJUSTED <5 yrs pneumonia OR 2.1 (95% CI 0.6-7.2) bronchitis OR 2.0 (0.7-6.3) flu OR 1.0 (0.3-3.1) cold OR 1.8 (0.5-6.3) throat infection OR 1.9 (0.6-6.1) middle-ear infection OR 1.7 (0.7-4.4) 5-17 yrs pneumonia OR 1.5 (0.6-4.0) bronchitis OR 1.5 (0.7-3.3) flu OR 1.0 (0.5-2.3) cold OR 1.0 (0.5-2.0) throat infection OR 1.5 (0.8-3.0) middle-ear infection OR 1.2 (0.6-2.3) | Age of house, ventilation/purification, crowding, heating, household smoker, mold | Increased risk for respiratory infection among children living in homes heated exclusively with wood stoves compared to homes heated exclusively with fuel oil, but associations not statistically significant |
| Robin et al. 1996 | Cooked with wood-burning stove, respirable particle concentration ≥65 μg/m3 | ALRI | UNADJUSTED OR any wood vs. gas/electricity alone OR 5.0 (95% CI 0.6-42.8); respirable particle concentration ≥65 μg/m3 vs. lower concentration of respirable particles OR 7.0 (0.9-56.9) | Access to clinic/hospital, no. of rooms, no. of children in home, caretaker other than mother, running water, smoker, ceremonial herbs, home type | Increased ALRIs was associated (although wide CI) with cooking with wood-burning stoves and higher indoor air concentrations of respirable particles |
| Singleton et al. 2016 | Woodstove for primary heat source | Cough between colds, health provider ever said child had asthma, wheeze between colds | ADJUSTED Cough between colds: woodstove for primary heat source OR 3.2 (P=0.03) | Household crowding, mold in child’s bedroom, piped water/sewer system, RH, smoker | Higher risk for cough between colds associated with primary wood heat |
| Other | |||||
| Bulkow et al. 2012 | Mold | LRTI | UNADJUSTED OR 1.21 (95% CI 0.74-1.97) | Bottle fed, medically high-risk, vomiting after feeding, woodstove, rooms with sinks | Hospitalizations for LRTI not associated with homes with visible mold |
| Surdu et al. 2006 | Mold | Asthma | UNADJUSTED OR 0.83 (90% CI 0.30-2.29) | See above | No relationship found between asthma and mold in homes |
| Ware et al. 2014 | Mold | Bronchitis, cold, flu, middle-ear infection, pneumonia, throat infection | UNADJUSTED <5 yrs bronchitis OR 1.7 (95% CI 0.6-4.3), cold OR 2.4 (0.6-8.7), flu OR 2.5 (1.0-6.1), middle-ear infection OR 2.2 (1.0-5.0), pneumonia OR 1.3 (0.5-3.5), throat infection OR 1.8 (0.7-4.5); 5-17 yrs bronchitis OR 1.6 (0.9-3.0), cold OR 1.7 (0.9-3.3), flu OR 2.0 (1.1-3.7), middle-ear infection OR 1.8 (1.2-2.7), pneumonia OR 1.6 (0.5-2.3), throat infection OR 1.6 (1.0-2.8) | Age of house, ventilation/ purification, crowding, heating, household smoker | Non-significant elevated prevalence of reported respiratory infections associated with reported concerns about indoor mold |
| Petersen et al. 2003 | Mold | CRD | UNADJUSTED n=9/22 interviewees had opinions of mold as a contributing factor of CRD | Allergy, genetic/familial, inhalant abuse, diet, SE conditions | - |
| Goldcamp et al. 2006 (J Agric Saf Health) | Living on farm | Non-fatal injury | UNADJUSTED AI youth 24 injuries/1,000 youth (95% CI ±4.4), 50.9% of total injuries among racial minorities; injury rate among all racial minority youth: 12 (±1.7); rate ratio between injury rates for AI youth for work injuries and non-work injuries:1.3 (17.8/1,000 youth, 95% CI 12.7-22.9; 13.8/1,000 youth, 11.8-15.85) | Age, farm type, race, sex, work status | AI youth living on farms had almost double the overall injury among all racial minority household youth. Work-related injury rates for AI youth living on farms were greater than non-work injuries. |
| Goldcamp et al. 2006 (J Agromedicine) | Living on farm | Non-fatal injury | UNADJUSTED 83% of AI youth living on livestock farm types 27 injuries/1,000 youth, rest on crop farms 17% 18.9 injuries/1,000 youth | Age, farm type, sex, work status | More than half of all injuries to AI household youth on AI-operated farms were due to livestock operations. |
| Morris et al. 1990 | Dirt floor | LRTI (bronchiolitis or pneumonia) | Too few controls with a dirt floor for OR | Asthma family history, respiratory illness exposure, running water, wood-burning stove | - |
| Surdu et al. 2006 | Garage attached to home | Asthma | UNADJUSTED OR 1.31 (90% CI 0.39-4.43) | Asthma history; smoking; premature birth; breastfed; burn-barrel near home; insects in house; day-care; moist walls, ceilings, carpets, furniture; mold; pets | Non-significant increased risk of asthma associated with families with a garage attached to house |
| Ware et al. 2014 | House built before 1985 | Bronchitis, cold, flu, middle-ear infection, pneumonia, throat infection | UNADJUSTED <5 yrs bronchitis OR 0.2 (95% CI 0.1-0.6), cold OR 0.2 (0.1-0.7), flu OR 1.2 (0.5-2.6), middle-ear infection OR 0.6 (0.3-1.3), pneumonia OR 1.2 (0.4-3.1), throat infection OR 1.3 (0.6-3.0); 5-17 yrs bronchitis OR 0.6 (0.3-1.1), cold OR 0.9 (0.5-1.6), flu OR 1.2 (0.7-2.0), middle-ear infection OR 0.9 (0.5-1.3), pneumonia OR 1.2 (0.6-2.5), throat infection OR 0.9 (0.6-1.5) | Age of house, ventilation/ purification, crowding, heating, household smoker, mold | Lower reported prevalence of bronchitis among children less than 5 years associated with residence in an older home (built before 1985) |
| Petersen et al. 2003 | Steam baths/housing sand dust | CRD | UNADJUSTED n=22/22 interviewees reported steam from outdoor baths and n=16/22 interviewees: dust from river sandbars, building sand pads, or roads with motorized traffic as contributing factors | See above | - |
β = beta; μg/m3 - micrograms per cubic meter of air; AI - American Indian; ALRI - acute lower respiratory illness; BTEX - benzene, toluene, ethylbenzene, o-xylene, and m,p-xylene; CI - confidence interval; CO2 - carbon dioxide; CRD - chronic respiratory disease; hMPV - human metapneumovirus; hPIV - human parainfluenza virus; IPD - invasive pneumococcal disease; LRTI - lower respiratory tract infection; OR - odds ratio; PM - particulate matter; PPM - parts per million; RH - relative humidity; RR - relative rate; RSV - respiratory syncytial virus; SE - socioeconomic; VOC - volatile organic compound.
The two remaining studies examined adverse birth outcomes (27) from exposure to potential hazardous waste and waste disposal methods (19) among mothers living near open dumpsites in Alaska-Native villages who gave birth. Gilbreath and Kass (19) found mothers from villages with high hazard dumpsite rankings were more likely to live in villages with some households or no households with piped water compared to mothers from villages with low hazard dumpsite rankings. And, a significantly greater risk of low birth weight (OR: 1.35, 95% CI 1.06-1.72), very low birth weight (OR: 2.13, 95% CI 1.21-3.75), and preterm birth (OR: 1.27, 95% CI 1.07-1.51) was observed for infants from mothers who lived in villages with some households with piped water versus infants from mothers residing in villages with all households receiving piped water (27).
Household use of wood for heating or cooking
Six studies identified the use of wood for heating or cooking in the home as a non-chemical stressor. In five of these studies, an increased risk of respiratory illness was observed due to a woodstove or wood-burning stove [(30): greatest OR for cough between colds=3.18, p=0.027; (28): greatest OR for pneumonia among those aged less than 5 years=2.1, 95% CI 0.6-7.2; (21): OR for LRTIs=2.21, 95% CI 1.20-4.10; (29): OR for acute lower respiratory infections=5.0, 95% CI 0.6-42.8); (24): OR for LRTIs =4.85, 95% CI 1.69-12.91]. The remaining study did not find any association from household use of wood for heating ((20): relative risk for LRTIs=1.00, 95% CI 0.95-1.07].
Mold
The presence of mold was identified as a non-chemical stressor in four studies, one of which collected information about factors contributing to chronic respiratory disease (18). For the three studies with available effect estimates, two studies found an increased risk of respiratory illness due to mold ((28): unadjusted OR for cold among those aged less than 5 years=2.4 (95% CI: 0.6-8.7); (21): unadjusted OR for LRTIs=1.21 (95% CI: 0.74-1.97)). The third study found a lower risk of having asthma [(31): unadjusted OR=0.83 (90% CI: 0.30-2.29)]. None of these associations were statistically significant.
Farm operations
Two studies targeted the occurrence of injuries among household youth on minority-operated farms (32) and then only among a sub-cohort of AI-operated farms (33). In the Minority Farm Operator Childhood Agricultural Injury Survey, Goldcamp et al. (32) found that AI youth had almost double the rate of non-fatal injuries (24.0 injuries per 1000 youth, 95% CI ± 4.4) compared to injury rates among other minority youth living on farms (Asian: 4.6, 95% CI ± 2.2; Black: 6.4, 95% CI ± 2.4, Other: 12.3, 95% CI ± 3.7). AI youth had a greater rate of work-related injuries (17.8 per 1000 youth, 95% CI 12.7-22.9) compared to non-work-related injuries (13.8, 95% CI 11.8-15.8). Among AI-operated farms, Goldcamp et al. (33) observed more than half of AI youth (74%) lived on livestock farms and for AI youth that sustained injuries, 83% were due to livestock operations.
Natural environment
Residential proximity to polluted landscapes
Nineteen studies reported the same non-chemical stressor: residential proximity to polluted landscapes mainly contaminated by hazardous waste (Tables 2 and 4). Twelve of the 19 studies evaluated the impact of this non-chemical stressor on developmental outcomes (cognitive function, thyroid function, sexual maturation, lung function, and birth outcomes). Thirteen studies used biomarkers to characterize exposure to this stressor, with eight obtaining environmental measurements or employing other exposure characterization methods (19), (27), (41), (44), (46), (49), (50), (51) (Table 4). For these sample populations, 14 of the 19 studies reported on the same cohort living in the same territory in NY; investigators targeted youth (11 studies) and mothers (eight studies).
Table 4.
Study findings for chemical stressors and health outcomes.
| Study | Chemical(s) of interest (media) |
Health outcome(s) |
Effect estimate/ measure of association/ summary measure |
Other variables considered |
Association(s) between chemical and health outcome(s) |
|---|---|---|---|---|---|
| Youth (cohort from same territory) | |||||
| Schell et al. 2004 | p,p’-DDE, HCB, mirex, PCBs, lead (blood) | Thyroid function | ADJUSTED Sum of 8 persistent PCBs: TSH β=0.29 P≤0.05 FT4 β=−0.30 P≤0.05 T4 β=−0.35 P≤0.01 T3 β=−0.08; p,p’-DDE: TSH β=0.09 FT4 β=−0.01 T4 β=−0.03 T3 β=0.07; HCB: TSH β=−0.02 FT4 β=−0.08 T4 β=−0.02 T3 β=−0.04; mirex: TSH β=−0.04 FT4 β=−0.01 T4 β=−0.09 T3 β=−0.16; lead: TSH β=0.02 FT4 β=0.03 T4 β=0.02 T3 β=0.24 P≤0.05 | Age, lipids, other toxicants (p,p’-DDE, HCB, lead, mirex), sex, sum of 8 persistent PCBs as measure of PCB body burden, time of sample collection | Decreased levels of measures of thyroid function were significantly associated with increased persistent PCBs levels |
| Schell et al. 2008 | p,p’-DDE, HCB, PCBs, lead, mercury (blood) | Thyroid hormone levels | TSH levels: persistent 8 PCBs β=0.43 P=0.004, p,p’-DDE β=−0.076 P=0.488, HCB β=0.084 P=0.426, lead β=−0.017 P=0.58, mercury β=−0.026 P=0.628; FT4 persistent PCBs β=−0.099 P=0.015, p,p’-DDE β=−0.003 P=0.93, HCB β=−0.027 P=0.35, lead β=0.001 P=0.89, mercury β=0.007 P=0.62 | Age, breastfeeding, cholesterol, duration between interview and blood draw, sex, time of blood collection, triglycerides | Decreased levels of measures of thyroid function were significantly associated with increased persistent PCB levels |
| Schell et al. 2009 | p,p’-DDE, HCB, PCB groups by chlorination and structure (blood) | Thyroid function | TPOAb levels among those who were breastfed: PCBs detected in >50% of sample β=0.43 (P=0.01); p,p’-DDE β=0.34 (P=0.05); HCB β=0.05 (P=0.76); mirex β=0.09 (P=0.56) | Age, BMI, breastfeeding, diet, education, height, material well-being, sex, tobacco/alcohol use, weight | Increased TPOAb levels associated with significantly higher levels of all PCB groupings (except non-persistent PCBs) and levels of p,p’-DDE |
| Newman et al. 2006 | PCBs (sum of 16 PCB congeners detected in 50% or more of samples) (blood) | Cognitive function | ADJUSTED Summary PCB levels (ΣPCB50%) and long term memory measures with P<0.05: Delayed Recall Index β=−3.6 Long Term Retrieval β=−6.9 Comprehension-Knowledge β=−4.6 | Mother (e.g., cognition, smoking); adolescent (e.g., breastfeeding, other toxicants (p,p’-DDE, HCB, lead, mercury, mirex)) | Decreased test scores for long-term memory and comprehension-knowledge associated with increased concentrations of PCBs |
| Newman et al. 2009 | PCBs (congeners grouped by structure: dioxin-like or non-dioxin-like and by persistence: high or low) (blood) | Cognitive function | ADJUSTED Relationships with P-values <0.05: Σdioxin-like PCBs50%: measure of intellectual ability and reasoning skills (Ravens) β=−0.16 r2=18%, Delayed Recall β=−0.16 r2=13%, Long Term Retrieval β=−0.16 r2=11%; Σnon-dioxin-like PCBs50%: Ravens β=−0.08 r2=17%, Delayed Recall β=−0.21 r2=13%, Long Term Retrieval β=−0.25 r2=11%; Σpersistent PCBs50%: Delayed Recall β=−0.22 r2=13%, Long Term Retrieval β=−0.20 r2=12%, Auditory Processing β=−0.23 r2=12%; Σlow-persistent PCBs50%: Delayed Recall β=−0.16 r2=13% Long Term Retrieval β=−0.22 r2=12% | Age, BMI, breastfeeding, cholesterol/ triglyceride level, other toxicants (HCB, p,p’-DDE, mirex, blood lead, mercury), maternal BMI/ cognitive scores, smoking during pregnancy, SE status, sex | Decreased test scores for long-term memory associated with increased concentrations of PCB groupings by persistence and dioxin-like/non-dioxin-like congeners |
| Newman et al. 2014 | PCBs (sum of persistent congeners that may have been active throughout participants’ lives or prenatally, found in at least 50% of participants) (blood) | ADHD | ADJUSTED Only association with P-value <0.05 between summary measure of persistent PCBs and ADHD score (Impulsive-hyperactive Conners parent T-scores) β=−3.84 | Age, BMI, lipids, maternal factors (BMI, breastfeeding duration, cognitive ability, pregnancy, SE status, smoking during pregnancy), other toxicants (p,p’-DDE, lead, HCB, mercury), tobacco/ alcohol use | No evidence of adverse effects of persistent PCB levels on ADHD-like behavior |
| Denham et al. 2005 | p,p’-DDE, HCB, mirex, PCBs, lead, mercury | Timing of menarche | Pre- or post-menarcheal status (binary logistic regression) mean-centered levels: p,p’-DDE (ppb) β=−0.37 (P=0.66), group of estrogenic PCBs (ppb) β=2.13 (P=0.04), HCB (ppb) β=0.12 (P=0.93), lead (μg/dL) β=−1.29 (P=0.01), mercury (μg/dL) β=0.16 (P=0.78) | Age, BMI, SE status | Lower probability of reaching menarche significantly associated with higher lead levels and earlier age at menarche with higher PCB levels |
| Ernst et al. 1986 | Fluoride from aluminum smelter (urine) | Lung function | Only significant association of lung function with exposure: among boys, lung function CV/VC% high exposure adjusted mean 8.25 (SEE=1.02) low exposure mean=5.36 (SEE=1.07) P-value for differences=0.05; only significant association of lung function with urinary fluoride: among boys, CV/VC% slope 4.78 (P=0.02); among girls, CV/VC% 4.40 (P=0.01) | Age, height, smoking, time since last cold, weight | Increased closing volume (may indicate small airway abnormalities) significantly associated with living near smelter 60% of lifetime among boys and increasing levels of urinary fluoride |
| Gallo et al. 2011 | p,p’-DDE, HCB, PCBs (blood) | - | Geometric mean concentrations breastfed/non-breastfed: total PCBs 0.87/0.78 (P=0.04); Σ14 PCB50% 0.47/0.40 (P=0.02); Σ9 persistent PCBs 0.35/0.29 (P<0.01)); Σ5 non-persistent PCBs 0.10/0.11 (P=0.41); p,p’-DDE 0.33/0.32 (P=0.69); HCB 0.03/0.03 (P=0.27) | Age, BMI, breastfeeding, diet, education, medications, recreational/traditional activities, SE status, sex, tobacco/ alcohol use | - |
| Schell et al. 2003 | p,p’-DDE, HCB, mirex, PCBs, lead, mercury (blood) | - | Breastfed/non-breastfed ratios geometric mean levels: total PCBs 1.13=1.74/1.53 P≤0.001; ΣPCB50% (congeners with ≥50% detection rate) 1.28=0.76/0.59 P≤0.001; ΣPCB75% 1.32=0.60/0.45 P≤0.001; Σpersistent PCBs 1.39=0.46/0.33 P≤0.001; p,p’-DDE 1.45=0.45/0.31 P≤0.001; HCB 1.07=0.04/0.03; mirex 1.26~0.02/0.02 P=0.06; lead 1.03=0.72/0.70 P=0.89; mercury 0.96~0.09/0.09 P=0.64 | - | - |
| Youth (other AI cohort) | |||||
| Malcoe et al. 2002 | Lead from former uranium mining region (blood concentrations and residential environmental levels in dust, paint, soil, water) | - | Median blood lead levels (mg/kg) for AIs (5.0) and Caucasians (5.0) (P=0.48); median mean soil levels (mg/kg) for AIs (103) and Caucasians (148) (P=0.03) mean soil correlation 0.32 (P<0.001) front yard soil 0.32 (P<0.001) back yard soil 0.27 (P<0.001) mean sill dust 0.19 (P=0.005) mean floor dust 0.34 (P<0.001) child’s bedroom floor dust 0.24 (P<0.001) exterior paint index 0.12 (P=0.080) interior paint index 0.13 (P=0.051) water −0.01 P=0.92; associations with P-values <0.05 mean soil lead β=0.74 mean floor dust lead loading β=0.45 | Caregiver education, child’s hygiene, mouthing, poverty | - |
| Mothers/infants (cohort from same territory) | |||||
| Fitzgerald et al. 1998 | PCBs (total PCBs - summed each of 68 PCB-containing zones or peaks) (breast milk) | - | ADJUSTED Geometric mean breast milk total PCB concentration (ppm, fat basis) AI and control (rural White) mothers who gave birth 1986-1989: 0.602 vs 0.375 (P<0.01); 1990: 0.352 vs 0.404; 1991-1992 0.254 vs 0.318. Geometric mean breast milk concentrations (ppb, fat basis) for greatest concentrations of PCB congeners 1986-1989 AI vs control mothers, all pairwise comparisons had P-values less than 0.05: #138 53.5 vs 29.9; #153 49.8 vs 32.8; #99 32.9 vs 14.8. | Alcohol consumption/ antibiotic use before pregnancy, maternal age, previous breastfeeding | - |
| Fitzgerald et al. 2004 | PCBs (air, blood among pregnant women, local fruit and vegetable, local meat, soil, wild duck concentrations) | - | UNADJUSTED Geometric mean (median) of total PCBs (summed congener concentrations of 68 PCB containing zones or peaks) in serum of pregnant women: 1.2 ppb (maximum: 7.8); geometric means of 3 leading serum PCB congener concentrations (ppb) IUPAC Nos.: #153 0.092; #138 0.0345; #180 0.0142; surface soil total PCB average (range) concentration (ppb): 62 (<0.2-886); local meat: 20 (<0.2-69); wild duck: 481 (<0.2-5970); local fruits and vegetables: 5.33 (<0.2-150); average total PCB in air in winter: ≤1 ng/m3 and maximum averages in spring/summer: 9.2-10.8. | - | - |
| Hong et al. 1994 | PCBs (breast milk) | - | UNADJUSTED Mean (range) total coplanar PCB (sum of 12 non-ortho- and mono-ortho-substituted PCBs) milk levels fat: 49 ng/g (3-178) for AIs vs. 55 ng/g (8-179) controls (P=0.47). Main contributions of individual non-ortho- and mono-ortho-substituted PCB congeners to total calculated toxic equivalent values were PCB congeners #118 (25.8 pg/g lipid); #126 (25 pg/g lipid); #105 (10.8 pg/g lipid); and 156 (7.4 pg/g lipid). |
- | - |
| Fitzgerald et al. 2001 | p,p’-DDE, HCB, mirex (breast milk) | - | ADJUSTED Geometric mean breast milk levels (ppb, fat basis) of AI vs. control (rural White) mothers: 1986-1989: p,p’-DDE: 420 vs. 198 (P<0.05), HCB: 1.8 vs. 1.7, mirex: 2.6 vs. 1.2 (P<0.10); 1990: p,p’-DDE: 198 vs. 113 (P<0.05), HCB: 8.7 vs. 11.0, mirex: 2.3 vs. 1.0 (P<0.10); 1991-1992: p,p’-DDE: 183 vs. 190, HCB: 12.5 vs. 14.4, mirex: 3.0 vs. 1.4 (P<0.05) | Antibiotic use before pregnancy, BMI, breastfeeding, education, maternal age/height, occupation, parity, tobacco/ alcohol use | - |
| Mothers/infants (other AI/AN cohorts) | |||||
| Gilbreath et al. 2006a | Potential exposure to hazardous waste and waste disposal methods from maternal residence in village with open dumpsite(s) at time of birth | Fetal/neonatal deaths, congenital anomalies | ADJUSTED All deaths: high hazard dumpsite contents rate ratio: 2.04 (95% CI 0.48-8.57) vs. moderate hazard dumpsite contents; other congenital anomalies: high hazard dumpsite contents rate ratio: 4.27 (1.76-10.36) compared to moderate hazard dumpsite contents | Gender, healthcare options, interpregnancy interval, maternal age/education, missing values, piped water, prenatal care, race, tobacco/alcohol use | Infants from mothers in villages containing open dumpsites with high hazard dumpsite contents were more likely to have other congenital defects |
| Gilbreath et al. 2006b | Potential exposure to hazardous waste and waste disposal methods from maternal residence in village with open dumpsite(s) at time of birth | Adverse birth outcomes | ADJUSTED Low birthweight: high hazard dumpsite ranking OR 2.06 (95% CI 1.28-3.32), intermediate dumpsite hazard OR 1.73 (1.06-2.84) compared to infants from mothers in villages with low hazard dumpsite rankings; IGR: high hazard dumpsite OR 3.98 (1.93-8.21), intermediate hazard dumpsite OR 4.38 (2.20-8.77); preterm birth: high hazard OR 1.24 (0.89-1.74), intermediate hazard OR 0.77 (0.52-1.12) | Gender, health care options, interpregnancy interval, maternal age/education, missing values, parity, piped water, prenatal care, race, tobacco/alcohol use | Higher proportion of infants from mothers in villages with high or intermediate hazard dumpsites had low birth weight or intrauterine growth retardation compared to infants from mothers in villages containing low ranked dumpsites |
| Orr et al. 2002 | COIs, inorganic compounds, nitrates/nitrites, pesticides, VOCs from mother’s residence at time of delivery in same census tract as hazardous waste sites | Birth defect | ADJUSTED Greatest OR among AI/ANs for spina bifada (OR 7.35, 95% CI 1.01-53.44), NTDs (OR 5.51, 0.74-40.87), oral clefts (OR 2.45, 0.70-8.56); greatest OR among Hispanic/Latinos for anencephaly 1.70 (0.69-4.18); greatest OR among Black/African Americans for integument 1.19 (0.77-1.83); greatest OR among Asian/Pacific Islanders for anencephaly 4.30 (1.42-13.03); greatest ORs between potential exposure and any birth defect for all groups for COIs (OR 1.30, 1.02-1.67), nitrates/nitrites (OR 1.27, 0.68-2.36), pesticides (OR 1.18, 0.97-1.43) | Maternal age, prenatal care | Greatest increased risk of adverse birth outcomes among AI/Alaska Natives from potential exposure to contaminants vs. controls (same counties, no birth defect) |
| Shields et al. 1992 | Radiation from former uranium mining region | Adverse birth outcomes | Only statistical significant association when mother lived near tailings/mine dump and adverse birth outcome (group of outcomes included hip dysplasias and dislocations OR 2.71, 95% CI 1.09-7.64). Significant associations found when mother (OR 2.05, 1.16-3.76) or father (OR 2.56, 1.14-6.28) worked at electronics plant (worker exposures included variety of chemicals/solvents) and all adverse birth outcomes and for outcomes as above when mother worked at plant (OR 2.71, 1.09-7.64). | Mother’s co-morbidities (e.g., tobacco/alcohol use), period of birth by 6-yr intervals | Increased risk of adverse birth outcomes significantly with mother living near mine tailings/dumps; also independently associated when either parent worked at electronics plant |
| Monheit et al. 2008 | Herbicide (fluridone concentrations in aquatic vegetation, sediment, water) | - | Maximum fluridone concentration in vegetation 3.4 ppb sediment 65 ppb water 0.3 ppb; hazard quotient (compared ADD with non-carcinogenic reference dose) for child “worse-case” scenario for vegetation: sub-chronic (1 yr): 4.0E-05, chronic (6 yrs): 2.5E-04; sediment sub-chronic: 1.1E-06, chronic: 6.8E-06; water sub-chronic: 2.5E-07, chronic: 1.5E-06. Found little to no hazard from consuming vegetation. | Tule vegetation harvesting | - |
| Xue et al. 2014 | PCBs (blood) | - | ADJUSTED Among 12-≤30 years, A/P/N/M had highest total PCB concentrations (0.6 ng/g) compared to other racial/ethnic groups. Also highest daily average fish consumption for A/P/N/M = 0.3 g/kg. Pearson correlation coefficient=0.07 (P<0.01) between fish intake and total blood PCB levels | Age, gender, region, other racial/ethnic groups, survey periods | - |
| Outdoor air pollution | |||||
| Surdu et al. 2006 | Burn-barrel near home | Asthma | UNADJUSTED OR 1.56 (90% CI 0.52-4.74) | See above | Non-significant increased risk of asthma with burn-barrel near house |
| Ware et al. 2014 | Outdoor smoke | Bronchitis, cold, flu, middle-ear infection, pneumonia, throat infection | UNADJUSTED <5 yrs bronchitis OR 1.8 (95% CI 0.6-4.8), cold OR 0.9 (0.2-3.7), flu OR 1.3 (0.5-3.6), middle-ear infection OR 1.9 (0.7-5.4), pneumonia OR 1.8 (0.6-5.6), throat infection OR 1.4 (0.5-4.4); 5-17 yrs bronchitis OR 1.4 (0.7-2.8), cold OR 2.0 (0.8-4.5), flu OR 1.3 (0.7-2.4), middle-ear infection OR 1.4 (0.7-2.9), pneumonia OR 1.5 (0.6-3.7), throat infection OR 1.0 (0.5-1.8) | Age of house, change ventilation/ purification, crowding, heating, household smoker, mold | Non-significant elevated prevalence of reported respiratory infections associated with reported concerns about outdoor sources of smoke |
| Indoor woodstove use for primary heat source | |||||
| Singleton et al. 2016 | VOCs, CO2, PM2.5 | Cough between colds, health provider ever said child had asthma, wheeze between colds | ADJUSTED Cough between colds: BTEX>100 μg/m3 OR 4.4 (P<0.001), PM2.5>25 μg/m3 OR 2.2 (P=0.03); wheezing between colds OR 1.9 (P=0.07) for BTEX>100 μg/m3; ever said had asthma OR 3.02 (P=0.03) for BTEX>100 μg/m3, OR 0.38 (P=0.11) for CO2>1000 ppm | Household crowding, mold in child’s bedroom, piped water/sewer system, RH, smoker | Higher risk for cough between colds associated with VOCs > 100 μg/m3, and PM2.5 > 25 μg/m3; higher risk of wheezing between colds and asthma diagnosis associated with VOCs > 100 μg/m3 |
β = beta; p,p’-DDE - p,p’- dichlorophenyldichloroethylene; r2- coefficient of determination; ADD - average daily dose; ADHD - attention deficit hyperactivity disorder; AI/AN - American Indian/Alaska Native; A/P/N/M - Asian/Pacific/Native American/Other Multiracial; BMI - body mass index; CI - confidence interval; COI - cytochrome oxidase inhibitor; CV/VC% = closing volume as percent of vital capacity; FT4 - free thyroxine; HCB - hexachlorobenzene; IGR - intrauterine growth retardation; MUS - musculoskeletal; NTD - neural tube defect; OR - odds ratio; PCB - polychlorinated biphenyl; PPM - parts per million; PPB - parts per billion; SE - socioeconomic; SEE - standard error of estimate; T3 - triidothyronine; T4 - total thyroxine; TPOAb - anti-thyroid peroxidase; TSH - thyroid stimulating hormone; VOC - volatile organic compound.
AI youth
Among the 11 studies with AI youth cohorts, ten studies examined exposures of youth living in the same territory in NY adjacent to three hazardous waste sites (one National Priority Superfund site and two NY State Superfund sites) and an aluminum smelter (41) to PCBs, p,p′-DDE, HCB, mirex, fluoride, lead, and mercury. The remaining study examined childhood lead exposures from a former uranium mining region in Oklahoma (44).
Toxicant levels.
A study by Schell et al. (43) found youth (n=271 in NY, between 10 and 17 years) who were breastfed to have on average 1.3 times the levels of total PCB blood concentrations, persistent PCBs, and other toxicants (p,p′-DDE and mirex) compared to youth who were not breastfed (Table 4). Levels of HCB, lead, and mercury were similar across both breastfed and not-breastfed youth (43). When a sub-sample of these youth were between 17 and 20 years old (n=152), both groups (those who were breastfed and not-breastfed) had lower total PCB geometric mean concentrations than when they were sampled at a younger age (42). Levels of p,p′-DDE and HCB were similar to levels when sampled at an earlier age.
Cognitive function.
Newman et al. (37), (38), (39) conducted additional studies among the NY youth cohort to investigate relationships between PCB concentrations and cognitive function. Newman et al. (37) observed among 271 youth (between 10 and 17 years) that as PCB concentrations increased, long-term memory (Delayed Recall Index β=−3.6, p=0.019, long-term retrieval β=−6.9, p=0.004) and comprehension and knowledge (β=−4.6, p=0.043) scores decreased. Further research by Newman et al. (38) found decreased scores for long-term memory from increased PCB concentrations (all PCB congener groups: dioxin-like, non-dioxin-like, persistence, and low-persistence). These associations were observed between PCBs grouped by structure (dioxin-like PCBs and nonverbal Ravens’ test scores) and persistence (persistent PCBs and auditory processing scores, low-persistent PCBs and scores for comprehension and knowledge). Additional work by Newman et al. (39) did not find evidence of adverse effects of persistent PCB levels on ADHD-like behavior.
Thyroid function.
Among 232 adolescents in NY, Schell et al. (35) observed a significant reduction in levels of thyroid function measures with higher persistent PCB concentrations (similar findings were found in a preliminary study by Schell et al. (34) among a smaller cohort of 115 adolescents). And, Schell et al. (36) found those with elevated anti-thyroid peroxidase levels (suggesting an elevated risk of autoimmune disease) among 47 adolescents who were breastfed had significantly greater levels of all PCB groupings (except non-persistent PCBs) and levels of p,p′-DDE.
Other outcomes.
In a study evaluating multi-chemical exposures among adolescent girls (40), only higher lead levels were significantly associated with a delay in attaining menarche (β=−1.29, p=0.01) and a group of potentially estrogenic PCB congeners were associated with reaching menarche earlier (β=2.13, p=0.04). Another study (41) examined lung function among 253 adolescents who lived near an aluminum smelter. Ernst et al. (41) found significant associations between increased closing volume (may be indicative of lung abnormalities in small airways) only among boys who had lived near the smelter for 60% or more of their lives versus those who had lived farther away for 60% or more of their lives (high exposure mean CV/VC%=8.25, SEE=1.02 vs. low exposure mean CV/VC%=5.36, SEE=1.07; p=0.05).
Mothers
For the six studies including mothers, four studies examined maternal exposure to PCBs and DDT compounds. The two remaining studies investigated mother, father, and grandparent exposure to radiation from residence and/or work locations in a former uranium mining region in New Mexico (50) and maternal exposures to contaminants around hazardous waste sites in California (49).
Pregnant women.
Among 111 pregnant AI women living in NY from 1992 to 1995, Fitzgerald et al. (46) observed the total PCB geometric mean blood concentrations to be 1.2 ppb (maximum 7.8). PCB congeners detected at the greatest concentrations were 153 (geometric mean 0.09 ppb), 138 (0.03 ppb), and 180 (0.01 ppb).
Mothers who breastfed their infants
For AI mothers who gave birth between 1986 and 1989, the total PCB geometric mean breast milk concentration was 0.6 ppm (fat basis) (45). After 1989, their total PCB geometric mean breast milk concentration was lower (0.35 ppm in 1990 and 0.25 ppm for 1991-1992). Compared to Caucasian mothers (controls) who gave birth around the same time, their total PCB geometric mean breast milk concentration was lower than that of AI mothers only for the earliest period of study (1986-1989): 0.38 ppm (p<0.01). After 1989, though, the control mothers’ total PCB geometric mean breast milk concentration was slightly greater than that of the AI mothers: 0.40 ppm in 1990 and 0.32 ppm for 1991-1992.
The PCB congeners detected at the greatest concentrations in breast milk were identical among AI and control mothers. According to Fitzgerald et al. (45), they were PCB congeners 138 (geometric mean: 53.5 ppb, fat basis among AI mothers vs. 29.9 ppb among control mothers), 153 (49.8 ppb vs. 32.8 ppb), and 99 (32.9 ppb vs. 14.8 ppb). Another study by Hong et al. (47) examined the same cohort of breastfeeding mothers who gave birth between 1988 and 1990 and found PCB congeners to be the main contributors to their total PCB calculated toxic equivalent values. These congeners were 118 (25.8 pg/g lipid), 126 (25 pg/g), and 105 (10.8 pg/g).
For other toxicants that were examined among mothers who breastfed, geometric mean breast milk concentrations among AI mothers were all greater than those for Caucasian mothers only for the earliest period of study (1986-1989): p,p′-DDE: 420 ppb (fat basis) vs. 198 ppb; HCB: 2.6 vs. 1.2 ppb; and mirex: 1.8 vs. 1.7 ppb (48). After 1989, the geometric mean breast milk concentration (ppb) for HCB (1990: 8.7 vs. 11.0 and 1991-1992: 12.5 vs. 14.4) and p,p′-DDE (1991-1992: 183 vs. 190) was lower than those for control mothers.
Developmental outcomes.
Among other cohorts, an increased risk of adverse birth outcomes was observed when mothers lived near uranium mine dumps (tailings) in New Mexico (50), near Superfund hazardous waste site(s) in California (49), and near open dumpsites in AN villages (19), (27). Shields et al. (50) found a significant increase for a group of birth outcomes (n=113) (OR 2.71, 95% CI 1.09-7.64), including outcomes such as hip dysplasias/dislocations and mental retardation, when the mother lived near tailings/dumps. An increased significant association for all birth outcomes was also found when the mother (OR 2.05, 1.16-3.76) or father (OR 2.56, 1.14-6.28) worked at an electronics plant, confounding the previous association because these workers were also exposed to a variety of chemicals and solvents (50). Orr et al. (49) found the greatest OR among AI/ANs for birth defects and exposure potential of mothers to contaminants at hazardous waste sites (OR 1.19, 0.62-2.27) (vs. mothers who were not exposed) compared to associations observed among Hispanics/Latinos (OR 1.15, 0.95-1.38), Black/African Americans (OR 0.95, 0.70-1.28), and Asian/Pacific Islanders (OR 1.13, 0.84-1.53). Gilbreath and Kass (19) reported the only significant predictor for adverse birth outcomes was infants born with anomalies classified as other defects from mothers in villages with high hazard rankings for their dumpsite contents (compared to moderate rankings) (rate ratio 4.27, 95% CI 1.76-10.36). For other adverse birth outcomes, Gilbreath and Kass (27) found a significantly higher proportion of infants from mothers born with low birthweight or intrauterine growth retardation in villages with high hazard dumpsites (OR 2.06, 1.28-3.32; OR 3.98, 1.93-8.21, respectively) or intermediate hazards (OR 1.73, 1.06-2.84; OR 4.38, 2.20-8.77, respectively) compared to low hazard dumpsites.
Dietary consumption.
In two studies, we identified fluridone and PCBs as chemical stressors from aquatic vegetation (51) or dietary consumption (52), respectively (Table 2). Monheit et al. (51) observed very low levels of fluridone (an herbicide) in sediment, vegetation, and water, and found herbicide application methods posed little to no adverse effects to AI/AN children from fluridone exposure through consumption of the vegetation (Table 4). Xue et al. (52) found NHANES “Asian, Pacific Islander, Native American or multiracial” participants between 12 and 30 years had the highest total blood PCB concentrations [~0.6 nanograms per gram (ng/g)] when compared to total blood PCB concentrations among non-Hispanic-Caucasian (>0.4 ng/g), other Hispanic (>0.4 ng/g), non-Hispanic-Black (~0.4 ng/g), and Mexican-American (<0.4 ng/g) participants for the years 2001 and 2004. A linkage between PCB blood concentrations and dietary consumption information collected for the same NHANES participants found a positive correlation between fish consumption and total PCB blood concentrations (Pearson coefficient: 0.07, p<0.01) (52).
Outdoor/indoor air pollution.
Two studies identified stressors from outdoor air [concerns of outdoor smoke (28) and a burn-barrel near the home (31)] and indoor air pollution [use of woodstove for heating (30)]. For outdoor air pollution, both observed an increased risk for respiratory illness, but were not statistically significant [(28): greatest OR for colds among those 5-17 years=2.0, 95% CI 0.8-4.5; (31): OR for asthma=1.56, 90% CI 0.52-4.74]. From the indoor use of a woodstove, an increased risk was found for: cough between colds when VOC levels were greater than 100 μg/m3 (OR=4.4, p<0.001), levels of PM2.5 were greater than 25 μg/m3(OR=2.2, p=0.03); and an asthma history when VOC levels were higher than 100 μg/m3 (OR=3.02, p=0.03).
Discussion
To the best of our knowledge, this is the first review to focus on chemical and non-chemical stressors from AI/AN children’s built and natural environments and how these stressors may impact their health and well-being. The paucity of data on this research topic shows a significant information gap associated with understanding AI/AN children’s health and well-being. Additionally, 70% of the information we identified was collected from AI/AN populations in NY and Alaska.
Despite a paucity of information, this review suggests a potential increase in respiratory illness from the use of wood for heating or cooking or lack of running water. Among the 21 studies that identified stressors from the natural environment, 14 shared one cohort from the same territory in NY and the main chemical stressors identified were PCBs, p,p′-DDE, HCB, lead, and mercury.
When we compared stressors that we identified for AI/AN children to those for other non-urban children in the U.S., distinct chemical or non-chemical stressors were found from their built and natural environments (53), (54), (55), (56), (57). For instance, Loewenherz et al. (53) found children living in households with pesticide applicators and near pesticide-treated orchards in Washington State, had greater organophosphorus pesticide exposures compared to children without a household pesticide applicator and a greater distance from agricultural pesticide spraying.
For non-chemical stressors, the use of farm equipment, primarily tractors (54) followed by wagons, combines, and forklifts, posed a risk for injuries among children living around farms (55). Included in this review were two studies targeting minority youth living on farms (32), (33); a large proportion of these children’s non-fatal injuries were attributed to livestock operations. In another study among children up to 9 years old with farm-related injury deaths (in Wisconsin and Illinois) between 1979 and 1985, 55% were due to tractors, wagons, and trucks and 15% from drownings (56). Other chemical and non-chemical stressors included living on farms that raised swine (asthma prevalence of 44% among rural Iowa children, p=0.01) and raising swine and adding antibiotics to feed (56%, p=0.01) (57).
For stressors from the built environment that we identified for AI/AN children, similar stressors were observed for other children living in non-urban settings. These included inadequate plumbing for running water and wastewater disposal services. Among 188 rural low-income households with 320 children under 7 years that received water from a well in two western U.S. counties, 27% of households detected at least one contaminant, including total coliforms (18%), arsenic (6%), synthetic organic chemicals (6%), nitrates (2%), fluoride (2%), and Escherichia coli (<1%) (58). Also, in a study by Borchardt et al. (2003) among children less than 19 years old in Wisconsin, diarrhea was associated with drinking from a household well contaminated with fecal enterococci (OR=6.18, 95% CI: 1.22-31.46).
For other children living in rural settings, stressors were identified related to access to resources and impact of nature on life stress, which were not identified as part of our review. According to the 2001 National Household Travel Survey, rural households traveled farther than urban residents to access health care; rural residence was associated with a trip of 30 road miles or more (OR=2.67, 95% CI: 1.39-5.15) (59). Another study found rural residence to lower levels of life stress among children in grades three to five who lived in higher levels of nearby nature (vegetation near residence) compared to children with little nearby nature (60).
Limitations
The main limitation of this review was the few identified relevant studies (<40). This limitation ruled out a quantitative review (i.e. meta-analysis) due to the variety of study designs, stressors, and outcomes. Few studies had matching methodology for the same stressor and outcome, so we opted to qualitatively compare studies for similar stressors and outcomes. We also compared effect estimates from community/village-level analyses and effect estimates from individual-level analyses for similar stressors and outcomes.
Other limitations were due to sample diversity and publication bias. A lack of diversity was noted among our studies’ sample cohorts; 14 studies reported on the same cohort living in the same territory in NY. These studies mainly identified the same stressor (living around hazardous waste/polluted sites and exposure to PCBs). Another limitation was that we relied on published literature, perhaps limiting us to studies that generated findings with distinctive or significant associations as non-significant associations are not as frequently published. We did not have access to unpublished literature investigating other possible stressors and outcomes.
Future research
This review identified an information gap concerning research outside of AI/AN children’s social environment. Almost all 35 studies from this review targeted stressors where AI/AN children lived, in mostly non-urban settings. According to Tulve et al.’s (6) conceptual framework, a child’s total environment needs to be considered to examine the interrelationships between chemical and non-chemical stressors, inherent characteristics, and children’s activities and behaviors in influencing their health and well-being. The total environment includes chemical and non-chemical stressors from environments where children live, learn, and play (6). Future studies need to consider chemical and non-chemical stressors from the built environment outside the household (e.g. school, daycare) and in urban settings. Other elements of the natural environment (e.g. access to green space, parks) need to also be considered in influencing health and well-being; this population may be receiving one of the largest exposures to green space (e.g. forests)/bluespace (e.g. lakes). Linkages between chemical and non-chemical stressors from AI/AN children’s built, natural, and social environments can then be performed in order to evaluate the total environment and their impacts on AI/AN children’s health and well-being.
Conclusion
This review provides information about the nature of chemical and non-chemical stressors from the built and natural environments that may influence AI/AN children’s health and well-being. The findings from this review can be used as a guide to promote healthy environments for AI/AN children in particular for household use of wood for heating or cooking and access to indoor running water. This work identified a major research gap, which may help direct future research initiatives to develop studies to consider stressors outside the household and other elements of the natural environment.
Acknowledgments:
The authors wish to thank Lindsay Stanek, Tim Wade, and Ferdouz Cochran (U.S. EPA) for their technical review contributions.
Funding: The United States Environmental Protection Agency (EPA) through its Office of Research and Development wholly funded the research described here. This research was supported by an appointment to the Internship/Research Participation Program at the U.S. Environmental Protection Agency, Office of Research and Development, National Exposure Research Laboratory, administered by the Oak Ridge for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Conflicts of interest: None
References
- 1.Cohen Hubal EA, Sheldon LS, Burke JM, McCurdy TR, Berry MR, Rigas ML, et al. Children’s exposure assessment: a review of factors influencing children’s exposure, and the data available to characterize and assess that exposure. Environ Health Perspect 2000;108(6):475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ Health Perspect 2000;108(Suppl 1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss B Vulnerability of children and the developing brain to neurotoxic hazards. Environ Health Perspect 2000;108(Suppl 3):375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman LR. Children-unique and vulnerable. Environmental risks facing children and recommendations for response. Environ Health Perspect 1995;103(Suppl 6):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Environmental Protection Agency. Guidelines for developmental toxicity risk assessment. Risk Assessment Forum, 1991. [Google Scholar]
- 6.Tulve NS, Ruiz JDC, Lichtveld K, Darney SP, Quackenboss JJ. Development of a conceptual framework depicting a child’s total (built, natural, social) environment in order to optimize health and well-being. J Environ Health Sci 2016;2(2):1–8. [Google Scholar]
- 7.U.S. Environmental Protection Agency. Concepts, Methods and data sources for cumulative health risk assessment of multiple chemicals, exposures and effects: a resources document, 2007.
- 8.U.S. Environmental Protection Agency. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Risk Assessment Forum, 2005. [Google Scholar]
- 9.Indian Health Service (U.S. Department of Health and Human Services). Trends in Indian Health. 2014 edition. Available at: https://www.ihs.gov/dps/index.cfm/publications/trends2014/ Accessed: July 31, 2018.
- 10.Indian Health Service (U.S. Department of Health and Human Services). Environmental Health Services. Available at: https://www.ihs.gov/newsroom/factsheets/environmentalhealthservices/.
- 11.Burnette CEP, Figley CRP. Risk and protective factors related to the wellness of American Indian and Alaska Native youth: a systematic review. Intl Public Health J 2016;8:137–54. [Google Scholar]
- 12.Green HJ. Risks and attitudes associated with extra-cultural placement of American Indian children: a critical review. J Am Acad Child Psychiatry 1983;22(1):63–7. [DOI] [PubMed] [Google Scholar]
- 13.Dinges NG, Trimble JE, Hollenbeck AR. American Indian adolescent socialization: a review of the literature. J Adolesc 1979;2(4):259–96. [DOI] [PubMed] [Google Scholar]
- 14.Stevens DC, Hanson J, Prasek JL, Elliott A. Breastfeeding: a review of the benefits for American Indian women. S D Med 2009;61:448–51. [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau. Cartographic Boundary Shapefiles - American Indian/Alaska Native Areas/Hawaiian Home Lands 2017. Available at: https://www.census.gov/geo/maps-data/data/cbf/cbf_aiannh.html.
- 17.U.S. Census Bureau. TIGER/Line Shapefile, 2017, Nation, U.S., Current American Indian/Alaska Native/Native Hawaiian Areas National (AIANNH) National. Available at: https://catalog.data.gov/dataset/tiger-line-shapefile-2017-nation-u-s-current-american-indian-alaska-native-native-hawaiian-area.
- 18.Petersen KM, Singleton RJ, Leonard L. A qualitative study of the importance and etiology of chronic respiratory disease in Alaska Native children. Alaska Med 2003;45(1):14–20. [PubMed] [Google Scholar]
- 19.Gilbreath S, Kass PH. Fetal and neonatal deaths and congenital anomalies associated with open dumpsites in Alaska Native villages. Int J Circumpolar Health 2006;65(2):133–47. [DOI] [PubMed] [Google Scholar]
- 20.Bruden DJT, Singleton R, Hawk CS, Bulkow LR, Bentley S, Anderson LJ, et al. Eighteen years of respiratory syncytial virus surveillance changes in seasonality and hospitalization rates in southwestern Alaska Native children. Pediatr Infect Dis J 2015;34(9):945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulkow LR, Singleton RJ, DeByle C, Miernyk K, Redding G, Hummel KB, et al. Risk factors for hospitalization with lower respiratory tract infections in children in rural Alaska. Pediatrics 2012;129(5):E1220–7. [DOI] [PubMed] [Google Scholar]
- 22.Gessner BD. Lack of piped water and sewage services is associated with pediatric lower respiratory tract infection in Alaska. J Pediatr 2008;152(5):666–70. [DOI] [PubMed] [Google Scholar]
- 23.Hennessy TW, Ritter T, Holman RC, Bruden DL, Yorita KL, Bulkow L, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health 2008;98(11):2072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris K, Morgenlander M, Coulehan JL, Gahagen S, Arena VC. Wood-burning stoves and lower respiratory tract infection in American Indian children. Am J Dis Child 1990;144(1):105–8. [DOI] [PubMed] [Google Scholar]
- 25.Reisman J, Rudolph K, Bruden D, Hurlburt D, Bruce MG, Hennessy T. Risk factors for pneumococcal colonization of the nasopharynx in Alaska Native adults and children. J Pediatric Infect Dis Soc 2014;3(2):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, et al. Invasive pneumococcal disease in Alaskan children impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J 2010;29(3):251–6. [DOI] [PubMed] [Google Scholar]
- 27.Gilbreath S, Kass PH. Adverse birth outcomes associated with open dumpsites in Alaska native villages. Am J Epidemiol 2006;164(6):518–28. [DOI] [PubMed] [Google Scholar]
- 28.Ware DN, Lewis J, Hopkins S, Boyer B, Montrose L, Noonan CW, et al. Household reporting of childhood respiratory health and air pollution in rural Alaska Native communities. Int J Circumpolar Health 2014;73:24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robin LF, Lees PSJ, Winget M, Steinhoff M, Moulton LH, Santhosham M, et al. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr Infect Dis 1996;15(10):859–65. [DOI] [PubMed] [Google Scholar]
- 30.Singleton R, Salkoski AJ, Bulkow L, Fish C, Dobson J, Albertson L, et al. Housing characteristics and indoor air quality in households of Alaska Native children with chronic lung conditions. Indoor Air 2016;27:478–86. [DOI] [PubMed] [Google Scholar]
- 31.Surdu S, Montoya LD, Tarbell A, Carpenter DO. Childhood asthma and indoor allergens in Native Americans in New York. Environ Health 2006;5:22. doi: 10.1186/1476-069X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldcamp EM, Hendricks KJ, Layne LA, Myers JR. Nonfatal injuries to household youth on racial minority-operated farms in the U.S. 2000. J Agric Saf Health 2006;12(4):315–24. [DOI] [PubMed] [Google Scholar]
- 33.Goldcamp EM, Hendricks KJ, Layne LA, Myers JR. Nonfatal injuries to household youth on Native American operated farms in the U.S. 2000. J Agromedicine 2006;11(3-4):61–9. [DOI] [PubMed] [Google Scholar]
- 34.Schell LM, Gallo MV, DeCaprio AP, Hubicki L, Denham M, Ravenscroft J. Thyroid function in relation to burden of PCBs, p,p′-DDE, HCB, mirex and lead among Akwesasne Mohawk youth: a preliminary study. Environ Toxicol Pharmacol 2004;18(2):91–9. [DOI] [PubMed] [Google Scholar]
- 35.Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p′-DDE, and other toxicants in Akwesasne Mohawk youth. Environ Health Perspect 2008;116(6):806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ Res 2009;109(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman J, Aucompaugh AG, Schell LM, Denham M, DeCaprio AP, Gallo MV, et al. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol Teratol 2006;28(4):439–45. [DOI] [PubMed] [Google Scholar]
- 38.Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD, et al. Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology 2009;30(4):686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman J, Behforooz B, Khuzwayo AG, Gallo MV, Schell LM, Akwesasne task force on the environment. PCBs and ADHD in Mohawk adolescents. Neurotoxicol Teratol 2014;42:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics 2005;115(2):e127–34. [DOI] [PubMed] [Google Scholar]
- 41.Ernst P, Thomas D, Becklake MR. Respiratory survey of North American Indian children living in proximity to an aluminum smelter. Am Rev Respir Dis 1986;133(2):307–12. [DOI] [PubMed] [Google Scholar]
- 42.Gallo MV, Schell LM, DeCaprio AP, Jacobs A. Levels of persistent organic pollutant and their predictors among young adults. Chemosphere 2011;83(10):1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schell LM, Hubicki LA, DeCaprio AP, Gallo MV, Ravenscroft J, Tarbell A, et al. Organochlorines, lead, and mercury in Akwesasne Mohawk youth. Environ Health Perspect 2003;111(7):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malcoe LH, Lynch RA, Kegler MC, Skaggs VJ. Lead sources, behaviors, and socioeconomic factors in relation to blood lead of Native American and White children: a community-based assessment of a former mining area. Environ Health Perspect 2002;110:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald EF, Hwang S-A, Bush B, Cook K, Worswick P. Fish consumption and breast milk PCB concentrations among Mohawk women at Akwesasne. Am J Epidemiol 1998;148(2):164–72. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald EF, Hwang S-A, Langguth K, Cayo M, Yang B- Z, Bush B, et al. Fish consumption and other environmental exposures and their associations with serum PCB concentrations among Mohawk women at Akwesasne. Environ Res 2004;94(2): 160–70. [DOI] [PubMed] [Google Scholar]
- 47.Hong CS, Xiao J, Casey AC, Bush B, Fitzgerald EF, Hwang SA. Mono-ortho-and non-ortho-substituted polychlorinated biphenyls in human milk from Mohawk and control women: effects of maternal factors and previous lactation. Arch Environ Contam Toxicol 1994;27(3):431–7. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald EF, Hwang S-A, Deres DA, Bush B, Cook K, Worswick P. The association between local fish consumption and DDE, mirex, and HCB concentrations in the breast milk of Mohawk women at Akwesasne. J Expo Sci Environ Epidemiol 2001;11(5):381. [DOI] [PubMed] [Google Scholar]
- 49.Orr M, Bove F, Kaye W, Stone M. Elevated birth defects in racial or ethnic minority children of women living near hazardous waste sites. Int J Hyg Environ Health 2002;205(1-2):19–27. [DOI] [PubMed] [Google Scholar]
- 50.Shields LM, Wiese W, Skipper B, Charley B, Banally L. Navajo birth outcomes in the Shiprock uranium mining area. Health Phys 1992;63(5):542–51. [DOI] [PubMed] [Google Scholar]
- 51.Monheit SG, Leavitt RC, Akers P, Wong E. Health hazard assessment for Native Americans exposed to the herbicide fluridone via the ingestion of tules at Clear Lake, California, USA. Hum Ecol Risk Assess 2008;14(5):1056–69. [Google Scholar]
- 52.Xue J, Liu SV, Zartarian VG, Geller AM, Schultz BD. Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J Expo Sci Environ Epidemiol 2014;24(6):615–21. [DOI] [PubMed] [Google Scholar]
- 53.Loewenherz C, Fenske RA, Simcox NJ, Bellamy G, Kalman D. Biological monitoring of organophosphorus pesticide exposure among children of agricultural workers in central Washington state. Environ Health Perspect 1997;105(12):1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorann S Fatal unintentional injuries among Kentucky farm children: 1979 to 1985. J Rural Health 1989;5(3):246–56. [Google Scholar]
- 55.Rivara FP. Fatal and nonfatal farm injuries to children and adolescents in the United States. Pediatrics 1985;76(4):567–73. [PubMed] [Google Scholar]
- 56.Salmi LR, Weiss HB, Peterson PL, Spengler RF, Sattin RW, Anderson HA. Fatal farm injuries among young children. Pediatrics 1989;83(2):267–71. [PubMed] [Google Scholar]
- 57.Merchant JA, Naleway AL, Svendsen ER, Kelly KM, Burmeister LF, Stromquist AM, et al. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect 2005;113(3):350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Postma J, Butterfield PW, Odom-Maryon T, Hill W, Butterfield PG. Rural children’s exposure to well water contaminants: implications in light of the American Academy of Pediatrics’ recent policy statement. J Am Acad Nurse Pract 2011;23(5):258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Probst JC, Laditka SB, Wang J-Y, Johnson AO. Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res 2007;7(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells NM, Evans GW. Nearby nature: a buffer of life stress among rural children. Environ Behav 2003;35(3):311–30. [Google Scholar]


