Abstract
Background
Extranodal natural killer (NK) / T cell lymphoma is a subtype of non-Hodgkin’s lymphoma (NHL) that usually has an aggressive clinical course. It is the predominant trigger of lymphoma-associated hemophagocytic syndrome (LAHS), which is highly lethal and with extremely poor prognosis. This study is aiming to characterize the associated clinical features and prognostic factors of the disease.
Patients and methods
Twenty-eight patients with extranodal NK/T cell lymphoma associated hemophagocytic lymphohistiocytosis (HLH) were retrospectively analyzed. The clinical records were collected, and the associations between clinical or laboratory parameters and overall survival (OS) were assessed.
Results
The most frequently clinical characteristics were fever (96.4%), and splenomegaly (81.5%). Concerning the laboratory findings, the most common features were hyperferritinemia (91.7%), grade III/IV thrombocytopenia (64.3%), hypertriglyceridemia (48%), severe anemia (46.4%), hypofibrinogenemia (45%), and grade III/IV neutropenia (32.1%). The interval between the diagnosis of NK/T LAHS and death / last contact was between 4 to 701 days with the median interval of 15 days. We found that higher serum lactic dehydrogenase (LDH) at HLH, hypofibrinogenemia, and splenomegaly were significantly associated with worse survival (P=0.002, 0.003, 0.003). Furthermore, Eastern Cooperative Oncology Group (ECOG) score, extra-upper aerodigestive tract NK/T cell lymphoma (EUNKTL) and cutaneous involvement were risk factors of HLH.
Conclusion
Our data indicated that levels of LDH, fibrinogen, and presence of splenomegaly were prognostic factors of the disease. Higher ECOG scores, EUNKTL and cutaneous involvement were risk factors of NK/T LAHS. Additional independent, prospective clinical trials will be needed to explore optimal treatment.
Keywords: NK/T-cell lymphoma, hemophagocytic lymphohistiocytosis, risk factor, prognosis
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a severe life-threatening disorder, characterized by hyperactivation of macrophages due to uncontrolled hypersecretion of inflammatory cytokines. Patients with HLH have a wide clinical spectrum but typically present with high fever, splenomegaly, cytopenias, coagulation abnormalities, and tissue evidence of hemophagocytosis.1,2 HLH can be classified into two distinct forms: familial hemophagocytic lymphohistiocytosis (primary HLH) and secondary hemophagocytic lymphohistiocytosis (secondary HLH).3,4 Secondary HLH is associated with various conditions including infections, connective tissue diseases, and hematological malignancies, mainly non-Hodgkin’s lymphoma (NHL).5,6 Large studies have suggested that natural killer (NK)/T-cell lymphoma is the predominant trigger of lymphoma-associated hemophagocytic syndrome (LAHS) in Asia.7,8 NK/T-cell lymphoma includes two subtypes: upper aerodigestive tract NK/T-cell lymphoma (UNKTL) and extra-upper aerodigestive tract NK/T-cell lymphoma (EUNKTL).9 Briefly, UNKTL comprised all lymphomas confined to nasal cavity, nasopharynx, and the upper aerodigestive tract, whereas EUNKTL included lymphomas occurring at all other sites. Due to the rarity and heterogeneity of HLH, there have been few investigations with large sample size of NK/T-cell LAHS. In this retrospective study, we analyzed the clinical records of 28 patients with extranodal NK/T-cell lymphoma-associated HLH, in an attempt to have better understanding of the clinical characteristics and prognostic factors of the disease.
Patients and methods
A total of 28 patients who were diagnosed as extranodal NK/T-cell lymphoma-associated HLH and treated in Shanghai Cancer Center, China from January 2006 to July 2017, were included in this retrospective study. This study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center. All patients signed separate informed consent forms for reviewing their medical records and research. All pathological results were reviewed by experienced pathologists in pathology department of Shanghai Cancer Center. All patients were pathologically confirmed of NK/T-cell lymphoma through biopsy samples according to the criteria of the WHO.10 HLH was diagnosed according to the criteria proposed by the Histiocyte Society in 2004.2 In the absence of a known gene mutation, a diagnosis of HLH can be made with at least five of the following eight criteria: fever, splenomegaly, cytopenias in two or more cell lines, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis, low or absent activity in NK cells, ferritin concentration >500 µg/L, or soluble CD25 concentration >2,400 U/mL. The demographic details and clinical and laboratory features of the patients are summarized in Table 1. For each patient, the following data before or at the time of HLH diagnosis were collected: patient demographics, UNKTL or EUNKTL, Ann Arbor stage, NK/T-cell lymphoma prognostic index (NKPI) score,11 Eastern Cooperative Oncology Group (ECOG) performance score, fever, bone marrow involvement, laboratory tests, type of treatment, and survival status.
Table 1.
Baseline characteristics of 28 patients with NK/T-cell LAHS
| Characteristics | No. of patients | % |
|---|---|---|
| Gender | ||
| Male | 18 | 64.3 |
| Female | 10 | 35.7 |
| Age at diagnosis (years) | ||
| >60 | 3 | 10.7 |
| ≤60 | 25 | 89.3 |
| ECOG | ||
| 0–1 | 13 | 46.4 |
| 2–3 | 15 | 53.6 |
| Ann Arbor stage | ||
| I + II | 19 | 67.9 |
| III + IV | 9 | 32.1 |
| Bone marrow involvement (n=13) | ||
| Yes | 3 | 23.1 |
| No | 10 | 76.9 |
| LDH level before HLH (n=22) | ||
| Normal | 13 | 59.1 |
| Elevated | 9 | 40.9 |
| Subtypes | ||
| UNKTL | 16 | 57.1 |
| EUNKTL | 12 | 42.9 |
| EBER positive (n=16) | ||
| Yes | 15 | 93.8 |
| No | 1 | 6.2 |
| Cutaneous involvement | ||
| Yes | 10 | 35.7 |
| No | 18 | 64.3 |
| NKPI | ||
| 0–1 | 18 | 64.3 |
| ≥2 | 10 | 35.7 |
| Neutropenia | ||
| I/II | 8 | 28.6 |
| III/IV | 9 | 32.1 |
| No | 11 | 39.3 |
| Anemia | ||
| I/II | 15 | 53.6 |
| III/IV | 13 | 46.4 |
| Fever | ||
| Yes | 27 | 96.4 |
| No | 1 | 3.6 |
| Splenomegaly (n=27) | ||
| Yes | 22 | 81.5 |
| No | 5 | 18.5 |
| Ferritin (ng/mL) (n=12) | ||
| ≥500 | 11 | 91.7 |
| <500 | 1 | 8.3 |
| Fibrinogen (g/L) (n=20) | ||
| ≤1 | 9 | 45 |
| >1 | 11 | 55 |
| Triglycerides (mmol/L) (n=25) | ||
| ≥3.0 | 12 | 48 |
| <3.0 | 13 | 52 |
| Thrombocytopenia | ||
| I/II | 10 | 35.7 |
| III/IV | 18 | 64.3 |
| LDH at HLH (U/L) (n=27) | ||
| >1,000 | 11 | 40.7 |
| ≤1,000 | 16 | 59.3 |
Abbreviations: EBER, EBV-encoded RNA; ECOG, Eastern Cooperative Oncology Group; EUNKTL, extra-upper aerodigestive tract NK/T-cell lymphoma; HLH, hemophagocytic lymphohistiocytosis; LDH, lactic dehydrogenase; NK, natural killer; NKPI, NK/T-cell lymphoma prognostic index; UNKTL, upper aerodigestive tract NK/T-cell lymphoma.
Statistical analyses
All analyses were performed using PASW statistics 18 (SPSS Inc., Chicago, IL, USA). Overall survival (OS) was measured from the date of diagnosis to the date of death owing to any cause or the date of last follow-up. Patients who died within 2 weeks after diagnosis of NK/T-cell LAHS were defined as short survival group, and those who survived >2 months were defined as long survival group. Chi-squared test was used to compare the clinical and laboratory data for shorter survival group and longer survival group. For survival analyses, Kaplan–Meier survival curves were constructed, and differences were tested by the log-rank test. All P-values were two-sided, and the results were considered significant if P<0.05.
Results
Patient characteristics
All 28 cases were from hospitalized patients, of whom 18 (64.3%) were men and 10 (35.7%) were women. The median age was 42 years (range 19–74 years) at the time of HLH diagnosis. According to Ann Arbor staging criteria, 19 (67.9%) patients were at stage I/II, and 9 (32.1%) were at stage III/IV. NKPI score (including the presence of B symptoms, stage III or IV, elevated lactic dehydrogenase [LDH] level, and lymph node involvement) was available for all patients, and accordingly, 18 patients had NKPI score of 0 or 1 and 10 patients had NKPI score of ≥2. Twelve patients (42.9%) were diagnosed as EUNKTL. Ten patients (35.7%) had skin involvement. Symptoms of NK/T-cell LAHS in our study were non-specific including, most commonly, fever (96.4%) and splenomegaly (81.5%). Most patients were EBER positive (93.8%). Concerning the laboratory findings, the most common were hyperferritinemia (91.7%), grade III/ IV thrombocytopenia (64.3%), hypertriglyceridemia (48%), severe anemia (46.4%), hypofibrinogenemia (45%), and grade III/IV neutropenia (32.1%). Thrombocytopenia and neutropenia were assessed according to CTCAE version 4.0, and grade III/IV thrombocytopenia represented platelet count of <50*10E9/L. Grade III/IV neutropenia represented neutrophilic granulocyte count of <1*10E9/L. Basic characteristics of the patients concerning the clinical and laboratory parameters are recorded (Table 1).
Treatment and response
After the diagnosis of extranodal NK/T-cell lymphoma, 16 patients received DICE (ifosfamide, etoposide, cisplatine, and dexamethasone) regimen with or without involved-field radiotherapy as first-line chemotherapy. Six patients received asparaginase-based chemotherapy regimen, and the other patients received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) regimen. The complete response rate was achieved in 14 (50%) patients. Six (21.4%) patients achieved partial response giving an objective response rate of 71.4% according to the response assessment criteria proposed by Cheson et al.12 Thirteen patients with refractory or relapsing disease were treated with asparaginase-based regimen as salvage therapy achieved an ORR of 28.6%. The median time from diagnosis of NK/T-cell lymphoma to the onset of HLH was 9 months (1–228 months). After the onset of HLH, patients were treated with chemotherapy following the HLH-2004 guideline proposed by the Histiocyte Society. The remission rate of the treatment was 23.8% (5/21), and statistically significant difference was found between clinical response to treatment and patients’ OS (P=0.00021). One patient in our study received allo-allogeneic hematopoietic stem cell transplant after treatment of HLH and was still alive at the time of last follow-up. The interval between the diagnosis of NK/T-cell LAHS and death/last contact was between 4 and 701 days with the median interval of 15 days (Figure 1).
Figure 1.
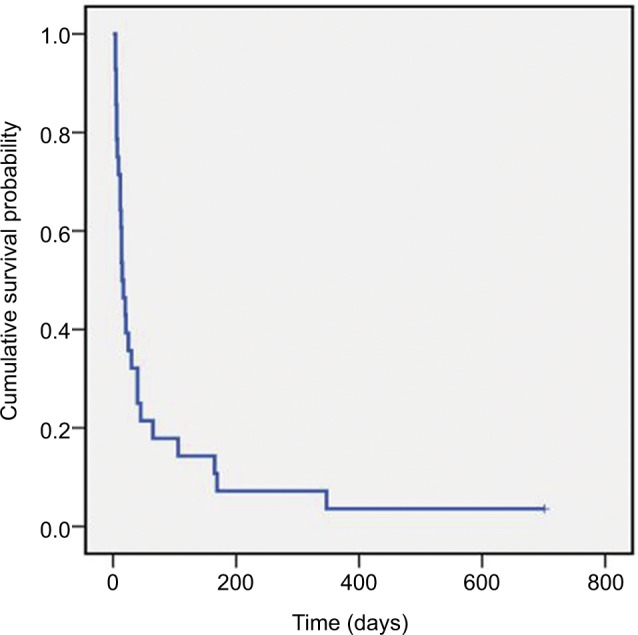
Survival of NK/T-cell LAHS patients
Note: The overall survival of NK/T LAHS patients.
Abbreviations: NK, natural killer; LAHS, lymphoma-associated hemophagocytic syndrome.
Comparison of patients’ characteristics between the longer survival group and the shorter survival group
In our study, we divided patients into two groups: patients died within 2 weeks after diagnosis of NK/T-cell LAHS were defined as short survival group (n=13), and those who survived >2 months after diagnosis of NK/T-cell LAHS were defined as long survival group (n=6). We noticed that splenomegaly was detected more in short survival group, and the difference is of statistically significance (P<0.001). We also found that hypofibrinogenemia and LDH >1,000 U/L at diagnosis of HLH were also statistically significant different (P=0.026, P=0.007) between the two groups. Other factors such as gender, age at diagnosis of HLH, ECOG, stage, EBV-encoded RNA (EBER) status, anemia, and thrombocytopenia were not statistically different between two groups (Table 2).
Table 2.
Characteristics of patients with NK/T LAHS between longer survival group and shorter survival group
| Characteristics | Shorter group | Longer group | P-value |
|---|---|---|---|
| Gender | |||
| Male | 8 | 4 | 0.829 |
| Female | 5 | 2 | |
| Age at diagnosis | |||
| Mean ± SD | 41.42±14.34 | 39.29±3.63 | 0.876 |
| Age at HLH | |||
| Mean ± SD | 42.37±13.82 | 41.82±5.08 | 0.876 |
| ECOG | |||
| 0–1 | 5 | 4 | 0.252 |
| 2–3 | 8 | 2 | |
| Ann Arbor stage | |||
| I + II | 10 | 4 | 0.637 |
| III + IV | 3 | 2 | |
| LDH level before HLH | |||
| Normal | 6 | 3 | 1 |
| Elevated | 4 | 2 | |
| Subtypes | |||
| UNKTL | 7 | 4 | 0.599 |
| EUNKTL | 6 | 2 | |
| EBER positive | |||
| Yes | 6 | 4 | 0.428 |
| No | 1 | 0 | |
| Cutaneous involvement | |||
| Yes | 6 | 3 | 0.876 |
| No | 7 | 3 | |
| Anemia | |||
| I/II | 7 | 4 | 0.599 |
| III/IV | 6 | 2 | |
| Splenomegaly | |||
| Yes | 12 | 1 | <0.001 |
| No | 0 | 5 | |
| Ferritin (ng/mL) | |||
| ≥500 | 1 | 4 | 0.121 |
| <500 | 1 | 0 | |
| Fibrinogen (g/L) | |||
| ≤1 | 6 | 0 | 0.026 |
| >1 | 3 | 4 | |
| Triglycerides (mmol/L) | |||
| ≥3.0 | 6 | 1 | 0.091 |
| <3.0 | 4 | 5 | |
| Thrombocytopenia | |||
| I/II | 4 | 3 | 0.419 |
| III/IV | 9 | 3 | |
| LDH at HLH (U/L) | |||
| >1,000 | 8 | 0 | 0.007 |
| ≤1,000 | 4 | 6 | |
Abbreviations: EBER, EBV-encoded RNA; ECOG, Eastern Cooperative Oncology Group; EUNKTL, extra-upper aerodigestive tract NK/T-cell lymphoma; HLH, hemophagocytic lymphohistiocytosis; LDH, lactic dehydrogenase; NK, natural killer; NKPI, NK/T-cell lymphoma prognostic index; UNKTL, upper aerodigestive tract NK/T-cell lymphoma.
The impact of patients’ clinical and laboratory parameters on OS
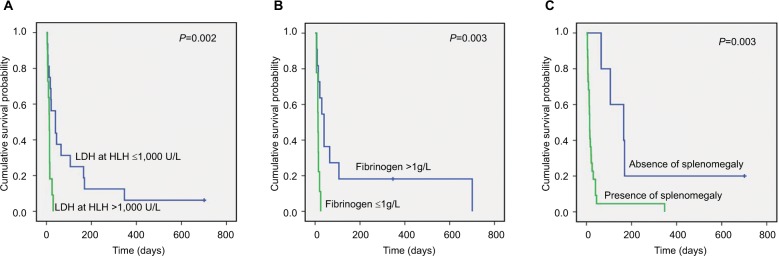
Kaplan–Meier analysis indicated that patients with higher LDH at diagnosis of HLH had a lower OS compared to those with lower levels of LDH (P=0.002, Figure 2A). An association was found between hypofibrinogenemia and a worse OS (P=0.003; Figure 2B). Presence of splenomegaly was also correlated with a poor OS (P=0.003; Figure 2C).
Figure 2.
Correlations between levels of LDH, fibrinogen, and presence of splenomegaly and patient OS.
Note: Kaplan–Meier analysis illustrating the association between levels of LDH (A), fibrinogen (B), presence of splenomegaly (C), and OS in NK/T-cell LAHS (meanings of blue and green lines are indicated in each panel).
Abbreviations: LAHS, lymphoma-associated hemophagocytic syndrome; LDH, lactic dehydrogenase; NK, natural killer; OS, overall survival.
Discussion
The onset of HLH during or after the treatment of NK/T-cell lymphoma is a rare, but often catastrophic event. In most cases even intensive therapeutic measures cannot prevent the lethal outcome.13 Therefore, it would be highly valuable to rapidly identify clinical and/or laboratory risk factors of the disease. Here, in this retrospective study, we concentrated on the clinical and laboratory features, and prognostic factors of NK/T-cell lymphoma patients diagnosed with HLH.
The mortality rate within 1 month after diagnosis of NHL-related HLH was reported as high as 20%.14 NK/T-cell lymphoma had a worse prognosis than B-cell lymphoma, with 5-year survival rate of <15%.15 In our study, mortality rate was 96.4% with only one patient survived, and the median survival was 15 days, which underscores the rapid progression and poor prognosis of the disease.
To clarify the increased risk for NK/T-cell lymphoma-associated HLH, we compared the patients’ characteristics in our cohort with some previous studies.9,11 Table 3 indicates that increased risk for HLH was associated with higher ECOG scores (ECOG 2–3; P<0.001, P<0.001), primary site of involvement (EUNKTL; P<0.001, P=0.006), and cutaneous involvement (P<0.001). Previous studies have reported that splenomegaly and elevated LDH levels affect patient prognosis.16,17 Besides, in our study, we also found that hypofibrinogenemia was an adverse prognostic factor of HLH.
Table 3.
Comparison of patient characteristics in our study with two previous published studies
| Characteristics | Our study (FUSCC China) | Lee et al,9 Korea | χ2 | P-value | Kim et al,11 Korea | χ2 | P-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| N=28 | N=262 | N=208 | |||||
|
| |||||||
| Gender | |||||||
| Male | 18 | 170 | 0.004 | 0.950 | 131 | 0.018 | 0.893 |
| Female | 10 | 92 | 77 | ||||
| Age at diagnosis (years) | |||||||
| >60 | 3 | 55 | 1.670 | 0.196 | 141 | 33.796 | <0.001 |
| ≤60 | 25 | 207 | 67 | ||||
| ECOG | |||||||
| 0–1 | 13 | 228 | 29.688 | <0.001 | 187 | 36.082 | <0.001 |
| 2–3 | 15 | 34 | 21 | ||||
| Ann Arbor stage | |||||||
| I + II | 19 | 200 | 0.984 | 0.321 | 145 | 0.040 | 0.841 |
| III + IV | 9 | 62 | 63 | ||||
| LDH before HLH | |||||||
| Normal | 13 | 166 | 0.159 | 0.69 | 125 | 0.008 | 0.927 |
| Elevated | 9 | 96 | 83 | ||||
| Subtypes | |||||||
| UNKTL | 16 | 222 | 13.085 | <0.001 | 167 | 7.592 | 0.006 |
| EUNKTL | 12 | 40 | 41 | ||||
| NKPI | |||||||
| 0–1 | 18 | 128 | 0.35 | 0.554 | 110 | 1.292 | 0.25 |
| ≥2 | 10 | 91 | 98 | ||||
| Cutaneous involvement | |||||||
| Yes | 10 | 7 | 50.049 | <0.001 | NA | ||
| No | 18 | 255 | NA | ||||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EUNKTL, extra-upper aerodigestive tract NK/T-cell lymphoma; FUSCC, Fudan University Shanghai Cancer Center; HLH, hemophagocytic lymphohistiocytosis; LDH, lactic dehydrogenase; NKPI, NK/T-cell lymphoma prognostic index; UNKTL, upper aerodigestive tract NK/Tcell lymphoma.
Treatment for NK/T-cell LAHS remains a challenge. In our study, we mainly carried out treatment according to HLH-04 guidelines. We found there was statistically significant difference between treatment response and patients’ OS (P=0.00021), which was consistent with other previous reports.5,18 Previous reports also recommended that those who respond well with NK/T-cell LAHS initial induction therapy should be actively engaged in HSCT.19,20 However, even with the currently recommended therapy, HLH is frequently a fatal condition with a high rate of mortality. Also some investigators suggested doctors should simultaneously consider controlling high inflammatory cytokine storm and treating NK–T-cell lymphoma.21 Further clinical trials should be carried out to explore better therapeutic procedures, especially for those who do not respond to first line treatment regimens.
There are some limitations of our study. First, because of the long period of study, the number of copies of EBV-DNA was not routinely detected; the association between the levels of EBV-DNA and patients’ OS were not analyzed. Second, we only enrolled and analyzed hospitalized patients, the number of cases in this study was relatively small, and only one patient received HSCT.
Conclusion
In summary, NK/T-cell LAHS is highly lethal and with extremely poor prognosis. Our data indicated that the levels of LDH, fibrinogen, and presence of splenomegaly were prognostic factors of the disease. Higher ECOG scores, EUNKTL, and cutaneous involvement were risk factors of NK/T-cell LAHS. Additional independent, prospective clinical trials will be needed to explore optimal treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38(1):20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 2.Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Aricò M, Elinder G, Imashuku S, Janka G. Familial hemophagocytic lymphohistiocytosis. Primary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998;12(2):417–433. doi: 10.1016/s0889-8588(05)70520-7. [DOI] [PubMed] [Google Scholar]
- 4.Janka G, Imashuku S, Elinder G, Schneider M, Henter JI. Infection-and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998;12(2):435–444. doi: 10.1016/s0889-8588(05)70521-9. [DOI] [PubMed] [Google Scholar]
- 5.Han AR, Lee HR, Park BB, et al. Lymphoma-associated hemophagocytic syndrome: clinical features and treatment outcome. Ann Hematol. 2007;86(7):493–498. doi: 10.1007/s00277-007-0278-6. [DOI] [PubMed] [Google Scholar]
- 6.Majluf-Cruz A, Sosa-Camas R, Pérez-Ramírez O, Rosas-Cabral A, Vargas-Vorackova F, Labardini-Méndez J. Hemophagocytic syndrome associated with hematological neoplasias. Leuk Res. 1998;22(10):893–898. doi: 10.1016/s0145-2126(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi N, Miura I, Chubachi A, Miura AB, Nakamura S. A clinicopathological study of 20 patients with T/natural killer (NK)-cell lymphoma-associated hemophagocytic syndrome with special reference to nasal and nasal-type NK/T-cell lymphoma. Int J Hematol. 2001;74(3):303–308. doi: 10.1007/BF02982065. [DOI] [PubMed] [Google Scholar]
- 8.Han L, Li L, Wu J, et al. Clinical features and treatment of natural killer/T cell lymphoma associated with hemophagocytic syndrome: comparison with other T cell lymphoma associated with hemophagocytic syndrome. Leuk Lymphoma. 2014;55(9):2048–2055. doi: 10.3109/10428194.2013.876629. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 10.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SJ, Oh SY, Hong JY, et al. When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol. 2010;21(5):1058–1063. doi: 10.1093/annonc/mdp412. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmberg K, Nichols KE, Henter JI, et al. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica. 2015;100(8):997–1004. doi: 10.3324/haematol.2015.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivière S, Galicier L, Coppo P, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127(11):1118–1125. doi: 10.1016/j.amjmed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Ishii E, Ohga S, Imashuku S, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65. doi: 10.1532/IJH97.07012. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Zhang L, Liu J, et al. A clinical study of 21 patients with hemophagocytic syndrome in 295 cases diagnosed with nasal type, extra-nodal nature killer/T cell lymphoma. Cancer Biol Ther. 2017;18(4):252–256. doi: 10.1080/15384047.2017.1295176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia J, Song Y, Lin N, et al. Clinical features and survival of extranodal natural killer/T cell lymphoma with and without hemophagocytic syndrome. Ann Hematol. 2016;95(12):2023–2031. doi: 10.1007/s00277-016-2805-9. [DOI] [PubMed] [Google Scholar]
- 18.Jin Z, Wang Y, Wang J. Multivariate analysis of prognosis for patients with natural killer/T cell lymphoma-associated hemophagocytic lymphohistiocytosis. Hematology. 2018;23(4):228–234. doi: 10.1080/10245332.2017.1385191. [DOI] [PubMed] [Google Scholar]
- 19.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005. doi: 10.1182/blood-2013-01-453233. [DOI] [PubMed] [Google Scholar]
- 20.Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42(3):175–180. doi: 10.1038/bmt.2008.133. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Li P, Zhang R, et al. Identification of clinical features of lymphoma-associated hemophagocytic syndrome (LAHS): an analysis of 69 patients with hemophagocytic syndrome from a single-center in central region of China. Med Oncol. 2014;31(4):902. doi: 10.1007/s12032-014-0902-y. [DOI] [PubMed] [Google Scholar]