Abstract
Background
Exercise-induced laryngeal obstruction (EILO) is common in athletes and presents with dyspnoea, chest tightness, inspiratory stridor and sometimes panic reactions. The evidence for conservative treatment is weak, but case reports suggest effects from inspiratory muscle training (IMT). We aimed to explore effects from IMT used in athletes with EILO.
Method
Twenty-eight athletes, mean age 16.4 years, diagnosed with EILO at our clinic, participated in a 6-week treatment programme, using a resistive flow-dependent IMT device (Respifit S). Four athletes competed at international level, 13 at national and 11 at regional levels. Video-recorded continuous transnasal flexible laryngoscopy was performed from rest to peak exercise (continuous laryngoscopy exercise (CLE) test) and scored before and 2–4 weeks after the training period. Ergospirometric variables were obtained from this CLE set-up. Lung function was measured according to guidelines. Symptom scores and demographic variables were obtained from a questionnaire.
Results
After the treatment period, symptoms had decreased in 22/28 (79%) participants. Mean overall CLE score had improved after treatment (p<0.001), with the scores becoming normal in five athletes but worse in two. Most of the improvement was explained by changes at the glottic laryngeal level (p=0.009). Ergospirometric variables revealed significantly higher peak minute ventilation explained by higher tidal volumes and were otherwise unchanged.
Conclusion
This explorative study underlines the heterogeneous treatment response of EILO and suggests that IMT may become an efficient conservative treatment tool in subgroups, possibly contributing to better control of the vocal folds. The signals from this study should be tested in future controlled interventional studies.
Keywords: vocal cord dysfunction, exercise, EILO, continuous laryngoscopy exercise test, CLE, larynx, supraglottic, glottic, inspiratory muscle training
What are the new findings?
This explorative study of athletes with exercise-induced laryngeal obstruction (EILO) suggests that respiratory symptoms and scores from continuous laryngoscopy exercise (CLE) tests can improve after a treatment period with inspiratory muscle training (IMT).
The IMT treatment period primarily seemed to improve the glottic CLE subscores.
IMT may be a tool to treat subgroups of athletes with EILO; however, controlled trials are needed to establish the scientific evidence.
How might it impact on clinical practice in the future?
The findings further substantiate that larynx should be visualised from rest to peak exertion in all athletes who suffer from symptoms suggesting EILO.
The findings highlight the heterogeneity of EILO, and that future treatment is likely to be individually tailored and based on a more comprehensive phenotyping than today.
The study provides data that can serve as basis for setting up controlled studies on IMT used to treat EILO in athletes.
Providing the findings are verified in future controlled studies, IMT may become a conservative treatment tool for subgroups of EILO, possibly contributing to better control of the vocal fold movements.
Introduction
Exercise-induced inspiratory symptoms (EIIS) are common in young athletes,1 and primarily characterised by prolonged and/or noisy inspiration and shortness of breath.2 EIIS usually occurs during vigorous exercise when the ventilation requirement is high and resolves spontaneously shortly after termination of the exertion. EIIS are most often related to obstruction of the larynx, involving either supraglottic laryngeal structures and/or the vocal folds, and should then be labelled exercise-induced laryngeal obstruction (EILO).3 4 The prevalence of EILO in unselected young Scandinavian populations has been reported to be 5.7% and 7.5%,5 6 and as high as 35.2% in athletes.1
Despite widely different pathophysiology, EILO is often confused with exercise-induced bronchoconstriction (EIB) which is an unfortunate situation that sometimes leads to prescription of high doses of asthma medication.1 2 6–9 The literature highlights that EILO should be diagnosed by means of objective methodology, visualising the laryngeal structures from rest to peak exertion.3 In most cases, the obstruction seems to originate in supraglottic structures (including the epiglottis) during the inspiratory phase of the breathing cycles, increasing as the ventilatory requirements increase with increasing exercise load,9 10 before glottic structures become involved, seemingly as a secondary phenomenon; however, this is not always so.2 11 12 This observed heterogeneity in the laryngeal findings suggests that one treatment modality is unlikely to work in all phenotypes of EILO.
So far, the evidence base for EILO treatment has been weak, generally consisting of small studies or single case reports conducted in poorly defined patient groups (EILO phenotypes), often with vaguely defined outcome measures.4 Historically, there are case reports suggesting effects from inspiratory muscle training (IMT).12–15 However, the mechanisms involved are unclear, for example, we do not know if IMT would influence glottic and supraglottic EILO in different ways, information that would aid the planning of further studies of this treatment. Thus, aiming to expand our knowledge on non-invasive treatment tools for EILO, we embarked on this explorative study, addressing effects from IMT offered to motivated athletes consecutively presenting at our EILO clinic, irrespective of phenotype.
Methods
Participants and study design
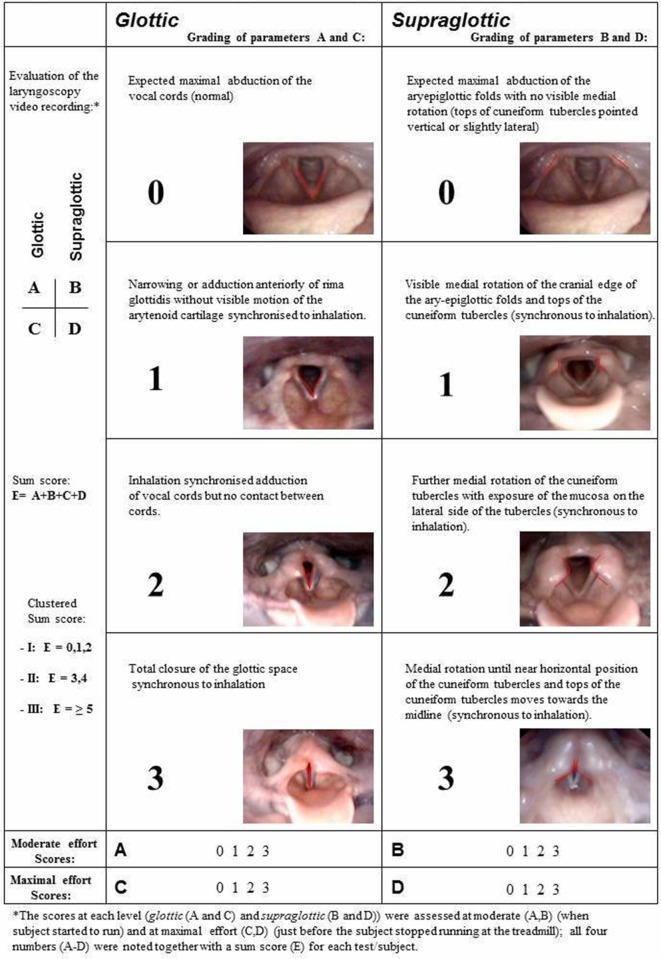
Within an explorative study design, all athletes consecutively referred for work-up of EIIS and diagnosed with EILO at the outpatient clinic at the Pediatric Department of Haukeland University Hospital in Western Norway between May 2012 and May 2014 were offered a structured treatment period with IMT, providing a device was available (ie, convenience-based inclusion within a given time frame). The inclusion criteria required that the severity scores of EILO obtained during a continuous laryngoscopy exercise (CLE) test16 were graded as two or more, either at the supraglottic level or at the vocal folds (ie, the glottic level)17 (regarding CLE scores, see figure 1). Moreover, there should be no evidence that EIB could explain the patient’s symptoms. Asthma per se was not an exclusion criterion, but in cases where asthma was present or suspected, EIB was excluded as cause of symptoms by performing a standardised test as described by Carlsen et al, either at our institution or by the referring institution.18 CLE scores, lung function and ergospirometry data were collected before and 2–4 weeks after the IMT treatment period, as were symptom scores obtained using a standardised questionnaire that also served to obtain demographic background variables. All athletes answered the question “When you are physically active, how much are you bothered by your breathing difficulties?”, using a numeric rating scale from 1 to 5 (1=nothing, 2=a bit, 3=pretty much, 4=a lot and 5=disabling). The study was approved by the Committee on Medical Research Ethics of Western Norway (REK number 2009/2111), and informed written consent was obtained from the participants.
Figure 1.
Grading system according to Maat et al 17 reproduced with permission.
CLE test
An integrated set-up for continuous video-recorded laryngoscopy throughout a maximal cardiopulmonary exercise test, coupled with video recordings of the upper part of the body and sound recordings, was applied as previously described.16
A transnasal flexible fiberoptic laryngoscope (Olympus ENF-P3, Tokyo, Japan), diameter 3.5 mm, was introduced after applying a decongestive nasal spray (Rhinox) and local anaesthesia (Xylocaine), and secured in a position allowing for a good view of the laryngeal entrance, including both supraglottic structures and the vocal folds. Continuous video recordings from the laryngoscope, a film of the upper part of the body and breath sounds were obtained simultaneously throughout the exercise test and stored in one single file for later evaluation. Laryngeal movements were scored as described previously17; at moderate (ie, when the test person changed from walking to running) and at maximal exercise intensity at glottic (labelled A and C, respectively) and supraglottic (labelled B and D, respectively) levels, and additionally a total sum score labelled E (figure 1). A CLE subscore of 1 (glottic or supraglottic) was regarded as normal.2 All assessments were done by two experienced raters (HHC and ODR) who were exposed to anonymised video recordings from all participants, blinded to the circumstances under which the video had been obtained, that is, if the video had been obtained before or after the treatment period. An uninvolved photographer presented the two films from each participant in random order. Disagreements were solved by consensus. Clinical symptoms during the CLE test were recorded in a questionnaire.
Pulmonary function and exercise test
Spirometry was performed with a Vmax 22 (SensorMedics, Yorba Linda, California, USA) according to guidelines,19 recording FVC, FEV1 and forced expiratory flow at 50% of FVC (FEF50%), and reported as percentages of predicted.20 Forced inspiratory volume in first second (FIV1) and forced inspiratory flow at 50% (FIF50%) of forced inspiratory volume capacity were recorded, and FEF50/FIF50 and FEV1/FIV1 were calculated and classified as abnormal if exceeding 1.5.8 The configuration of the flow-volume loops (FVL) was classified as normal or abnormal by an experienced respiratory physiologist (ODR), based on flattening or truncation of the inspiratory limbs.21 The patients ran on a treadmill (Woodway ELG 70, Weil am Rhein, Germany) using a computerised and modified Bruce protocol,22 increasing speed and/or elevation every minute, aiming to reach maximum exercise capacity after 6–12 min. Variables of gas exchange were measured breath by breath using a facemask (Hans Rudolph, Kansas City, Missouri, USA) connected to a Jaeger Oxycon Pro Cardiopulmonary Exercise testing system (Viasys Health Care, Yorba Linda, California, USA). The test was considered successful if the patient continued until exhaustion, preferably supported by a plateau in oxygen consumption and/or the heart rate response, or until stopped by respiratory distress. Duration and distance on treadmill running were recorded.
Inspiratory muscle training
Respifit S (Biegler GmbH, Mauerbach, Austria) was used to measure the maximal inspiratory mouth pressure (PImax), using the best value of 10 according to guidelines23 in order to provide exact settings of the resistance during the IMT sessions. The same device was used for the 6-week training sessions. To promote correct breathing technique, the participants were instructed to inhale using the diaphragm and to minimise movements of the shoulders in the cranial direction. Two modes of inspiratory muscle training were tested in accordance with the manual supplied by the manufacturer, that is, (1) inspiratory muscle strength training (IMST) with the resistance set to produce mouth pressures ≥80% of PImax and (2) moderate resistance or inspiratory muscle endurance training (IMET) with the resistance set at 60%–80% of PImax. In the IMST sessions, subjects performed five maximal inhalations repeated three times, separated by a 1 min break. In the IMET sessions, subjects were instructed to breathe in and out 12–16 times for 1 min. The frequency and power were guided by an animation program ensuring correct use of the device, and data from each training session were stored by a memory card measuring treatment compliance. The participants trained every day, in cycles of 2 days with IMET followed by 1 day of IMST, for a total of 6 weeks.
Statistical methods
This was a descriptive study, with main outcomes being the CLE scores and symptom scores obtained before versus after the IMT treatment period, compared using Gosset’s paired sample t-test.24 Means, SD, 95% CIs and ranges were calculated, as appropriate. The CLE scores are by nature ordinal and categorical, ranging from 0 to 3. Due to the few number of categories, the data were reported as mean values and mean differences with 95% CI, as this is considered to provide more information than medians and IQRs.25 Mixed linear model regression including interaction terms were applied to address if CLE-score changes (before vs after the treatment period) differed when obtained at moderate versus maximal exercise intensity and at the glottic versus supraglottic laryngeal level.26
All analyses were performed with SPSS V.24.
Results
Fifty-one eligible athletes presented to our clinic during the inclusion period, of whom 30 (59%) were included within the convenience-based sampling structure of the study. Two included athletes had to be excluded due to incomplete data sets. The age range of the remaining 28 was 12–25 years (mean 16.4), 4 males and 24 females. Four athletes were competing at international level, 13 at a national level and 11 at a regional level, and they were engaged in various endurance sports (table 1). Lung function was normal in all.
Table 1.
Characteristics of 28 included athletes consecutively referred for work-up of EIIS and diagnosed with EILO at the outpatient clinic at the Pediatric Department of Haukeland University Hospital in Bergen, Norway between May 2012 and May 2014 who were offered a structured treatment period with IMT
| Variable | N=28 |
| Category | Statistic |
| Female, n (%) | 24 (85.7) |
| Age in years, mean (SD) | 16.4 (2.8) |
| Height in cm, mean (SD) | 169.4 (8.4) |
| Weight in kg, mean (SD) | 61.6 (10.9) |
| Type of sports, n | |
| Soccer | 6 |
| Handball | 6 |
| Cross-country skiing | 4 |
| Biathlon | 4 |
| Athletics | 2 |
| Cycling | 2 |
| Swimming | 2 |
| Running | 1 |
| Kayaking | 1 |
| Competition level, n | |
| International | 4 |
| National | 13 |
| National regional | 11 |
| Level of laryngeal obstruction* | |
| Supraglottic>glottic | 7/28 |
| Glottic>supraglottic | 2/28 |
| Supraglottic=glottic | 19/28 |
| Solely glottic | 0/28 |
*At maximum exercise, before IMT.
EIIS, exercise-induced inspiratory symptom; EILO, exercise-induced laryngeal obstruction; IMT, inspiratory muscle training.
Subjective scores
After the IMT treatment period, 22/28 (79%) responded yes to a question if they felt their EILO problem had improved during the treatment period. Mean (SD) ratings obtained before versus after the IMT treatment period to the question “how much are you bothered by your breathing difficulties” were 3.63 (0.79) versus 2.93 (1.07), respectively (p<0.001). After the IMT treatment period, 12/28 (43%) athletes responded nothing or a bit to this question, whereas one reported that symptoms had increased.
Laryngeal findings during exercise (CLE scores)
At diagnosis, before the treatment period at maximum exercise, none had a solely glottic obstruction, 7/28 had supraglottic score exceeding the glottic score, 2/28 had glottic score exceeding the supraglottic score and 19/28 had similar supraglottic and glottic score (table 1). After the IMT treatment period, 5/28 (18%) athletes had no signs of laryngeal obstruction, presenting with CLE sum scores 0 or 1. In 23/28 (82%) athletes, the CLE sum scores had improved, eight of whom had a decrease in CLE sum score of two or more, whereas 3/28 had the same CLE sum scores before and after the treatment period. In two athletes, the CLE sum score had increased, of whom one was judged by both raters as a classical supraglottic phenotype of EILO and the other as a typical glottic EILO phenotype, characterised by anxiety and panic.
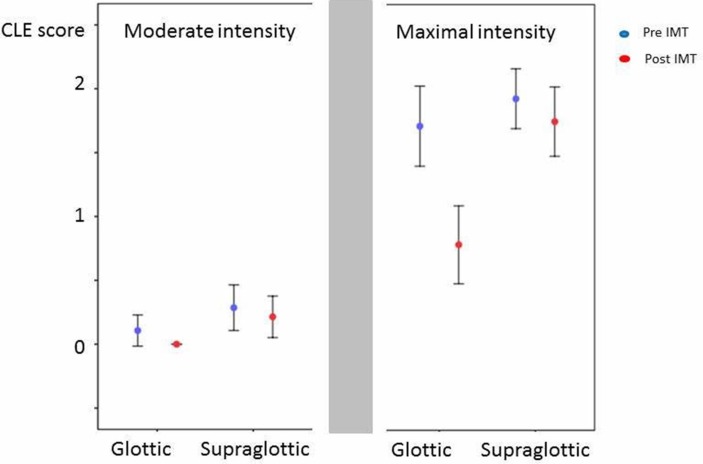
The pre-treatment versus the post-treatment differences in the CLE scores are given in table 2. A significant difference was observed only at maximal exercise intensity at the glottic laryngeal level. In the mixed model linear regression of CLE scores obtained pre-treatment versus post-treatment at moderate versus maximal exercise intensity at the glottic versus the supraglottic laryngeal level, the three-way interaction was found to be highly significant (F-test p=0.009) (table 2), confirming that CLE-score changes at maximal exercise intensity at the glottic laryngeal level explained most of the overall effect. The mean CLE scores with 95% CIs are presented in figure 2.
Table 2.
Mean CLE score differences as obtained at glottic and supraglottic laryngeal levels at moderate and maximal exercise intensity before versus after the IMT treatment period in 28 included athletes consecutively referred for work-up of EIIS and diagnosed with EILO at the outpatient clinic at the Pediatric Department of Haukeland University Hospital in Bergen, Norway between May 2012 and May 2014 as estimated from mixed model linear regression analysis
| Exercise intensity | Glottic/supraglottic | CLE score differences | SE | 95% CI | F-test p value* |
| Moderate | Glottic | 0.11 | 0.13 | −0.16 to 0.37 | 0.426 |
| Moderate | Supraglottic | 0.07 | 0.13 | −0.19 to 0.34 | 0.596 |
| Maximal | Glottic | 0.93 | 0.13 | 0.66 to 1.19 | <0.001 |
| Maximal | Supraglottic | 0.18 | 0.13 | −0.09 to 0.44 | 0.186 |
| Overall | 0.32 | 0.07 | 0.19 to 0.54 | <0.001 |
*P value for three-way interaction: 0.009.
CLE, continuous laryngoscopy exercise; EIIS, exercise-induced inspiratory symptom; EILO, exercise-induced laryngeal obstruction; IMT, inspiratory muscle training.
Figure 2.
CLE scores before and after treatment with IMT of 28 athletes consecutively referred for work-up of EIIS and diagnosed with EILO at the outpatient clinic at the Pediatric Department of Haukeland University Hospital in Bergen, Norway between May 2012 and May 2014. Dots represent mean values, and vertical lines represent 95% confidence intervals. CLE-scores, Continuous laryngoscopy exercise test (0-3); IMT, Inspiratory Muscle training; EIIS, Exercise induced inspiratory symptoms; EILO, Exercise induced laryngeal obstruction.
Spirometric and cardiopulmonary outcomes
The shape of the inspiratory limb of the flow-volume loop could be assessed in 25/28 athletes. The configuration was deemed abnormal in 5/28 (17.9%); however, FEF50/FIF50 and FEV1/FIV ratios exceeding 1.5 were not seen in any. Still, the FEF50/FIF50 ratio was significantly lower after versus before the IMT treatment period (table 3).
Table 3.
Physiological outcomes in 28 included athletes consecutively referred for work-up of EIIS and diagnosed with EILO at the outpatient clinic at the Pediatric Department of Haukeland University Hospital in Bergen, Norway between May 2012 and May 2014
| Pre-IMT n=28 | Post-IMT n=28 | P value | |
| PEF % predicted | 105.6 (13.6) | 108.0 (13.0) | 0.020 |
| FVC % predicted | 111.9 (11.4) | 112.2 (12.1) | 0.685 |
| FEV1 % predicted | 113.8 (11.0) | 113.3 (10.5) | 0.636 |
| FEV1/FIV1 | 0.98 (0.1) | 0.97 (0.1) | 0.432 |
| FEF50/FIF50 | 0.98 (0.2) | 0.90 (0.2) | 0.017 |
| Distance on treadmill (m) | 778.2 (159.8) | 800.5 (174.7) | 0.136 |
| Time on treadmill (min) | 10.9 (1.28) | 11.0 (1.36) | 0.248 |
| Heart rate max (bpm) | 187 (10) | 187 (8) | 0.853 |
| VO2 peak (mL/min) | 3143 (654) | 3135 (644) | 0.856 |
| VO2 peak (mL/min/kg) | 51.6 (9.2) | 51.1 (8.9) | 0.415 |
| VE (L/min) | 105.1 (26.0) | 109.6 (24.2) | 0.036 |
| Breathing reserve (%) | 17.5 (13.0) | 14.2 (11.6) | 0.057 |
| Breathing rate (breaths/min) | 50 (9) | 48 (7) | 0.224 |
| Vt (L/breath) | 2.14 (0.50) | 2.31 (0.51) | 0.003 |
EIIS, exercise-induced inspiratory symptom; EILO, exercise-induced laryngeal obstruction; FEF50, forced expiratory flow at 50%; FEV1, forced expiratory volume in first second; FIF50, forced inspiratory flow at 50%; FIV1, forced inspiratory volume in first second; IMT, inspiratory muscle training; PEF, peak expiratory flow; VE, Minute ventilation; VO2 peak, peak oxygen uptake; Vt, Tidal volume.
The distance completed, the time spent running on the treadmill, the obtained peak VO2 and the maximal heart rate did not differ before versus after the IMT treatment period. The minute ventilation (VE) and the tidal volume (Vt) increased significantly during the IMT training period, and the breathing rate was numerically (although not significantly) slightly reduced (table 3).
Discussion
After a 6-week training period with IMT, most participating athletes (22/28 or 79%) reported subjective improvement. The CLE scores improved in 23 participants (82%), had become normal in five (18%) but worse in two (7%). Improvements were linked to a change at the glottic laryngeal level at peak exercise. Ergospirometric variables revealed significantly higher peak VE explained by higher Vt but were otherwise unchanged (eg, no changes in peak VO2). The findings suggest that controlled studies should be incited, hypothesising that IMT can become an efficient treatment modality in subgroups of EILO, with particular focus on studying patients with glottic EILO.
Strengths and weaknesses of the study
The major strength of this study was the application of the best available and also a verifiable outcome measure, that is, CLE scores obtained before and after the IMT treatment period. CLE scores are based on endoscopic visual evaluation of laryngeal structures from rest to peak exercise, which is the gold standard for diagnosing EILO.3 CLE testing has been successfully applied in numerous recent studies in this field of respiratory medicine.1 2 4–6 11 17 27–35 Studies addressing the validity and reliability of the CLE scoring system have reached somewhat variable conclusions.17 36 37 It nevertheless seems reasonable to conclude that experience is a factor that influences these issues. In this study, two highly experienced raters, both having assessed CLE recordings form approximately 2000 patients, performed all scoring procedures according to a strict system designed to handle the expressed concerns to the extent possible. Previous studies addressing effects from treatments of EILO have mostly relied on symptom reports. As symptoms reported by patients with exercise-related breathing problems have been found inadequate as a diagnostic tool in several studies,1 2 5 6 11 38 39 a similar inadequacy seems likely to expect also in a context addressing effects from treatments. Thus, in this respect, this study represents an improvement. The convenience-based sampling process with relatively few participants with a strong preponderance of young female participants are obvious weaknesses. There are no published well-designed epidemiological studies of EILO in athletes; however, most, but not all,6 clinical studies report age and sex distributions similar to this present study.2 5 40 Finally, the lack of a control group or some sort of ‘sham treatment’ clearly weakens the conclusions, as this leaves the study open for unknown effects from participants becoming familiarised with the CLE procedure, and thus might have become more relaxed at the second test performed after the treatment period. Having in mind the explorative study design, these weaknesses should incite designing properly controlled studies of IMT in EILO, focusing particularly on the glottic components.
Inspiratory muscle training
The theoretical basis for testing effects from IMT in patients suffering from EILO is based on the phasic relationship that exists between the diaphragm and the posterior cricoarytenoid (PCA) muscle, which is the main abductor of the vocal folds.41 42 Signalled via the vagal nerve, the PCA muscle contracts slightly before the diaphragm, abducting the larynx before air starts entering the airways.43 By using MRI, How et al demonstrated that also upper airway dilator muscles, external muscles as genioglossus and geniohyoid, are activated in response to IMT, providing evidence for IMT being able to condition upper airway muscles.44 We have previously reported how various IMT modes influenced the larynx in healthy subjects, with lower resistance loads seemingly targeting abduction better than higher loads30 suggesting that IMT applied as low-intensity endurance training (IMET) would be better suited as treatment for EILO than IMST. Since this present study was the first to apply IMT in a systematic way in patients with EILO, we nonetheless chose the training program prescribed by the manual provided by the manufacturer. Previous studies on other groups of patients have tended to use resistance load set from 40% to 90% of PImax.13 14 45 46 We need a randomised control study to solve this issue with IMST versus IMET.
The goal of any treatment for EILO is to achieve a more effective and better controlled laryngeal abduction during exercise, and thereby facilitate air flowing through the laryngeal inlet at an increasing speed without causing an undue increase of resistance. Our study showed a significant improvement in minute ventilation, necessarily reflecting also increased airflow through the larynx. This outcome was related to a significant increase in Vt and a tendency for lower breath rates (not significant); however, it was somewhat inconsistent from test to test and therefore difficult to interpret physiologically. Peak VO2 did not differ, suggesting a relieved (improved) breathing pattern at peak exercise. Regrettably, we do not have access to blood gases to properly substantiate such potential ventilatory effects. Resting spirometry obtained before and after the treatment period confirmed low sensitivity in relation to diagnosing EILO,47 but a significant decreased FEF50/FIF50 ratio after treatment suggests that inspiratory resistance was reduced, also at rest.
CLE scores as outcome measure in EILO treatment studies
The evidence base for effects of different treatment options for EILO is limited, and a general lack of verifiable outcome measures complicates interpretations of previous studies. PImax is the most commonly used IMT outcome measure, but in patients with EILO, PImax seems irrelevant since the treatment targets coordination of inspiratory laryngeal movements more than the strength of the diaphragm.30 Reports of subjective improvements certainly provide information that is important, but has serious sources of error,48 and do not enhance our causal understanding. The use of verifiable visual laryngeal outcome measures such as CLE scores16 17 can provide information that serve to understand this prevalent condition better, for example, contribute to distinguishing psychological from organic breathing problems, and to understand what structures incite and perpetuate the pathology of a malfunctioning larynx in a patient with EILO. Studies have questioned inter-rater reliability of CLE scores,37 and measures should be taken to ensure a high level of experience among raters, with disagreements solved by consensus, as in this study. We have reported highly divergent responses to IMT in a previous case report,12 further substantiated in this study with two participants deteriorating after the treatment period. Effects seemed linked to changes at the glottic level during maximum intensity suggesting that IMT may be most likely to improve EILO where glottic structures are most heavily involved. Moreover, one should probably be careful applying IMT in patients with a predominant supraglottic EILO. However, these are data revealed in an uncontrolled and explorative study, and we need well-designed in-depth controlled studies to investigate these signals further.
Conclusion
This study underlines the heterogeneity of EILO and suggests that future treatment is likely to be individually tailored and based on a more comprehensive phenotyping than today. The study suggests that IMT may become an efficient conservative treatment tool in subgroups of EILO, and that IMT might contribute to the patient achieving a better control of particularly the vocal fold movements. The signals from this explorative study should be tested in future controlled interventional studies. The cliche ‘more studies are needed’ certainly applies to this large and under-studied group of patients.
Acknowledgments
Previously presented abstract and poster at the American Thoracic Society meeting in San Francisco, 2012.
Footnotes
Contributors: AS: main author, substantial contributions to the conception of the work, data collection and drafting the manuscript. TA: substantial contributions to the conception of the work, critically revising the work and final approval of the version to be published. HHC: substantial contributions to the conception of the work, data collection, critically revising the work and final approval of the version to be published. MH: substantial contributions to critically revising the work and final approval of the version to be published. MV: substantial contributions to the conception of the work, data collection, critically revising the work and final approval of the version to be published. J-HH: substantial contributions to critically revising the work and interpretation of data, final approval of the version to be published. GEE: substantial contributions to statistical analysis and interpretation of the work, final approval of the version to be published. TH: substantial contributions to the conception or design of the work, interpretation of data and revising the manuscript, final approval of the version to be published. ODR: substantial contributions to the conception or design of the work, data collection, interpretation of data and revising the manuscript, final approval of the version to be published.
Funding: Major funding institutions: Haukeland University Hospital and University of Bergen.
Competing interests: Haukeland University Hospital owns parts of US patent No. 11/134551, protecting the commercial rights of the CLE test.
Patient consent for publication: Obtained.
Ethics approval: The study was approved by the Committee on Medical Research Ethics of Western Norway (REK no. 2009/2111).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Deidentified participant data such as ergospirometry data, questionnaire and images/film of the laryngeal obstruction are available by reasonable request to the main author.
References
- 1. Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013;45:2030–5. 10.1249/MSS.0b013e318298b19a [DOI] [PubMed] [Google Scholar]
- 2. Røksund OD, Maat RC, Heimdal JH, et al. Exercise induced dyspnea in the young. Larynx as the bottleneck of the airways. Respir Med 2009;103:1911–8. 10.1016/j.rmed.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 3. Christensen PM, Heimdal J-H, Christopher KL, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions: TABLE 1. Eur Respir Rev 2015;24:445–50. 10.1183/16000617.00006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50:1602221 10.1183/13993003.02221-2016 [DOI] [PubMed] [Google Scholar]
- 5. Christensen PM, Thomsen SF, Rasmussen N, et al. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 2011;268:1313–9. 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 6. Johansson H, Norlander K, Berglund L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2015;70:57–63. 10.1136/thoraxjnl-2014-205738 [DOI] [PubMed] [Google Scholar]
- 7. Rice SG, Bierman CW, Shapiro GG, et al. Identification of exercise-induced asthma among intercollegiate athletes. Ann Allergy 1985;55:790–3. [PubMed] [Google Scholar]
- 8. Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest 2003;123:468–74. 10.1378/chest.123.2.468 [DOI] [PubMed] [Google Scholar]
- 9. Hull JH. Not all wheeze is asthma: time for patients to exercise their rights. Thorax 2015;70:7–8. 10.1136/thoraxjnl-2014-206096 [DOI] [PubMed] [Google Scholar]
- 10. Christopher KL, Wood RP, Eckert RC, et al. Vocal-cord dysfunction presenting as asthma. N Engl J Med 1983;308:1566–70. 10.1056/NEJM198306303082605 [DOI] [PubMed] [Google Scholar]
- 11. Buchvald F, Phillipsen LD, Hjuler T, et al. Exercise-induced inspiratory symptoms in school children. Pediatr Pulmonol 2016;51:1200–5. 10.1002/ppul.23530 [DOI] [PubMed] [Google Scholar]
- 12. Clemm HSH, Sandnes A, Vollsæter M. The heterogeneity of exercise-induced laryngeal obstruction. Am J Respir Crit Care Med 2018;197:1068–9. 10.1164/rccm.201708-1646IM [DOI] [PubMed] [Google Scholar]
- 13. Ruddy BH, Davenport P, Baylor J, et al. Inspiratory muscle strength training with behavioral therapy in a case of a rower with presumed exercise-induced paradoxical vocal-fold dysfunction. Int J Pediatr Otorhinolaryngol 2004;68:1327–32. 10.1016/j.ijporl.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 14. Mathers-Schmidt BA, Brilla LR. Inspiratory muscle training in exercise-induced paradoxical vocal fold motion. J Voice 2005;19:635–44. 10.1016/j.jvoice.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 15. Dickinson J, Whyte G, McConnell A. Inspiratory muscle training: a simple cost-effective treatment for inspiratory stridor. Br J Sports Med 2007;41:694–5. 10.1136/bjsm.2006.033654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heimdal JH, Roksund OD, Halvorsen T, et al. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope 2006;116:52–7. 10.1097/01.mlg.0000184528.16229.ba [DOI] [PubMed] [Google Scholar]
- 17. Maat RC, Røksund OD, Halvorsen T, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol 2009;266:1929–36. 10.1007/s00405-009-1030-8 [DOI] [PubMed] [Google Scholar]
- 18. Carlsen KH, Engh G, Mørk M. Exercise-induced bronchoconstriction depends on exercise load. Respir Med 2000;94:750–5. 10.1053/rmed.2000.0809 [DOI] [PubMed] [Google Scholar]
- 19. Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- 20. Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow–volume curve with growth and aging. Am Rev Respir Dis 1983;127:725–34. 10.1164/arrd.1983.127.6.725 [DOI] [PubMed] [Google Scholar]
- 21. Boris M, Goldblatt A, Krigsman A. Laryngeal dysfunction: a common cause of respiratory distress, often misdiagnosed as asthma and responsive to antireflux therapy. Allergy Asthma Proc 2002;23:133–9. [PubMed] [Google Scholar]
- 22. Cumming GR, Everatt D, Hastman L. Bruce treadmill test in children: normal values in a clinic population. Am J Cardiol 1978;41:69–75. 10.1016/0002-9149(78)90134-0 [DOI] [PubMed] [Google Scholar]
- 23. American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 24. Student The probable error of a mean. Biometrika 1908;6:1–25. 10.1093/biomet/6.1.1 [DOI] [Google Scholar]
- 25. Lydersen S. Statistical review: frequently given comments. Ann Rheum Dis 2015;74:323–5. 10.1136/annrheumdis-2014-206186 [DOI] [PubMed] [Google Scholar]
- 26. West BT WK, Galecki AT, Model LM. A practical guide using statistical software. London: Taylor & Francis Group, 2007. [Google Scholar]
- 27. Hilland M, Røksund OD, Sandvik L, et al. Congenital laryngomalacia is related to exercise-induced laryngeal obstruction in adolescence. Arch Dis Child 2016;101:443–8. 10.1136/archdischild-2015-308450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maat RC, Hilland M, Røksund OD, et al. Exercise-induced laryngeal obstruction: natural history and effect of surgical treatment. Eur Arch Otorhinolaryngol 2011;268:1485–92. 10.1007/s00405-011-1656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maat RC, Roksund OD, Olofsson J, et al. Surgical treatment of exercise-induced laryngeal dysfunction. Eur Arch Otorhinolaryngol 2007;264:401–7. 10.1007/s00405-006-0216-6 [DOI] [PubMed] [Google Scholar]
- 30. Sandnes A, Andersen T, Hilland M, et al. Laryngeal movements during inspiratory muscle training in healthy subjects. J Voice 2013;27:448–53. 10.1016/j.jvoice.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 31. Norlander K, Johansson H, Jansson C, et al. Surgical treatment is effective in severe cases of exercise-induced laryngeal obstruction: a follow-up study. Acta Otolaryngol 2015;135:1152–9. 10.3109/00016489.2015.1062548 [DOI] [PubMed] [Google Scholar]
- 32. Christensen P, Thomsen SF, Rasmussen N, et al. [Exercise-induced inspiratory stridor. An important differential diagnosis of exercise-induced asthma]. Ugeskr Laeger 2007;169:4047–50. [PubMed] [Google Scholar]
- 33. Walsted ES, Swanton LL, van van Someren K, et al. Laryngoscopy during swimming: a novel diagnostic technique to characterize swimming-induced laryngeal obstruction. Laryngoscope 2017;127:2298–301. 10.1002/lary.26532 [DOI] [PubMed] [Google Scholar]
- 34. Olin JT, Clary MS, Fan EM, et al. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J 2016;48:1192–200. 10.1183/13993003.00160-2016 [DOI] [PubMed] [Google Scholar]
- 35. Hargrove AE, Hargrove MB, Olin JT. Therapeutic laryngoscopy during exercise for treatment of refractory exercise-induced laryngeal obstruction. A personal experience. Ann Am Thorac Soc 2017;14:444–7. 10.1513/AnnalsATS.201612-948OR [DOI] [PubMed] [Google Scholar]
- 36. Norlander K, Christensen PM, Maat RC, et al. Comparison between two assessment methods for exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2016;273:425–30. 10.1007/s00405-015-3758-7 [DOI] [PubMed] [Google Scholar]
- 37. Walsted ES, Hull JH, Hvedstrup J, et al. Validity and reliability of grade scoring in the diagnosis of exercise-induced laryngeal obstruction. ERJ Open Res 2017;3:00070-2017 10.1183/23120541.00070-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu-Hasan M, Tannous B, Weinberger M. Exercise-induced dyspnea in children and adolescents: if not asthma then what? Ann Allergy Asthma Immunol 2005;94:366–71. 10.1016/S1081-1206(10)60989-1 [DOI] [PubMed] [Google Scholar]
- 39. Weinberger MM. Etiology of exercise-induced dyspnea: not just exercise-induced asthma or vocal cord dysfunction. J Allergy Clin Immunol 2008;121:269 10.1016/j.jaci.2007.08.062 [DOI] [PubMed] [Google Scholar]
- 40. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin North Am 2010;43:43–66. 10.1016/j.otc.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 41. Brancatisano T, Collett PW, Engel LA. Respiratory movements of the vocal cords. J Appl Physiol Respir Environ Exerc Physiol 1983;54:1269–76. 10.1152/jappl.1983.54.5.1269 [DOI] [PubMed] [Google Scholar]
- 42. Bartlett D. Respiratory functions of the larynx. Physiol Rev 1989;69:33–57. 10.1152/physrev.1989.69.1.33 [DOI] [PubMed] [Google Scholar]
- 43. Petcu LG, Sasaki CT. Laryngeal anatomy and physiology. Clin Chest Med 1991;12:415–23. [PubMed] [Google Scholar]
- 44. How SC, McConnell AK, Taylor BJ, et al. Acute and chronic responses of the upper airway to inspiratory loading in healthy awake humans: an MRI study. Respir Physiol Neurobiol 2007;157(2-3):270–80. 10.1016/j.resp.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 45. Baker SE, Sapienza CM, Martin D, et al. Inspiratory pressure threshold training for upper airway limitation: a case of bilateral abductor vocal fold paralysis. J Voice 2003;17:384–94. 10.1067/S0892-1997(03)00066-3 [DOI] [PubMed] [Google Scholar]
- 46. Illi SK, Held U, Frank I, et al. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med 2012;42:707-24 10.2165/11631670-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 47. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest 1999;116:1676–82. 10.1378/chest.116.6.1676 [DOI] [PubMed] [Google Scholar]
- 48. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41:1073–93. 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]