Abstract
One of the main priorities of rehabilitation after anterior cruciate ligament reconstruction (ACLR) surgery is the restoration of knee extensor muscle strength. Residual deficits in knee extensor muscle size and strength after injury are linked to poor biomechanics, reduced knee function, increased knee osteoarthritis risk, as well as heightened risk of re-injury upon return to sport. Most studies indicate that knee extensor muscle strength is typically not resolved prior to return to sport. This clinical commentary discusses strategies to optimize and accelerate the recovery of knee extensor strength post-surgery, with the purpose to support the clinician with evidence-based strategies to implement into clinical practice. Principally, two strategies exist to normalize quadriceps strength after surgery, 1) limiting strength loss after injury and surgery and 2) maximizing and accelerating the recovery of strength after surgery. Optimal preparation for surgery and a focused attempt to resolve arthrogenic muscle inhibition are essential in the pre and post-operative period prior to the inclusion of a periodized strength training program. Often voluntary strengthening alone is insufficient to fully restore knee extensor muscle strength and the use of electrical stimulation and where necessary the use of blood flow restriction training with low loads can support strength recovery, particularly in patients who are significantly load compromised and experience pain during exercise. Resistance training should employ all contraction modes, utilize open and closed kinetic chain exercise of both limbs, and progress from isolated to functional strength training, as part of a periodized approach to restoring neuromuscular function. Furthermore, thinking beyond the knee musculature and correcting core and hip dysfunction is also important to ensure an optimal knee extension strengthening program. The purpose of this clinical commentary is to provide a series of evidenced based strategies which can be implemented by clinicians responsible for the rehabilitation of patients after ACLR.
Level of evidence
5
Keywords: Anterior cruciate ligament reconstruction, functional recovery, injury prevention, rehabilitation, sports medicine
INTRODUCTION
One of the main priorities of rehabilitation after anterior cruciate ligament reconstruction (ACLR) surgery is the restoration of knee extensor muscle strength. After ACL injury and subsequent surgery, there is often considerable pain, swelling/inflammation, reduced function, muscle atrophy and strength loss. Residual deficits in knee extensor muscle size and strength after injury are linked to poor biomechanics,1 reduced knee function and increased knee osteoarthritis risk,2 poorer outcomes and heightened risk of re-injury upon RTS.3 For example, those who reported a limb symmetry index (LSI) less than 90% were at nearly three times greater risk of sustaining a subsequent knee injury than who achieved more than 90% LSI (33 vs 12%).3 For every 1%-point increase in quadriceps symmetry there was a 3% reduction in re-injury rate. Early return to sport (RTS), without sufficient neuromuscular recovery is associated with early knee osteoarthritis changes only one year after surgery.4
Most researchers have indicated that knee extensor muscle strength is typically not achieved by six-months after surgery or at the time of return to play.2,5-8 Furthermore, recent research suggests that the conventional LSI may underestimate the deficits in knee extensor muscle strength post-surgery.8 Only 29% of patients achieved a LSI less than 10% when the reconstructed limb was compared to pre-injury injured limb values at six-months post ACL surgery, compared to 57% who achieved this marker when using the conventional LSI (when injured limb is compared to contralateral limb).8 As such deficits in knee extensor strength are possibly even more marked than previously thought, with only one in three to one in four achieving this marker prior to medical discharge and RTS. Importantly, the restoration of knee extensor muscle strength represents a mid-stage rehabilitation marker, one which should be achieved prior to restoring movement quality, functional strength, power and explosive muscle strength as well as subsequent sport-specific re-training and RTS.9,10 Therefore, the inability to restore knee extensor muscle strength in a timely fashion is likely resulting in incomplete recovery in other important rehabilitation factors (e.g., explosive strength and motor patterning). In order to achieve complete functional recovery and optimise the late-stage rehabilitation process, there is a need to first optimize and accelerate the recovery of knee extensor muscle strength to i) provide an optimal platform on which late-stage rehabilitation can commence and ii) actually allow time in most clinical cases for late-stage rehabilitation and an appropriate RTS process.
The aim of this clinical commentary/narrative review is to discuss strategies to optimize and accelerate the recovery of quadriceps strength post ACLR surgery. This will provide practitioners working with individuals after with important theoretical and practical information which can be applied to their functional recovery framework to help optimise their patient outcomes.
WHAT ARE THE REASONS FOR QUADRICEPS WEAKNESS AFTER ACLR?
Determining the reasons for quadriceps weakness after ACLR are essential to design strategies to optimize patient functional recovery. Typically, there is limited consideration of the notion that if one fails to overcome muscle inhibition one will be unable to optimally restore muscle mass and strength. Arthrogenic muscle inhibition (AMI) is hypothesized to be present after ACLR and contribute to the ever-present post-traumatic knee extensor muscle strength deficit.2,11-14 Loss of mechanoreceptors from the ACL is thought to disrupt the ligamentous–muscular reflex between the ACL and the quadriceps, leading to an inability to actively recruit high-threshold motor units during voluntary quadriceps contractions. Furthermore, pain and swelling both result in neuromuscular inhibition via the AMI process and resultant muscle atrophy and weakness.6,15 AMI typically limits the ability to achieve desired intensity levels and neuromuscular activation, and is often present bilaterally following unilateral ACLR, and in some cases, can be equivalent to the injured limb.16
Muscle strength is influenced by both neural and morphological factors. The loss of function and significant neural inhibition can result in marked muscle atrophy which contribute to loss of strength and function. Williams et al.17 reported that quadriceps atrophy and activation failure together account for approximately 62% of the variance in the quadriceps weakness of ACL-deficient non-copers, suggesting atrophy also plays a significant role in reducing quadriceps strength. As such, the resolution of muscle mass and neural activation are key aspects of ACL rehabilitation and strategies to restore them are of considerable importance.
Maximizing knee extensor strength recovery after ACL surgery
Two strategies exist to normalize quadriceps strength after surgery. These include:
Limit strength loss after injury and surgery and
Maximize and accelerate the recovery of strength after surgery.
STRATEGIES TO LIMIT STRENGTH LOSS AFTER ACL RECONSTRUCTION
The greater the degree of muscle atrophy and larger strength deficits post-surgery the longer time it will take to restore these deficits. In terms of this approach there are two strategies to consider a) the level of atrophy and strength loss prior to surgery and b) the degree of atrophy and strength loss post-surgery.
Optimally prepare for surgery
Optimally preparing for surgery and resolving deficits in muscle mass and strength would be expected to enhance post-operative function. The research available indicates that prehabilitation (a five to six-week program focusing on restoration of muscle strength, quadriceps hypertrophy and hop performance) results in superior knee function post operatively.18-20 Recent research shows that patients with better pre-operative quadriceps activation demonstrated greater post-operative activation, whilst patients with better pre-operative strength also demonstrated better post-operative strength.21 There is no consensus on the optimal level of pre-surgery function.22 Grindem et al.18 recommended patients should have a LSI of 90% for muscle strength and hop performance prior to ACL reconstruction, which may not be plausible for all patients.
Resolve Arthrogenic Muscle Inhibition (AMI) quickly post-surgery
After injury or surgery, there is often considerable pain and swelling/ inflammation. Acute injury management should adhere to the principles of POLICE, (protection, optimal loading, ice, compression and elevation)23 to ensure joint protection and healing, removal of pain and swelling but maintenance and gradual restoration of function through optimal load application. Pain and swelling both result in neuromuscular inhibition via the AMI process and resultant muscle atrophy and weakness.6,15 The clinician should utilize a variety of interventions to combat pain, swelling and AMI to be able to progress through the remainder of the rehabilitation program optimally.
a) Use Anaesthetics. Local anaesthetics may reverse AMI through the reduction of pain and may also reduce AMI by blocking other afferents contributing to the inhibition. AMI persists once pain has subsided and can be induced in the absence of pain (e.g., the effusion model does not cause pain but results in AMI),24 therefore, rehabilitation strategies effective in removing AMI, should not be focused solely on removing painful stimuli.
b) Use Ice. Use of cryotherapy (ice), compression and elevation are standard practices as part of acute injury management, in line with the POLICE23 recommendations. Cooling of the knee joint may also may serve to decrease AMI24 and facilitate increased quadriceps activation. The effects are thought to be maintained after the removal of cryotherapy and as such, may serve as a strategy to temporarily reduce AMI and increase quadriceps recruitment prior to exercise.
c) Utilize transcutaneous electrical nerve stimulation. Transcutaneous electrical nerve stimulation (TENS) of the cutaneous nerves has been shown to reduce presynaptic inhibition,25 which is a contributor to AMI.26 Hopkins et al.24 demonstrated that 30 mins of TENS treatment reversed the inhibitory effects of induced knee effusion. However, this was temporary as the inhibition returned to baseline levels after the machine was turned off. As such, the greatest effect of TENS appears as a supplement to active exercise with an effect to minimize AMI and promote quadriceps recruitment.15,27
Optimal load to preserve quadriceps strength
Optimal loading may be defined as the load applied to structures that maximizes physiological adaptation.28 Additionally, in the context after injury, it can also be considered as the load which ‘minimizes adaptation’ (e.g., muscle strength loss and atrophy due to functional limitations).
Achieving optimal loading is challenging. It is essential that in the early periods after surgery, the rehabilitation program incorporates progressive optimal loading to prevent muscle atrophy and strength loss and subsequently facilitate functional recovery. Use of electrical stimulation can support strength preservation, through providing a stimulus to activate the motor units, which may be inhibited due to AMI. The use of electrical stimulation and voluntary isometric contractions can support muscle mass and strength preservation in the early phase.29 Monitoring pain and joint effusion particularly during the early phases of rehabilitation are important to ensure that the applied training stimulus is not excessive and causing tissue overload. Measurement of pain via the use of the visual analog scale should be taken regularly and recorded. Swelling can be measured with limb girth daily. Measurement of knee circumference at the patella has been shown to have strong intra-tester reliability and good sensitivity to change.30 Within, the knee, change greater than one centimeter was shown to be clinically significant.
Strategies to maximise and accelerate the recovery of strength after ACLR
Incorporate a periodized strength training program
Following the satisfactory resolution of pain, swelling and AMI, it is important to incorporate a periodized strength training program to fully restore neuromuscular function of the knee extensors, as well as other muscles. Restoration of quadriceps function requires the application of strength and conditioning principles applied to the injured athlete,31 and can be considered as optimal re-conditioning. Key strategies after ACLR are to restore muscle mass, strength (across the force-velocity curve), explosive strength (rate of force development), power and coordination (e.g., ability to use this strength in sport-specific movements). A significant challenge for rehabilitation specialists is designing optimal training programmes that facilitate neural and musculotendon adaptations whilst been mindful of biological healing constraints, and safety.32,33 To fully restore neuromuscular performance after ACLR it is important to incorporate a periodized neuromuscular training program, respecting tissue healing times and the patients individualised functional recovery.
Periodization can be defined as the planned manipulation of training variables (load, sets and repetition) in order to maximize training adaptations and prevent over-training.33 There is a lack of evidence concerning the best periodization approach after ACLR, but it is the authors view and that of others31 that the use of periodization in rehabilitation is superior to non-periodized approaches and the use of non-linear approaches, respecting the phases of rehabilitation is important. When designing the program, it is important to have an understanding of how training variables can manipulate training outcome. This entails understanding how changes in load/ intensity, volume and set configurations can influence strength adaptations (and their associated mechanism) after ACLR, placed alongside the functional recovery process.
Important considerations in terms of resistance training are i) the mechanical tension on the muscle; ii) the metabolic stress induced through training and iii) the extent of muscle damage. Mechanical tension refers to the loading of muscle and is proposed to disrupt skeletal muscle structures, compromizing the integrity of individual muscle fibres and leading to cellular responses via stimulation of the mTOR pathway.34 Local metabolic stress involves the accumulation of metabolic by-products such as hydrogen ions, and blood lactate from fast glycolysis,35,36 which then stimulate catabolism; while muscle damage is proposed to lead to hypertrophic responses secondary to muscle damage, subsequent inflammation and upregulation of muscle synthesis to repair the tissue. The manipulation of various resistance training variables can influence muscle strength and size and include training volume, loading of exercise intensity, training frequency, training to failure, exercise variation, contraction type and recovery between efforts. Considering these training variables are important when designing an optimal resistance training program for ACL patients to RTS quickly and optimally.
In general, it appears that high volume resistance training is necessary to bring about increased strength and muscle size. Schoenfeld et al.37 concluded that high volume resistance training produces greater gains in muscle mass than low volume training. It is thought that high volume training may enhance muscle mass gains due to prolonged metabolic stress.38 There is a balance however, between a high training volume and an excessive volume which may lead to over-training and potential joint stress and tissue overload. Amirthalingam et al.39 found no significant difference in muscle hypertrophy when training with 5 sets of 10 repetition versus 10 sets of 10 repetitions over six weeks of training. As such, it is thought that volume and muscle adaptation are not linearly related but instead follows an inverted ‘U’ shape40 with an optimal training volume to elicit muscle hypertrophy and strength. This exact value is not known and may relate to the individual, the training history, recruitment, recovery strategies as well as lifestyle outside of the clinic (e.g., sufficient recovery practices, sleep, nutrition and rest etc.), and possible unresolved biological consequences of injury (e.g., pain, swelling and AMI).
It is thought that the mechanical tension or load, typically presented as a percentage of maximal load that can be lifted (one repetition maximum, 1RM) is important for maximising muscle hypertrophy and strength. This is because the increased load results in increased mechanical tension on the muscle which is an important stimulus. The American College of Sports Medicine (ACSM) recommends loads of 60-70% 1RM for the development of muscle strength and 70-85% for hypertrophy.41 Traditionally, it was thought that very high loads were necessary to bring about activation of all type II motor units based on the Henneman size principle42 and achieve full and complete muscle hypertrophy (targeted at all motor units). However, it is suggested that more low-load training also recruits fast-twitch muscle fibres, provided the working set is continued close to volitional fatigue.43 There appears to be no difference or at most a small trend for higher muscle hypertrophy with higher load resistance training compared to low-load training in terms of muscle hypertrophy.37 Training set intensity however, can have marked effects on other variables such as maximal eccentric strength and rate of force development (RFD). For example, conventional resistance training using loads of 70% maximal has been shown to enhance maximal muscle strength and muscle hypertrophy41,44 but results in a reduction in the relative RFD (scaled to maximal voluntary force) and as such no change in RFD.44-46 The importance for RFD in the rehabilitation program has recently been discussed,10 and it is apparent that following full and complete restoration of muscle strength after ACLR, there are still significant 30% deficits in RFD.47 RFD was only restored following a subsequent period of power training 12 months after surgery. Recently, Mangine et al.46 showed that moderate intensity resistance training with loads at 70% 1RM over eight weeks resulted in no change in RFD, whereas strength training using high loads (90% maximal) elicited large increases in RFD (+70%). As such, each training intensity may bring about specific underlying adaptations and evoke differing alterations in mechanical variables (strength, power, RFD). Obviously, high load strength training (>85-90% 1RM) can only be implemented following satisfactory recovery of range of motion, pain and swelling, AMI and sufficient muscle mass to tolerate these high forces.48
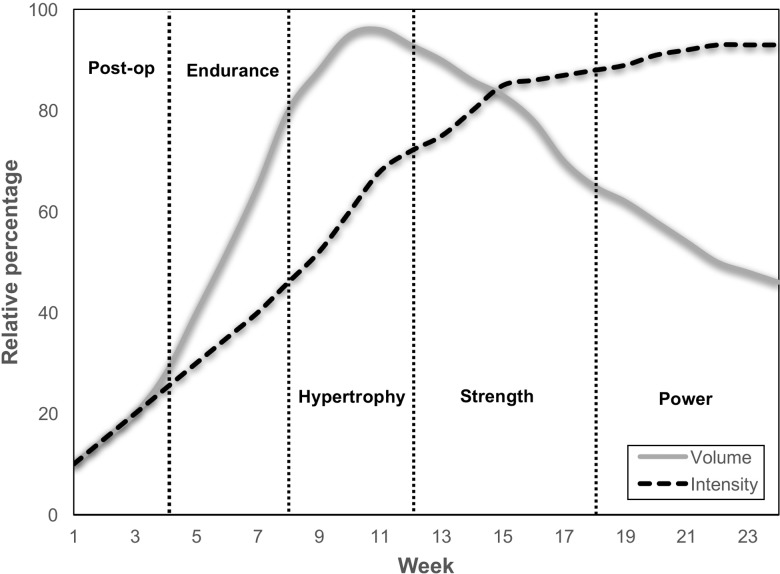
It is recommended to utilize a periodized resistance training program throughout the ACL rehabilitation program, beginning with optimal post-operative recovery, prior to moderate to high volume low to moderate loads resistance training until failure to promote initial strength gains and hypertrophy of all motor units (achieved largely through metabolic stimuli), when the joint is more load compromised and cannot likely tolerate high forces; followed by a period of moderate to high intensity (70-80% 1RM) resistance training with moderate to high volume (5-8 sets) with the goal to fully restore muscle size and maximise strength; finishing with very high intensity strength training (90%, / 5RM) and lower volumes in the latter phases of rehabilitation to target maximal voluntary activation, eccentric maximal muscle strength and restore power and explosive strength (Figure 1).10 Obviously, it is important to respect tissue healing, joint response and individual adaptations, and produce a minimum stimulus in the post-operative recovery period to preserve muscle mass, but not overload the joint.
Figure 1.
A graphical representation of a general outline of a periodised resistance training program after anterior cruciate ligament reconstructive surgery (the time lines are for a patient returning to sport at around 6 months). The program begins with low intensity and volume, to preserve muscle mass and strength as much as possible in the post-operative period. It has a gradual increase in intensity and volume to promote muscle hypertrophy, muscle endurance and strength recovery at low to moderate loads, when the athlete is still load compromised. It progresses to moderate to high intensity strength training with moderate to high volume in the third stage prior to very high intensity and lower volumes in the final stage prior to return to sport.
Open chain or closed chain, isolated or functional?
There is no consensus among the existing published evidence as to whether closed kinetic chain (CKC) or open kinetic chain (OKC) exercises should be the intervention of choice following ACLR.49 There are doubts about safety of OKC exercises, which are arguably unsupported by substantial published evidence.49 OKC exercises can be useful as they isolate the muscle and limit the involvement of other muscle groups and thus, can ensure higher and more complete activation and fatigue of the target muscle. Some studies have shown that OKC exercises or OKC plus CKC exercises are more effective than CKC exercises alone in improving quadriceps strength after ACL reconstruction50,51 and in patients who are ACL deficient.52 Others have found no differences in patients’ quadriceps strength when comparing the two types of exercises.51,53 Available clinical research suggests that cautiously incorporating OKC exercises into ACL rehabilitation will improve quadriceps function and the authors advocate the use of both OKC and CKC exercise.54
A particular consideration with knee extensor strengthening after ACLR, is minimizing patellofemoral joint (PFJ) stress, given the high prevalence of patients who go on to develop patellofemoral pain syndrome (PFPS) after ACLR surgery.55-57 In OKC exercises such as knee extensions, quadriceps muscle force and PFJ stress are greatest near full extension.58,59 Conversely, in CKC exercises such as lunges and the leg press, quadriceps muscle force and PFJ stress are highest near full flexion.55,56 As such, it is recommended to initially restrict high load OKC strengthening between 40-90 degrees of knee flexion, and CKC between 0-80 degrees, which collectively can enable complete strengthening through the arc of motion, at reduced PFJ stress. Each can be implemented at a similar time (typically 4 weeks after surgery, but with an initial focus on control as opposed to load), respecting the principles of optimal load progressions.
When there are residual deficits in knee extensor strength (typical after ACLR surgery), it is essential to implement isolated strength techniques as opposed to functional exercises such as squat with load. This is because, significant strength deficits result in biomechanical compensatory strategies i.e., cheating where the hip extensors are utilised instead of the knee extensors.60 Therefore, functional exercises alone are an inadequate means for resolving quadriceps weakness and restoring normal quadriceps strength.61 As isolated quadriceps strength increases, a gradual increase in the use of functional strength training techniques such as squat, deadlift, lunge and their derivatives can be implemented.
Functional strength refers to the ability to produce force in movements in which the muscles are typically used62 and is essential for athletic performance.63,64 Functional strength is also important for optimal movement quality and force dissipation. For example, landing from a jump results in 1.5-2 times body mass transferred through each limb.65-,67 An inability of the neuromuscular system to tolerate these forces, i.e. low functional eccentric strength of the kinetic chain would result in these forces been either off-loaded from the sagittal plane to the frontal plane and/or absorbed via the ligaments, tendon and joints, thus potentially lead to joint or tendon overload.68-69 As such, it is essential to have both a sufficient knee extension strength and global functional strength capacity to ensure that the neuromuscular system can adequately generate and ‘accept’ high ground reaction forces during sporting type movements.
The authors suggest that isolated machine based strengthening techniques be the main strategy to restore knee extensor strength in the early to middle phases of rehabilitation (e.g., week 4 to week 12 after surgery as an example) and once the patient has resolved at least 80% of the knee extensors strength of their contralateral limb70 (assessed through isokinetic testing of the knee extensors, typically at 90-120 days post-surgery in our patients). In general, it is advised to initially implement isolated OKC and CKC early in the rehabilitation period, using initially isometric contractions (with additional modalities, see subsequent text), with specific joint angle restrictions to limit PFJ stress. Motor patterning and muscle imbalance correctives at adjacent joints should accompany this work to prepare for functional strengthening. Once the patient has restored knee extensors strength to within 20% of the contralateral side, moderate to high load strength training using both isolated (high load, 5RM) and functional (moderate load, 8RM) techniques can be used.
Target the VMO?
PFP is common following ACLR with 30-50% still experiencing PFP at 1 year after surgery,55-57 and is problematic for the recovery of quadriceps strength after ACLR as it will result in PF inhibition, and limit quadriceps activation. Often practitioners work around patients PFP and lower the loads to a point of no pain (as pain is a potent inhibitor and contributor to AMI), but this often results in a load which is insufficient to elicit sufficient stimulus for muscle hypertrophy and strength adaptation. The resolution of PFP is essential to full and complete knee extensor strength recovery. There is a lack of consensus on the source of pain in relation to PFP.71 However, patellar maltracking including increased lateral patellar translation,72-74 tilt72 and spin,74 as well as increased lateral PFJ stress75,76 may associate with PFPS. As the vastus medialis oblique (VMO) has the ability to control lateral patellar tracking, delay or weakness of VMO is considered a key biomechanical risk factor for patellar maltracking.77 As such, rehabilitation specialists and researchers often advocate selective strengthening on the VMO, to help restore normal patellofemoral biomechanics and reduce pain, thus supporting more optimal quadriceps recovery. There is however, debate, as to whether it is possible to selectively strengthen the VMO, with current evidence suggesting that voluntary strengthening techniques will not specifically recruit the VMO.78-81 In those with patella maltracking, the use of electromyography (EMG) biofeedback measures during neuromuscular contractions can provide auditory or visual feedback signals, designed to increase awareness and voluntary control of muscle activation. When utilized in conjunction with strength training, EMG biofeedback aimed at increasing VMO activation while maintaining constant vastus lateralis (VL) activity has been shown to improve VMO/VL activation ratios.82 Additionally, taping of the patella may be an effective strategy to transiently optimise patella tracking. Using taping techniques to control patella tracking during resistance exercise have found increased patient tolerance to knee joint loading, increased VMO activity, and improved onset of the VMO in relation to the VL muscles.83-86
Think beyond the knee
Typically, early and mid-phase programs focus exclusively on resolving knee mechanics. It is becoming accepted that weakness of core and hip muscles are risk factors in lower extremity injury risk87-90 and in particular ACL injuries.91,92 A systematic review by Petersen et al.93 revealed deficits in hip muscle strength after ACLR. Beyond injury prevention, proximal dysfunction is associated with high risk movement biomechanics and linked to PFP.87,90 Strong evidence currently exists that patients with anterior knee pain have deficits in hip abduction, hip extension and external rotation strength.94 Hip muscle strengthening is effective in reducing the intensity of pain and improving functional capabilities in patients with PFP95 and should form part of the ACLR rehabilitative program focused on resolving knee extensor strength deficits.
Utilize the powers of electrical stimulation
Neuromuscular electrical stimulation (NMES) appears to be a promising intervention for use after ACLR. NMES allows for the direct activation of the motor axon, and could allow for the direct recruitment of the inhibited motoneurons. Muscle activation by means of NMES allows for the recruitment of a greater proportion of type II muscle fibers when compared with voluntary contractions of a similar intensity.96-98 Furthermore, although, during voluntary contractions there is a logical order of recruitment beginning with the smallest motor units and progressing to the largest motor units,42 NMES results in a reversal of the order of motor unit recruitment.99 The activation of type II motor units are essential to achieve a higher level of quadriceps force production, as well as sufficient power and RFD. As such, their recruitment by means of NMES undoubtedly should aid in the quest to achieve complete recovery of quadriceps strength. A recent meta-analysis reported that use of NMES in addition to standard physical therapy appears to significantly improve quadriceps strength and physical function in the early post-operative period compared to standard physical therapy alone.29
Incorporate blood flow restriction training in selective patients
In the load compromised patient (early after surgery) or in those patients who experience PFP and subsequent quadriceps inhibition and as such, cannot achieve the required load and activation to bring about the necessary stimulus for adaptation, blood flow restriction (BFR) training may be an effective therapy. Low-intensity resistance training with BFR can result in in greater strength and muscle hypertrophy when compared to resistance training with the same intensity under normal flow100-103 and comparable to gains with moderate to high intensity resistance training.104 Under ischemic conditioning, fast twitch fibres are recruited even under low intensity activity, as type I motor units fatigue rapidly which allows for the recruitment of type II units earlier. BFR training may also serve as an effective stimulus during an unloading phase for patients because it results in a positive training adaptation, although causing little to no muscle damage102 and thus, can be used sparingly throughout the rehabilitation cycle. Low-load BFR was shown to be superior at improving functional capacity and pain in patients with PFP compared to moderate intensity resistance training with BFR.104 Sub-group analysis revealed that in those with pain on resisted knee extension there was considerable benefits in enhancing function, but is similar to resistance training at moderate to high loads (70% 1RM) in those without pain on resisted knee extension.104 As such, BFR therapy at low loads can may be a useful tool to develop muscle strength in patients who are unable to perform high-resistance exercise or patients who have persistent extremity weakness despite traditional therapy, or maybe used sparingly as part of a periodized strength training program.
Don't forget the other leg
An ACL injury has recently been suggested as a single leg injury, but a double leg problem.105 Deficits in knee extensor strength, neuromuscular control and proprioception, which are prevalent in the injured limb are also present in the contralateral uninjured limb.106-108 As discussed, this lower than optimal level of strength in the contralateral limb can result in an overestimation of knee extensor strength of the injured when examining the limb symmetry index in the conventional manner (injured versus uninjured).8 As such, it is advised to ensure that rehabilitation target both limbs. Additionally, cross-education training, which is the increase in muscle force on the untrained side after resistance training of the contralateral homologous limb muscle,109 has been suggested to accelerate the recovery of the injured limbs strength after ACLR and augment the LSI, 110 although this is not a consistent finding.111
SUMMARY AND IMPLEMENTATION
The restoration of knee extensor muscle size, activation and strength forms essential components of rehabilitation after ACLR. AMI can limit the desired activation values during resistance training and limit strength recovery after surgery. It is recommended that optimal preparation for surgery, the adoption of POLICE and focused attempt to resolve AMI are essential in the pre and post-operative period prior to the inclusion of a periodised strength training program. Appreciation of strength and conditioning principles including load, volume, rest and recovery (between sets and sessions) are important. Often voluntary strengthening alone is insufficient to fully restore knee extensor muscle strength and the use of electrical stimulation and where necessary the use of BFR training with low loads can support strength recovery, particularly in patients who are significantly load compromised and experience pain during exercise. Resistance training should employ all contraction modes, utilise OKC and CKC exercise, begin with isolated strength tasks and finish and progress to functional strength training and agility type exercises to prepare for sporting practice. Restoring balance between the quadricep muscles and resolving possible patellar tracking issues, through manual therapy, biofeedback training and stretching are important additional considerations. Finally, thinking beyond the knee and correcting core and hip dysfunction may be important to ensure an optimal knee extension strengthening program. Optimizing the use of disinhibitory techniques (e.g., ICE, massage) and activation techniques (pre-activation exercises, electrical stimulation, TENS) may support more optimised training outcomes. It is hoped that these recommendations may support practitioners who have the responsibility to rehabilitate patients after ACLR, and support the quest to enhance patient outcomes (RTS rates, long term knee joint health and re-injury risk) after ACLR surgery.
Table 1.
A schematic layout of an example periodized resistance training approach for the athlete after anterior cruciate ligament reconstruction. The program involves one pre-operative and five post-operative stages aligned with the functional recovery status of the athlete after surgery. The particular goal, strategy and approach are outlined discussing the factor relevant within the text. The program is a typical approach (and allocated time) to a professional athlete, who was able to return to team training at six months after reconstructive surgery. Time lines are dependent upon the injury (e.g., concomitant injury, such as cartilage, medial collateral ligament), and individual healing and progression time lines. Criteria and not time should be used to transition between stages.
| Stage | Weeks | Goal | Strategy | Knee extension exercises | Supplementary modalities (injured) |
|---|---|---|---|---|---|
| 0 | 6 weeks pre-op to surgery |
|
|
|
|
| 1 | 0-4 |
|
|
|
|
| 2 | 5-8 |
|
|
|
|
| 3 | 9-12 |
|
|
|
|
| 4 | 13-18 |
|
|
|
|
| 5 | 19-24+ |
|
|
|
|
AMI = arthrogenic muscle inhibition; CKC = closed kinetic chain; OKC = open kinetic chain; iso = isometric; ecc = eccentric; con = concentric; BFRT = blood flow restriction training; POLICE = protection, optimal loading, ice, compression and elevation; RM = repetition maximum; BL = bilateral; UL = unilateral; OFR = on-field rehabilitation; TENS = transcutaneous electrical stimulation
References
- 1.Palmieri-Smith RM Lepley LK. Quadriceps strength asymmetry following ACL reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43:1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder-Mackler L Delitto A Bailey SL, et al. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166-73. [DOI] [PubMed] [Google Scholar]
- 3.Grindem H Snyder-Mackler L Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50:804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culvenor AG Patterson BE Guermazi A, et al. Accelerated return to sport after anterior cruciate ligament reconstruction and early knee osteoarthritis features at 1 year: An exploratory study. Phys Med Rehabil. 2017; 2018;10:349-56. [DOI] [PubMed] [Google Scholar]
- 5.Lepley AS Gribble PA Thomas AC, et al. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: A 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25:828-39. [DOI] [PubMed] [Google Scholar]
- 6.Palmieri-Smith RM Thomas AC Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008; 27:405-24. [DOI] [PubMed] [Google Scholar]
- 7.Thomee R Kaplan Y Kvist Y, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1798-1805. [DOI] [PubMed] [Google Scholar]
- 8.Wellsandt E Failia MS Synder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Ther. 2017;47:334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckthorpe M. Entering a player back into training after long term rehabilitation. In: Roi GS, Della Villa S (Eds). The future of football medicine. Calzetti Mariucci Editore, Torgiano, 2017, pp. 538.
- 10.Buckthorpe M Roi GS. The time has come to incorporate a greater focus on rate of force development training in the sports injury rehabilitation process. Muscle Tendon Ligament J. 2017;7:435-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley MV Jones DW Wilson D, et al. Rehabilitation of quadriceps inhibition due to isolated rupture of the anterior cruciate ligament. J Orthop Rheumatol. 1992;5:145–54. [Google Scholar]
- 12.Snyder-Mackler L Delitto A Stralka S, et al. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–7. [DOI] [PubMed] [Google Scholar]
- 13.Urbach D Nebelung W Weiler HT, et al. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31:1691–6. [DOI] [PubMed] [Google Scholar]
- 14.Urbach D Nebelung W Becker R, et al. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83:1104–10. [DOI] [PubMed] [Google Scholar]
- 15.Stokes M Shakespeare D Sherman K, et al. Transcutaneous nerve stimulation and post-meniscectomy quadriceps inhibition. Int J Rehabil Res. 1985;8:248. [Google Scholar]
- 16.Shelbourne KD Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18:292–9. [DOI] [PubMed] [Google Scholar]
- 17.Williams GN Snyder-Mackler L Barrance PJ, et al. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005; 38:685-93. [DOI] [PubMed] [Google Scholar]
- 18.Grindem H Granen LP Risberg MA, et al. How does combined preoperative and postoperative rehabilitation programme influence the outcome of ACL reconstruction two years after surgery? A comparison between patients in the Delaware-Oslo ACL Cohort and the Norwegian National Knee Ligament Registry. Br J Sports Med. 2015; 49:385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaarani SR O'Hare C Quinn A, et al. Effect of prehabilitation on the outcome of anterior cruciate ligament reconstruction. Am J Sports Med. 2013; 41:2117-27. [DOI] [PubMed] [Google Scholar]
- 20.de Valk EJ Moen MH Winters M, et al. Preoperative patient and injury factors of successful rehabilitation after anterior cruciate ligament reconstruction with single-bundle techniques. Arthrosc. 2013;29:1879-95. [DOI] [PubMed] [Google Scholar]
- 21.Lepley LK Palmieri-Smith RM. Pre-operative quadriceps activation is related to post-operative activation, not strength, in patient's post-acl reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016; 24:236-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hägglund M Waldén M Thomeé R. Should patients reach certain knee function benchmarks before anterior cruciate ligament reconstruction? Does intense ‘prehabilitation’ before anterior cruciate ligament reconstruction influence outcome and return to sports? Br J Sports Med. 2015;49:1423-4. [DOI] [PubMed] [Google Scholar]
- 23.Bleakley CM Glasgow P MacAuley DC. PRICE needs updating, should we call the POLICE? Br J Sports Med. 2012;46:220-1. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins JT Ingersoll CD Edwards JE, et al. Cryotherapy and TENS decrease arthrogenic muscle inhibition of the vastus medialis following knee joint effusion. J Athl Train. 2002;37:25-32. [PMC free article] [PubMed] [Google Scholar]
- 25.Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia affarents from the human lower limb. J Physiol. 1996;491:197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmieri RM Tom JA Weltman A, et al. Quadriceps arthrogenic muscle inhibition is partially mediated by a presynaptic spinal mechanism. Knee Surg Sports Traumatol Artrosc. 2005;13:370-6. [DOI] [PubMed] [Google Scholar]
- 27.Arvidsson I Eriksson E. Postoperative TENS pain relief after knee surgery: objective evaluation. Orthopedics. 1986;9:1346–51. [DOI] [PubMed] [Google Scholar]
- 28.Glasgow P Phillips N Bleakley C. Optimal loading: key variables and mechanisms Br J Sports Med. 2015;49:278-9. [DOI] [PubMed] [Google Scholar]
- 29.Hauger AV Reiman MP Bjordal JM, et al. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc. 2018;26:399-410. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen T Christensen M Christensen S, et al. Reliability of knee joint range of motion and circumference measurements after total knee arthroplasty: does tester experience matter. Physiother Res Int. 2010;15:126-34. [DOI] [PubMed] [Google Scholar]
- 31.Reiman MP Lorenz DS. Integration of strength and conditioning principles into a rehabilitation program. Int J Sports Phys Ther. 2011;6:241-53. [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz D Morrison S. Current concepts in periodization of strength and conditioning for the sports physiotherapist. Int J Sports Phys Ther. 2015;10:734-47. [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz DS Reiman MP Walker JC. Periodization: current review and suggested implementation for athletic rehabilitation. Sports Health. 2010;2:509-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornberger TA Chu WK Mak YW, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signalling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103:4741-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godfrey RJ Madgwick Z Whyte GP. The exercise-induced growth hormone response in athletes. Sports Med. 2003;33:599-613. [DOI] [PubMed] [Google Scholar]
- 36.Tesch PA Colliander EB Kaiser P. Muscle metabolism during intense, heavy resistance exercise. Eur J Appl Phsiol Occup Physiol. 1986;55:362-6. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld BJ Wilson JM Lowery RP Krieger JW. Muscular adaptations in low-versus high load resistance training: A meta-analysis. Eur J Sport Sci. 2016;16:1-10. [DOI] [PubMed] [Google Scholar]
- 38.Goto K Ishii N Kizuka T, et al. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37:955-63. [PubMed] [Google Scholar]
- 39.Amirthalingam T Mavros Y Wilson GC, et al. Effects of a modified German volume training program on muscular hypertrophy and strength. J Strength Cond Res. 2017;31:3109-19. [DOI] [PubMed] [Google Scholar]
- 40.Schoenfeld BJ. Science and development of muscle hypertrophy. Champaign, IL: Human Kinetics; 2016. pp. 51-56. [Google Scholar]
- 41.American College of Sports Medicine. Position stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. [DOI] [PubMed] [Google Scholar]
- 42.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126;1345-7. [DOI] [PubMed] [Google Scholar]
- 43.Burd NA West DW Staples AW, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than low volume resistance exercise in young men. PLoS One. 2010;5:e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tillin NA Pain MTG Folland JP. Short-term unilateral resistance training affects the agonist antagonist but not the force-agonist activation relationship. Muscle Nerve. 2011;43:375-84. [DOI] [PubMed] [Google Scholar]
- 45.Andersen LL Andersen JL Zebis MK, et al. Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports. 2010;20:e162-9. [DOI] [PubMed] [Google Scholar]
- 46.Mangine GT Hoffman JR Wang R, et al. Resistance training intensity and volume affect changes in rate of force development in resistance-trained men. Eur J Appl Physiol. 2016; 116:2367–2374. [DOI] [PubMed] [Google Scholar]
- 47.Angelozzi M Madama M Corsica C, et al. Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42:772-80. [DOI] [PubMed] [Google Scholar]
- 48.Houglum PA. Therapeutic exercise for musculoskeletal injuries. Human Kinetics, Champaign, IL, 2010. [Google Scholar]
- 49.Jewiss D Ostman C Smart N. Open versus closed kinetic chain exercises following an anterior cruciate ligament reconstruction: a systematic review and meta-analysis. J Sports Med (Hindawi Publ Corp). 2017;2017:4721548. 10.1155/2017/4721548. Epub 2017 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bynum EB Barrack RL Alexander AH. Open versus closed chain kinetic exercises after anterior cruciate ligament reconstruction. A prospective randomized study. Am J Sports Med. 1995;23:401–6. [DOI] [PubMed] [Google Scholar]
- 51.Mikkelsen C Werner S Eriksson E. Closed kinetic chain alone compared to combined open and closed kinetic chain exercises for quadriceps strengthening after anterior cruciate ligament reconstruction with respect to return to sports: a prospective matched follow-up study. Knee Surg Sports Traumatol Arthrosc. 2000;8:337–42. [DOI] [PubMed] [Google Scholar]
- 52.Tagesson S Oberg B Good L, et al. A comprehensive rehabilitation program with quadriceps strengthening in closed versus open kinetic chain exercise in patients with anterior cruciate ligament deficiency: a randomized clinical trial evaluating dynamic tibial translation and muscle function. Am J Sports Med. 2008;36:298–307. [DOI] [PubMed] [Google Scholar]
- 53.Heijne A Werner S. Early versus late start of open kinetic chain quadriceps exercises after ACL reconstruction with patellar tendon or hamstring grafts: a prospective randomized outcome study. Knee Surg Sports Traumatol Arthrosc. 2007;15:402–14. [DOI] [PubMed] [Google Scholar]
- 54.Zanobbi M Buckthorpe M. Open and closed kinetic chain in the knee rehabilitation. In Roi GS Della Villa S. Football medicine strategies for joint and ligament injuries. Calzetti Mariucci Editore, Torgiano, 2014, pp. 33. [Google Scholar]
- 55.Culvenor AG Collins NJ Vicenzino B, et al. Predictors and effects of patellofemoral pain following hamstring-tendon ACL reconstruction. J Sci Med Sport. 2016;19:518-23. [DOI] [PubMed] [Google Scholar]
- 56.Culvenor AG Øiestad BE Holm I, et al. Anterior knee pain following anterior cruciate ligament reconstruction does not increase the risk of patellofemoral osteoarthritis at 15- and 20-year follow-ups. Osteoarthritis Cartilage. 2017;25:30-3. [DOI] [PubMed] [Google Scholar]
- 57.Feller JA Webster KE. A randomized comparison of patellar tendon and hamstring 300 tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564-73. [DOI] [PubMed] [Google Scholar]
- 58.Escamilla RF Fleisig GS Zheng N, et al. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Med Sci Sports Exerc. 1998; 30:556-9. [DOI] [PubMed] [Google Scholar]
- 59.Steinkamp LA Dillingham MF Markel MD, et al. Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med. 1993; 21:438-44. [DOI] [PubMed] [Google Scholar]
- 60.Salem GJ Salinas R Harding FV. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84:1211–6. [DOI] [PubMed] [Google Scholar]
- 61.Snyder-Mackler L Delitto A Bailey SL, et al. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1995;77:1167–73. [DOI] [PubMed] [Google Scholar]
- 62.Buckthorpe M Gimpel M Wright S, et al. Hamstring muscle injuries in elite football: translating research into practice. Br J Sports Med. 2018;52:628-9. [DOI] [PubMed] [Google Scholar]
- 63.Tillin NA Pain MTG Folland JP. Explosive force production during isometric squats correlates with athletic performance in rugby union players. J Sports Sci. 2013;31:66-76. [DOI] [PubMed] [Google Scholar]
- 64.Wisløff U Castagna C Helgerud J, et al. Strong correlation of maximal squat strength with sprint performance and vertical jump height in elite soccer players. Br J Sports Med. 2004;38:285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bates NA Ford KR Myer GD, et al. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cleather D Goodwin J Bull A. Hip and knee joint loading during vertical jumping and push jerking. Clin Biomech. 2013; 28:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNair PJ Prapavessis H. Normative data of vertical ground reaction forces during landing from a jump. J Sci Med Sport. 1999;2:86–8. [DOI] [PubMed] [Google Scholar]
- 68.Hewett TE Ford KR Hoogenboom BJ, et al. Understanding and preventing ACL injuries: current biomechanical and epidemiologic considerations - update 2010. N Am J Sports Phys Ther. 2010;5:234-51. [PMC free article] [PubMed] [Google Scholar]
- 69.Lipps DB Wojtys Em Ashton-Miller JA. Anterior cruciate ligament fatigue failures in knees subjected to repeated simulated pivot landings. Am J Sports Med. 2013;41:1058-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams D Logerstedt DS Hunter-Giordano A, et al. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42:601-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powers CM Bolgla LA Callaghan MJ, et al. Patellofemoral pain: proximal, distal, and local factors, 2nd International Research Retreat. J Orthop Sports Phys Ther. 2012;42:A1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Draper CE Besier TF Santos JM, et al. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J Orthop Res. 2009;27:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Souza RB Draper CE Fredericson M, et al. Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther. 2010;40:277–85. [DOI] [PubMed] [Google Scholar]
- 74.Wilson NA Press JM Koh JL, et al. In vivo non invasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am. 2009;91:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heino Brechter J Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Exerc. 2002;34:1582–93. [DOI] [PubMed] [Google Scholar]
- 76.Farrokhi S Keyak JH Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage 2011;19:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waryasz GR McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med 2008;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dursun N Dursun E Kilic Z. Electromyographic biofeedback-controlled exercise versus conservative care for patellofemoral pain syndrome. Arch. Phys. Med. Rehabil. 2001; 82:1692-5. [DOI] [PubMed] [Google Scholar]
- 79.Mirzabeigi E Jordan C Gronley JK. Isolation of the vastus medialis oblique muscle during exercise. Am J Sports Med. 1999;27:50-3. [DOI] [PubMed] [Google Scholar]
- 80.Syme G Rowe P Martin D, et al. Disability in patients with chronic patellofemoral pain syndrome: a randomised controlled trial of VMO selective training versus general quadriceps strengthening. Man Ther. 2009;14:252-63. [DOI] [PubMed] [Google Scholar]
- 81.Yip SLM Ng GYF. Biofeedback supplementation to physiotherapy exercise programme for rehabilitation of patellofemoral pain syndrome: a randomized controlled study. Clin. Rehabil. 2006;20:1050-7. [DOI] [PubMed] [Google Scholar]
- 82.Ng GY Zhang AQ Li CK. Biofeedback exercise improved the EMG activity ratio of the medial and lateral vasti muscles in subjects with patellofemoral pain syndrome. J Electromyogr Kinesiol. 2008; 18:128-33. [DOI] [PubMed] [Google Scholar]
- 83.Christou EA. Patellar taping increases vastus medialis oblique activity in the presence of patellofemoral pain. J Electromyogr Kinesiol. 2004;14:495-504. [DOI] [PubMed] [Google Scholar]
- 84.Cowan SM Bennell KL Hodges PW. Therapeutic patellar taping changes the timing of vasti muscle activation in people with patellofemoral pain syndrome. Clin J Sport Med. 2002;12:339-47. [DOI] [PubMed] [Google Scholar]
- 85.Powers CM Landel R Sosnick T, et al. The effects of patellar taping on stride characteristics and joint motion in subjects with patellofemoral pain. J Orthop Sports Phys Ther. 1997;26:286-91. [DOI] [PubMed] [Google Scholar]
- 86.Salsich GB Brechter JH Farwell D, et al. The effects of patellar taping on knee kinetics, kinematics, and vastus lateralis muscle activity during stair ambulation in individuals with patellofemoral pain. J Orthop Sports Phys Ther. 2002;32:3-10. [DOI] [PubMed] [Google Scholar]
- 87.Davis IS Powers CM. Patellafemoral pain syndrome: proximal, distal and local factors, an international retreat. April 30-May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther, 2010; 40:A1-16. [DOI] [PubMed] [Google Scholar]
- 88.Ireland ML Willson JD Ballantyne BT, et al. Hip strength in females with and without patellafemoral pain. J Orthop Sports Phys Ther. 2003;33:671-6. [DOI] [PubMed] [Google Scholar]
- 89.Leetun DT Ireland ML Willson JD, et al. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc. 2004;36:926-34. [DOI] [PubMed] [Google Scholar]
- 90.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42-51. [DOI] [PubMed] [Google Scholar]
- 91.Khayambashi K Ghoddosi N Straub RK, et al. Hip muscle strength predicts non-contact anterior cruciate ligament injury in male and female athletes: a prospective study. Am J Sports Med. 2016;44:355-61. [DOI] [PubMed] [Google Scholar]
- 92.Zakulak BT Hewett TE Reeves NP, et al. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35:1123-30. [DOI] [PubMed] [Google Scholar]
- 93.Petersen W Taheri P Forkel P, et al. Return to play following ACL reconstruction: a systematic review about strength deficits. Arch Orthop Trauma Surg. 2014;134:1417–28. [DOI] [PubMed] [Google Scholar]
- 94.Prins MR van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55:9-15. [DOI] [PubMed] [Google Scholar]
- 95.Santos TRT Oliveira BA Ocarino JM, et al. Effectiveness of hip muscle strengthening in patellofemoral pain syndrome patients: a systematic review. Braz J Phys Ther. 2015; 19:167-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Binder-Macleod SA Halden EE Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc. 1995;27:556–65. [PubMed] [Google Scholar]
- 97.Cabric M Appel HJ Resic A. Fine structural changes in electrostimulated human skeletal muscle. Evidence for predominant effects on fast muscle fibres. Eur J Appl Physiol Occup Physiol. 1987;57:1–5. [DOI] [PubMed] [Google Scholar]
- 98.Trimble MH Enoka RM. Mechanism underlying the training effects associated with neuromuscular electrical stimulation. Phys Ther. 1991;71:273–80. [DOI] [PubMed] [Google Scholar]
- 99.Bickel CS Gregory CM Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol. 2011;111:2399-407. [DOI] [PubMed] [Google Scholar]
- 100.Moore DR Burgomaster KA Schofield LM Gibala MJ Sale DG Phillips SM. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol. 2004;92(4–5):399–406. [DOI] [PubMed] [Google Scholar]
- 101.Sumide T Sakuraba K Sawaki K Ohmura H Tamura Y. Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport. 2009;12:107–12. [DOI] [PubMed] [Google Scholar]
- 102.Takarada Y Nakamura Y Aruga S, et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000; 88:61–5. [DOI] [PubMed] [Google Scholar]
- 103.Takarada Y Sato Y Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308-14. [DOI] [PubMed] [Google Scholar]
- 104.Giles L Webster KE McClelland J, et al. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br J Sports Med. 2017;51:1688-94. [DOI] [PubMed] [Google Scholar]
- 105.Trulsson A. Additional perspectives on ‘ACL rupture is a single leg injury but a double leg problem…’ Br J Sports Med Published Online First 28 April 2018. 10.1136/bjsports-2017-098974. [DOI] [PubMed]
- 106.Chung KS Ha JK Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Two-year follow up after reconstruction. Am J Sports Med. 2015;43:3101-21. [DOI] [PubMed] [Google Scholar]
- 107.Lepley AS Gribble PA Thomas AC, et al. Quadriceps neural alterations in anterior cruciate ligament reconstruction patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25:828-39. [DOI] [PubMed] [Google Scholar]
- 108.Zult T Gokeler A van Raay JJ, et al. An anterior cruciate ligament injury does not affect the neuromuscular function of the non-injured leg except for dynamic balance and voluntary quadriceps activation. Knee Surg Sports Traumatol Arthrosc. 2017;25:172-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carrol TJ Herbert RD Munn J, et al. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101:1514-22. [DOI] [PubMed] [Google Scholar]
- 110.Harput G Ulusoy B Tildiz TI, et al. Cross-education improves quadriceps strength recovery after ACL reconstruction: a randomized controlled trial. Knee Surg Traumatol Arthrosc. 2018. Jun 29. 10.1007/s00167-018-5040-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Zult T Gokeler A van Raay JJ, et al. Cross-education does not accelerate the rehabilitation of neuromuscular functions after ACL reconstruction: a randomised controlled clinical trial. Eur J Appl Physiol. 2018;118:1609-23. [DOI] [PMC free article] [PubMed] [Google Scholar]