Abstract
The resistance mechanisms of bacteria and protozoans have evidenced the need of discover new compounds with potential pharmaceutical activity against pathogenic microorganisms. Medicinal plants have been for centuries a promising alternative as sources of new drugs. The objective of this work was to evaluate the chemical composition, antimicrobial and antileishmanial activities of Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils. Chemical analysis was performed by gas chromatography-mass spectrometry. Antimicrobial activity was performed by disk diffusion and minimum inhibitory concentration (MIC) test. Antileishmanial activity was performed against antipromastigote and intracellular amastigote of Leishmania amazonensis. Cytotoxic and nitrite production were realized in BALB/c peritoneal macrophages. The major compounds of the essential oils were cinnamic aldehyde (46.30%) in C. zeylanicum, cis-p-menth-2-en-1-ol (33.88%) and linalyl acetate (13.90%) in O. vulgare, and turmerone (55.43%) in C. longa. The MIC showed significant antimicrobial activity of C. longa essential oil against S. aureus (83.3 ± 14.43 µg/mL). Antipromastigote activity showed IC50 values >500 µg/mL to C. zeylanicum, 308.4 ± 1.402 µg/mL to O. vulgare, and 405.5 ± 1.119 µg/mL to C. longa essential oil. Activity against intracellular amastigote of L. amazonensis showed IC50 of 63.3 ± 1.369 µg/mL and cytotoxic was not observed, resulting in selectivity index higher than 15.79 to parasite. C. longa essential oil decreased nitrite production in peritoneal macrophages, but not in Leishmania-infected cells. The chemical composition of the three essential oils is directly associated to its potential biological action, as the antimicrobial activity. C. longa presented a potent antileishmanial activity against promastigote and intracellular amastigote of L. amazonensis, although this activity is not linked to nitric oxide, since C. longa essential oil inhibits its production.
1. Introduction
Hospital-acquired infections are directly linked to Gram-positive pathogens as Staphylococcus aureus, and Gram-negative pathogens as Escherichia coli and Pseudomonas aeruginosa. It is estimated that in the United States in the 1990s hospital infections cost $4.5 billion and contributed to more than 88,000 deaths [1]. The main treatment adopted to combat hospital infections is the indiscriminate use of antimicrobials that generated methicillin-resistant S. aureus. Frequent use of antibiotics is cited as the cause of various bacteria resistance to a range of commonly available antibiotics, especially penicillin [2].
Drugs used during treatment of infections are generally associated with adverse effects on the patient, including hypersensitivity, hepatotoxicity, and nefrotoxicity. The treatment for leishmaniasis, a complex of disease that affects millions of people around the world, is one of the treatments where cases of resistance and toxicity are constantly reported [3–5]. In the face of these side effects and the resistance that pathogenic microorganisms have acquired, research has been focused with attention on essential oils, extracts, and biologically active compounds isolated from plants species used in traditional medicine.
The genus Cinnamomum includes approximately 250 species; among these is Cinnamomum zeylanicum Blume (Lauraceae) commonly called cinnamon. It is widely used as a seasoning in cooking because of its characteristic flavor. It is cultivated mainly in countries like India, Sri Lanka, and China. Extracts, essential oils, and cinnamon isolates have excellent applications in food, cosmetics, and pesticides, due to antimicrobial, antioxidant, and antifungal properties [6, 7]. In traditional medicine, it has wide use as a remedy for indigestion, diabetes, acne, respiratory, and urinary problems, etc. [8].
Turmeric (Curcuma longa L.) is a plant native to Southeast Asia that belongs to the Zingiberaceae family. As a powder called saffron, it has been used continuously as a seasoning in vegetarian and nonvegetarian food preparations and also has digestive properties. Its pyriform rhizomes, often short and branched, are home remedies used in folk medicine. The therapeutic properties of turmeric or its compounds include antimicrobial [9], antifungal, and antioxidant [10] activity. Antileishmanial activity of molecules isolated from C. longa has been described in trial assays to promastigote forms, but there is no data about its effectiveness against intracellular amastigote, the form found in mammalian hosts [11].
Oregano or marjoram (Origanum vulgare Linn.) is the most variable species of the genus Origanum, characterized by morphological and chemical diversity. Used worldwide since ancient times in traditional and popular medicine, oregano is spread all over the world and is particularly abundant in the Mediterranean, Eurasian, and North African area. Several studies have shown that the plant has a wide variety of secondary metabolites, most of them phenolic compounds such as flavonoids, terpenoids, phenolic acids and alkaloids, and fatty acids, among others, which are the main components responsible for its action [12].
Due to the wide use of these plants, associated with the need to discover new compounds with potential pharmaceutical activity against pathogens, this work aimed to evaluate the chemical composition and antimicrobial and antileishmanial activities of Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils.
2. Material and Methods
2.1. Plant Material
The leaves of Cinnamomum zeylanicum were collected in the city of São Luis, Maranhão, Brazil. The taxonomic identification was made by Ana Zelia Silva in the Seabra Ático Herbarium of the Department of Botany of the Universidade Federal do Maranhão, registry number 1153. Aerial parts of Origanum vulgare and Curcuma longa rhizome were purchased in the Central Market of São Luís. All three plants were dried for 48 hours and sprayed in an electric knife mill at the Laboratório de Controle de Qualidade de Alimentos e Água of the Universidade Federal do Maranhão.
2.2. Essential Oils Extraction
The essential oils were extracted by hydrodistillation using Clevenger system. A quantity of 100g of dry leaves diluted in water at a ratio of 1:10 was boiled at 100°C for 3 hours. The oil was dried over anhydrous sodium sulfate and kept in an amber bottle under refrigeration [13]. For in vitro biological assay, the essential oils were dissolved in dimethylsulfoxide (DMSO) at 100 times the highest concentration of use and subsequently diluted in an appropriate medium to a final concentration of less than 1% of DMSO.
2.3. Gas Chromatography-Mass Spectrometry Analyses (GC-MS)
The essential oils under study were dissolved in ethyl acetate 1 mg/mL and analyzed on Shimadzu QP 5000 gas chromatograph with ZB-5 ms capillary column (5% phenyl arylene 95% dimethylpolysiloxane) coupled at 70 eV (40-500 Da) HP 5MS mass selective detector of electronic impact with a transference temperature of 280°C. Chromatographic conditions were volume injection of 0.3 μL of ethyl acetate; helium carrier gas (99.99%); injector temperature: 280°C, split mode (1:10); initial temperature of 40°C and a final temperature of 300°C; initial time 5 min and final time 7.5 min at 8°C/min [14].
2.4. Bacterial Culture
Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 12600), and Pseudomonas aeruginosa (ATCC 27853) strains were cultured in Brain Heart Infusion (BHI) broth for 24 h at 37°C and diluted to 108 CFU/mL following the MacFarland scale [15].
2.5. Antimicrobial Assays
Disk diffusion tests were performed with filter paper impregnated with 75 μL of essential oil placed on the surface of Mueller-Hinton agar seeded with 100 μL of each bacterium. Gentamycin, 10μg per disk (Laborclin, Brazil), was used as reference. The plates were incubated at 35°C and after 24 hours the inhibition halo was measured with a millimeter ruler [16]. The minimum inhibitory concentration (MIC) was determined using the broth dilution methodology and performed in triplicate with the same bacterium used in disk diffusion test. An aliquot of the essential oil prepared in DMSO was transferred to a test tube containing BHI broth. Serial dilutions of essential oils were then performed resulting in concentrations of 5–2000 μg/mL. Amoxicillin, gentamycin, and polymyxin B (0.015 a 128 μg/mL) were used as reference. Microbial suspensions containing 1.5x108 CFU/mL of the bacteria were added at each concentration of essential oil or antibiotic drug and incubated at 35°C for 24 h. Tubes without bacteria were used as control of broth sterility and bacterial growth. The MIC was defined as the lowest concentration which visibly inhibited bacterial growth observed by the absence of visible turbidity. BHI broth was subjected to the inoculum microbial seeding test on the surface of plate-count agar to confirm growth inhibition [17].
2.6. Parasites
Leishmania amazonensis (MHOM/BR/76/MA-76) promastigote forms were cultured at 26°C in Schneider's Insect medium (Sigma, USA) supplemented with 10% fetal bovine serum (Gibco-USA), 100U/mL of penicillin (Gibco, USA), and 100 μg/mL of streptomycin (Sigma, USA) [18]. Cultures with a maximum of seven in vitro passages were used.
2.7. Animals
Female BALB/c mice, 4–6 weeks old, were purchased from Instituto de Ciência e Tecnologia em Biomodelos of Fundação Oswaldo Cruz, Rio de Janeiro, and maintained in accordance with National Council for Control of Animal Experimentation (Conselho Nacional de Controle de Experimentação Animal–CONCEA). The local Ethics Committee on Animal Care and Utilization approved all procedures involving the animals (CEUA/IOC – L053/2016).
2.8. Cell Culture
Peritoneal macrophages were obtained from BALB/c mice elicited with 3 mL 3% thioglycolate for 72 h, and maintained in RPMI 1640 (Sigma, USA) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL), at 37°C and 5% CO2 [18].
2.9. Antipromastigote Assay
L. amazonensis promastigote forms from a 2-4-day-old culture were placed in 96-well plates with different concentrations of essential oils (3.9–500 μg/mL) or amphotericin B (0.01–2.5 μg/mL), at a final volume of 100 μL per well for 72 h. Wells without parasites were used as blanks and wells with parasites and DMSO 1% were used as controls. Parasite viability was evaluated by the modified colorimetric method with tetrazolium-dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) [19]. 10 μL of MTT (5 mg/mL) was added to each well and after five hours 150 μL of DMSO was added to dissolve the formazan crystals. Absorbance was read on a spectrophotometer at a wavelength of 570 nm. The data was normalized, with absorbance value of control used as 100%, and the results were used to calculate the 50% inhibition of parasite growth (IC50).
2.10. Cytotoxicity Assay
Peritoneal macrophages were cultured in 96-well plates (5×105 cells/mL) with different concentrations of essential oils (7.8–1000 μg/mL) or amphotericin B (0.07–10 μg/mL) up to a final volume of 100 μL per well, at 37°C and 5% CO2. Wells without cells were used as blanks and wells with cells and DMSO 1% only were used as controls. After 24 h, the cells were analyzed by MTT colorimetric method [19]. Briefly, 10 μL of MTT (5 mg/mL) was added to each well and after two hours then the medium was discarded, and 100 μL of DMSO was added to dissolve the formazan crystals. Absorbance values were normalized, with absorbance value of control used as 100%, and the results were used to calculate the 50% cell cytotoxicity (CC50).
2.11. Activity against Intracellular Amastigote of L. amazonensis and Selectivity Index (SI)
Peritoneal macrophages from BALB/c were cultured in 24-well plates (5x105 cells/well), with coverslips, at 37°C and 5% CO2. The cells were infected with L. amazonensis promastigote forms, 10:1 parasite/cell, for 6 h and afterwords washed to remove noninternalized parasites. The infected cells were treated with different essential oil concentrations (62.5–500 µg/mL) for 24 h. The coverslips with the infected and treated cells were fixed with Bouin, stained with Giemsa, and examined by light microscopy. The IC50 was calculated from the total of intracellular amastigote from 200 cells. The percentage of infected cells was obtained from the number of infected cells divided by two. The mean number of amastigotes per cell was obtained from the number of intracellular amastigotes in 200 cells divided by the number of infected cells [20]. Amphotericin B was used as the reference drug. The selectivity index (SI) was obtained from the ratio of BALB/c peritoneal macrophages CC50 and intracellular amastigote IC50.
2.12. Nitrite Quantification of Peritoneal Macrophages Treated with C. longa Essential Oil
BALB/c peritoneal macrophages (5x106 cells/mL) were treated with C. longa essential oil (400 µg/mL) and/or stimulated with L. amazonensis (5x107 parasites/mL). After 48 hours, the supernatant was collected and nitrite quantification performed with Griess reagent. 100µL of culture supernatant was added to 100 µL of Griess reagent (50 µL of sulfanilamide 1% in 2.5% H3PO4 solution and 50 µL of N-(1-naphthyl)ethylenediamine 0.1% solution) in 96-well plates and after 10 min read at 570nm on the spectrophotometer. The nitrite values were obtained from the standard curve of sodium nitrite (1.5–100 µM) [21].
2.13. Statistical Analysis
The numerical results were expressed as mean ± standard deviation and were organized into tables or plotted in graphs. The IC50 and CC50 were obtained from a nonlinear regression curve of concentration log versus normalized response. Comparison between IC50 values was performed by one-way ANOVA and Tukey's multiple comparisons test. Analyses were performed with the software GraphPad Prism 7.00 and differences were considered significant when p<0.05.
3. Results
3.1. Chemical Composition of C. zeylanicum, C. longa, and O. vulgare Essential Oils
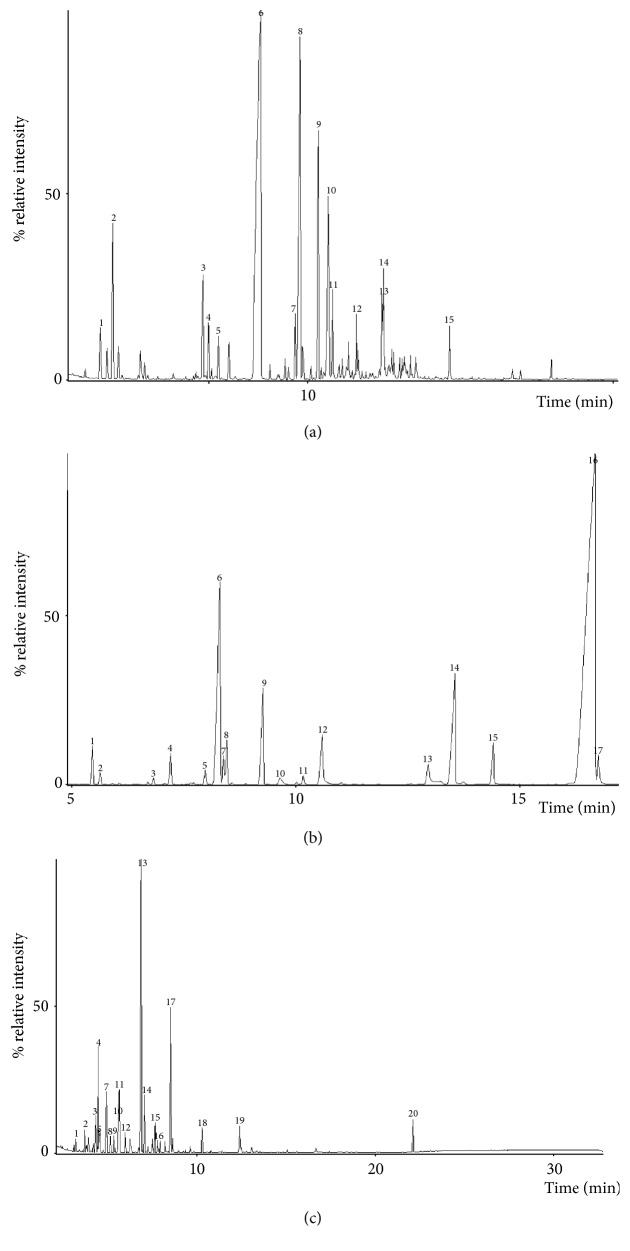
The essential oil yield of O. vulgare, C. zeylanicum, and C. longa was 0.73%, 0.9%, and 3.4%, respectively. GC-MS was used to identify, quantify, and evaluate the chemical compounds present in leaves C. zeylanicum, in the aerial parts of O. vulgare, and in the rhizome of C. longa, as shown in Table 1 and Figure 1. Compounds present in essential oils were enumerated according to elution order and retention time. Fifteen compounds were identified in C. zeylanicum, with peaks 6, 8, and 9 being identified as cinnamic aldehyde (46.30%), α-copaene (16.35%), and trans-β-Caryophyllene (8.26%), respectively, representing its main constituents. C. longa essential oil showed 17 compounds with turmerone (55.43%), β-turmerone (12.02%), and γ-curcumene (6.96) as major compounds. O. vulgare essential oil presented 20 compounds, with cis-p-Menth-2-en-1-ol (33.88%) and linalyl acetate (13.90%) identified as the main constituents.
Table 1.
Compounds identified in essential oils from leafs of Cinnamomum zeylanicum, Curcuma longa, and Origanum vulgare by CG-MS.
| Peak a | Cinnamomum zeylanicum | Curcuma longa | Origanum vulgare | ||||||
| t RET b | Compounds | %A c | t RET | Compounds | %A | t RET | Compounds | %A | |
|
| |||||||||
| 1 | 3.207 | α-pinene | 1.47 | 5.464 | α-pinene | 1.15 | 3.221 | α-pinene | 0.80 |
| 2 | 3.618 | Benzaldehyde | 4.16 | 5.638 | Myrcene | 0.37 | 3.733 | Bicyclo[3.1.0]hexane | 1.73 |
| 3 | 6.568 | 3-phenylpropionaldehyde | 2.95 | 6.823 | Vinyl propionate | 0.20 | 4.330 | (+)-4-Carene | 3.08 |
| 4 | 6.748 | Borneol | 1.06 | 7.206 | p-Cymene | 1.01 | 4.469 | p-Cymene | 8.29 |
| 5 | 7.077 | α-terpineol | 0.87 | 7.206 | Bisabolone | 0.55 | 4.533 | Cyclohexene | 1.23 |
| 6 | 8.459 | Cinnamic Aldehyde | 46.30 | 8.304 | β-Turmerone | 12.02 | 4.561 | β-Phellandrene | 2.73 |
| 7 | 9.593 | 3-Phenyl-1-propanol | 1.46 | 8.385 | 1,8-cineole | 1.01 | 4.954 | p-Menth-2-en-1-ol | 4.62 |
| 8 | 9.744 | α-Copaene | 16.35 | 8.461 | Camphor | 1.24 | 5.171 | 1,4-cyclohexadiene | 1.21 |
| 9 | 10.346 | trans-β-Caryophyllene | 8.26 | 9.267 | α-Terpineol | 4.13 | 5.361 | cis-Sabinene hydrate | 1.29 |
| 10 | 10.669 | (e)-cinnamyl acetate | 7.54 | 9.645 | Terpinolene | 0.43 | 5.598 | Terpinolene | 3.11 |
| 11 | 10.809 | α-Humulene | 2.16 | 10.167 | α-Zingiberene | 0.29 | 5.651 | 1,6-octadien-3-ol | 5.69 |
| 12 | 11.593 | delta-cadienene | 1.42 | 10.587 | β-Sesquiphellandrene | 2.67 | 5.996 | trans-Sabinene hydrate | 1.59 |
| 13 | 12.422 | (-)-Spathulenol | 2.09 | 12.945 | β-Caryophyllene | 1.00 | 6.877 | cis-p-Menth-2-en-1-ol | 33.88 |
| 14 | 12.481 | Caryophyllene oxide | 2.80 | 13.544 | γ-Curcumene | 6.96 | 7.079 | 3-Cyclohexen-1-ol | 5.26 |
| 15 | 14.645 | benzyl benzoate | 1.12 | 14.397 | ar-Curcumene | 1.58 | 7.669 | (+)-α-Terpineol | 2.61 |
| 16 | – | – | – | 16.664 | Turmerone | 55.43 | 7.817 | Carvacrol methyl ether | 0.94 |
| 17 | – | – | – | 16.739 | β-Sesquiphellandrene | 1.10 | 8.543 | Linalyl acetate | 13.90 |
| 18 | – | – | – | – | – | – | 10.307 | Thymol | 2.41 |
| 19 | – | – | – | – | – | – | 12.400 | trans-β-Caryophyllene | 2.46 |
| 20 | – | – | – | – | – | – | 22.115 | 1H-Cycloprop(E)azulen-7-ol | 3.16 |
a: peak number according to the order of column elution. b: retention time (minutes) of the compounds in column. c: percentage of normalized area which indicates the relative distribution of the compounds in the sample.
Figure 1.
Chromatograms of Cinnamomum zeylanicum (a), Curcuma longa (b), and Origanum vulgare (c) essential oils.
3.2. Antimicrobial Activity of C. zeylanicum, C. longa, and O. vulgare Essential Oils
The disc diffusion method showed that the largest halo was observed for C. zeylanicum essential oil against E. coli, whereas O. vulgare and C. longa essential oil presented better inhibition against S. aureus. The MIC trial showed significant antimicrobial activity of the essential oils studied, with C. longa essential showing the better activity against S. aureus (Table 2).
Table 2.
Diameters of inhibition zones and minimum inhibitory concentration of different bacteria culture after 24 hours of treatment with Cinnamomum zeylanicum, Curcuma longa, or Origanum vulgare essential oil.
| Compounds | Bacteria strain | |||
| Escherichia coli | Staphylococcus aureus | Pseudomonas aeruginosa | ||
|
| ||||
| Inhibition zones (mm) | Cinnamomum zeylanicum | 15.00 ± 1.000 | 14.67 ± 0.577 | 10.33 ± 0.577 |
| Curcuma longa | 12.67 ± 0.577 | 15.33 ± 0.577 | 9.66 ± 0.577 | |
| Origanum vulgare | 14.67 ± 0.577 | 15.67 ± 0.577 | 12.00 ± 1.000 | |
| gentamycin | 14.33 ± 0.577 | 20.67 ± 0.577 | 16.67 ± 0.577 | |
|
| ||||
| MIC (μg/mL) | Cinnamomum zeylanicum | 133.3 ± 14.43 | 216.7 ± 28.87 | 550.0 ± 0.00 |
| Curcuma longa | 216.7 ± 28.87 | 83.3 ± 14.43 | 383.3 ± 57.74 | |
| Origanum vulgare | 266.7 ± 28.87 | 166.7 ± 28.87 | 483.3 ± 28.87 | |
| amoxicillin | 16.0 ± 0.00 | 8.0 ± 0.00 | n.d. | |
| gentamycin | n.d. | 2.0 ± 0.00 | n.d. | |
| polymyxin B | n.d. | n.d. | 16.0 ± 0.00 | |
To determine the inhibition zones, 75μL of each essential oil was used in the disk diffusion test. n.d.: not determined. Data represents mean ± standard deviation of experiment realized in triplicate.
3.3. Antileishmanial Activity, Cytotoxicity, and SI of C. zeylanicum, O. vulgare, and C. longa Essential Oils
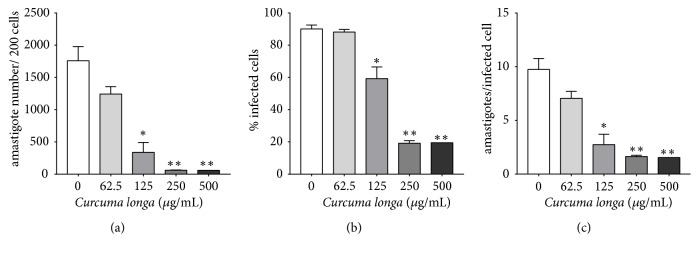
Data of the antileishmanial activity, cytotoxicity, and SI are described in Table 3. C. longa presented the better antipromastigote activity between the three essential oils, while C. zeylanicum showed the worst activity (Figure 2). As the C. longa showed the most potent antipromastigote activity between the three essential oils (p<0.0001), the intracellular amastigote activity was performed only with their essential oil, which showed a 4.87-fold decrease in the intracellular amastigote IC50 value when compared to the promastigote IC50. The analysis of infection parameters showed that at 125 µg/mL there was a statistically significant reduction of all parameters of infection, with decrease of amastigote number in 200 cells and percentage of infected cells of 80.73% and 40.75%, respectively, and mean of amastigotes per cell reduction from 9.74 to 2.74. The decrease in infection parameters was even further reduced at 250 and 500 µg/mL (Figure 3). The decrease in intracellular amastigote number, percentage of infected cells, and mean of amastigotes per infected cell induced by C. longa essential oil treatment are observed in Figure 4. None of the three essential oils presented cytotoxicity at the analyzed concentrations. The SI showed that C. longa essential oil has activity 15.79-fold more selective to intracellular amastigote of L. amazonensis than to BALB/c peritoneal macrophage. The reference drug amphotericin B showed antileishmanial activity and cytotoxicity as expected.
Table 3.
Antileishmanial activity against Leishmania amazonensis and cytotoxicity against BALB/c peritoneal macrophages of Cinnamomum zeylanicum, Curcuma longa, and Origanum vulgare essential oils.
| Essential oils/ compounds | L. amazonensis IC50 | Cytotoxicity CC50 | SI | |
| Promastigote (µg/mL) | Intracellular amastigote (µg/mL) | Peritoneal macrophage (µg/mL) | ||
|
| ||||
| Cinnamomum zeylanicum | >500 a,b,c | n.d. | >1000 | n.d. |
| Curcuma longa | 308.4 ± 1.402 a,d,e | 63.3 ± 1.369 a | >1000 | >15.79 |
| Origanum vulgare | 405.5 ± 1.119 b,d,f | n.d. | >1000 | n.d. |
| Amphotericin B | 0.764 ± 0.139 c,e,f | 1.045 ± 0.145 a | 37.22 ± 1.834 | 35.6 |
Data represents mean ± standard deviation of at least two experiments realized in triplicate. CC50: cytotoxic concentration for 50% of cells; IC50: inhibitory concentration for 50% of parasites; SI: selectivity index; n.d.: not determined. Equal letters in the same column mean statistical difference between IC50 (p<0.0001) by one-way ANOVA and Tukey's multiple comparisons test.
Figure 2.
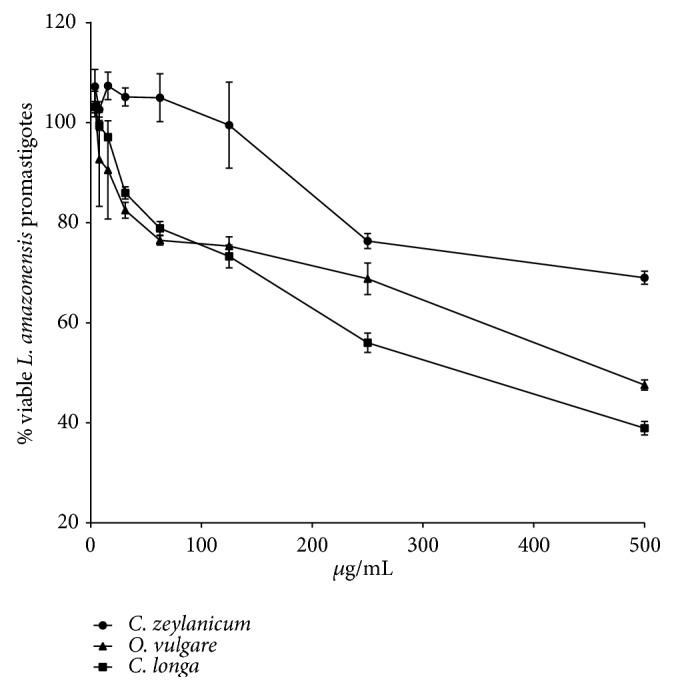
Viability of Leishmania amazonensis promastigotes treated for 72 hours with Cinnamomum zeylanicum, Curcuma longa, and Origanum vulgare essential oils.
Figure 3.
Infection parameters of BALB/c peritoneal macrophages infected with Leishmania amazonensis and treated with Curcuma longa essential oil. Data represent mean ± standard deviation of two independent experiments realized in triplicate. ∗p<0.05 and ∗∗p<0.01 when compared with the untreated group by Kruskal-Wallis followed by Dunn's multiple comparisons test.
Figure 4.
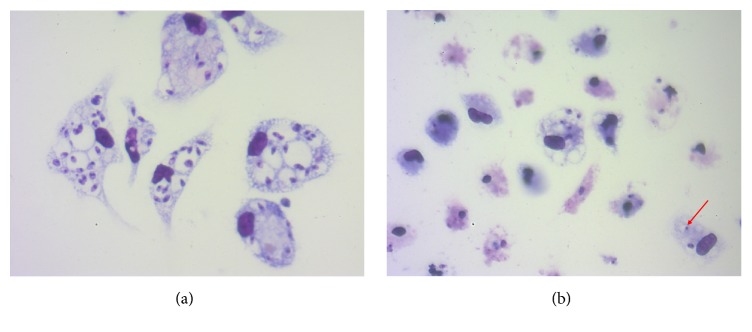
BALB/c peritoneal macrophages infected with Leishmania amazonensis and treated with Curcuma longa essential oil at 125 µg/mL for 24 hours. (a) Untreated infected macrophages. (b) Treatment decreased intracellular amastigote number and induced loss of intracellular amastigote integrity (red arrow). Images are representative of two experiments realized in triplicate. Giemsa, 40x objective.
3.4. C. longa Essential Oil Decreased Nitrite Production in Peritoneal Macrophages
Nitrite quantification in supernatant of BALB/c peritoneal macrophage treated with C. longa essential oil (400 µg/mL) showed a significant decrease in nitrite amount from 3.31 µM to 1.06 µM. The same decrease pattern was observed slightly in L. amazonensis infected macrophages treated with C. longa essential oil when compared with untreated and infected macrophages, from 3.46 µM to 1.66 µM (Figure 5).
Figure 5.
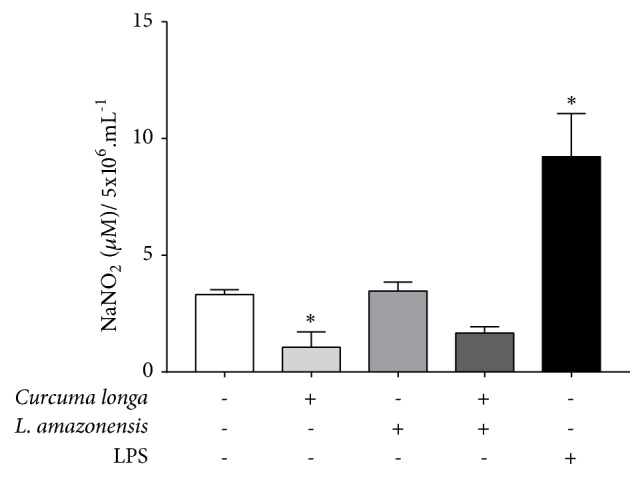
Nitrite quantification in supernatant of BALB/c peritoneal macrophage treated with Curcuma longa (400 µg/mL) and stimulated or not with Leishmania amazonensis. Data represents media ± standard deviation of experiment realized in sextuplicate; ∗p<0.05 when compared with untreated and unstimulated macrophage by Kruskal–Wallis and Dunn's multiple comparison test.
4. Discussion
In order to analyze the chemical composition and antimicrobial and antileishmanial activity of C. zeylanicum, O. vulgare, and C. longa essential oils, we evaluated the chemical constituents, activity against E. coli, S. aureus, and P. aeruginosa, activity against promastigote and intracellular amastigote of L. amazonensis, and cytotoxicity in BALB/c peritoneal macrophage.
Different investigations have identified and quantified several chemical compounds of the essential oils of C. zeylanicum, C. longa, and O. vulgare. The essential oils yield obtained in our extraction was similar to that described in literature [22–24], as well as chemical composition, where cinnamaldehyde is generally the main component of C. zeylanicum essential oil [6, 8, 25, 26], and turmerone the major compound of C. longa essential oil [27, 28]. On the other hand, there are different chemotypes described to O. vulgare revealing different chemical profile, as the chemotype rich in linalool/linalyl acetate with predominant presence of linalyl acetate, the chemotype rich in carvacrol and c-terpinene, and chemotype rich in thymol [29, 30].
The disc diffusion method was used to evaluate the ability of the antibacterial activity of the essential oils of C. zeylanicum, O. vulgare, and C. longa to form inhibition halos against the growth of Gram-positive bacteria (S. aureus) and Gram-negative strains (E. coli and P. aeruginosa). Antimicrobial susceptibility tests of the three essential oils demonstrated active against the standard strains. C. zeylanicum essential oil presented larger halos against Gram-positive, and cinnamaldehyde was reported to be responsible for their antimicrobial action [6, 31]. Antimicrobial activity against Gram-positive bacteria has also been reported for C. longa [1], and O. vulgare has demonstrated efficacy for both Gram-positive and Gram-negative strains [32].
The yield and quality of essential oils vary with genetics, agroclimatic conditions, cultivation techniques, soil conditions, harvest time, etc. Therefore, chemical compositions and major compounds of essential oils vary in different habitats, and their bioactivity is closely associated with changes in their composition. Cinnamic aldehyde, for example, showed MIC ranging from 0.5 to 1000 μg/mL against 20 strains of P. aeruginosa [33] and therapeutic potential by inhibiting infections related to biofilm production by S. aureus [34]. The turmerone compound found in C. longa demonstrated activity against E. coli [35] while showed no activity against Gram-positive bacterial strains [36].
Antileishmanial assays showed that C. zeylanicum essential oil did not inhibit growth of L. amazonensis promastigotes at analyzed concentrations while O. vulgare essential oil showed high IC50 value. There are few data about antileishmanial activity of these essential oils. An evaluation of C. zeylanicum essential oil from Iran against L. major promastigotes displayed a significant reduction in the number of parasites [37]. Colombian O. vulgare essential oil exhibited activity against promastigotes of L. panamensis (IC50: 42.23 ± 2.04), L. braziliensis (IC50:204.36 ± 21.56), L. major (IC50: >640 ± 0.0), and L. guyanensis (IC50: 171.8 ± 20.64) [38]. While there are no data about the chemical composition of Colombian O. vulgare essential oil that presented antileishmanial activity, its origin indicates a possible difference in chemical composition compared to O. vulgare essential oil that we evaluated. In Iranian C. zeylanicum essential oil, the presence of 83.47% of (E)-cinnamaldehydein, compared with 46.30% of cinnamic aldehyde observed in our C. zeylanicum essential oil, can be a variable responsible for the difference in activity observed between both oils.
C. longa essential oil presented the better activity among the three oils, and in literature there are several constituents isolated and related to its antileishmanial activity, with curcumin being the most described. Curcumin has shown an average IC50 of 5.3 µM against promastigotes of various leishmanial strains [39]. Curcumin 1, demethoxycurcumin 2, and bis-demethoxycurcumin 3, isolated from the rhizomes of C. longa, showed moderate activity against L. major promastigote, with IC50 of 7.8, 14.1, and 21.5 µg/mL, respectively [40]. Synthetic derivatives of curcumin were ten times more efficient than the original curcumin against L. amazonensis promastigotes [41]. Four curcuminoid analogs showed IC50 values less than 5 mM to promastigotes of L. major and axenic culture of L. mexicana amastigotes, with the majority presenting values higher to amastigote axenic than promastigote [42].
As most of the published studies with curcuminoids isolated from C. longa against Leishmania have been performed against the promastigote form, it is not clear if the activity described in the literature reflects the real leishmanicidal properties of these molecules [11]. Thus, we proceeded to the activity against infected macrophages, a more appropriate model to demonstrate the antileishmanial potential in vitro.
First, we performed the cytotoxic analysis against BALB/c peritoneal macrophages, where cytotoxicity was not observed at tested concentrations for any of three essential oils. Then, intracellular amastigote activity was performed and showed that C. longa essential oil not only maintained the antileishmanial activity observed against promastigote form but also presented higher efficacy against intracellular amastigote. Trans-dibenzalacetone, a synthetic monoketone analog of curcumin, demonstrated antiproliferative effect on the intracellular amastigotes of L. donovani (IC50 7.43 ± 1.88 μg/mL) that also was significantly lower than the IC50 value determined for promastigotes (17.80 ± 1.42 μg/mL) [43]. Selectivity index demonstrated the selective antileishmanial activity of C. longa essential oil for intracellular amastigote with similar value of trans-dibenzalacetone, SI value of 15.34 for intracellular amastigotes against the BALB/c mouse cell line J774A.1 [43].
The enhancement of activity observed by C. longa essential oil against intracellular amastigote suggests that antileishmanial activity may be related to not only direct action against the parasite, but also indirect mechanism. Mechanisms of direct action on Leishmania have already been described for the analog of curcumin trans-dibenzalacetone. It alters the general ultrastructure and the mitochondrial physiology of the parasite and triggers apoptotic cell death in L. donovani [43]. Macrophage activation is one of the more important mechanisms of indirect action, as induction of reactive oxygen species and reactive oxygen nitrogen species. Several plant materials have antileishmanial activity associated with induction of macrophage activation [18, 44], especially inducing nitric oxide (NO) [21]. Therefore, we evaluated the nitrite production, an indirect measurement to quantify NO, in supernatant of BALB/c peritoneal macrophages infected or not with L. amazonensis and treated with C. longa essential oil.
Nitrite quantification showed that C. longa essential oil inhibited NO production in peritoneal macrophages. The anti-inflammatory activity of C. longa (turmeric) has been described for a long time [45], and inhibition of NO production has already been described in a macrophage cell RAW 264.7 stimulated by LPS [46, 47]. Altough we observed an increase of nitrite in peritoneal macrophages stimulated with L. amazonensis and treated with C. longa essential oil when compared with unstimulated and treated cells, there was no significant enhancement in its NO production due to treatment. Indeed, there is a description of curcumin that overcomes the inhibitory effect of nitric oxide on L. major and L. donovani. As an antioxidant, curcumin is capable of blocking the action of both NO and NO congeners on the Leishmania parasite by acting on S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and DETANONOate, which release NO, 3-morpholino-sydnonimine hydrochloride (SIN-1), which releases NO and superoxide, and peroxynitrite, which is formed from the reaction of NO with superoxide [48]. The inability of inducing NO production in BALB/c peritoneal macrophages reveals that probably there are other possible mechanisms involved in intracellular amastigote activity of C. longa essential oil which should be elucidated in further studies.
5. Conclusions
The major compounds in the essential oils were cinnamic aldehyde (C. zeylanicum), turmerone (C. longa), and cis-p-Menth-2-en-1-ol (O. vulgare). All three essential oils exhibited antimicrobial action, highlighting C. longa essential oil against S. aureus. Antileishmanial activity against promastigote forms of L. amazonensis was observed to C. longa essential oil, but not to C. zeylanicum and O. vulgare essential oil. C. longa essential oil activity against intracellular amastigote is the first description of activity against the parasite form found in mammal host, ensuring their potential antileishmanial activity. C. longa essential oil did not induce NO production in BALB/c peritoneal macrophages, suggesting that intracellular amastigote activity may be related to other mechanisms.
Acknowledgments
The present study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES) [Finance Code 001], Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro [E-26/111.252/2014], for Kátia da Silva Calabrese, the Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão [APP-00844/09 and Pronex-241709/2014], Conselho Nacional de Desenvolvimento Científico e Tecnológico [407831/2012.6 and 309885/2017-5] for Ana Lucia Abreu-Silva and [312765/2016-9] for Fernando Almeida-Souza, and CNPq/SECTI/FAPEMA [DCR03438/16] for Fernando Almeida-Souza.
Abbreviations
- DMSO:
Dimethyl sulfoxide
- GC-MS:
Gas chromatography-mass spectrometry analyses
- CFU:
Colony-forming unit
- MIC:
Minimum inhibitory concentration
- BHI:
Brain heart infusion
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IC50:
Inhibitory concentration of 50%
- CC50:
50% cell cytotoxicity
- SI:
Selectivity index
- NO:
Nitric oxide
- LPS:
Lipopolysaccharide.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Gupta A., Mahajan S., Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnology Reports. 2015;6:51–55. doi: 10.1016/j.btre.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma S., Joshi S., Chitnis V., Hemwani N., Chitnis D. Growing problem of methicillin resistant staphylococci--Indian scenario. Indian Journal of Medical Sciences. 2000;54(12):535–540. [PubMed] [Google Scholar]
- 3.Pimentel M. I. F., Baptista C., Rubin É. F., et al. American cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis resistant to meglumine antimoniate, but with good response to pentamidine: A case report. Journal of the Brazilian Society of Tropical Medicine. 2011;44(2):254–256. doi: 10.1590/S0037-86822011000200026. [DOI] [PubMed] [Google Scholar]
- 4.Borges M. M., da Silva Pranchevicius M. C., Noronha E. F., Romero G. A. S., Carranza-Tamayo C. O. Efficacy and safety of amphotericin B deoxycholate versus N-methylglucamine antimoniate in pediatric visceral leishmaniasis: An open-label, randomized, and controlled pilot trial in Brazil. Journal of the Brazilian Society of Tropical Medicine. 2017;50(1):67–74. doi: 10.1590/0037-8682-0455-2016. [DOI] [PubMed] [Google Scholar]
- 5.Masmoudi A., Maalej N., Mseddi M., et al. Glucantime injection: benefit versus toxicity. Médecine et Maladies Infectieuses. 2005;35(1):42–45. doi: 10.1016/j.medmal.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Unlu M., Ergene E., Unlu G. V., Zeytinoglu H. S., Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food and Chemical Toxicology. 2010;48(11):3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Guoruoluo Y., Zhou H., Wang W., Zhou J., Aisa H. A., Yao G. Chemical constituents from the immature buds of Cinnamomum cassia (Lauraceae) Biochemical Systematics and Ecology. 2018;78:102–105. doi: 10.1016/j.bse.2018.04.008. [DOI] [Google Scholar]
- 8.Procopio F. R., Oriani V. B., Paulino B. N., et al. Solid lipid microparticles loaded with cinnamon oleoresin: Characterization, stability and antimicrobial activity. Food Research International. 2018;113:351–361. doi: 10.1016/j.foodres.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Marini E., Di Giulio M., Magi G., et al. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of Mycobacterium abscessus. Phytotherapy Research. 2018;32(3):488–495. doi: 10.1002/ptr.5994. [DOI] [PubMed] [Google Scholar]
- 10.Avanço G. B., Brugnari T., Abreu Filho B. A. D., Mikcha J. M. G., Machinski M. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 2017;73:806–813. doi: 10.1016/j.foodcont.2016.09.032. [DOI] [Google Scholar]
- 11.Haddad M., Sauvain M., Deharo E. Curcuma as a parasiticidal agent: A review. Planta Medica. 2011;77(6):672–678. doi: 10.1055/s-0030-1250549. [DOI] [PubMed] [Google Scholar]
- 12.Beltrán J. M. G., Espinosa C., Guardiola F. A., Esteban M. Á. In vitro effects of Origanum vulgare leaf extracts on gilthead seabream (Sparus aurata L.) leucocytes, cytotoxic, bactericidal and antioxidant activities. Fish & Shellfish Immunology. 2018;79:1–10. doi: 10.1016/j.fsi.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Roby M. H. H., Sarhan M. A., Selim K. A., Khalel K. I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Industrial Crops and Products. 2013;44:437–445. doi: 10.1016/j.indcrop.2012.10.012. [DOI] [Google Scholar]
- 14.Abdelhady M. I., Aly H. A. H. Antioxidant antimicrobial activities of callistemon comboynensis essential oil. Free Radicals and Antioxidants. 2012;2(1):37–41. doi: 10.5530/ax.2012.2.8. [DOI] [Google Scholar]
- 15.CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 4th. WAYNE, Pa, USA: CLSI; 2009. [Google Scholar]
- 16.Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 17.da Silva V. D., Almeida-Souza F., Teles A. M., et al. Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Industrial Crops and Products. 2018;119:201–208. doi: 10.1016/j.indcrop.2018.04.005. [DOI] [Google Scholar]
- 18.Almeida-Souza F., de Oliveira A. E., Abreu-Silva A. L., da Silva Calabrese K. In vitro activity of Morinda citrifolia Linn. fruit juice against the axenic amastigote form of Leishmania amazonensis and its hydrogen peroxide induction capacity in BALB/c peritoneal macrophages. BMC Research Notes. 2018;11(1) doi: 10.1186/s13104-018-3555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira I. d., Moragas Tellis C. J., Chagas M. d., et al. Carapa guianensis aublet (andiroba) seed oil: chemical composition and antileishmanial activity of limonoid-rich fractions. BioMed Research International. 2018;2018:10. doi: 10.1155/2018/5032816.5032816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida-Souza F., de Souza C. D. S. F., Taniwaki N. N., et al. Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c. Nitric Oxide: Biology and Chemistry. 2016;58:51–58. doi: 10.1016/j.niox.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Morshedloo M. R., Salami S. A., Nazeri V., Maggi F., Craker L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Industrial Crops and Products. 2018;119:183–190. doi: 10.1016/j.indcrop.2018.03.049. [DOI] [Google Scholar]
- 23.S. K., Kujur A., Prakash B. Assessment of preservative potential of Cinnamomum zeylanicum Blume essential oil against food borne molds, aflatoxin B1 synthesis, its functional properties and mode of action. Innovative Food Science and Emerging Technologies. 2016;37:184–191. doi: 10.1016/j.ifset.2016.08.018. [DOI] [Google Scholar]
- 24.Xiang H., Zhang L., Xi L., et al. Phytochemical profiles and bioactivities of essential oils extracted from seven Curcuma herbs. Industrial Crops and Products. 2018;111:298–305. doi: 10.1016/j.indcrop.2017.10.035. [DOI] [Google Scholar]
- 25.Echegoyen Y., Nerín C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chemistry. 2015;170:30–36. doi: 10.1016/j.foodchem.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Kačániová M., Terentjeva M., Vukovic N., et al. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharmaceutical Journal. 2017;25(8):1108–1116. doi: 10.1016/j.jsps.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Correa H. A., Paula J. T., Kayano A. C. A. V., et al. Composition and antimalarial activity of extracts of Curcuma longa L. obtained by a combination of extraction processes using supercritical CO2, ethanol and water as solvents. The Journal of Supercritical Fluids. 2017;119:122–129. doi: 10.1016/j.supflu.2016.08.017. [DOI] [Google Scholar]
- 28.Angel G. R., Menon N., Vimala B., Nambisan B. Essential oil composition of eight starchy Curcuma species. Industrial Crops and Products. 2014;60:233–238. doi: 10.1016/j.indcrop.2014.06.028. [DOI] [Google Scholar]
- 29.Mastro G. D., Tarraf W., Verdini L., Brunetti G., Ruta C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chemistry. 2017;235:1–6. doi: 10.1016/j.foodchem.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Padilla A. M., Bustamante J. M., Tarleton R. L. CD8+ T cells in Trypanosoma cruzi infection. Current Opinion in Immunology. 2009;21(4):385–390. doi: 10.1016/j.coi.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao S. C., Young D. G., Oberg C. J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. Journal of Essential Oil Research. 2000;12(5):639–649. doi: 10.1080/10412905.2000.9712177. [DOI] [Google Scholar]
- 32.Stefanakis M. K., Touloupakis E., Anastasopoulos E., Ghanotakis D., Katerinopoulos H. E., Makridis P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control. 2013;34(2):539–546. doi: 10.1016/j.foodcont.2013.05.024. [DOI] [Google Scholar]
- 33.Utchariyakiat I., Surassmo S., Jaturanpinyo M., Khuntayaporn P., Chomnawang M. T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complementary and Alternative Medicine. 2016;16(1, article 158) doi: 10.1186/s12906-016-1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kot B., Wierzchowska K., Grużewska A., Lohinau D. The effects of selected phytochemicals on biofilm formed by five methicillin-resistant Staphylococcus aureus. Natural Product Research. 2017;32(11):1299–1302. doi: 10.1080/14786419.2017.1340282. [DOI] [PubMed] [Google Scholar]
- 35.Hoi-Seon L. Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Science and Biotechnology. 2006;15(4):559–563. [Google Scholar]
- 36.Joycharat N., Thammavong S., Voravuthikunchai S. P., et al. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Natural Product Research. 2014;28(23):2169–2172. doi: 10.1080/14786419.2014.924934. [DOI] [PubMed] [Google Scholar]
- 37.Jorjani O., Raeisi M., Hezarjaribi H. Z., Soltani M., Soosaraei M. Studying the chemical composition in vitro activity of Cinnamomum zeylanicum and Eugenia caryophyllata essential oils on Leishmania major. Journal of Pharmaceutical Sciences and Research. 2017;9(8):1300–1304. [Google Scholar]
- 38.Sanchez-Suarez J., Riveros I., Delgado G. Evaluation of the leishmanicidal and cytotoxic potential of essential oils derived from ten colombian plants. Iranian Journal of Parasitology. 2013;8(1):129–136. [PMC free article] [PubMed] [Google Scholar]
- 39.Saleheen D., Ali S. A., Ashfaq K., Siddiqui A. A., Agha A., Yasinzai M. M. Latent activity of curcumin against leishmaniasis in vitro. Biological & Pharmaceutical Bulletin. 2002;25(3):386–389. doi: 10.1248/bpb.25.386. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen H. B., Christensen S. B., Kvist L. P., Karazmi A. A simple and efficient separation of the curcumins, the antiprotozoal constituents of Curcuma longa. Planta Medica. 2000;66(4):396–398. doi: 10.1055/s-2000-8533. [DOI] [PubMed] [Google Scholar]
- 41.Gomes D., Alegrio L., Lim M., Leon L., Araújo C. Synthetic derivatives of curcumin and their activity against Leishmania amazonensis. Arzneimittel-Forschung/Drug Research. 2002;52(02):120–124. doi: 10.1055/s-0031-1299867. [DOI] [PubMed] [Google Scholar]
- 42.Changtam C., de Koning H. P., Ibrahim H., Sajid M. S., Gould M. K., Suksamrarn A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. European Journal of Medicinal Chemistry. 2010;45(3):941–956. doi: 10.1016/j.ejmech.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 43.Chauhan I. S., Rao G. S., Shankar J., Chauhan L. K., Kapadia G. J., Singh N. Chemoprevention of Leishmaniasis: In - vitro antiparasitic activity of dibenzalacetone, a synthetic curcumin analog leads to apoptotic cell death in Leishmania donovani. Parasitology International. 2018;67(5):627–636. doi: 10.1016/j.parint.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Da Silva B. J. M., Da Silva R. R. P., Rodrigues A. P. D., Farias L. H. S., Do Nascimento J. L. M., Silva E. O. Physalis angulata induces death of promastigotes and amastigotes of Leishmania (Leishmania) amazonensis via the generation of reactive oxygen species. Micron. 2016;82:25–32. doi: 10.1016/j.micron.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Arora R. B., Kapoor V., Basu N., Jain A. P. Anti-inflammatory studies on Curcuma longa (turmeric) The Indian Journal of Medical Research. 1971;59(8):1289–1295. [PubMed] [Google Scholar]
- 46.Kawasaki K., Okuda-Hanafusa C., Aoyagi M., et al. Inhibitory effect of the compounds from the water extract of Curcuma longa on the production of PGE2 and NO in a macrophage cell line stimulated by LPS. Bioscience, Biotechnology, and Biochemistry. 2018;82(12):1–9. doi: 10.1080/09168451.2018.1511366. [DOI] [PubMed] [Google Scholar]
- 47.Yuan T., Zhang C., Qiu C., et al. Chemical constituents from Curcuma longa L. and their inhibitory effects of nitric oxide production. Natural Product Research. 2017;32(16):1887–1892. doi: 10.1080/14786419.2017.1354185. [DOI] [PubMed] [Google Scholar]
- 48.Chan M. M.-Y., Adapala N. S., Fong D. Curcumin overcomes the inhibitory effect of nitric oxide on Leishmania. Parasitology Research. 2005;96(1):49–56. doi: 10.1007/s00436-005-1323-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.