Abstract
Objective
The objective was to investigate the short-term risk of major adverse cardiovascular events (MACEs) or congestive heart failure (CHF) in patients with psoriatic arthritis (PsA) or psoriasis initiating a biological therapy.
Methods
Screening for the study was carried out using MEDLINE, Cochrane and Embase, from the inception of the database to December 2017. Randomised controlled trials (RCTs) of anti-tumour necrosis factor (TNF), anti-interleukin (IL)12/23, anti-IL23 and anti-IL17 agents for the treatment of PsA or psoriasis were included. Two investigators independently extracted MACEs or CHF data reported during the placebo-controlled phase. The primary outcome measures were the incidence of MACEs or CHF.
Results
Of 753 references screened, 62 articles were selected, and 12 articles were added by manual searches. Accordingly 77 RCTs were included in the meta-analysis (MA) (10 174 patient-years (P-Y)). No significant difference was observed in MACE incidences in patients receiving anti-TNF, anti-IL12/23, anti-IL23 or anti-IL17 agents in comparison to the placebo. However, 10 MACEs were observed in the anti-IL12/23 group (1150 P-Y) compared with 1 in the placebo group (652 P-Y), with 0.01 −0.00 to 0.02 event/P-Y risk difference, which is not statistically significant. This trend was not observed in the anti-IL23 group. No significant difference was observed in CHF incidence in patients receiving biological agents in comparison to placebo.
Conclusion
This MA of 77 RCTs did not reveal any significant change in the short-term risk of MACE or CHF in patients with PsA or psoriasis initiating a biological therapy.
Keywords: Psoriatic arthritis, psoriasis, biological agent, major cardiovascular event, congestive heart failure
Key messages.
What is already known about this subject?
Both psoriatic arthritis (PsA) and psoriasis have been associated with an increased prevalence of systemic and vascular inflammation and clinical atherosclerosis.
Biological therapies, approved for the treatment of PsA or psoriasis, have demonstrated anti-inflammatory effects. They could theoretically prevent atherosclerosis and therefore decrease the long-term risk of cardiovascular diseases.
What does this study add?
This meta-analysis did not reveal any significant change in the risk of major adverse cardiovascular events or congestive heart failure in patients with PsA or psoriasis initiating biological therapy.
How might this impact on clinical pratice?
In a short-term perspective, the results should reassure the physicians about the cardiovascular safety of biological therapies.
In a long-term perspective, studies involving a larger number of patients as well as a longer duration of treatment exposure are needed to evaluate the impact of biological therapies on the cardiovascular risk of patients with PsA or psoriasis.
Introduction
Both psoriatic arthritis (PsA) and psoriasis have been associated with an increased prevalence of systemic and vascular inflammation and clinical atherosclerosis.1
A recent meta-analysis (MA) of observational studies showed a 43% increased risk of cardiovascular (CV) diseases in patients with PsA, while morbidity risks for myocardial infarction, cerebrovascular diseases and heart failure were increased by 68%, 22% and 31%, respectively, compared with the general population.2
Notwithstanding, a MA of observational studies revealed that morbidity risks for stroke and myocardial infarction were increased by 10% and 20%, respectively in patients with mild psoriasis, while the risks of stroke, myocardial infarction and CV death were increased by 38%, 70% and 37%, respectively in patients with severe psoriasis, compared with the general population.3
It is a matter of debate whether the increased risk of CV morbidity and mortality observed in patients with PsA or psoriasis represents a causal association or a predisposition due to the underlying standard CV risk factors exhibited by these patients, but one hypothesis is that the inflammatory cascade activated in patients with severe PsA or psoriasis may contribute to the development of atherosclerosis.1 4 5
On the one hand, various experimental studies demonstrated that inhibition of proinflammatory cytokines, such as tumour necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, had beneficial effects on cardiac function and outcome.6 On the other hand, several experimental studies showed that IL-12 family cytokines, including IL-12, IL-23, IL-27 and IL-35, were involved in the crosstalk between major immune cell types that drive the proinflammatory and anti-inflammatory responses in atherosclerosis.7 Such pleiotropic role in atherosclerosis was also reported for IL-17, with some experimental studies suggesting a proatherogenic effect, while the others proposed an atheroprotective role.8
Several biological therapies are currently approved for moderate-to-severe PsA or psoriasis, when a conventional systemic therapy fails to achieve disease control or when a patient is unable to tolerate the conventional systemic therapy because of adverse effects.5 9 These biological therapies include anti-TNF agents, approved for PsA and psoriasis (adalimumab, etanercept and infliximab) or only for PsA (certolizumab and golimumab); anti-IL12/23 agents, approved for PsA and psoriasis (ustekinumab) or previously assessed in psoriasis (briakinumab); anti-IL23 agents approved for psoriasis (guselkumab) with promising results in PsA (guselkumab and risankizumab) and psoriasis (tildrakizumab) and anti-IL17A agents, approved for PsA and psoriasis (ixekizumab and secukinumab) or only for psoriasis, with promising results in PsA (brodalumab).5
These biological therapies, which have demonstrated anti-inflammatory effects in inflammatory rheumatic and skin diseases, could theoretically prevent atherosclerosis, and therefore decrease the long-term risk of CV diseases.10 However, the short-term CV safety profile of these medicines is still a matter of debate, both in PsA and psoriasis.4 11 12 This is especially the case since clinicians are cognizant of the withdrawal of the application for approval of briakinumab in 2011, because of major adverse cardiovascular events (MACEs) reported during phase II and phase III clinical trials in subjects with psoriasis.13
The objective of this systematic review and MA of randomised controlled trials (RCTs) was to investigate the short-term risk of MACEs or congestive heart failure (CHF) in patients with PsA or psoriasis initiating a biological therapy.
Materials and methods
This MA was performed on RCTs from MEDLINE (PubMed), Cochrane and EMBASE data bases. We restricted the searches to the English language. All RCTs on anti-tumor necrosis factor (anti-TNF), anti-interleukin IL12/23, anti-IL23 and anti-IL17 agents used in the treatment of PsA, chronic plaque and pustuloplantar psoriasis were reviewed. Manual searches in American college of Rheumatology (ACR), European League Against Rheumatism (EULAR), American Academy of Dermatology (AAD) and Psoriasis Gene to Clinic (PGC) abstract archives were included in the review.
The searches were conducted using the following key words: (“Psoriasis” OR “Arthritis, Psoriatic”) AND (“Adalimumab” OR “Certolizumab” OR “Etanercept” OR “Golimumab” OR “Infliximab” OR “Briakinumab” OR “Ustekinumab” OR “Guselkumab” OR “Risankizumab” OR “Tildrakizumab” OR “Brodalumab” OR “Ixekizumab” OR “Secukinumab”).
On the EMBASE and Cochrane data bases, the searches were restricted to “trials”. On PubMed, we restricted the research to “clinical trials”. On Cochrane data base, the selection of studies was restricted to the same key words, using (“Stroke” OR “Cardiovascular diseases”) in addition.
We included studies from the inception of the data base to December 2017. All the randomised, placebo-controlled, double-blind studies, that assess the efficacy and safety of anti-TNF, anti-IL12/23, anti-IL23 or anti-IL17 agents in adult patients with PsA and/or psoriasis were included if safety data concerning MACEs (defined as myocardial infarction, stroke or CV death) or CHF (defined as global cardiac failure with signs of right and left cardiac decompensation) were available.
Two authors (BC and ArC) separately screened titles and article abstracts and reviewed all the safety data on MACEs or CHF in selected articles.
For each study selected, the following data were extracted and listed:
Study design/characteristics: study design, trial duration.
Patient characteristics: number of patients in each arm, age, gender ratio, disease duration, proportion of patients with PsA, baseline Psoriasis Area and Severity Index score and baseline body surface area score.
Intervention: treatment arm (biological therapy or placebo).
Primary outcomes: number of MACEs or CHF in each arm during the placebo-controlled phase.
To take into consideration the duration of the placebo-controlled phase and the number of patients in each arm, the results were expressed in patient-year (P-Y) in the biological group and the control group to enable the comparison between RCTs.
The Jadad Scale was used for quality assessment for RCTs.
Primary outcome measures were the incidence of MACEs or CHF during the placebo-controlled phase of biological treatment. We included all the events that occurred in patients who received at least 1 dose of study agent or placebo. MACEs or CHF that occurred during the follow-up open-label period were not considered.
The results of each selected study were presented as risk differences with a 95% CI. This MA was carried out using the inverse variance method whereby estimates of each study were pooling using a fixed or random effects model according to the level and significance of heterogeneity. Heterogeneity was tested with Cochran’s Q-test and evaluated by I2 statistic with the following classification: 0% to 30% indicated negligible heterogeneity, 30% to 50% moderate heterogeneity, 50% to 75% substantial heterogeneity and high heterogeneity for values above 75%. For the Q-test, a p value less than 0.10 was considered as significant, and a random-effects model was used.
Analysis involved use of RevMan V.5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) for the MA calculations. P values less than 0.05 were considered statistically significant.
Results
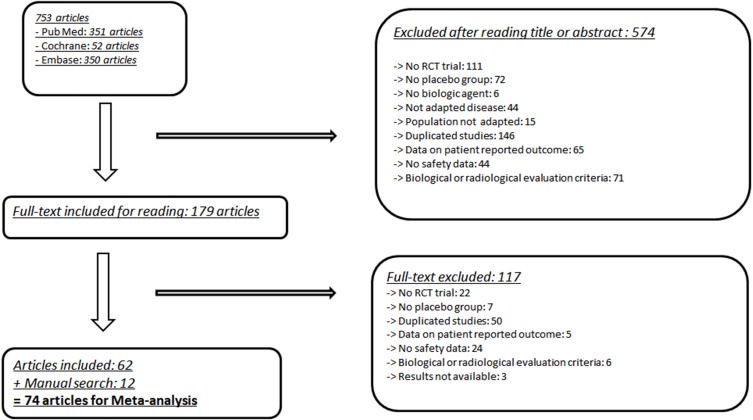
About 753 references met the selection criteria for this MA, which was carried out until December 2017.
Finally, 62 articles met the inclusion criteria, and 12 articles were added after manual searches. The details of the selection process are available in the flow chart (eg, figure 1).
Figure 1.
Flow chart of studies included in the MA. MA, meta-analysis; RCT, randomised controlled trial.
The CV safety data were extracted from 74 articles, corresponding to 77 RCTs14–86 (3 articles that investigated safety data in 2 RCTs). The summary of the RCTs included in the MA is presented in (eg, (online supplementary table 1)).
rmdopen-2018-000763supp001.doc (4.9MB, doc)
The main baseline characteristics of patients included in the RCTs selected for this MA are summarised in (eg, (online supplementary table 2)).
The JADAD Scale for each RCT is presented in (eg, (online supplementary table 3)).
Primary outcome measure: MACEs
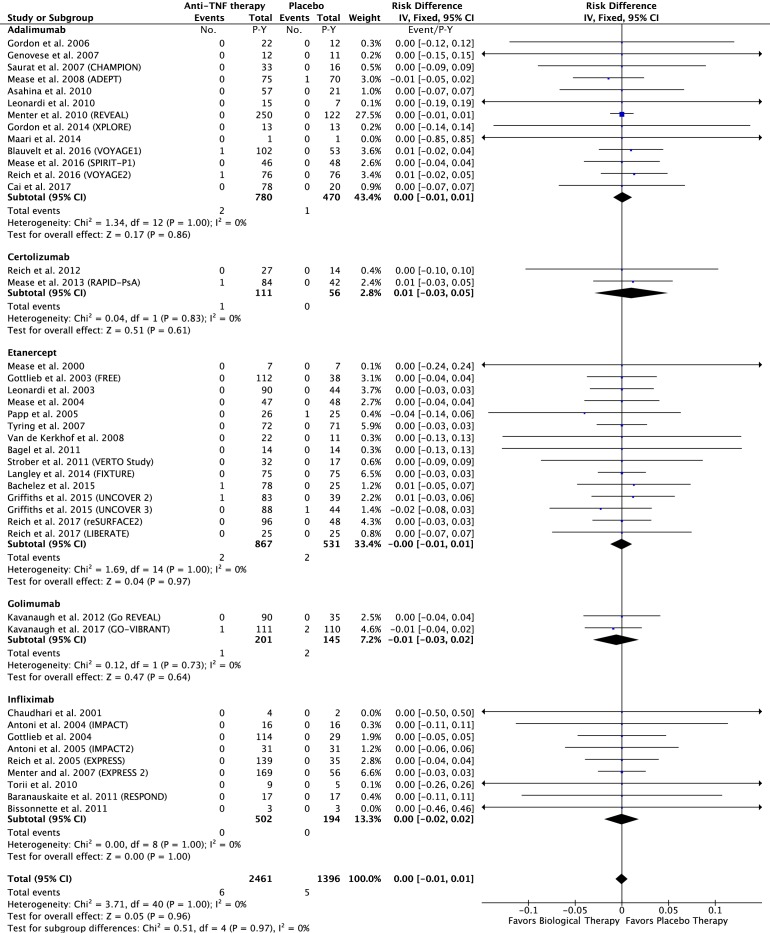
In the anti-TNF group: 6 of the 2461 P-Y in the anti-TNF group and 5 of the 1396 P-Y in the placebo group had a MACE. The risk difference was 0.00; 95% CI −0.01 to 0.01 event/P-Y; p=0.96 (figure 2).
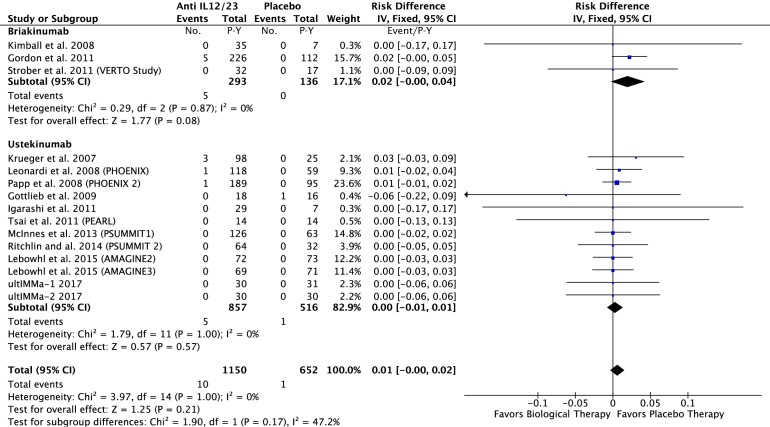
In the anti-IL12/23 group: 10 of the 1150 P-Y in the anti-IL12/23 and 1 of the 652 P-Y in the placebo group had a MACE. The risk difference was 0.01; 95% CI −0.00 to 0.02 event/P-Y; p=0.21 (figure 3).
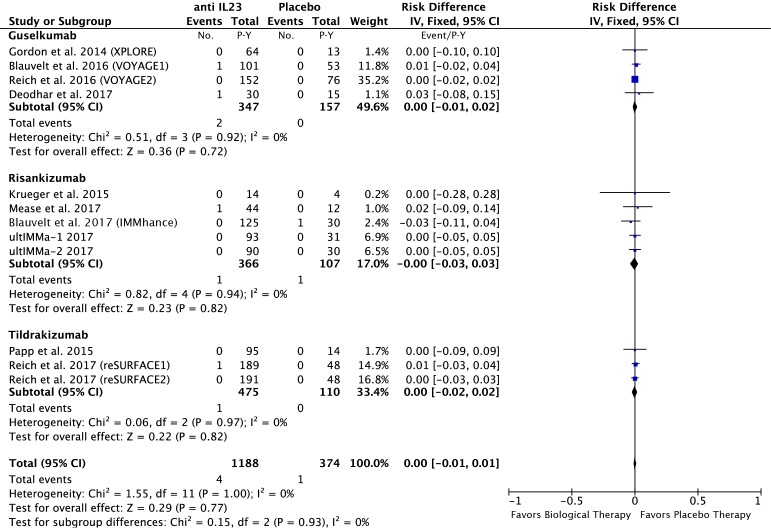
In the anti-IL23 group: 4 of the 1188 P-Y in the anti-IL23 and 1 of the 374 P-Y in the placebo group had a MACE. The risk difference was 0.00; 95% CI −0.01 to 0.01 event/P-Y; p=0.77 (figure 4).
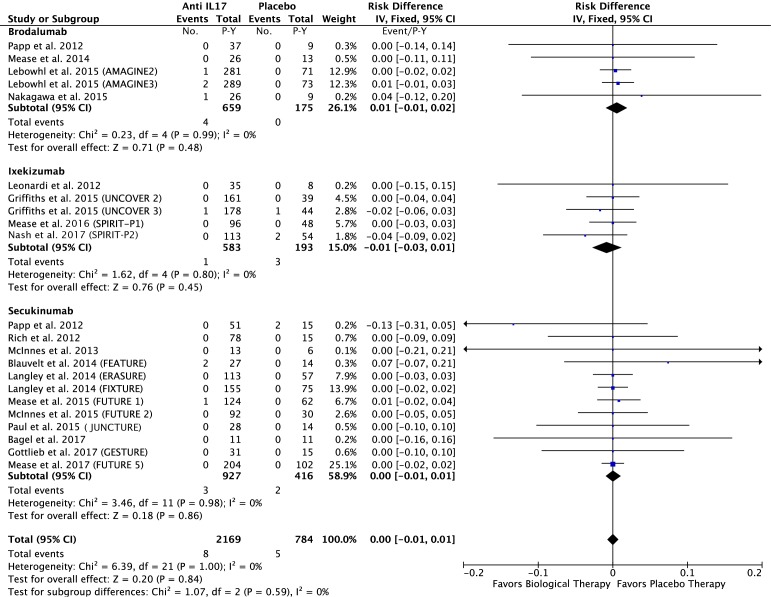
In the anti-IL17 group: 8 of the 2169 P-Y of the anti-TNF group and 5 of the 784 P-Y in the placebo group had a MACE. The risk difference was −0.00; 95% CI −0.01 to 0.01 event/P-Y; p=0.84 (eg, figure 5).
Figure 2.
Difference in the risk of MACEs in patients treated with anti-TNF agents compared with the placebo in RCTs. MACEs, major adverse cardiovascular events; RCTs, randomised controlled trials; TNF, tumour necrosis factor.
Figure 3.
Difference in the risk of MACEs in patients treated with anti-IL12/23 agents compared with the placebo in RCTs. IL, interleukin; MACEs, major adverse cardiovascular events; RCTs, randomised controlled trials.
Figure 4.
Difference in the risk of MACEs in patients treated with anti-IL23 agents compared with the placebo in RCTs. IL, interleukin; MACEs, major adverse cardiovascular events; RCTs, randomised controlled trials.
Figure 5.
Difference in the risk of MACEs in patients treated with anti–IL17 agents compared with the placebo in RCTs. IL, interleukin; MACEs, major adverse cardiovascular events; RCTs, randomised controlled trials.
Co-primary outcome measure: CHF
In the anti-TNF group: 0 of the 2461 P-Y in anti-TNF group and 0 of the 1396 P-Y in the placebo group had a CHF. The risk difference was 0.00; 95% CI −0.01 to 0.01 event/P-Y; p>0.99(eg, (online supplementary figure 6)).
In the anti-IL12/23 group: 0 of the 1150 P-Y in the anti-IL12/23 and 1 of 652 P-Y in the placebo group had a CHF. The risk difference was −0.00; 95% CI −0.01 to 0.01 event/P-Y; p=0.98 (eg, (online supplementary figure 7)).
In the anti-IL23 group: 0 of the 1188 P-Y in the anti-IL23 and 0 of 374 P-Y in the placebo group had a CHF. The risk difference was 0.00; 95% CI −0.01 to 0.01 event/P-Y; p>0.99 (eg, (online supplementary figure 8)).
In the anti-IL17 group: 1 of the 2169 P-Y of the anti-TNF group and 0 of the 784 P-Y in the placebo group had a CHF. The risk difference was 0.00; 95% CI −0.01 to 0.01 event/P-Y; p=0.99 (eg, (online supplementary figure 9)).
Discussion
In this MA of RCTs, there was no significant change in the short-term risk of MACEs or CHF in patients with PsA or psoriasis initiating a biological therapy such as anti-TNF, anti-IL12/23, anti-IL23 or anti-17 compared with the placebo. However, there was a trend for an increased risk of MACEs in patients exposed to anti-IL12/23.
Our MA showed no statistically significant difference in the short-term risk of MACEs in patients with PsA or psoriasis initiating a biological therapy.
The first MA published in 2011, which focused on psoriasis, included 15 RCTs on anti-TNF (adalimumab, etanercept and infliximab) and 9 RCTs on anti-IL12/23 (ustekinumab and briakinumab) agents and showed no significant difference in the rate of MACEs observed in patients receiving anti-TNF or anti-IL12/23 agents, in comparison to the placebo.12
A second MA published in 2012, which focused on psoriasis, included 9 RCTs on anti-IL12/23 (ustekinumab and briakinumab) agents and showed an increased rate of MACEs in patient who received anti-IL12/23 agents in comparison to the placebo.87
A third MA published in 2015, which focused on PsA and psoriasis, included six observational studies on systemic therapies (mixed biological therapies, non-biological therapies, corticosteroids and NSAIDs), and concluded that there was a decreased risk of CV events with systemic therapies in comparison to no systemic therapy or topical treatment.13
A fourth MA, published in 2016, which included 35 RCTs on anti-TNF (infliximab, etanercept and adalimumab), anti-IL12/23 (ustekinumab) and anti-IL17 (secukinumab and ixekizumab) agents, showed no statistically significant difference in the rate of MACEs observed in patients receiving biological therapies in comparison to the placebo.4
Our results are consistent with those of the first and fourth MA, but differ from those of the second MA,87 which showed a statistically significant increase in the rate of MACEs in patients who received anti-IL12/23 therapies. Differences in methods of statistical analysis used to calculate the risk of MACE could explain these discordant results. Our MA was carried out using the inverse variance method while the MA by Tzellos et al 87 used the Peto OR method which indicates that trials with no events in both arms were automatically given zero weight and excluded from the analysis.88 89 Several methodological issues explain the discordant results between our MA and the MA by Roubille et al 13 which included a few observational studies and both conventional synthetic and biological therapies.
However, even though our MA shows no statistically significant difference in terms of MACE incidence in patients receiving anti-IL12/23 biological Disease Modifying AntiRheumatic Drug (DMARDs) in comparison to the placebo, we observed a trend of increased risk of MACEs in patients initiating a treatment with briakinumab and to a lesser extent with ustekinumab in comparison to the placebo. It is interesting to note that briakinumab was withdrawn from further clinical development in 2011 because MACE were reported during phase II and phase III clinical trials in subjects with psoriasis.12 Further randomised trials involving bDMARDs targeting IL12/23 pathway could increase the number of events in treated patients compared with placebo and then improve the power of the statistical test. If this trend was confirmed in further studies, the observed difference might become statistically significant in future MA.
No increase in the risk of MACEs was observed in the anti-IL23 (guselkumab, risankizumab and tildrakizumab) group compared with the placebo group in our MA.
Our MA shows no statistically significant difference in the short-term risk of CHF in patients with PsA or psoriasis initiating a biological therapy. No previous MA has assessed the short-term risk of CHF at the initiation of biological therapies in patients with PsA or psoriasis.
Several limitations should be considered when interpreting the findings of our MA. First, MACEs were not clearly defined in all the RCTs. Second, the short duration of the placebo-controlled phase of the RCTs only enabled a short-term evaluation of the impact of biological therapies on the risk of MACEs or CHF. Third, the non-inclusion criteria of RCTs usually excluded patients at high risk of MACEs and CHF.
Our MA has certain strengths in comparison to previous MAs. First, it assessed the risk of MACEs in both PsA and psoriasis, based on the assumption that in both of these chronic inflammatory diseases, there is an increase in the risk of MACEs and they both require several biological therapies. This resulted in a higher number of patients being included in this MA in comparison to the previous MAs. Second, it investigated the risk of CHF, which represents another aspect of CV events that affects patients with chronic inflammatory diseases. Third, it assessed the risk of MACEs or CHF in patients exposed to a new class of biological therapy, selectively targeting anti-IL23 (guselkumab, risankizumab and tildrakizumab) in PsA or psoriasis.4 12 87
While both PsA and psoriasis have been associated with an increased risk of CV morbidity and mortality,2 3 the potential impact of DMARDs on the risk of CV events should be taken into consideration by rheumatologists and dermatologists in terms of treatment decisions in clinical practice. In a short-term perspective, the results of our MA, which did not reveal any significant change in the risk of MACE or CHF in patients with PsA or psoriasis initiating a bDMARD, should reassure the physicians about the CV safety of these therapeutic agents. In a long-term perspective, the use of anti-TNF agents was associated with a lower CV event risk in patients with psoriasis in comparison with methotrexate90 or phototherapy91 and with a lower risk of myocardial infarction in patients with rheumatoid arthritis in comparison with csDMARDs.92 93 Studies involving a larger number of patients as well as a longer duration of treatment exposure are needed to evaluate the impact of anti-IL12/23, anti-IL23 or anti-IL17 agents on the risk of MACEs in patients with PsA or psoriasis in clinical practice.94
Our MA, which is focused on the placebo-controlled phase of RCTs, did not reveal any significant change in the short-term risk of MACEs or CHF in patients with PsA or psoriasis initiating an anti-TNF, anti-IL12/23, anti-IL23 or anti-IL17 agent in comparison to the placebo. Data from the long-term extension phases of these RCTs and from the long-term follow-up of patients with PsA and psoriasis included in biological therapy registries are required to further characterise the long-term impact of biological therapies on the risk of MACEs or CHF.
Acknowledgments
We are grateful to the professors and organisers of ASLER seminary (for Systematic Analysis of the Litterature in Rheumatology) for their useful advice in the writing of this manuscript. We wish to thank AbbVie who provided logistic support in the organisation of sessions about the implementation of meta-analysis and remained independent of the collection, analysis and interpretation of data. We are grateful to Mrs Weill C. who helped us in the EMBASE bibliographic research.
Footnotes
Contributors: BC, ArC: contributed to the conception of the study, the article selection process, the data collection, the data analysis, the results interpretation and the manuscript writing and approval. AR-W: contributed to the conception of the study, results interpretation and manuscript writing and approval. YD, AC: contributed to the conception of the study, results interpretation and manuscript approval. TB: was in charge of the statistical analyses. All authors take responsibility for the integrity of the work as a whole, from inception to published article, and they should indicate that they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. They give permission to reproduce published material, report sensitive personal information, to use illustrations of identifiable persons or to name persons for their contributions.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The ethics committee approvals of each trial were obtained for all the studies selected in this meta-analysis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This study involved only published reports and none unpublished information. No database was used for this study.
References
- 1. Raychaudhuri SP, Wilken R, Sukhov AC, et al. . Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 2017;76:21–37. 10.1016/j.jaut.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 2. Polachek A, Touma Z, Anderson M, et al. . Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res 2017;69:67–74. 10.1002/acr.22926 [DOI] [PubMed] [Google Scholar]
- 3. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and cardiovascular events: updating the evidence. Arch Dermatol Res 2017;309:225–8. 10.1007/s00403-016-1712-1 [DOI] [PubMed] [Google Scholar]
- 4. Rungapiromnan W, Yiu ZZN, Warren RB, et al. . Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2017;176:890–901. 10.1111/bjd.14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greb JE, Goldminz AM, Elder JT, et al. . Psoriasis. Nat Rev Dis Primers 2016;2:16082 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- 6. Hartman MHT, Groot HE, Leach IM, et al. . Translational overview of cytokine inhibition in acute myocardial infarction and chronic heart failure. Trends Cardiovasc Med 2018;28:369–79. 10.1016/j.tcm.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 7. Chistiakov DA, Bobryshev YV, Orekhov AN. Heterogeneity of Tregs and the complexity in the IL-12 cytokine family signaling in driving T-cell immune responses in atherosclerotic vessels. Mol Immunol 2015;65:133–8. 10.1016/j.molimm.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 8. Allam G, Abdel-Moneim A, Gaber AM. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed Pharmacother 2018;106:1412–8. 10.1016/j.biopha.2018.07.110 [DOI] [PubMed] [Google Scholar]
- 9. Gossec L, Smolen JS, Ramiro S, et al. . European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 10. Langley RG, Papp K, Gottlieb AB, et al. . Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol 2013;27:1252–61. 10.1111/j.1468-3083.2012.04705.x [DOI] [PubMed] [Google Scholar]
- 11. Tousoulis D, Oikonomou E, Economou EK, et al. . Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J 2016;37:1723–32. 10.1093/eurheartj/ehv759 [DOI] [PubMed] [Google Scholar]
- 12. Ryan C, Leonardi CL, Krueger JG, et al. . Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 2011;306:864–71. 10.1001/jama.2011.1211 [DOI] [PubMed] [Google Scholar]
- 13. Roubille C, Richer V, Starnino T, et al. . The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. 10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon KB, Langley RG, Leonardi C, et al. . Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol 2006;55:598–606. 10.1016/j.jaad.2006.05.027 [DOI] [PubMed] [Google Scholar]
- 15. Genovese MC, Mease PJ, Thomson GT, et al. . Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040–50. [PubMed] [Google Scholar]
- 16. Saurat JH, Stingl G, Dubertret L, et al. . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558–66. 10.1111/j.1365-2133.2007.08315.x [DOI] [PubMed] [Google Scholar]
- 17. Mease PJ, Gladman DD, Ritchlin CT, et al. . Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. 10.1002/art.21306 [DOI] [PubMed] [Google Scholar]
- 18. Asahina A, Nakagawa H, Etoh T, et al. . Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010;37:299–310. 10.1111/j.1346-8138.2009.00748.x [DOI] [PubMed] [Google Scholar]
- 19. Leonardi C, Langley RG, Papp K, et al. . Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol 2011;147:429–36. 10.1001/archdermatol.2010.384 [DOI] [PubMed] [Google Scholar]
- 20. Menter A, Gordon KB, Leonardi CL, et al. . Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol 2010;63:448–56. 10.1016/j.jaad.2009.09.040 [DOI] [PubMed] [Google Scholar]
- 21. Gordon KB, Duffin KC, Bissonnette R, et al. . A phase 2 Trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015;373:136–44. 10.1056/NEJMoa1501646 [DOI] [PubMed] [Google Scholar]
- 22. Maari C, Bolduc C, Nigen S, et al. . Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatolog Treat 2014;25:57–60. 10.3109/09546634.2012.713458 [DOI] [PubMed] [Google Scholar]
- 23. Blauvelt A, Papp KA, Griffiths CE, et al. . Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- 24. Mease PJ, van der Heijde D, Ritchlin CT, et al. . Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reich K, Armstrong AW, Foley P, et al. . Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017;76:418–31. 10.1016/j.jaad.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 26. Cai L, Gu J, Zheng J, et al. . Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol 2017;31:89–95. 10.1111/jdv.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reich K, Ortonne JP, Gottlieb AB, et al. . Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol 2012;167:180–90. 10.1111/j.1365-2133.2012.10941.x [DOI] [PubMed] [Google Scholar]
- 28. Mease PJ, Fleischmann R, Deodhar AA, et al. . Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mease PJ, Goffe BS, Metz J, et al. . Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–90. 10.1016/S0140-6736(00)02530-7 [DOI] [PubMed] [Google Scholar]
- 30. Gottlieb AB, Matheson RT, Lowe N, et al. . A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003;139:1627–32. 10.1001/archderm.139.12.1627 [DOI] [PubMed] [Google Scholar]
- 31. Leonardi CL, Powers JL, Matheson RT, et al. . Etanercept as monotherapy in patients with psoriasis. N Engl J Med Overseas Ed 2003;349:2014–22. 10.1056/NEJMoa030409 [DOI] [PubMed] [Google Scholar]
- 32. Mease PJ, Kivitz AJ, Burch FX, et al. . Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. 10.1002/art.20335 [DOI] [PubMed] [Google Scholar]
- 33. Papp KA, Tyring S, Lahfa M, et al. . A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304–12. 10.1111/j.1365-2133.2005.06688.x [DOI] [PubMed] [Google Scholar]
- 34. Tyring S, Gottlieb A, Papp K, et al. . Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006;367:29–35. 10.1016/S0140-6736(05)67763-X [DOI] [PubMed] [Google Scholar]
- 35. van de Kerkhof PC, Segaert S, Lahfa M, et al. . Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol 2008;159:1177–85. 10.1111/j.1365-2133.2008.08771.x [DOI] [PubMed] [Google Scholar]
- 36. Bagel J, Lynde C, Tyring S, et al. . Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol 2012;67:86–92. 10.1016/j.jaad.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 37. Strober BE, Crowley JJ, Yamauchi PS, et al. . Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011;165:661–8. 10.1111/j.1365-2133.2011.10419.x [DOI] [PubMed] [Google Scholar]
- 38. Langley RG, Elewski BE, Lebwohl M, et al. . Secukinumab in plaque psoriasis — results of two phase 3 trials. N Engl J Med Overseas Ed 2014;371:326–38. 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 39. Bachelez H, van de Kerkhof PCM, Strohal R, et al. . Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. The Lancet 2015;386:552–61. 10.1016/S0140-6736(14)62113-9 [DOI] [PubMed] [Google Scholar]
- 40. Griffiths CE, Reich K, Lebwohl M, et al. . Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–51. 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 41. Reich K, Papp KA, Blauvelt A, et al. . Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390:276–88. 10.1016/S0140-6736(17)31279-5 [DOI] [PubMed] [Google Scholar]
- 42. Reich K, Goodfield M, Green L. Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment in the LIBERATE study. J Am Acad Dermatol 2017;76:AB224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kavanaugh A, McInnes IB, Mease P, et al. . Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014;73:1689–94. 10.1136/annrheumdis-2013-204902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kavanaugh A, Husni ME, Harrison DD, et al. . Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol 2017;69:2151–61. 10.1002/art.40226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaudhari U, Romano P, Mulcahy LD, et al. . Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357:1842–7. 10.1016/S0140-6736(00)04954-0 [DOI] [PubMed] [Google Scholar]
- 46. Antoni CE, Kavanaugh A, Kirkham B, et al. . Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. 10.1002/art.20967 [DOI] [PubMed] [Google Scholar]
- 47. Gottlieb AB, Evans R, Li S, et al. . Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004;51:534–42. 10.1016/j.jaad.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 48. Antoni C, Krueger GG, de Vlam K, et al. . Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. 10.1136/ard.2004.032268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reich K, Nestle FO, Papp K, et al. . Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005;366:1367–74. 10.1016/S0140-6736(05)67566-6 [DOI] [PubMed] [Google Scholar]
- 50. Menter A, Feldman SR, Weinstein GD, et al. . A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007;56:e1–15. 10.1016/j.jaad.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 51. Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010;59:40–9. 10.1016/j.jdermsci.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 52. Baranauskaite A, Raffayová H, Kungurov NV, et al. . Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 2012;71:541–8. 10.1136/ard.2011.152223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bissonnette R, Poulin Y, Guenther L, et al. . Treatment of palmoplantar psoriasis with infliximab: a randomized, double-blind placebo-controlled study. J Eur Acad Dermatol Venereol 2011;25:1402–8. 10.1111/j.1468-3083.2011.03984.x [DOI] [PubMed] [Google Scholar]
- 54. Kimball AB, Gordon KB, Langley RG, et al. . Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol 2008;144:200–7. 10.1001/archdermatol.2007.63 [DOI] [PubMed] [Google Scholar]
- 55. Gordon KB, Langley RG, Gottlieb AB, et al. . A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol 2012;132:304–14. 10.1038/jid.2011.304 [DOI] [PubMed] [Google Scholar]
- 56. Krueger GG, Langley RG, Leonardi C, et al. . A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007;356:580–92. 10.1056/NEJMoa062382 [DOI] [PubMed] [Google Scholar]
- 57. Leonardi CL, Kimball AB, Papp KA, et al. . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). The Lancet 2008;371:1665–74. 10.1016/S0140-6736(08)60725-4 [DOI] [PubMed] [Google Scholar]
- 58. Papp KA, Griffiths CE, Gordon K, et al. . Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol 2013;168:844–54. 10.1111/bjd.12214 [DOI] [PubMed] [Google Scholar]
- 59. Gottlieb A, Menter A, Mendelsohn A, et al. . Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. 10.1016/S0140-6736(09)60140-9 [DOI] [PubMed] [Google Scholar]
- 60. Igarashi A, Kato T, Kato M, et al. . Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol 2012;39:242–52. 10.1111/j.1346-8138.2011.01347.x [DOI] [PubMed] [Google Scholar]
- 61. Tsai TF, Ho JC, Song M, et al. . Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011;63:154–63. 10.1016/j.jdermsci.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 62. McInnes IB, Kavanaugh A, Gottlieb AB, et al. . Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- 63. Ritchlin C, Rahman P, Kavanaugh A, et al. . Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lebwohl M, Strober B, Menter A, et al. . Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015;373:1318–28. 10.1056/NEJMoa1503824 [DOI] [PubMed] [Google Scholar]
- 65.ultIMMa-1 AbbVie. Data on File, RRTI65191, NCT.
- 66.ultIMMa-2 AbbVie. Data on File, RRTI65192, NCT.
- 67. Deodhar AA, Gottlieb AB, Boehncke WH. Efficacy and safety results of guselkumab in patients with active psoriatic arthritis over 56 weeks from a phase 2a, randomized, double-blind, placebo-controlled study [abstract]. Arthritis Rheumatol 2017;69(suppl 10). [Google Scholar]
- 68. Krueger JG, Ferris LK, Menter A, et al. . Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2015;136:116–24. 10.1016/j.jaci.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 69. Mease PJ, Kellner H, Morita A. Efficacy and safety results from a phase 2 trial of risankizumab, a selective IL- 23p19 inhibitor, in patients with active psoriatic arthritis [abstract]. Arthritis Rheumatol 2017;69(suppl 10). [Google Scholar]
- 70. Blauvelt A, Papp KA, Gooderham M. Efficacy and safety of risankizumab, an interleukin-23 inhibitor, in patients with moderate-to-severe chronic plaque psoriasis: 16-week results from the phase III IMMhance trial. Br J Dermatol 2017;177:e248. [Google Scholar]
- 71. Papp K, Thaçi D, Reich K, et al. . Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol 2015;173:930–9. 10.1111/bjd.13932 [DOI] [PubMed] [Google Scholar]
- 72. Papp KA, Leonardi C, Menter A, et al. . Brodalumab, an anti–interleukin-17–receptor antibody for psoriasis. N Engl J Med Overseas Ed 2012;366:1181–9. 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
- 73. Mease PJ, Genovese MC, Greenwald MW, et al. . Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014;370:2295–306. 10.1056/NEJMoa1315231 [DOI] [PubMed] [Google Scholar]
- 74. Nakagawa H, Niiro H, Ootaki K, et al. . Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci 2016;81:44–52. 10.1016/j.jdermsci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 75. Leonardi C, Matheson R, Zachariae C, et al. . Anti–Interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med Overseas Ed 2012;366:1190–9. 10.1056/NEJMoa1109997 [DOI] [PubMed] [Google Scholar]
- 76. Nash P, Kirkham B, Okada M, et al. . Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017;389:2317–27. 10.1016/S0140-6736(17)31429-0 [DOI] [PubMed] [Google Scholar]
- 77. Papp KA, Langley RG, Sigurgeirsson B, et al. . Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol 2013;168:412–21. 10.1111/bjd.12110 [DOI] [PubMed] [Google Scholar]
- 78. Rich P, Sigurgeirsson B, Thaci D, et al. . Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol 2013;168:402–11. 10.1111/bjd.12112 [DOI] [PubMed] [Google Scholar]
- 79. McInnes IB, Sieper J, Braun J, et al. . Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014;73:349–56. 10.1136/annrheumdis-2012-202646 [DOI] [PubMed] [Google Scholar]
- 80. Blauvelt A, Prinz JC, Gottlieb AB, et al. . Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015;172:484–93. 10.1111/bjd.13348 [DOI] [PubMed] [Google Scholar]
- 81. Mease PJ, McInnes IB, Kirkham B, et al. . Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med Overseas Ed 2015;373:1329–39. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- 82. McInnes IB, Mease PJ, Kirkham B, et al. . Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 83. Paul C, Lacour JP, Tedremets L, et al. . Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015;29:1082–90. 10.1111/jdv.12751 [DOI] [PubMed] [Google Scholar]
- 84. Bagel J, Duffin KC, Moore A, et al. . The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol 2017;77:667–74. 10.1016/j.jaad.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 85. Gottlieb A, Sullivan J, van Doorn M, et al. . Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol 2017;76:70–80. 10.1016/j.jaad.2016.07.058 [DOI] [PubMed] [Google Scholar]
- 86. Mease PJ, van der Heijde D, Landewé RBM. Subcutaneous secukinumab inhibits radiographic progression in psoriatic arthritis: primary results from a large randomized, controlled, double-blind phase 3 study [abstract]. Arthritis Rheumatol 2017;69(suppl 10). [Google Scholar]
- 87. Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol 2013;27:622–7. 10.1111/j.1468-3083.2012.04500.x [DOI] [PubMed] [Google Scholar]
- 88. Dommasch ED, Troxel AB, Gelfand JM. Major cardiovascular events associated with anti-IL 12/23 agents: a tale of two meta-analyses. J Am Acad Dermatol 2013;68:863–5. 10.1016/j.jaad.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 89. Tzellos T, Kyrgidis A, Trigoni A, et al. . Association of anti-IL-12/23 biologic agents ustekinumab and briakinumab with major adverse cardiovascular events. J Eur Acad Dermatol Venereol 2013;27:1586–7. 10.1111/jdv.12126 [DOI] [PubMed] [Google Scholar]
- 90. JJ W, Guérin A, Sundaram M. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. janv 2017;76:81–90. [DOI] [PubMed] [Google Scholar]
- 91. Wu JJ, Sundaram M, Cloutier M, et al. . The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: An observational cohort study. J Am Acad Dermatol 2018;79:60–8. 10.1016/j.jaad.2018.02.050 [DOI] [PubMed] [Google Scholar]
- 92. Ljung L, Askling J, Rantapää-Dahlqvist S, et al. . The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther 2014;16:R127 10.1186/ar4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Low AS, Symmons DP, Lunt M, et al. . Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:654–60. 10.1136/annrheumdis-2016-209784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Caiazzo G, Fabbrocini G, Di Caprio R, et al. . Psoriasis, cardiovascular events, and biologics: lights and shadows. Front Immunol 2018;9:1668 10.3389/fimmu.2018.01668 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000763supp001.doc (4.9MB, doc)