Summary
Background
Non-fatal health outcomes from diseases and injuries are a crucial consideration in the promotion and monitoring of individual and population health. The Global Burden of Disease (GBD) studies done in 1990 and 2000 have been the only studies to quantify non-fatal health outcomes across an exhaustive set of disorders at the global and regional level. Neither effort quantified uncertainty in prevalence or years lived with disability (YLDs).
Methods
Of the 291 diseases and injuries in the GBD cause list, 289 cause disability. For 1160 sequelae of the 289 diseases and injuries, we undertook a systematic analysis of prevalence, incidence, remission, duration, and excess mortality. Sources included published studies, case notification, population-based cancer registries, other disease registries, antenatal clinic serosurveillance, hospital discharge data, ambulatory care data, household surveys, other surveys, and cohort studies. For most sequelae, we used a Bayesian meta-regression method, DisMod-MR, designed to address key limitations in descriptive epidemiological data, including missing data, inconsistency, and large methodological variation between data sources. For some disorders, we used natural history models, geospatial models, back-calculation models (models calculating incidence from population mortality rates and case fatality), or registration completeness models (models adjusting for incomplete registration with health-system access and other covariates). Disability weights for 220 unique health states were used to capture the severity of health loss. YLDs by cause at age, sex, country, and year levels were adjusted for comorbidity with simulation methods. We included uncertainty estimates at all stages of the analysis.
Findings
Global prevalence for all ages combined in 2010 across the 1160 sequelae ranged from fewer than one case per 1 million people to 350 000 cases per 1 million people. Prevalence and severity of health loss were weakly correlated (correlation coeffi cient –0·37). In 2010, there were 777 million YLDs from all causes, up from 583 million in 1990. The main contributors to global YLDs were mental and behavioural disorders, musculoskeletal disorders, and diabetes or endocrine diseases. The leading specific causes of YLDs were much the same in 2010 as they were in 1990: low back pain, major depressive disorder, iron-deficiency anaemia, neck pain, chronic obstructive pulmonary disease, anxiety disorders, migraine, diabetes, and falls. Age-specific prevalence of YLDs increased with age in all regions and has decreased slightly from 1990 to 2010. Regional patterns of the leading causes of YLDs were more similar compared with years of life lost due to premature mortality. Neglected tropical diseases, HIV/AIDS, tuberculosis, malaria, and anaemia were important causes of YLDs in sub-Saharan Africa.
Interpretation
Rates of YLDs per 100 000 people have remained largely constant over time but rise steadily with age. Population growth and ageing have increased YLD numbers and crude rates over the past two decades. Prevalences of the most common causes of YLDs, such as mental and behavioural disorders and musculoskeletal disorders, have not decreased. Health systems will need to address the needs of the rising numbers of individuals with a range of disorders that largely cause disability but not mortality. Quantifi cation of the burden of non-fatal health outcomes will be crucial to understand how well health systems are responding to these challenges. Effective and affordable strategies to deal with this rising burden are an urgent priority for health systems in most parts of the world.
Funding
Bill & Melinda Gates Foundation.
Introduction
Non-fatal health outcomes from diseases and injuries are a crucial consideration in the promotion and monitoring of individual and population health. In an era in which the Millennium Development Goals (MDGs) have refocused global health attention on prevention of mortality from selected disorders, it is important to emphasise that health is about more than avoiding death. Individuals, households, and health systems devote enormous resources to the cure, prevention, and amelioration of non-fatal sequelae of diseases and injuries. Some form of periodic accounting about the burden of non-fatal illness in populations, and how it is changing, should therefore be available for policy making and planning. Quantification of the burden of non-fatal health outcomes was one of the main goals in launching the Global Burden of Disease study (GBD) in the 1990s.1 The study introduced the disability-adjusted life-year (DALY) as a time-based measure of health that enables commensurable measurement of years of life lost due to premature mortality (YLLs) with years of life lived in less than ideal health (years lived with disability [YLDs]). The amalgamation of both components of individual and population health under a comprehensive framework for measuring population health can provide important insights into a broader set of causes of disease burden than can consideration of mortality alone.
To our knowledge, the various revisions of the GBD are the only effort to quantify non-fatal health outcomes across an exhaustive set of disorders at the global and regional level.2‒8 Many national burden of disease studies and subnational studies have analysed local patterns of YLDs as well.9‒16 Publication of the GBD 1990 results raised awareness about a range of disorders that primarily cause ill health and not death, such as unipolar major depression, bipolar disorder, asthma, and osteoarthritis.17‒19 This attention has led to greater policy debate and action on mental health and other non-communicable diseases at WHO,4,20,21 in non–governmental organisations, and in many countries.22 The burden of non-fatal illness attributed to some parasitic diseases has also been an important issue highlighted by the GBD findings.23‒26
Despite the unique role of the GBD in provision of comparative quantification of the burden of non-fatal health outcomes, there have been important limitations. The evidence on MDG-related diseases has been regularly revised and incorporated into updates of the GBD, but many disorders have not been systematically analysed since 1990. Global Health Statistics, a companion volume to the original Global Burden of Disease and Injuries book, provided estimates of incidence, prevalence, remission, and case fatality for 483 sequelae, by age and sex, for eight regions of the world.27 The GBD 2000 revisions included 474 sequelae. A substantial number, but not all, of these sequelae were revised since GBD 1990. Those that were not revised were approximated with constant relations between YLLs and YLDs or YLD rates estimated from the GBD 1990. Even when revisions were undertaken, however, many were not based on systematic analyses of published studies and unpublished sources. The epidemiological inputs to YLD estimates such as prevalence have been released for only 40 sequelae. The most important limitation of both the GBD 1990 and 2000 efforts is that YLDs have not been estimated with uncertainty. Uncertainty can come from many sources, including heterogeneity in the empirical data that are available and uncertainty in the indirect estimation models used to make predictions for populations with little or no data. Because the empirical basis for estimating prevalence or incidence is much weaker for some sequelae than it is for others, uncertainty is likely to vary substantially across sequelae and across countries and regions for the same sequelae.8,28
The Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) provided an important opportunity to address the key limitations of past burden of disease assessments, including a more standardised approach to evidence synthesis, epidemiological estimation with uncertainty, and assessment of comorbidity. In this Article, we describe the approach to undertaking these analyses with the available evidence, and discuss key comparative results. Subsequent disease-specific and injury-specific papers are planned that will provide much more detail on data, methods, and results for various disorders of interest.
Methods
Overview
Details of the GBD 2010 hierarchical cause list, the 21 epidemiological regions (and combinations of these into seven super-regions), the 20 age groups, and the relation between different components of GBD 2010 are published elsewhere.29 For the GBD 2010, YLDs are computed as the prevalence of a sequela multiplied by the disability weight for that sequela without age weighting or discounting. The YLDs arising from a disease or injury are the sum of the YLDs for each of the sequelae associated with that disease. Across the 291 diseases and injury causes in the study, 289 cause disability—for these causes there were 1160 sequelae that captured the major outcomes of these diseases and injuries.29,30 The key analytical task for the study was to estimate the prevalence with uncertainty of each of the 1160 sequelae for 20 age groups, both sexes, and 21 regions for 1990, 2005, and 2010. See panel for terminology used in GBD 2010.
For each disease or injury, we identified the key sequelae from that cause. Sequelae could include the disease itself, such as diabetes, or the outcomes associated with that disease such as diabetic foot, neuropathy, or retinopathy. Some clinical disorders were classified as a disease but also can be a consequence of another disease—eg, chronic kidney disease secondary to diabetes is a consequence of diabetes but was classified as a disease. Any given outcome appears in the GBD cause and sequela list only once to avoid double counting of the associated burden. Across the 1160 sequelae, we identified 220 unique health states, representing a parsimonious list providing enough detail to describe the large variations between health states while still a manageable number for which we were able to derive disability weights by survey. In principle, we estimated YLDs at the level of an individual and then assigned individual health loss to all the contributing sequelae present in an individual. The analysis can be divided into seven specific steps (figure 1) which are briefly described below.
Figure 1:
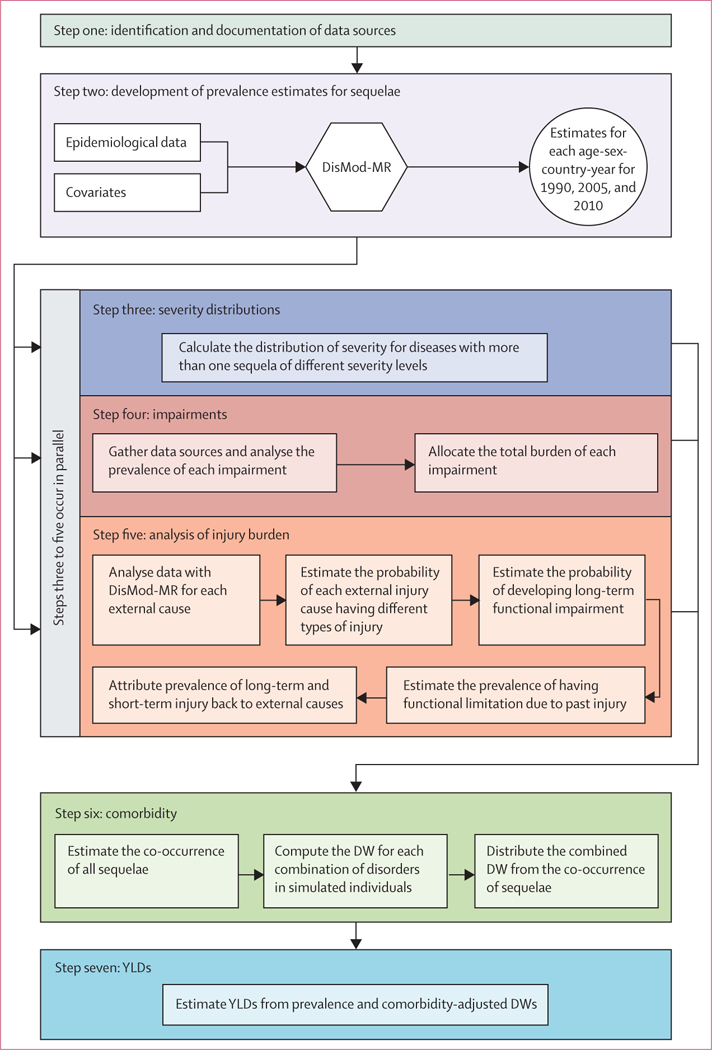
Overview of the seven steps in the estimation of prevalence and years lived with disability (YLDs) DW=disability weight.
Identification and documentation of data sources
The analysis for each sequela began with the identification and documentation of sources of data for incidence, prevalence, remission, duration, and excess mortality. We used nine types of data sources. First, contributors to the GBD have undertaken systematic reviews for disease sequelae. For example, for epilepsy we retrieved: 230 prevalence studies from 83 countries in all 21 world regions, a further 97 studies of incidence, 25 studies of the mortality risk in people with epilepsy, and only one study on remission meeting inclusion criteria. For other disease sequelae, only a small fraction of the existing data appear in the published literature, and other sources predominate such as local surveys of schistosomiasis prevalence or antenatal clinic serosurveillance for HIV/AIDS. Second, reports to governments of cases have been used for African trypanosomiasis, measles, pertussis, tuberculosis, leprosy, dengue, cholera, and yellow fever. Use of these data for burden of disease assessment required explicit modelling of the case detection rate for every disease. Third, we used population-based disease registry data for cancers,32‒40 chronic kidney diseases, multiple sclerosis,41 Parkinson’s disease,42,43 and congenital anomalies.44 Cancer registries have been established in many developed countries and are being rapidly established in developing countries. For example, by the end of 2010, cancer registries had expanded in China to 149 registries covering 31 provinces;45 India now has 23 registries.46 Fourth, many countries, in collaboration with UNAIDS and WHO, have established networks of antenatal clinics that test women presenting for antenatal care for HIV, syphilis, and other disorders. Fifth, for 43 countries, we obtained hospital discharge data coded to ICD9 or ICD10. Use of these data required an explicit model of selection bias to take into account variations in access to care. Additionally, for chronic diseases, we had to estimate the average number of admissions to hospital per person per year with a disease to interpret the results. We analysed datasets with unique identifiers for every patient for seven US states from 2003 to 2007 for cirrhosis and pneumoconiosis. Hospital discharge data were an important source for acute disorders such as stroke, myocardial infarction, appendicitis, or pancreatitis, and for injuries. Sixth, for skin diseases and other mental and behavioural disorders, outpatient data collected in health systems with nearly complete or at least representative samples of ambulatory data47‒55 have also been used after taking into account selection bias. Seventh, we used interview questions, direct measurements (eg, hearing, vision, and lung function testing), serological measurements, and anthropometry from the re-analysis of multiple household surveys. Surveys of selected populations such as school children for intellectual disability,56 nursing home residents for dementia,57 or mental health clinic attendees for schizo-phrenia58 have also been used after taking into consideration selection bias. Eighth, re-analysis of cohort or follow-up studies has been used for some causes such as impairment due to injury. We also used cohort studies to provide information about remission rates, duration, and mortality risks for many chronic disorders. Finally, we used indirect prevalence studies as an input to estimate the total number of drug users.59 These estimates were produced from a combination of multiplier, capture-recapture, and back- projection methods combining data from treatment centres, police records, court records, and survey data.
Developing prevalence estimates for sequelae
Meta-analysis or meta-regression of descriptive epidemiological studies60‒63 poses many challenges. First, for many regions and for many sequelae data are scarce. Predictions of prevalence need to take advantage of relations with covariates in a meta-regression or default to the average of a region, super-region, or the world. Second, in settings with multiple measurements, study results can be highly heterogeneous because of much non-sampling error. Sources of non-sampling error include selection bias in the population studied, study design, implementation issues in data collection, widely varying case definitions across studies, and the use of different diagnostic technologies or laboratory techniques. Third, available studies have often used diverse age groups like 17–38 years or 15 years and above. Fourth, data for various disorders were collected for many different outcomes such as incidence, prevalence, remission, excess mortality, or cause-specific mortality. The mix of data varies across diseases and across regions for a disease. All of these sources provide some relevant information for the estimation of prevalence. Fifth, within regions or countries, the true prevalence of a sequela can vary enormously. Sixth, on the basis of biology or clinical series, there might be strong prior views on the age pattern of incidence or prevalence for a disorder that should be reflected in the results. For instance, we would not expect prevalence of Alzheimer’s disease before age 40 years and diagnostic rules stipulate that the onset of attention deficit and hyperactivity disorder cannot occur before age 4 years or after age 8 years.64
To address these challenges, we have developed a Bayesian meta-regression method, DisMod-MR, which estimates a generalised negative binomial model for all epidemioiogical data. The model includes the following: covariates that predict variation in true rates; covariates that predict variation across studies because of measurement bias; super-region, region, and country random intercepts; and age-specific fixed effects. When appropriate, the rates were assumed to have been constant over time, which allowed data for incidence, prevalence, excess mortality, and cause-specific mortality to inform prevalence estimates. The differential equations governing the relation between the parameters of incidence, remission, mortality, prevalence, and duration are well charac-terised.65,66 DisMod-MR can use data reported for any age group to inform the maximum likelihood estimate. We used a large set of 179 covariates that have been appropriately imputed so that the data provide a complete time series for all 187 countries in the analysis (see the appendix for details of the estimation equations used for DisMod-MR and the approach to numerical solution, as well as an example of its application).29
For cancer incidence and prevalence, we used the approach applied by Forouzanfar and colleagues67 to breast and cervical cancers. We estimated the mortality- to-incidence ratio for each cancer for all country, age, and sex groups using data from all high-quality registries that reported on both incidence and mortality. We developed separate models for both sexes. Cause of death estimates for each cancer by country, year, age, and sex68 were divided by the predicted mortality-to-incidence ratio to generate incidence estimates. To estimate the prevalence of each of four sequelae of cancer including: diagnosis or treatment phase, remission, recurrence, and terminal phase, we estimated the natural history of incident cases using a calculated 5 year survival and relative duration of each cancer phase. We also used a variant of this approach to estimate incidence and prevalence for visceral leishmaniasis.
We used four sets of alternative methods for some disorders because of variation in the types of data available and the complexity of their spatial and temporal distributions (see appendix for further details). For HIV/ AIDS, we used the UNAIDS natural history model developed with the Spectrum platform.69,70 Detailed estimates of prevalence and mortality with uncertainty by age and sex have been provided based on the 2012 revision of HIV/AIDS epidemiology. We developed natural history models for measles and pertussis. For ascariasis, trichuriasis, hookworm, and schistosomiasis, prevalence of the disease has been estimated with geospatial estimation methods.71‒73 For diphtheria, tetanus, and rabies, we have used systematic reviews of data for case-fatality rates with estimates of mortality to estimate incidence—the mortality estimates for these diseases are described elsewhere.68 For these disorders, DisMod-MR was used as a meta-regression method to estimate the case-fatality rate by age, sex, and region. For tuberculosis and dengue, the key source of information was registered cases. We developed statistical models that simultaneously model the expected rates as a function of covariates and the undercount of cases as a function of health system access.
Severity distributions
For 41 diseases, the sequelae of the disease have been linked to more than one health state including stroke, anxiety, major depressive disorder, symptomatic heart failure, and chronic obstructive pulmonary disease (COPD). After analysing the prevalence of the overall disorder, we estimated the distribution of these prevalent cases across severity levels. Disability weights were measured in population surveys30 for individuals without comorbidity. Two estimates are needed to calculate YLDs: the disability weight for individuals with a single sequela and the disability weight for individuals with multiple sequelae, which is a common occurrence. The prevalence of comorbid disorders can be estimated with micro-simulation. However, we needed to estimate the distribution of severity controlling for comorbidity, otherwise the severity distribution would be systematically biased towards more severe symptoms caused by comorbidity. For example, if individuals with depression are also likely to have anxiety and substance-use disorders, the reported distribution of functional health status would be shifted towards the more severe end.
Data for severity distributions are often scarcer and of poorer quality than are data for prevalence of disorders, with some exceptions.74,75 Approaches to severity classification are inconsistent across disorders.76 Because of the heterogeneity of the available evidence for disease severity, we supplemented disease specific reviews with an analysis of three data sources: the US Medical Expenditure Panel Survey (MEPS) 2000–09,77 the US National Epidemiological Survey on Alcohol and Related Conditions (NESARC) 2000–01 and 2004–05,78 and the Australian National Survey of Mental Health and Wellbeing of Adults (AHS) 1997.79 These sources allow the assessment of the severity distributions taking into account co morbidity (see appendix for more details of this analysis). For some diseases for which data are available for the distribution of severity by age, sex, and region, we pooled proportions in each health state using DisMod-MR or simple meta-analysis methods.
Impairments
For selected impairments, we have constrained the estimates for sequelae related to that impairment to sum to estimates of the impairment prevalence from independent sources of data. For example, nine dis orders have blindness as a sequela. We have analysed all available blindness survey data and we constrain the prevalence of all blindness sequelae to sum to blindness prevalence. We did this impairment prevalence analysis for anaemia, blindness, low vision, hearing impairment, infertility, heart failure, epilepsy, and intellectual disability (appendix).
Analysis of injury burden
The analysis of YLDs from injuries needed careful consideration because injuries are classified in the cause list according to the external cause such as a road injury, animal bite, or drowning, whereas the functional limitations after injury are determined by the nature of injury such as brain trauma, femur fracture, or spinal cord transection. We did the injuries analysis in five steps, which are briefly outlined here with further details in the appendix. First, we analysed household survey and hospital discharge data using DisMod-MR for each external cause to generate estimates of incidence by age, sex, country, and year. Survey data included recall of injuries warranting admission to hospital as well as injuries that warranted medical attention but not admission to hospital. The metaregression included a covariate for whether an individual was admitted to hospital or not, which we used to generate predictions both for injury warranting hospital admission and injury warranting outpatient care. Second, we analysed hospital data from 28 countries that had dual coding of discharges by external cause and nature of injury after ICD9 and ICD10, using negative binomial models to estimate the probability of different groups of nature of injury as a function of age, sex, and an indicator variable for developed versus developing countries. Separate models were created for injury warranting hospital admission and injury warranting other health care. Third, for each nature of injury we estimated the probability of individuals developing long-term functional impairment. We re-analysed follow-up data from four studies using data from the Dutch Injury Surveillance system (LIS),80 the South Carolina Traumatic Brain Injury Follow-up Registry (SCTBIFR),81 the National Study on Costs and Outcomes of Trauma (NSCOT),82 and MEPS.77 Fourth, we used DisMod-MR to estimate the prevalence of individuals in the population who are likely to have functional limitation because of a previous injury. Prevalence was estimated from incidence assuming zero remission and a relative risk of death compared with the general population based on available studies. In the fifth step, the YLDs due to prevalent cases of long-term injury were attributed back to external causes in proportion with the contributions of these causes to every type of injury.
Comorbidity
Comorbidity was taken into account in the calculation of YLDs, which needed three analytical steps (appendix). First, we estimated the co-occurrence of all the sequelae for each age, sex, country, and year. Co-occurrence is a function of the prevalence of each sequela and whether the probabilities of co-occurrence are independent of, or dependent on, each other.83 We could not identify sufficiently large datasets to estimate these dependent probabilities reliably within age groups. We therefore adopted the simplifying assumption of independence. For each age-sex-country-year, we used a Monte Carlo simulation of 20 000 individuals to estimate the cooccurrence of sequelae. To capture uncertainty in the prevalences of each of the sequelae, for each age-sex- country-year, we ran 1000 different micro-simulations of 20 000 individuals.
Second, we calculated the combined disability weight for the estimated individuals with every combination of disorders. For all combinations of disorders generated in the micro-simulation, the combined disability weight for a simulated individual with two or more disorders is one minus the product of one minus each disability weight. Tests on real data such as MEPS as well as other studies suggest that this multiplicative model was the most appropriate.84,85 To propagate uncertainty in disability weights into the YLD estimates, each computation was based on a draw from the uncertainty distribution of each disability weight. Third, the combined disability weight from the co-occurrence of sequelae was apportioned to each of the contributing sequelae in proportion to the disability weight of a sequela on its own.
We tested the validity of our assumption of independence within an age-sex-country-year using the MEPS data (described above), which includes both individual-level measurement of functional status using SF‒12 and ICD‒ coded diagnoses. We applied the GBD approach assuming multiplicative disability weights and independent disorder probabilities to estimate YLDs and we computed directly from the MEPS data taking into account actual comorbid patterns at the individual level. The correlation coefficient for the two approaches was 0.999.
YLDs from residual categories
There are nine causes on the cause list such as other neglected tropical diseases, other neurological disorders, or other congenital anomalies that are groupings of a large number of often rare disorders. We approximate the YLDs for these disorders using the ratio of YLDs to YLLs for similar or related disorders to then estimate YLDs for these residual categories from YLLs that have been directly estimated.68
Ranking lists
For the presentation of leading causes of YLDs, the level at which causes are ranked is subject to debate. We have opted to use the level of disaggregation that seems most relevant for public health decision making. For example, we have chosen to include diarrhoeal diseases, lower respiratory infections, maternal disorders, stroke, liver cancer, cirrhosis, drug use, road injury, exposure to mechanical forces, animal contact, interpersonal violence, and congenital anomalies in the ranking list.
Decomposing changes in YLDs into demographic and epidemiological factors
To help understand the drivers of change in the number of YLDs by cause or region, we have estimated the proportion of the change from 1990 to 2010 due to growth in total population, change in population age-structure and sexstructure, and change in age-specifi c and sex-specifi c rates. We computed two counterfactual sets of YLDs. First, a population growth scenario computed as the number of YLDs expected in 2010 if only total population numbers increased to the level of 2010 but the age-sex structure of population stayed the same as in 1990 and age-sex specifi c rates remained at 1990 levels. Second, a popu lation growth and population ageing scenario computed as the number of YLDs expected in 2010, using 1990 age-specifi c and sex-specifi c rates and 2010 age-specifi c and sexspecific population numbers. The diff erence between 1990 numbers and the population growth scenario is the change in YLDs due strictly to the growth in total population. The change from the population growth scenario to the population growth and ageing scenario is the number of YLDs due to ageing of the population. The diff erence between 2010 YLDs and the population growth and ageing scenario is the difference in YLDs due to epidemiological change in age-specific and sex-specific YLDs per person. Each of these three differences is also presented as a percentage change with reference to the 1990 YLD estimate. Further details about the data and methods used for specific causes of YLDs are available on request from the corresponding author.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and the final responsibility to submit for publication.
Results
Global prevalence for all ages combined in 2010 across the 1160 sequelae varied from fewer than one case per 1 million people to 350 000 cases per 1 million people. 58 sequelae each affected more than 1% of the global population. Table 1 shows the global prevalence of the 50 most common sequelae in 2010. Of these sequelae, four were oral health disorders (dental caries of permanent teeth, chronic periodontitis, dental caries of baby teeth, and edentulism). Four skin diseases were also very common: fungal skin disease, acne vulgaris, pruritus, and eczema; collectively these disorders affected 2·1 billion individuals (table 1). The number of individuals affected by tension-type headaches or migraine was also very large—these neurological causes respectively ranked as the second and third most common. Low back pain, neck pain, other musculoskeletal, and osteoarthritis of the knee were also very common (table 1). Hearing loss affected 1·3 billion people and vision loss affected 661 million people. Two mental and behavioural disorders, anxiety and major depressive disorder, were in the top 30 most common causes. Two respiratory disorders, COPD and asthma, were also highly prevalent. Although prevalences varied substantially across communities, iron-deficiency anaemia affected 14·9% and infection with schistosomiasis affected 3·5% of the world’s population. Five of the top 50 most common sequelae affected only one sex: genital prolapse, uterine fibroids, benign prostatic hyperplasia, premenstrual syndrome, and polycystic ovarian disease. Table 1, however, shows prevalences at the level of only sequelae and not at the level of disease or injuries. Disorders such as chronic kidney diseases (CKD) does not appear in the top 30 causes because, at the sequelae level, we have separate estimates for CKD from hypertension, CKD from diabetes, and CKD from other causes.
Table 1:
Global prevalence of the 50 most common sequelae o
| Prevalence (both sexes) |
Male prevalence |
Female prevalence |
||||
|---|---|---|---|---|---|---|
| Total (thousands) |
Proportion of population (%) |
Total (thousands) |
Proportion of population (%) |
Total (thousands) |
Proportion of population (%) |
|
| Dental caries of permanent teeth | 2431636 | 35. 29% | 1 194 051 | 34.37% | 1 237 585 | 36.23% |
| Tension-type headache | 1431 067 | 20.77% | 655 937 | 18.88% | 775131 | 22.69% |
| Migraine | 1012944 | 14.70% | 371 072 | 10.68% | 641 873 | 18.79% |
| Fungal skin diseases | 985 457 | 14.30% | 516 167 | 14.86% | 469 291 | 13.74% |
| Other skin and subcutaneous diseases | 803597 | 11.66% | 417 129 | 12.01% | 386 468 | 11.32% |
| Chronic periodontitis | 743 187 | 1079% | 378 407 | 10.89% | 364 780 | 10.68% |
| Mild hearing loss with perinatal onset due to other hearing loss | 724 689 | 1.0.52% | 386 147 | 11.11% | 338 543 | 9.91% |
| Acne vulgaris | 646 488 | 9.38% | 311 349 | 8.96% | 335 140 | 9.81% |
| Low back pain | 632 045 | 9.17% | 334 793 | 9. 64% | 297 252 | 8.70% |
| Dental caries of baby teeth | 621 507 | 9.02% | 352 085 | 10.13% | 269 421 | 7.89% |
| Moderate iron-deficiency anaemia | 608 915 | 8.84% | 269 596 | 7.76% | 339 319 | 9.93% |
| Other musculoskeletal disorders | 560 978 | 8.14% | 262779 | 7.56% | 298 199 | 8.73% |
| Near sighted due to other vision loss | 459 646 | 6.67% | 235 052 | 6.77% | 224 593 | 6.58% |
| Mild iron-deficiency anaemia | 375 438 | 5.45% | 152 523 | 4.39% | 222915 | 6.53% |
| Asthma | 334 247 | 4.85% | 160 346 | 4.61% | 173 901 | 5.09% |
| Neck pain | 332 049 | 4.82% | 135 134 | 3. 89% | 196 915 | 5.77% |
| Chronic obstructive pulmonary disease | 328 615 | 4.77% | 168 445 | 4.85% | 160170 | 4.69% |
| Genital prolapse | 316 897 | 4.55% | .. | .. | 316 897 | 9.28% |
| Major depressive disorder | 298 441 | 4.33% | 111 441 | 3.21% | 187 000 | 5.48% |
| Pruritus | 280 229 | 4.07% | 117758 | 3.39% | 162471 | 4.76% |
| Anxiety disorders | 272 777 | 3.96% | 95 731 | 2.76% | 177 046 | 5.18% |
| Mild anaemia due to hookworm disease | 260254 | 3.78% | 149 572 | 4.30% | 110 681 | 3.24% |
| Osteoarthritis of the knee | 250 785 | 3.64% | 88 885 | 2.56% | 161 900 | 4.74% |
| Schistosomiasis | 238 366 | 3.46% | 124 289 | 3.58% | 114 077 | 3.34% |
| Eczema | 229761 | 3.33% | 104 259 | 3.00% | 125 502 | 3.67% |
| Uncomplicated diabetes mellitus | 227 588 | 3.30% | 114 817 | 3.30% | 112 771 | 3.30% |
| Uterine fibroids | 225 259 | 3.23% | .. | .. | 225 259 | 6.60% |
| Sexually transmitted chlamydial diseases | 215 621 | 3.13% | 85 675 | 2.47% | 129 946 | 3.80% |
| Benign prostatic hyperplasia | 210 142 | 3.05% | 210 142 | 6.05% | .. | .. |
| Premenstrual syndrome | 199 072 | 2.89% | .. | .. | 199 072 | 5.83% |
| Moderate hearing loss with perinatal onset due to other hearing loss | 189 919 | 2.76% | 103 629 | 2.98% | 86 290 | 2.53% |
| Goitre due to iodine deficiency | 187 181 | 2.72% | 69 752 | 2.01% | 117 429 | 3.44% |
| Lacerations, multiple wounds, other dislocations, and eye injuries due to falls | 185 700 | 2.70% | 110 263 | 3.17% | 75 438 | 2.21% |
| Edentulism | 158 284 | 2.30% | 67 264 | 1.94% | 91 020 | 2.66% |
| Trichomoniasis | 152232 | 2.21% | 49731 | 1.43% | 102501 | 3.00% |
| Chronic urolithiasis | 144346 | 2.10% | 90446 | 2.60% | 53901 | 1.58% |
| Mild hearing loss due to otitis media | 141600 | 2.06% | 79359 | 2.28% | 62241 | 1.82% |
| Mild anaemia due to sickle cell disorders | 141419 | 2.05% | 64343 | 1.85% | 77075 | 2.26% |
| Impetigo | 140495 | 2.04% | 67464 | 1.94% | 73031 | 2.14% |
| Diabetic neuropathy | 131930 | 1.91% | 63509 | 1.83% | 68421 | 2.00% |
| Other cardiovascular and circulatory diseases | 127990 | 1.86% | 48040 | 1.38% | 79950 | 2.34% |
| Molluscum contagiosum | 122601 | 1.78% | 65841 | 1.89% | 56760 | 1.66% |
| Otitis media (chronic) | 117881 | 1.71% | 55891 | 1.61% | 61989 | 1.81% |
| Polycystic ovarian syndrome | 116730 | 1.68% | .. | .. | 116730 | 3.42% |
| Angina due to ischaemic heart disease | 111705 | 1.62% | 59683 | 1.72% | 52022 | 1.52% |
| Dysthymia | 105520 | 1.53% | 43863 | 1.26% | 61657 | 1.81% |
| Scabies | 100625 | 1.46% | 51736 | 1.49% | 48889 | 1.43% |
| Mild anaemia due to thalassaemias | 95731 | 1.39% | 44362 | 1.28% | 51370 | 1.50% |
We detected a huge range of severity across sequelae with similar prevalence when comparing prevalence rate per 100 000 individuals on a log scale for each sequela compared with the average disability weight (appendix p 36). In general, more severe disorders were less common than less severe disorders, but there were notable exceptions. The variation in prevalence across disorders extended by more than a factor of 100 000. A weak relation exists when the more common sequelae are milder than the less common sequelae (correlation coefficient –0·37). The lack of a strong association between prevalence and severity plus the substantial number of highly prevalent, but mild, disorders draws attention to why consideration of prevalence of disorders alone is insufficient in quantifying burden of disease. To understand which causes contribute the greatest burden, we need to take into account both prevalence and severity of the health states. The disability weights collected from the general public provide the mechanism by which the highly diverse set of sequelae can be compared by adjusting for severity.30
In 2010, there were a total of 777 million YLDs globally, implying an average health loss of 0·11 years per person. By sex, the YLD rate was 10 819 per 100 000 male individuals and 11 755 per 100 000 female individuals, with female individuals accounting for 51·6% of all YLDs globally. Disaggregated into three broad cause groups, 15·3% of YLDs in 2010 were due to communicable, maternal, neonatal, and nutritional disorders, 78·6% to non-communicable diseases, and 6·1% to injuries. The heavy preponderance of YLDs from non-communicable diseases is substantially different from the distribution of years of life lost because of premature mortality (YLLs; 42·8%).68
We detected a characteristic pattern of the prevalence of disease adjusted for severity by age and sex at the global level in 2010 (figure 2). This figure provides an analysis using the 21 mutually exclusive and collectively exhaustive cause categories at the second level in the GBD cause list for male and female individuals. In children younger than 5 years, leading causes of YLDs included neonatal disorders, nutritional deficiencies, diarrhoea, lower respiratory infections, other infectious diseases, and neglected tropical diseases and malaria. Beginning at age 10 years and extending to age 65 years, mental and behavioural disorders were a major cause, contributing as much as 36% at age 20–29 years. Nearly as important but with an older age distribution, the other dominant cause was musculoskeletal disorders. The third most important factor in adults was other non-communicable diseases, which includes congenital anomalies, skin diseases, sense organ disorders, and oral disorders (figure 2). Diabetes, urogenital, blood, and endocrine diseases made a progressively larger contribution with age. Neurological disorders (Alzheimer’s disease and Parkinson’s disease in particular) started to make a major contribution in individuals aged 80 years or older. Chronic respiratory disorders made a substantial contribution in individuals aged 10 years and older, whereas cardiovascular diseases seemed progressively more important at older ages. The long-term cumulative disability from unintentional injuries is also an important factor. This age-sex pattern of the leading causes was very different from the pattern for mortality by cause, which was dominated by causes such as cancers, cardiovascular diseases, HIV and tuberculosis, diarrhoea, pneumonia, and other infectious diseases.68
Figure 2:
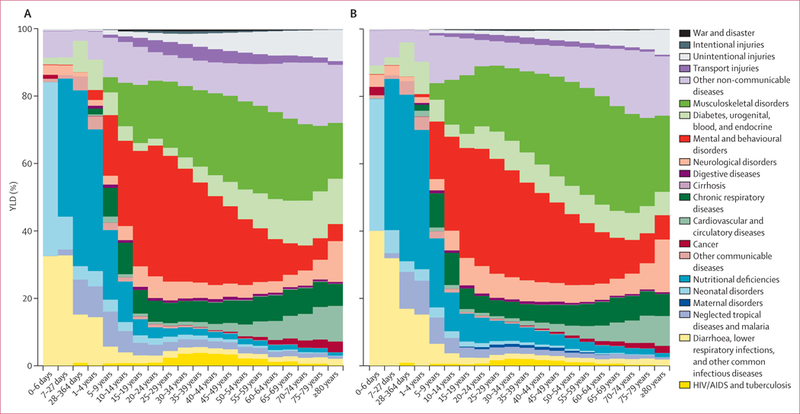
Percentage of years lived with disability (YLDs) in 2010, by cause and age
The GBD 2010 includes the assessment of 1160 sequelae, of which 600 are 40 different nature of injury sequelae (such as hip fracture or traumatic brain injury) for each of the 25 external causes of injury (such as falls or road injury). For simplicity of presentation, table 2 shows YLD estimates for all non-fatal health outcomes and some select groupings of sequelae. For example, we estimated YLDs for mild, moderate, and severe anaemia from a variety of causes but the table shows YLDs from all three forms of anaemia. For injuries we show only the YLDs by external cause without giving details for each nature of injury. Furthermore, we show results for both sexes combined for summary age groups (table 2) and the full age and sex detail for 2010 and 1990 (appendix pp 37–270). A substantial number of causes contribute to the overall YLDs at the global level (appendix pp 37–270). The leading causes were low back pain, which contributed 10·7% of total YLDs, and major depressive disorder, which contributed 8·1% of total YLDs. Within the broad category of communicable, maternal, neonatal, and nutritional disorders, the most important causes of YLDs included iron-deficiency anaemia, which accounted for 5·5% of all YLDs. Other causes within this group that caused 4 million or more YLDs included tuberculosis, HIV, diarrhoeal diseases, otitis media, malaria, intestinal nematodes, and neonatal disorders. Several neglected tropical diseases caused between 1 million and 4 million YLDs, including schistosomiasis, lymphatic filariasis, and food-borne trematodiases. Although major contributors to YLLs, the entire list of cancers caused a total of 4·5 million YLDs. Cardiovascular and circulatory diseases accounted for 2. 8% of all YLDs with ischaemic heart disease and stroke accounting for 60% of the total for the cardiovascular category and the rest distributed across a wide range of causes. Chronic respiratory diseases accounted for 6·3% of global YLDs with the largest contributor being COPD (29·4 million YLDs) followed by asthma with 13·8 million YLDs. YLD rates for COPD have risen since 1990 whereas asthma rates have decreased marginally in this period. Neurological disorders accounted for another 42·9 million YLDs—migraine accounted for more than half of these YLDs.
Table 2:
Global years lived with disability (YLDs) for a comprehensive set of 289 causes and select sequelae in 1990 and 2010, for all ages, both sexes combined, and per 100 000
| All ages YLDs (thousands) |
YLDs (per 100 000) |
|||||
|---|---|---|---|---|---|---|
| 1990 | 2010 | %Δ | 1990 | 2010 | %Δ | |
| All causes | 583 393 (484 649-694 406) | 777 401 (648 158-921 711) | 333% | 11 004 (9142-13 098) | 11 283 (9407-13 378) | 2.5% |
| Communicable, maternal, neonatal, and nutritional disorders | 113 925 (85 875-148 463) | 119 164 (91 399-152 096) | 46% | 2149 (1620-2800) | 1730 (1327-2207) | −19.5% |
| HIV/AIDS and tuberculosis | 7681 (5222-10 722) | 11 117 (7718-15 187) | 44.7% | 145 (99-202) | 161 (112-220) | 11.4% |
| Tuberculosis | 6085 (4020-8737) | 6774 (4500-9756) | 11.3% | 115 (76-165) | 98 (65-142) | −14.3% |
| HIV/AIDS | 1596 (1132-2125) | 4342 (3142-5629) | 172.2% | 30 (21-40) | 63(46-82) | 109.4% |
| HIV disease resulting in mycobacterial infection | 220 (143-314) | 1224 (793-1746) | 456.8% | 4(3-6) | 18 (12-25) | 328.4% |
| HIV disease resulting in other specified or unspecified diseases | 1376 (967-1857) | 3119 (2241-4107) | 126.7% | 26 (18-35) | 45 (33-60) | 74.4% |
| HIV pre-AIDS asymptomatic | 376 (227-569) | 889 (546-1338) | 136.8% | 7 (4-11) | 13(8-19) | 82.2% |
| HIV pre-AIDS symptomatic | 289 (193-411) | 531 (350-756) | 83.6% | 5 (4-8) | 8 (5-11) | 41.3% |
| AIDS with antiretroviral treatment | 0 (0-0) | 389 (251-578) | .. | 0 (0-0) | 6 (4-8) | .. |
| AIDS without antiretroviral treatment | 711 (483-958) | 1309 (913-1758) | 84.2% | 13 (9-18) | 19 (13-26) | 41.7% |
| Diarrhoea, lower respiratory infections, meningitis, and other common infectious diseases | 18 579 (13 419-25 301) | 19 921 (14 241-27 439) | 7.2% | 350 (253-477) | 289 (207-398) | −17.5% |
| Diarrhoeal diseases | 7654 (5135-10 855) | 8045 (5371-11 366) | 5.1% | 144 (97-205) | 117 (78-165) | −19.1% |
| Cholera | 115 (59-188) | 80 (42-134) | −30.1% | 2 (1-4) | 1 (1-2) | −46.2% |
| Other salmonella infections | 263 (150-410) | 341 (202-523) | 29.8% | 5(3-8) | 5(3-8) | −0.1% |
| Shigellosis | 703 (391-1111) | 744 (440-1147) | 5.8% | 13 (7-21) | 11 (6-17) | −18.6% |
| Enteropathogenic E coli infection | 972 (438-1652) | 845 (387-1416) | −13.0% | 18 (8-31) | 12 (6-21) | −33.1% |
| Enterotoxigenic E coli infection | 889 (520-1409) | 1065 (649-1643) | 19.8% | 17 (10-27) | 15 (9-24) | −7.8% |
| Campylobacter enteritis | 753 (407-1211) | 746 (416-1180) | −1.0% | 14 (8-23) | 11 (6-17) | −23.8% |
| Campylobacter enteritis | 753 (406-1211) | 746 (415-1181) | −0.9% | 14 (8-23) | 11 (6-17) | −23.8% |
| Guillain-Barre syndrome due to C enteritis | 1 (0-1) | 1 (1-2) | 35.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | 4.4% |
| Amoebiasis | 142 (84-217) | 205 (126-314) | 44.1% | 3 (2-4) | 3(2-5) | 10.9% |
| Cryptosporidiosis | 651 (312-1101) | 661 (316-1096) | 1.6% | 12 (6-21) | 10 (5-16) | −21.8% |
| Rotaviral enteritis | 1159 (624-1885) | 1269 (701-2006) | 9.5% | 22 (12-36) | 18 (10-29) | −15.8% |
| Other diarrhoeal diseases | 2007 (1027-3412) | 2089 (1054-3521) | 4.1% | 38 (19-64) | 30 (15-51) | −19.9% |
| Typhoid and paratyphoid fevers | 134 (25-348) | 172 (33-435) | 27.8% | 3 (0-7) | 2(0-6) | −1.7% |
| Typhoid and paratyphoid fevers | 124 (16-337) | 159 (20-423) | 27.8% | 2(0-6) | 2(0-6) | −1.6% |
| Liver abscess and cysts due to typhoid and paratyphoid fevers | 10 (7-15) | 13 (8-20) | 27.4% | <0.5 (0-0.5) | <0.5 (0-0.5) | −1.9% |
| Lower respiratory infections | 2113 (1444-2941) | 2331 (1592-3240) | 10.3% | 40 (27-55) | 34 (23-47) | −15.1% |
| Influenza | 510 (344-714) | 583 (393-815) | 14.2% | 10 (6-13) | 8(6-12) | −12.1% |
| Influenza | 510 (343-713) | 582 (392-814) | 14.2% | 10 (6-13) | 8(6-12) | −12.1% |
| Guillain-Barre syndrome due to influenza | 1 (1-2) | 2(1-3) | 34.3% | <0.5 (0-0.5) | <0.5 (0-0.5) | 3.3% |
| Pneumococcal pneumonia | 298 (203-414) | 367 (248-509) | 23.2% | 6 (4-8) | 5 (4-7) | −5.2% |
| H influenzae type B pneumonia | 216 (145-306) | 201 (134-286) | −6.6% | 4(3-6) | 3 (2-4) | −28.2% |
| Respiratory syncytial virus pneumonia | 52 (31-82) | 36 (21-55) | −31.3% | 1 (1-2) | 1 (0-1) | −47.2% |
| Other lower respiratory infections | 1037 (702-1459) | 1144 (779-1589) | 10.2% | 20 (13-28) | 17 (11-23) | −15.2% |
| Upper respiratory infections | 1438 (755-2542) | 1728 (911-3050) | 20.2% | 27 (14-48) | 25 (13-44) | −7.5% |
| Upper respiratory infections | 1437 (753-2541) | 1727 (910-3048) | 20.2% | 27 (14-48) | 25 (13-44) | −7.5% |
| Guillain-Barre syndrome due to upper respiratory infections | 1 (1-2) | 2(1-3) | 33.8% | <0.5 (0-0.5) | <0.5 (0-0.5) | 3.0% |
| Otitis media | 3794 (2456-5829) | 4436 (2887-6668) | 16.9% | 72 (46-110) | 64 (42-97) | −10.0% |
| Otitis media | 1359 (819-2150) | 1613 (979-2594) | 18.7% | 26 (15-41) | 23 (14-38) | −8.7% |
| Hearing loss due to otitis media | 2435 (1423-3929) | 2824 (1669-4533) | 16.0% | 46 (27-74) | 41 (24-66) | −10.8% |
| Meningitis | 2757 (1973-3732) | 2628 (1857-3643) | −4.7% | 52 (37-70) | 38 (27-53) | −26.7% |
| Pneumococcal meningitis | 920 (624-1298) | 886 (595-1254) | −3.7% | 17 (12-24) | 13(9-18) | −25.9% |
| Spneumoniae meningitis | 9 (5-14) | 11 (6-17) | 19.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | −8.0% |
| Long term sequelae due to S pneumoniae meningitis | 571 (324-899) | 488 (261-806) | −14.5% | 11 (6-17) | 7 (4-12) | −34.2% |
| Seizures due to S pneumoniae meningitis | 80 (52-118) | 79 (55-113) | −0.4% | 2(1-2) | 1 (1-2) | −23.4% |
| Hearing loss due to Spneumoniae meningitis | 261 (154-420) | 308 (185-500) | 18.2% | 5 (3-8) | 4 (3-7) | −9.0% |
| H influenzae type B meningitis | 646 (429-933) | 371 (247-524) | −42.6% | 12 (8-18) | 5 (4-8) | −55.8% |
| H influenzae type B meningitis | 10 (6-17) | 7 (4-12) | −32.0% | <0.5 (0-0.5) | <0.5 (0-0.5) | −47.7% |
| Long term sequelae due to H influenzae type B meningitis | 448 (253-715) | 233 (124-368) | −48.4% | 8(5-13) | 3(2-5) | −60.0% |
| Seizures due to H influenzae type B meningitis | 65 (38-110) | 31 (18-51) | −52.2% | 1(1-2) | <0.5 (0-1) | −63.3% |
| Hearing loss due to H influenzae type B meningitis | 123 (74-198) | 100 (60-160) | −18.5% | 2 (1-4) | 1 (1-2) | −37.3% |
| Meningococcal infection | 424 (302-597) | 403 (281-566) | −4.8% | 8 (6-11) | 6 (4-8) | −26.7% |
| Meningococcal infection | 6 (4-10) | 7 (4-12) | 13.0% | <0.5 (0-0.5) | <0.5 (0-0.5) | −13.0% |
| Long term sequelae due to meningococcal infection | 195 (115-306) | 165 (92-269) | −15.2% | 4(2-6) | 2 (1-4) | −34.8% |
| Seizures due to meningococcal infection | 35 (23-52) | 28 (18-40) | −21.4% | 1 (0-1) | <0.5 (0-1) | −39.5% |
| Hearing loss due to meningococcal infection | 187 (110-297) | 203 (119-325) | 8.6% | 4 (2-6) | 3(2-5) | −16.5% |
| Other meningitis | 733 (509-1036) | 930 (647-1361) | 27.0% | 14 (10-20) | 14 (9-20) | −2.3% |
| Other meningitis | 37 (24-53) | 49 (31-72) | 34.4% | 1 (0-1) | 1 (0-1) | 3.4% |
| Long term sequelae due to other bacterial meningitis infection | 283 (157-446) | 289 (149-476) | 2.2% | 5 (3-8) | 4 (2-7) | −21.4% |
| Seizures due to other bacterial meningitis infection | 48 (32-71) | 46 (31-66) | −4.0% | 1 (1-1) | 1 (0-1) | −26.1% |
| Hearing loss due to other bacterial meningitis infection | 365 (221-572) | 546 (322-883) | 49.6% | 7 (4-11) | 8 (5-13) | 15.1% |
| Encephalitis | 183 (120-260) | 205 (133-292) | 12.6% | 3 (2-5) | 3 (2-4) | −13.4% |
| Encephalitis | 5 (3-9) | 7 (4-12) | 35.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | 4.4% |
| Motor cognitive impairments due to encephalitis | 177 (117-253) | 198 (128-283) | 11.9% | 3(2-5) | 3 (2-4) | −13.9% |
| Diphtheria | <0'5 (0-2) | <0.5 (0-1) | −49.1% | <0.5 (0-0.5) | <0.5 (0-0.5) | −60.9% |
| Whooping cough | 181 (103-287) | 122 (70-195) | −32.5% | 3(2-5) | 2(1-3) | −48.0% |
| Tetanus | 78 (35-159) | 21(9-43) | −72.3% | 1 (1-3) | <0.5 (0-1) | −78.7% |
| Tetanus | 77 (35-158) | 21(9-43) | −72.3% | 1 (1-3) | <0.5 (0-1) | −78.7% |
| Long-term sequelae from neonatal tetanus | 1 (0-2) | <0.5 (0-1) | −73.2% | <0.5 (0-0.5) | <0.5 (0-0.5) | −79.4% |
| Measles | 106 (58-180) | 31 (17-51) | −70.8% | 2(1-3) | <0.5 (0-1) | −77.5% |
| Varicella | 142 (87-219) | 202 (124-308) | 42.0% | 3 (2-4) | 3 (2-4) | 9.3% |
| Chickenpox | 7 (2-16) | 7 (2-16) | −0.9% | <0.5 (0-0.5) | <0.5 (0-0.5) | −23.8% |
| Herpes zoster | 135 (84-209) | 195 (120-295) | 44.3% | 3 (2-4) | 3 (2-4) | 11.0% |
| Neglected tropical diseases and malaria | 23 491 (15 715-36 639) | 22 219 (15 693-31 544) | −5.4% | 443 (296-691) | 322 (228-458) | −27.2% |
| Malaria | 2662 (1257-4481) | 4070 (1853-6980) | 52.9% | 50 (24-85) | 59 (27-101) | 17.7% |
| Malaria | 433 (194-854) | 498 (218-933) | 14.8% | 8(4-16) | 7 (3-14) | −11.6% |
| Anaemia due to malaria | 2127 (833-3972) | 3367 (1312-6294) | 58.3% | 40 (16-75) | 49 (19-91) | 21.8% |
| Motor cognitive impairments due to malaria | 104 (41-273) | 211(81-556) | 102.8% | 2(1-5) | 3 (1-8) | 56.0% |
| Chagas disease | 324 (108-594) | 303(106-573) | −6.4% | 6(2-11) | 4(2-8) | −28.0% |
| Acute Chagas disease | 31 (7-62) | 28 (7-59) | −8.8% | 1(0-1) | <0.5 (0-1) | −29.8% |
| Chronic heart disease due to Chagas disease | 213 (38-429) | 195 (36-411) | −8.4% | 4(1-8) | 3 (1-6) | −29.5% |
| Chronic digestive disease due to Chagas disease | 73 (8-178) | 67 (7-157) | −8.5% | 1(0-3) | 1 (0-2) | −29.6% |
| Heart failure due to Chagas disease | 7 (4-10) | 13 (8-19) | 92.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | 48.2% |
| Leishmaniasis | 113 (53-215) | 124 (60-235) | 10.2% | 2 (1-4) | 2(1-3) | −15.2% |
| Visceral leishmaniasis | 8 (2-16) | 6(2-13) | −17.2% | <0.5 (0-0.5) | <0.5 (0-0.5) | −36.3% |
| Cutaneous leishmaniasis | 105 (47-206) | 118 (56-229) | 12.2% | 2 (1-4) | 2(1-3) | −13.7% |
| African trypanosomiasis | 33 (12-86) | 8(2-25) | −75.2% | 1(0-2) | <0.5 (0-0.5) | −80.9% |
| Schistosomiasis | 1797 (923-3413) | 2986 (1541-5666) | 66.2% | 34 (17-64) | 43 (22-82) | 27.9% |
| Schistosomiasis | 696 (229-1579) | 1148 (377-2607) | 65.0% | 13 (4-30) | 17 (5-38) | 27.0% |
| Mild diarrhoea due to schistosomiasis | 1 (0-1) | 1 (1-2) | 77.2% | <0.5 (0-0.5) | <0.5 (0-0.5) | 36.3% |
| Anaemia due to schistosomiasis | 433 (219-766) | 687 (344-1217) | 58.8% | 8 (4-14) | 10 (5-18) | 22.2% |
| Hepatomegaly due to schistosomiasis | 104 (47-200) | 185(84-355) | 77.8% | 2 (1-4) | 3(1-5) | 36.8% |
| Haematemesis due to schistosomiasis | 39 (26-55) | 69 (46-97) | 76.6% | 1(0-1) | 1 (1-1) | 35.9% |
| Ascites due to schistosomiasis | 31 (21-44) | 56 (38-77) | 78.9% | 1 (0-1) | 1(1-1) | 37.7% |
| Dysuria due to schistosomiasis | 174 (80-334) | 298 (135-572) | 71.0% | 3(2-6) | 4(2-8) | 31.5% |
| Bladder pathology due to schistosomiasis | 159 (72-304) | 268 (122-515) | 68.9% | 3 (1-6) | 4 (2-7) | 30.0% |
| Hydronephrosis due to schistosomiasis | 162 (74-310) | 277(126-533) | 71.5% | 3 (1-6) | 4(2-8) | 32.0% |
| Cysticercosis | 484 (378-600) | 457 (357-566) | −5.5% | 9(7-11) | 7 (5-8) | −27.3% |
| Echinococcosis | 89 (44-187) | 110 (55-228) | 23.9% | 2 (1-4) | 2 (1-3) | −4.7% |
| Chronic respiratory disease due to echinococcosis | 2 (1-6) | 3 (1-7) | 22.8% | <0.5 (0-0.5) | <0.5 (0-0.5) | −5.5% |
| Epilepsy due to echinococcosis | 13 (6-28) | 16 (8-32) | 24.1% | <0.5 (0-1) | <0.5 (0-0.5) | −4.5% |
| Abdominopelvic problems due to echinococcosis | 73 (36-155) | 91 (43-193) | 23.9% | 1 (1-3) | 1 (1-3) | −4.7% |
| Lymphatic filariasis | 2368 (1551-3399) | 2775 (1807-4000) | 17.2% | 45 (29-64) | 40 (26-58) | −9.9% |
| Lymphoedema | 955 (585-1454) | 1151 (698-1773) | 20.5% | 18 (11-27) | 17 (10-26) | −7.3% |
| Hydrocele due to lymphatic filariasis | 1414 (842-2103) | 1624 (981-2450) | 14.9% | 27 (16-40) | 24 (14-36) | −11.6% |
| Onchocerciasis | 512 (361-687) | 494 (360-656) | −3.5% | 10 (7-13) | 7 (5-10) | −25.7% |
| Skin disease due to onchocerciasis | 407 (277-559) | 352 (240-486) | −13.3% | 8(5-11) | 5 (3-7) | −33.3% |
| Vision loss due to onchocerciasis | 105 (79-134) | 142 (108-185) | 34.5% | 2(1-3) | 2 (2-3) | 3.5% |
| Trachoma | 144 (104-189) | 334 (243-438) | 132.5% | 3 (2-4) | 5 (4-6) | 78.9% |
| Dengue | 6(2-13) | 12 (6-23) | 103.9% | <0.5 (0-0.5) | <0.5 (0-0.5) | 56.9% |
| Dengue | 5(2-11) | 10 (5-20) | 105.9% | <0.5 (0-0.5) | <0.5 (0-0.5) | 58.5% |
| Post-dengue chronic fatigue syndrome | 1 (0-2) | 2 (0-4) | 92.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | 48.2% |
| Yellow fever | <0.5 (0-0.5) | <0.5 (0-0.5) | 15.1% | <0.5 (0-0.5) | <0.5 (0-0.5) | −11-4% |
| Rabies | <0.5 (0-1) | <0.5 (0-1) | −56.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | −66.7% |
| Intestinal nematode infections | 8741 (4778-15 094) | 4980 (2722-8442) | −43-0% | 165 (90-285) | 72 (40-123) | −56.2% |
| Ascariasis | 3950(2080-6805) | 1111 (618-1864) | −71'9% | 75 (39-128) | 16 (9-27) | −78.4% |
| Ascariasis infestation | 1995 (1091-3254) | 758 (419-1232) | −62.0% | 38 (21-61) | 11 (6-18) | −70.8% |
| Severe wasting due to ascariasis | 49 (32-72) | 43 (28-62) | −12.8% | 1 (1-1) | 1(0-1) | −32.9% |
| Mild abdominopelvic problems due to ascariasis | 1906 (871-3673) | 310 (139-598) | −83.7% | 36 (16-69) | 4(2-9) | −87.5% |
| Trichuriasis | 857 (465-1420) | 638 (349-1061) | −25.5% | 16 (9-27) | 9 (5-15) | −42.7% |
| Trichuriasis infestation | 677 (367-1104) | 504 (277-821) | −25.6% | 13 (7-21) | 7 (4-12) | −42.8% |
| Severe wasting due to trichuriasis | 9(5-13) | 9(5-13) | −0.5% | <0.5 (0-0.5) | <0.5 (0-0.5) | −23.4% |
| Mild abdominopelvic problems due to trichuriasis | 171 (77-327) | 126 (57-246) | −26.3% | 3 (1-6) | 2 (1-4) | −43.3% |
| Hookworm disease | 3934(2056-6983) | 3231(1695-5732) | −17.9% | 74 (39-132) | 47 (25-83) | −36.8% |
| Hookworm infestation | 1315 (718-2150) | 1011 (556-1655) | −23.1% | 25 (14-41) | 15(8-24) | −40.9% |
| Severe wasting due to hookworm disease | 34 (21-49) | 42 (27-61) | 23.4% | 1 (0-1) | 1(0-1) | −5.0% |
| Mild abdominopelvic problems due to hookworm disease | 241(110-462) | 217(98-422) | −10.0% | 5(2-9) | 3(1-6) | −30.8% |
| Anaemia due to hookworm disease | 2344 (983-4348) | 1962 (895-3672) | −16.3% | 44 (19-82) | 28 (13-53) | −35.6% |
| Food-borne trematodiases | 2394 (635-8501) | 1875 (708-4837) | −21.7% | 45 (12-160) | 27 (10-70) | −39.7% |
| Heavy clonorchiasis | 367 (95-1145) | 296 (100-822) | −19.4% | 7 (2-22) | 4(1-12) | −37.9% |
| Heavy fascioliasis | 32 (18-53) | 42 (26-65) | 32.5% | 1 (0-1) | 1(0-1) | 2.0%% |
| Heavy intestinal fluke infection | 101 (58-179) | 106 (64-170) | 4.9% | 2(1-3) | 2(1-2) | −19.3% |
| Heavy opisthorchiasis | 48 (29-77) | 60 (37-92) | 27.0% | 1 (1-1) | 1(1-1) | −2.3% |
| Cerebral paragonimiasis | 57 (7-245) | 43 (8-148) | −25.2% | 1 (0-5) | 1 (0-2) | −42.4% |
| Heavy paragonimiasis | 1789 (233-7696) | 1328 (280-4234) | −25.8% | 34 (4-145) | 19 (4-61) | −42.9% |
| Other neglected tropical diseases | 3825 (2517-6057) | 3690 (2556-5303) | −3.5% | 72 (47-114) | 54 (37-77) | −25.8% |
| Other neglected tropical disease | 1007 (533-2568) | 949 (657-1557) | −5.8% | 19 (10-48) | 14 (10-23) | −27.5% |
| Anaemia due to other neglected tropical diseases | 2873 (1920-4163) | 2800 (1857-4054) | −2.5% | 54 (36-79) | 41 (27-59) | −25.0% |
| Maternal disorders | 1394 (935-2271) | 1790 (1138-2936) | 28.4% | 26 (18-43) | 26 (17-43) | −1.2% |
| Maternal haemorrhage | 143 (84-234) | 98 (61-151) | −31.7% | 3 (2-4) | 1 (1-2) | −47.5% |
| Maternal haemorrhage | 29 (18-46) | 19 (12-29) | −34.2% | 1 (0-1) | <0.5 (0-0.5) | −49.4% |
| Anaemia due to maternal haemorrhage | 114(65-193) | 79 (47-124) | −31.1% | 2 (1-4) | 1 (1-2) | −47.0% |
| Maternal sepsis | 80 (46-128) | 42 (25-65) | −48.4% | 2(1-2) | 1 (0-1) | −60.3% |
| Hypertensive disorders of pregnancy | 69 (41-111) | 93 (53-151) | 33.2% | 1(1-2) | 1 (1-2) | 2.5% |
| Pre-eclampsia | 60 (33-100) | 83 (44-141) | 38.9% | 1(1-2) | 1 (1-2) | 6.9% |
| Eclampsia | 4 (1-7) | 3 (1-7) | −14.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | −34.3% |
| Long-term sequelae for hypertensive disorders of pregnancy | 6(1-15) | 7 (2-15) | 6.3% | <0.5 (0-0.5) | <0.5 (0-0.5) | −18.2% |
| Obstructed labour | 809 (458-1493) | 1182 (641-2194) | 46.0% | 15(9-28) | 17 (9-32) | 12.4% |
| Obstructed labour | 77 (40-140) | 34 (19-57) | −56.1% | 1(1-3) | <0.5 (0-1) | −66.3% |
| Fistula | 732 (390-1425) | 1148 (601-2138) | 56.8% | 14 (7-27) | 17 (9-31) | 20.6% |
| Abortion | 27 (15-52) | 32 (19-59) | 19.8% | 1 (0-1) | <0.5 (0-1) | −7.8% |
| Other maternal disorders | 264 (180-420) | 343 (225-526) | 30.1% | 5(3-8) | 5(3-8) | 0.1% |
| Neonatal disorders | 8422(6368-10706) | 9464 (7167-11 937) | 12.4% | 159 (120-202) | 137 (104-173) | −13.5% |
| Preterm birth complications | 2298 (1743-2895) | 2982 (2236-3716) | 29.7% | 43 (33-55) | 43 (32-54) | −0.2% |
| Impairment due to preterm birth complications | 2041 (1471-2613) | 2636 (1882-3359) | 29.1% | 39 (28-49) | 38 (27-49) | −0.7% |
| Retinopathy of prematurity due to preterm birth complications | 257 (154-376) | 347 (212-508) | 34.9% | 5 (3-7) | 5 (3-7) | 3.8% |
| Neonatal encephalopathy (birth asphyxia/trauma) | 5625 (4116-7298) | 6132 (4471-8030) | 9.0% | 106 (78-138) | 89 (65-117) | −16.1% |
| Sepsis and other infectious disorders of the newborn baby | 18 (9-32) | 23 (12-40) | 24.9% | <0.5 (0-1) | <0.5(0-1) | −3.9% |
| Other neonatal disorders | 481 (357-618) | 328 (244-417) | −31.8% | 9(7-12) | 5 (4-6) | −47.5% |
| Nutritional deficiencies | 49 887 (34 714-70 780) | 49 942 (34 705-70 350) | 0.1% | 941(655-1335) | 725 (504-1021) | −23.0% |
| Protein-energy malnutrition | 3200 (2071-4743) | 2720(1766-3972) | −15.0% | 60 (39-89) | 39 (26-58) | −34.6% |
| Kwashiokor or marasmus due to protein-energy malnutrition | 298 (155-520) | 197 (103-339) | −33.7% | 6 (3-10) | 3(1-5) | −49.0% |
| Severe wasting due to protein-energy malnutrition | 2906 (1803-4418) | 2530 (1604-3772) | −12.9% | 55 (34-83) | 37 (23-55) | −33.0% |
| Iodine deficiency | 3181 (2049-4912) | 3889(2468-6136) | 22.3% | 60 (39-93) | 56 (36-89) | −5.9% |
| Goitre due to iodine deficiency | 2902 (1823-4617) | 3767 (2382-5990) | 29.8% | 55 (34-87) | 55 (35-87) | −0.1% |
| Idiopathic intellectual disability due to iodine deficiency | 271 (181-386) | 113 (73-167) | −58.4% | 5 (3-7) | 2(1-2) | −68.0% |
| Heart failure due to iodine deficiency | 7(5-11) | 10 (6-14) | 33.3% | <0.5 (0-0.5) | <0.5 (0-0.5) | 2.6% |
| Vitamin A deficiency | 740 (565-941) | 806 (612-1037) | 9.0% | 14 (11-18) | 12 (9-15) | −16.1% |
| Iron-deficiency anaemia | 42 731 (28 506-61 896) | 42 494 (28 170-61 626) | −0.6% | 806 (538-1167) | 617 (409-894) | −23.5% |
| Iron-deficiency anaemia | 42 728 (28 497-61 897) | 42 505 (28 166-61 656) | −0.5% | 806 (538-1168) | 617(409-895) | −23.5% |
| Heart failure due to iron-deficiency anaemia | 17 (11-24) | 24 (16-36) | 46.7% | <0.5 (0-0.5) | <0.5(0-1) | 12.9% |
| Other nutritional deficiencies | 35 (31-44) | 32 (24-36) | −9.2% | 1 (1-1) | <0.5(0-1) | −30.1% |
| Other communicable, maternal, neonatal, and nutritional disorders | 4472 (3188-6195) | 4711(3352-6562) | 5.3% | 84 (60-117) | 68 (49-95) | −18.9% |
| Sexually transmitted diseases excluding HIV | 1111 (589-2072) | 1298 (704-2439) | 16.9% | 21 (11-39) | 19 (10-35) | −10.0% |
| Syphilis | 73 (3-156) | 91 (4-200) | 25.5% | 1(0-3) | 1 (0-3) | −3.4% |
| Sexually transmitted chlamydial diseases | 560 (268-1025) | 669 (324-1233) | 19.6% | 11 (5-19) | 10 (5-18) | −8.0% |
| Sexually transmitted chlamydial diseases | 507 (233-952) | 609 (281-1143) | 20.1% | 10 (4-18) | 9 (4-17) | −7.6% |
| Salpingitis, inflammatory disease of cervix, and other female pelvic inflammatory diseases due to sexually transmitted chlamydial diseases | 27 (16-43) | 25 (15-40) | −7.5% | 1(0-1) | <0.5(0-1) | −28.8% |
| Infertility due to sexually transmitted chlamydial diseases | 25 (9-53) | 35 (14-72) | 37.7% | <0.5 (0-1) | 1 (0-1) | 6.0% |
| Gonococcal infection | 184 (94-336) | 249 (123-450) | 35.1% | 3(2-6) | 4 (2-7) | 4.0% |
| Gonococcal infection | 147 (69-282) | 207(96-390) | 40.7% | 3(1-5) | 3 (1-6) | 8.3% |
| Salpingitis, inflammatory disease of cervix, and other female pelvic inflammatory diseases due to gonococcal infection | 20 (12-33) | 19 (11-31) | −7.7% | <0.5 (0-1) | <0.5 (0-0.5) | −29.0% |
| Infertility due to gonococcal infection | 17(7-35) | 23 (9-47) | 37.7% | <0.5 (0-1) | <0.5(0-1) | 5.9% |
| Trichomoniasis | 182 (0-549) | 167 (0-493) | −8.4% | 3 (0-10) | 2 (0-7) | −29.5% |
| Other sexually transmitted diseases | 112 (68-181) | 122 (74-200) | 9.3% | 2 (1-3) | 2 (1-3) | −15.9% |
| Other sexually transmitted diseases | 70 (42-111) | 64 (39-105) | −7.8% | 1 (1-2) | 1(1-2) | −29.0% |
| Infertility due to other sexually transmitted diseases | 42 (17-91) | 58 (23-125) | 37.7% | 1 (0-2) | 1 (0-2) | 5.9% |
| Hepatitis | 449 (230-810) | 542 (280-981) | 20.7% | 8 (4-15) | 8 (4-14) | −7.1% |
| Acute hepatitis A | 172 (85-294) | 185 (95-311) | 7.6% | 3(2-6) | 3(1-5) | −17.2% |
| Acute hepatitis B | 194 (24-456) | 248 (28-585) | 28.1% | 4(0-9) | 4(0-8) | −1.4% |
| Acute hepatitis C | 24 (4-50) | 39 (7-79) | 61.2% | <0.5 (0-1) | 1 (0-1) | 24.1% |
| Acute hepatitis E | 59 (20-121) | 69 (24-142) | 17.8% | 1 (0-2) | 1 (0-2) | −9.3% |
| Leprosy | 26 (12-48) | 6 (3-11) | −76.6% | <0.5 (0-1) | <0.5 (0-0.5) | −82.0% |
| Other infectious diseases | 2886 (1920-4175) | 2864 (1902-4141) | −0.8% | 54 (36-79) | 42 (28-60) | −23.6% |
| Other infectious diseases | 922 (615-1330) | 957 (635-1386) | 3.8% | 17 (12-25) | 14 (9-20) | −20.2% |
| Anaemia due to other infectious diseases | 2000 (1329-2897) | 1947 (1292-2812) | −2.7% | 38 (25-55) | 28 (19-41) | −25.1% |
| Guillain-Barré syndrome due to other infectious diseases | 1 (0-1) | 1 (0-1) | 35.8% | <0.5 (0-0.5) | <0.5 (0-0.5) | 4.5% |
| Non-communicable diseases | 435 400 (365 526-515 063) | 611 076 (512 645-721 956) | 40.3% | 8213(6895-9715) | 8869 (7440-10 478) | 8.0% |
| Neoplasms | 2540 (1876-3348) | 4483 (3324-5861) | 76.5% | 48 (35-63) | 65(48-85) | 35.8% |
| Oesophageal cancer | 61(42-86) | 75 (49-103) | 22.8% | 1 (1-2) | 1(1-1) | −5.5% |
| Stomach cancer | 204 (137-286) | 229 (150-323) | 12.3% | 4(3-5) | 3(2-5) | −13.6% |
| Liver cancer | 77 (53-104) | 140 (96-189) | 82.7% | 1 (1-2) | 2(1-3) | 40.6% |
| Liver cancer secondary to hepatitis B | 35 (23-49) | 64 (43-89) | 81.4% | 1 (0-1) | 1 (1-1) | 39.6% |
| Liver cancer secondary to hepatitis C | 19 (13-27) | 34 (23-47) | 80.5% | <0.5 (0-1) | <0.5 (0-1) | 38.9% |
| Liver cancer secondary to alcohol use | 15 (9-21) | 29 (19-42) | 100.2% | <0.5 (0-0.5) | <0.5 (0-1) | 54.1% |
| Other liver cancer | 8(4-12) | 12 (7-19) | 60.3% | <0.5 (0-0.5) | <0.5 (0-0.5) | 23.3% |
| Larynx cancer | 47 (26-77) | 63 (34-102) | 32.5% | 1 (0-1) | 1 (0-1) | 2.0% |
| Trachea, bronchus, and lung cancers | 227 (154-317) | 355 (234-493) | 56.5% | 4(3-6) | 5 (3-7) | 20.5% |
| Breast cancer | 504 (351-714) | 898 (623-1268) | 78.0% | 10 (7-13) | 13 (9-18) | 37.0% |
| Cervical cancer | 99 (59-146) | 111 (64-160) | 11.8% | 2(1-3) | 2(1-2) | −14.0% |
| Uterine cancer | 47(25-82) | 68 (30-107) | 44.5% | 1 (0-2) | 1 (0-2) | 11.2% |
| Prostate cancer | 165 (109-249) | 464 (298-729) | 181.3% | 3(2-5) | 7 (4-11) | 116.4% |
| Colon and rectum cancers | 307 (223-411) | 564 (408-759) | 83.9% | 6 (4-8) | 8(6-11) | 41.5% |
| Mouth cancer | 63 (44-84) | 101 (71-136) | 61.6% | 1 (1-2) | 1 (1-2) | 24.3% |
| Nasopharynx cancer | 15 (9-23) | 25 (14-39) | 64.1% | <0.5 (0-0.5) | <0.5 (0-1) | 26.3% |
| Cancer of other part of pharynx and oropharynx | 29 (16-42) | 45 (25-67) | 53.3% | 1 (0-1) | 1 (0-1) | 18.0% |
| Gallbladder and biliary tract cancer | 19 (12-29) | 35 (20-53) | 81.2% | <0.5 (0-1) | 1 (0-1) | 39.5% |
| Pancreatic cancer | 23 (15-33) | 37 (23-54) | 59.4% | <0.5 (0-1) | 1 (0-1) | 22.6% |
| Malignant melanoma of skin | 24 (15-40) | 45 (25-74) | 84.5% | <0.5 (0-1) | 1 (0-1) | 42.0% |
| Non-melanoma skin cancer | 115 (85-148) | 255 (192-326) | 122.2% | 2(2-3) | 4(3-5) | 71.0% |
| Ovarian cancer | 41 (27-56) | 63 (39-89) | 53.8% | 1 (1-1) | 1 (1-1) | 18.3% |
| Testicular cancer | 8(4-12) | 12 (7-20) | 64.2% | <0.5 (0-0.5) | <0.5 (0-0.5) | 26.4% |
| Kidney and other urinary organ cancers | 32 (21-48) | 79 (52-118) | 143.4% | 1 (0-1) | 1 (1-2) | 87.3% |
| Bladder cancer | 81 (57-110) | 125 (85-171) | 54.6% | 2(1-2) | 2(1-2) | 18.9% |
| Brain and nervous system cancers | 57 (34-84) | 94 (51-134) | 63.3% | 1 (1-2) | 1(1-2) | 25.6% |
| Thyroid cancer | 21 (13-32) | 48 (28-75) | 132.4% | <0.5 (0-1) | 1 (0-1) | 78.8% |
| Hodgkin's disease | 13 (8-20) | 17 (10-27) | 25.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | −3.3% |
| Non-Hodgkin's lymphoma | 60 (42-80) | 110 (77-147) | 83.5% | 1 (1-2) | 2 (1-2) | 41.2% |
| Multiple myeloma | 22 (13-34) | 36 (21-53) | 64.9% | <0-5 (0-1) | 1 (0-1) | 26.9% |
| Leukaemia | 79 (53-110) | 123 (83-170) | 57.2% | 1 (1-2) | 2(1-2) | 20.9% |
| Other neoplasms | 99 (67-139) | 266 (174-366) | 168.2% | 2(1-3) | 4(3-5) | 106.4% |
| Cardiovascular and circulatory diseases | 14 373 (11 094-18 134) | 21 985 (16 947-27 516) | 53.0% | 271(209-342) | 319 (246-399) | 17.7% |
| Rheumatic heart disease | 1150 (765-1709) | 1430 (944-2067) | 24.3% | 22 (14-32) | 21 (14-30) | −4.4% |
| Valvular disease due to rheumatic heart disease | 861 (477-1429) | 1009 (557-1646) | 17.3% | 16 (9-27) | 15 (8-24) | −9.8% |
| Heart failure due to rheumatic heart disease | 290 (191-412) | 420 (278-592) | 45.1% | 5 (4-8) | 6 (4-9) | 11.6% |
| Ischaemic heart disease | 5952 (3679-8768) | 8795 (5447-12 806) | 47.8% | 112 (69-165) | 128 (79-186) | 13.7% |
| Myocardial infarction due to ischaemic heart disease | 29 (15-45) | 42 (22-67) | 45.5% | 1 (0-1) | 1 (0-1) | 11.9% |
| Angina due to ischaemic heart disease | 5030 (2942-7567) | 7234 (4232-10 986) | 43.8% | 95 (55-143) | 105 (61-159) | 10.7% |
| Heart failure due to ischaemic heart disease | 894 (609-1236) | 1518 (1038-2128) | 69.9% | 17 (11-23) | 22 (15-31) | 30.8% |
| Cerebrovascular disease | 2328 (1864-2837) | 4346 (3476-5298) | 86.7% | 44 (35-54) | 63 (50-77) | 43.6% |
| Ischaemic stroke | 1857 (1489-2263) | 3384 (2705-4121) | 82.2% | 35 (28-43) | 49 (39-60) | 40.2% |
| Ischaemic stroke (acute) | 77 (52-107) | 133 (90-183) | 72.8% | 1(1-2) | 2 (1-3) | 32.9% |
| Ischaemic stroke (chronic) | 1780 (1416-2187) | 3251 (2583-3999) | 82.6% | 34 (27-41) | 47 (37-58) | 40.5% |
| Haemorrhagic and other non-ischaemic stroke | 471(373-585) | 961 (769-1178) | 104.1% | 9 (7-11) | 14 (11-17) | 57.0% |
| Haemorrhagic non-ischaemic stroke (acute) | 28 (18-38) | 56 (37-78) | 104.1% | 1 (0-1) | 1 (1-1) | 57.1% |
| Haemorrhagic non-ischaemic stroke (chronic) | 444 (345-558) | 905 (717-1121) | 104.1% | 8 (7-11) | 13 (10-16) | 57.0% |
| Hypertensive heart disease | 292 (202-412) | 460 (315-639) | 57.4% | 6 (4-8) | 7 (5-9) | 21.1% |
| Cardiomyopathy and myocarditis | 272 (183-378) | 394 (269-551) | 44.8% | 5 (3-7) | 6 (4-8) | 11.4% |
| Acute myocarditis | 1 (0-1) | 1 (0-1) | 30.9% | <0.5 (0-0.5) | <0.5 (0-0.5) | 0.7% |
| Heart failure due to cardiomyopathy and myocarditis | 271 (182-378) | 393 (268-551) | 44.8% | 5 (3-7) | 6 (4-8) | 11.4% |
| Atrial fibrillation and flutter | 1433 (970-1987) | 2425 (1631-3382) | 69.2% | 27 (18-37) | 35 (24-49) | 30.2% |
| Peripheral vascular disease | 256 (132-453) | 419 (218-744) | 63.7% | 5 (2-9) | 6 (3-11) | 26.0% |
| Endocarditis | 42 (28-60) | 62 (42-87) | 46.1% | 1 (1-1) | 1 (1-1) | 12.4% |
| Endocarditis | <0.5 (0-1) | 1 (0-1) | 87.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | 44.4% |
| Heart failure due to endocarditis | 42 (28-59) | 61 (42-87) | 45.8% | 1 (1-1) | 1 (1-1) | 12.2% |
| Other cardiovascular and circulatory diseases | 2646 (1448-4148) | 3655(2053-5581) | 38.1% | 50 (27-78) | 53 (30-81) | 6.3% |
| Heart failure due to other circulatory diseases | 183 (123-259) | 268 (180-372) | 46.3% | 3 (2-5) | 4 (3-5) | 12.6% |
| Other cardiovascular and circulatory diseases | 2463 (1271-3992) | 3388 (1783-5346) | 37.5% | 46 (24-75) | 49 (26-78) | 5.8% |
| Chronic respiratory diseases | 34 976 (24 536-47 579) | 49 303 (33 874-67 087) | 41.0% | 660 (463-897) | 716 (492-974) | 8.5% |
| Chronic obstructive pulmonary disease | 20 097 (13 793-28 248) | 29 373 (19 850-41 822) | 46.2% | 379 (260-533) | 426 (288-607) | 12.5% |
| Chronic obstructive pulmonary disease | 19 805 (13 571-27 835) | 28 893 (19 455-41 183) | 45.9% | 374 (256-525) | 419 (282-598) | 12.3% |
| Heart failure due to chronic obstructive pulmonary disease | 292 (195-410) | 480 (316-678) | 64.1% | 6 (4-8) | 7 (5-10) | 26.3% |
| Pneumoconiosis | 212 (104-477) | 445 (193-1377) | 109.9% | 4(2-9) | 6(3-20) | 61.5% |
| Silicosis | 76 (22-199) | 136 (38-408) | 79.8% | 1 (0-4) | 2 (1-6) | 38.3% |
| Asbestosis | 44 (4-253) | 130(8-974) | 192.4% | 1(0-5) | 2 (0-14) | 125.0% |
| Coal workers' pneumoconiosis | 24 (10-43) | 33 (13-64) | 41.1% | <0.5 (0-1) | <0.5 (0-1) | 8.6% |
| Other pneumoconiosis | 43 (12-108) | 101 (26-261) | 135.2% | 1(0-2) | 1 (0-4) | 80.9% |
| Heart failure due to pneumoconiosis | 25 (17-36) | 45 (31-63) | 77.1% | <0.5 (0-1) | 1 (0-1) | 36.3% |
| Asthma | 10 835 (7247-15 268) | 13 835 (9286-19 487) | 27.7% | 204 (137-288) | 201 (135-283) | −1.7% |
| Interstitial lung disease and pulmonary sarcoidosis | 111 (68-182) | 162 (99-268) | 45.8% | 2(1-3) | 2 (1-4) | 12.2% |
| Interstitial lung disease and pulmonary sarcoidosis | 69 (37-134) | 99 (52-191) | 44.6% | 1(1-3) | 1 (1-3) | 11.3% |
| Heart failure due to interstitial lung disease and pulmonary sarcoidosis | 42 (28-60) | 62 (42-90) | 47.9% | 1 (1-1) | 1 (1-1) | 13.8% |
| Other chronic respiratory diseases | 3722 (2529-5177) | 5488 (3773-7675) | 47.5% | 70 (48-98) | 80 (55-111) | 13.5% |
| Cirrhosis of the liver | 455 (309-630) | 613 (415-862) | 34.8% | 9 (6-12) | 9(6-13) | 3.7% |
| Cirrhosis of the liver secondary to hepatitis B | 156 (93-235) | 198 (120-298) | 26.7% | 3 (2-4) | 3 (2-4) | −2.5% |
| Cirrhosis of the liver secondary to hepatitis C | 113 (67-170) | 155(93-235) | 37.5% | 2 (1-3) | 2(1-3) | 5.8% |
| Cirrhosis of the liver secondary to alcohol use | 109 (65-163) | 160 (94-241) | 46.6% | 2 (1-3) | 2(1-3) | 12.8% |
| Other cirrhosis of the liver | 77(46-118) | 101 (61-150) | 30.2% | 1(1-2) | 1 (1-2) | 0.2% |
| Digestive diseases (except cirrhosis) | 4467 (3265-5979) | 5473 (3916-7380) | 22.5% | 84 (62-113) | 79 (57-107) | −5.7% |
| Peptic ulcer disease | 355 (224-570) | 311 (196-485) | −12.3% | 7 (4-11) | 5 (3-7) | −32.5% |
| Peptic ulcer disease | 190 (111-328) | 154 (93-254) | −18.8% | 4(2-6) | 2 (1-4) | −37.5% |
| Anaemia due to peptic ulcer disease | 165(89-325) | 157 (87-273) | −4.8% | 3(2-6) | 2 (1-4) | −26.7% |
| Gastritis and duodenitis | 820 (498-1253) | 855 (525-1386) | 4.2% | 15 (9-24) | 12 (8-20) | −19.8% |
| Gastritis and duodenitis | 178 (120-259) | 182 (121-264) | 2.3% | 3(2-5) | 3 (2-4) | −21.3% |
| Anaemia due to gastritis and duodenitis | 642 (368-1046) | 673 (389-1174) | 4.7% | 12 (7-20) | 10(6-17) | −19.4% |
| Appendicitis | 154 (99-226) | 176 (112-257) | 14.3% | 3 (2-4) | 3 (2-4) | −12.0% |
| Paralytic ileus and intestinal obstruction without hernia | 10 (4-24) | 12 (6-27) | 24.4% | <0.5 (0-0.5) | <0.5 (0-0.5) | −4.2% |
| Inguinal or femoral hernia | 333 (144-689) | 441 (189-861) | 32.5% | 6(3-13) | 6(3-12) | 1.9% |
| Non-infective inflammatory bowel disease | 1582 (1130-2151) | 1795 (1272-2460) | 13.5% | 30 (21-41) | 26 (18-36) | −12.7% |
| Non-infective inflammatory bowel disease due to ulcerative colitis | 932 (583-1492) | 1169 (734-1801) | 25.4% | 18 (11-28) | 17 (11-26) | −3.5% |
| Non-infective inflammatory bowel disease due to Crohn's disease | 541 (356-775) | 513 (334-733) | −5.2% | 10 (7-15) | 7 (5-11) | −27.0% |
| Non-infective inflammatory bowel disease severe episodes due to ulcerative colitis | 54 (32-89) | 64 (39-102) | 18.6% | 1 (1-2) | 1(1-1) | −8.7% |
| Non-infective inflammatory bowel disease severe episodes due to Crohn's disease | 54 (31-91) | 50 (30-81) | −8.5% | 1 (1-2) | 1 (0-1) | −29.6% |
| Vascular disorders of intestine | 6 (4-10) | 14 (8-20) | 121.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | 70.5% |
| Gallbladder and bile duct disease | 314 (215-440) | 453 (310-635) | 44.6% | 6 (4-8) | 7 (4-9) | 11.2% |
| Pancreatitis | 133 (85-198) | 206 (131-303) | 54.9% | 3 (2-4) | 3 (2-4) | 19.2% |
| Other digestive diseases | 761 (553-1025) | 1209 (852-1638) | 58.9% | 14 (10-19) | 18 (12-24) | 22.3% |
| Neurological disorders | 29 389 (23 635-35 837) | 42 943 (34 605-52 115) | 46.1% | 554(446-676) | 623(502-756) | 12.4% |
| Alzheimer's disease and other dementias | 3785 (2720-5007) | 6801 (4898-9043) | 79.7% | 71 (51-94) | 99 (71-131) | 38.3% |
| Parkinson's disease | 356 (231-560) | 606 (396-964) | 70.5% | 7 (4-11) | 9(6-14) | 31.2% |
| Epilepsy | 6415 (4993-7799) | 8740 (6762-10 594) | 36.2% | 121 (94-147) | 127 (98-154) | 4.8% |
| Multiple sclerosis | 373 (276-473) | 524 (379-660) | 40.8% | 7 (5-9) | 8 (5-10) | 8.3% |
| Migraine | 15 927 (10 394-22 023) | 22 362 (14 395-31 121) | 40.4% | 300 (196-415) | 325(209-452) | 8.0% |
| Tension-type headache | 1266 (754-2016) | 1779 (1056-2822) | 40.5% | 24 (14-38) | 26 (15-41) | 8.1% |
| Other neurological disorders | 1267 (958-1616) | 2129 (1619-2723) | 68.0% | 24 (18-30) | 31(23-40) | 29.3% |
| Other neurological disorders | 1399 (1056-1789) | 2353 (1785-3011) | 68.2% | 26 (20-34) | 34 (26-44) | 29.4% |
| Guillain-Barre syndrome due to other neurological disorders | 2 (1-3) | 3 (2-4) | 35.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | 4.4% |
| Mental and behavioural disorders | 129 377 (106 771-154 032) | 176 626 (145 613-209 122) | 36.5% | 2440 (2014-2905) | 2564 (2113-3035) | 5.0% |
| Schizophrenia | 9760 (6186-13 369) | 14 400 (9160-19 752) | 47.5% | 184 (117-252) | 209 (133-287) | 13.5% |
| Alcohol use disorders | 10 470 (7173-14 644) | 13 826 (9248-19 212) | 32.1% | 197 (135-276) | 201 (134-279) | 1.6% |
| Alcohol dependence | 10 385 (7086-14 556) | 13 735 (9164-19 108) | 32.3% | 196 (134-275) | 199 (133-277) | 1.8% |
| Fetal alcohol syndrome | 85 (49-133) | 91 (55-138) | 6.9% | 2(1-3) | 1 (1-2) | −17.7% |
| Drug use disorders | 11 764 (8388-15 468) | 16 412 (11 836-21 583) | 39.5% | 222 (158-292) | 238 (172-313) | 7.3% |
| Opioid use disorders | 4812 (3350-6281) | 7170 (5143-9257) | 49.0% | 91 (63-118) | 104 (75-134) | 14.6% |
| Cocaine use disorders | 800 (475-1214) | 1085 (633-1639) | 35.7% | 15 (9-23) | 16 (9-24) | 4.4% |
| Amphetamine use disorders | 1894 (1067-2955) | 2596 (1460-3957) | 37.1% | 36 (20-56) | 38 (21-57) | 5.5% |
| Cannabis use disorders | 1693 (1105-2418) | 2057 (1348-2929) | 21.5% | 32 (21-46) | 30 (20-43) | −6.5% |
| Other drug use disorders | 2565 (1583-3817) | 3503 (2108-5170) | 36.6% | 48 (30-72) | 51 (31-75) | 5.1% |
| Unipolar depressive disorders | 54 010 (40 381-68 450) | 74 264 (55 670-94 240) | 37.5% | 1019 (762-1291) | 1078 (808-1368) | 5.8% |
| Major depressive disorder | 46 139 (34 517-58 427) | 63 179 (47 779-80 891) | 36.9% | 870 (651-1102) | 917 (693-1174) | 5.4% |
| Dysthymia | 7871 (5266-10 858) | 11 084 (7297-15 447) | 40.8% | 148 (99-205) | 161 (106-224) | 8.4% |
| Bipolar affective disorder | 9129 (5757-13 169) | 12 867 (8084-18 654) | 40.9% | 172 (109-248) | 187 (117-271) | 8.5% |
| Anxiety disorders | 19 664 (13 868-26 820) | 26 826 (18 779-36 795) | 36.4% | 371(262-506) | 389 (273-534) | 5.0% |
| Eating disorders | 1120 (749-1554) | 1956 (1316-2742) | 74.6% | 21 (14-29) | 28 (19-40) | 34.3% |
| Anorexia nervosa | 95 (65-136) | 188 (125-265) | 97.1% | 2(1-3) | 3 (2-4) | 51.7% |
| Bulimia nervosa | 1025 (687-1417) | 1768 (1183-2480) | 72.5% | 19 (13-27) | 26 (17-36) | 32.7% |
| Pervasive development disorders | 5918 (4133-8130) | 7666 (5355-10 565) | 29.5% | 112 (78-153) | 111 (78-153) | −0.3% |
| Autism | 3088 (2119-4260) | 4007 (2752-5563) | 29.8% | 58 (40-80) | 58 (40-81) | −0.2% |
| Asperger's syndrome | 2830 (1917-4016) | 3659 (2463-5150) | 29.3% | 53 (36-76) | 53 (36-75) | −0.5% |
| Childhood behavioural disorders | 5472 (3277-8359) | 6245 (3785-9347) | 14.1% | 103 (62-158) | 91 (55-136) | −12.2% |
| Attention-deficit hyperactivity disorder | 424 (244-667) | 491(280-775) | 15.8% | 8 (5-13) | 7 (4-11) | −10.9% |
| Conduct disorder | 5047 (2960-7840) | 5753 (3428-8748) | 14.0% | 95 (56-148) | 84 (50-127) | −12.3% |
| Idiopathic intellectual disability | 1247 (746-1924) | 1043 (572-1687) | −16.4% | 24 (14-36) | 15 (8-24) | −35.7% |
| Other mental and behavioural disorders | 822 (485-1307) | 1121 (661-1774) | 36.4% | 16 (9-25) | 16 (10-26) | 5.0% |
| Diabetes, urogenital, blood, and endocrine diseases | 38 626 (28 236-51 159) | 56 924 (42 172-75 399) | 47.4% | 729 (533-965) | 826 (612-1094) | 13.4% |
| Diabetes mellitus | 12 412 (8403-17 524) | 20 758 (14 415-28 762) | 67.2% | 234 (158-331) | 301 (209-417) | 28.7% |
| Uncomplicated diabetes mellitus | 4260 (2420-6828) | 6569 (3684-10 380) | 54.2% | 80 (46-129) | 95 (53-151) | 18.7% |
| Diabetic foot | 209 (108-356) | 308 (160-518) | 47.1% | 4 (2-7) | 4(2-8) | 13.2% |
| Diabetic neuropathy | 7325 (4967-10 520) | 11 914 (7977-17 035) | 62.6% | 138 (94-198) | 173 (116-247) | 25.2% |
| Amputation due to diabetes mellitus | 392 (192-669) | 905 (481-1427) | 130.9% | 7 (4-13) | 13 (7-21) | 77.6% |
| Vision loss due to diabetes mellitus | 227 (166-307) | 1062 (795-1395) | 368.6% | 4 (3-6) | 15 (12-20) | 260.6% |
| Acute glomerulonephritis | 1 (0-2) | 1 (0-2) | 1.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | −21.8% |
| Chronic kidney diseases | 2558 (1900-3288) | 4018 (2972-5204) | 57.1% | 48 (36-62) | 58 (43-76) | 20.9% |
| Chronic kidney disease due to diabetes mellitus | 621 (453-796) | 1003 (740-1317) | 61.5% | 12 (9-15) | 15 (11-19) | 24.2% |
| Stage IV chronic kidney disease due to diabetes mellitus | 88 (58-126) | 141 (94-205) | 60.5% | 2(1-2) | 2(1-3) | 23.5% |
| End-stage renal disease due to diabetes mellitus | 388 (270-506) | 626 (436-817) | 61.5% | 7 (5-10) | 9(6-12) | 24.2% |
| Anaemia due to chronic kidney disease stage III from diabetes mellitus | 145 (79-237) | 235 (126-378) | 62.0% | 3 (1-4) | 3(2-5) | 24.7% |
| Chronic kidney disease due to hypertension | 550 (411-711) | 872 (645-1123) | 58.4% | 10 (8-13) | 13 (9-16) | 21.9% |
| Stage IV chronic kidney disease due to hypertension | 88 (59-128) | 138 (93-198) | 56.7% | 2(1-2) | 2(1-3) | 20.6% |
| End-stage renal disease due to hypertension | 326 (225-422) | 515(359-670) | 58.0% | 6 (4-8) | 7(5-10) | 21.6% |
| Anaemia due to chronic kidney disease stage III from hypertension | 136 (77-222) | 219 (124-354) | 60.6% | 3 (1-4) | 3(2-5) | 23.6% |
| Chronic kidney disease unspecified | 1386 (1032-1800) | 2143(1585-2779) | 54.6% | 26 (19-34) | 31(23-40) | 19.0% |
| Stage IV unspecified or other chronic kidney disease | 229 (154-335) | 352 (233-506) | 53.9% | 4(3-6) | 5 (3-7) | 18.4% |
| End-stage renal disease from unspecified or other chronic kidney disease | 799 (555-1042) | 1242 (872-1607) | 55.5% | 15 (10-20) | 18 (13-23) | 19.6% |
| Anaemia due to unspecified or other chronic kidney disease stage III | 359 (208-579) | 549 (314-889) | 53.1% | 7 (4-11) | 8(5-13) | 17.8% |
| Urinary diseases and male infertility | 4651 (3057-7025) | 8188 (5398-11 978) | 76.0% | 88 (58-133) | 119 (78-174) | 35.5% |
| Tubulointerstitial nephritis, pyelonephritis, and urinary tract infections | 156 (84-269) | 207 (109-360) | 33.2% | 3 (2-5) | 3(2-5) | 2.5% |
| Urolithiasis | 480 (306-842) | 716 (447-1425) | 49.1% | 9 (6-16) | 10 (6-21) | 14.7% |
| Urolithiasis episodes | 204 (107-526) | 225 (104-918) | 10.5% | 4 (2-10) | 3(2-13) | −15.0% |
| Chronic urolithiasis | 277 (156-443) | 491(276-785) | 77.5% | 5 (3-8) | 7 (4-11) | 36.6% |
| Benign prostatic hyperplasia | 3726 (2392-5645) | 6834 (4377-10 179) | 83.4% | 70 (45-106) | 99 (64-148) | 41.1% |
| Male infertility | 126 (50-270) | 173 (70-365) | 36.9% | 2 (1-5) | 3(1-5) | 5.3% |
| Other urinary diseases | 162 (103-267) | 258 (167-415) | 58.8% | 3 (2-5) | 4(2-6) | 22.2% |
| Gynaecological diseases | 7671(4880-11715) | 10 042(6226-15619) | 30.9% | 145 (92-221) | 146 (90-227) | 0.7% |
| Uterine fibroids | 2341 (1568-3340) | 3037 (1967-4551) | 29.7% | 44 (30-63) | 44 (29-66) | −0.2% |
| Uterine fibroids | 934 (401-1852) | 1527(664-3059) | 63.5% | 18 (8-35) | 22 (10-44) | 25.8% |
| Anaemia due to uterine fibroids | 1407 (943-2023) | 1509 (1000-2199) | 7.3% | 27 (18-38) | 22 (15-32) | −17.4% |
| Polycystic ovarian syndrome | 2027 (971-3786) | 2756 (1312-5212) | 35.9% | 38 (18-71) | 40 (19-76) | 4.6% |
| Polycystic ovarian syndrome | 1982 (931-3747) | 2694 (1245-5171) | 35.9% | 37 (18-71) | 39 (18-75) | 4.6% |
| Infertility due to polycystic ovarian syndrome | 45 (18-95) | 62 (25-128) | 37.5% | 1(0-2) | 1 (0-2) | 5.8% |
| Female infertility | 91 (36-189) | 125 (50-259) | 37.6% | 2 (1-4) | 2 (1-4) | 5.9% |
| Endometriosis | 404 (142-738) | 544 (188-1007) | 34.6% | 8 (3-14) | 8(3-15) | 3.5% |
| Endometriosis | 388 (129-715) | 522 (166-974) | 34.4% | 7 (2-13) | 8(2-14) | 3.4% |
| Infertility due to endometriosis | 16(6-35) | 22 (9-46) | 37.8% | <0.5 (0-1) | <0.5 (0-1) | 6.0% |
| Genital prolapse | 1339 (544-2686) | 1811 (741-3649) | 35.2% | 25 (10-51) | 26 (11-53) | 4.1% |
| Premenstrual syndrome | 983 (49-2592) | 1249 (63-3337) | 27.0% | 19 (1-49) | 18 (1-48) | −2.3% |
| Other gynaecological diseases | 485 (330-703) | 520 (345-759) | 7.3% | 9(6-13) | 8 (5-11) | −17.4% |
| Haemoglobinopathies and haemolytic anaemias | 8271 (5746-11276) | 10 197 (7166-13 843) | 23.3% | 156 (108-213) | 148 (104-201) | −5.1% |
| Thalassaemias | 3725 (2499-5279) | 4636 (3098-6621) | 24.4% | 70 (47-100) | 67(45-96) | −4.2% |
| β-thalassaemia major | 31 (17-56) | 33 (19-59) | 7.9% | 1 (0-1) | <0.5 (0-1) | −16.9% |
| Haemoglobin E/β-thalassaemia | 22 (14-34) | 24 (15-37) | 8.8% | <0.5 (0-1) | <0.5 (0-1) | −16.3% |
| Haemoglobin H/β-thalassaemia | 10 (6-16) | 10 (6-16) | 3.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | −20.2% |
| Anaemia due to thalassaemias | 3653 (2427-5219) | 4557(3024-6530) | 24.7% | 69 (46-98) | 66 (44-95) | −4.0% |
| Heart failure due to thalassaemias | 10 (6-14) | 13 (8-19) | 34.1% | <0.5 (0-0.5) | <0.5 (0-0.5) | 3.2% |
| Sickle cell disorders | 2647 (1829-3615) | 3665 (2612-4949) | 38.5% | 50 (34-68) | 53 (38-72) | 6.5% |
| Homozygous sickle cell and severe sickle cell/β-thalassaemia | 334 (238-441) | 546 (389-736) | 63.8% | 6 (4-8) | 8(6-11) | 26.1% |
| Haemoglobin sickle cell disorders | 78 (54-107) | 130 (88-182) | 65.5% | 1 (1-2) | 2(1-3) | 27.3% |
| Mild sickle cell/β-thalassaemia | 26 (18-37) | 38 (27-53) | 43.8% | <0.5 (0-1) | 1 (0-1) | 10.6% |
| Anaemia due to sickle cell disorders | 2211 (1439-3181) | 2954 (1957-4240) | 33.6% | 42 (27-60) | 43 (28-62) | 2.8% |
| G6PD deficiency | 120 (81-174) | 146 (97-210) | 22.1% | 2(2-3) | 2(1-3) | −6.1% |
| Anaemia due to G6PD deficiency | 116 (77-171) | 141(93-205) | 21.5% | 2(1-3) | 2(1-3) | −6.5% |
| Heart failure due to G6PD deficiency | 4 (2-6) | 5(3-8) | 40.2% | <0.5 (0-0.5) | <0.5 (0-0.5) | 7.8% |
| Other haemoglobinopathies and haemolytic anaemias | 1779 (1193-2565) | 1750 (1171-2503) | −1.6% | 34 (23-48) | 25 (17-36) | −24.3% |
| Anaemia due to other haemoglobinopathies and haemolytic anaemias | 1757 (1173-2545) | 1720 (1138-2483) | −2.1% | 33 (22-48) | 25 (17-36) | −24.7% |
| Heart failure due to other haemoglobinopathies and haemolytic anaemias | 22 (14-32) | 31(20-45) | 39.5% | <0.5 (0-1) | <0.5 (0-1) | 7.4% |
| Other endocrine, nutritional, blood, and immune disorders | 3063 (2256-4177) | 3721 (2713-5114) | 21.5% | 58 (43-79) | 54 (39-74) | −6.5% |
| Other endocrine, nutritional, blood, and immune disorders | 1254 (739-1952) | 1919 (1133-2991) | 53.0% | 24 (14-37) | 28 (16-43) | 17.7% |
| Anaemia due to other endocrine, nutritional, blood, and immune disorders | 1777 (1187-2562) | 1756 (1166-2542) | −1.2% | 34 (22-48) | 25 (17-37) | −23.9% |
| Heart failure due to other endocrine, nutritional, blood, and immune disorders | 33 (22-47) | 48 (33-69) | 46.1% | 1 (0-1) | 1 (0-1) | 12.4% |
| Musculoskeletal disorders | 114 719 (87 053-145 247) | 165 955 (126 364-208 779) | 44.7% | 2164 (1642-2740) | 2409 (1834-3030) | 11.3% |
| Rheumatoid arthritis | 2566 (1831-3381) | 3776 (2672-4954) | 47.1% | 48 (35-64) | 55(39-72) | 13.2% |
| Osteoarthritis | 10 449 (7100-14 788) | 17 135 (11 884-24 256) | 64.0% | 197 (134-279) | 249 (172-352) | 26.2% |
| Osteoarthritis of the hip | 1821 (1200-2616) | 2917 (1945-4389) | 60.2% | 34 (23-49) | 42 (28-64) | 23.2% |
| Osteoarthritis of the knee | 8627 (5929-12 276) | 14 218 (9809-19 968) | 64.8% | 163 (112-232) | 206 (142-290) | 26.8% |
| Low back and neck pain | 82 111 (56 962-110 433) | 116 704 (80 615-156 527) | 42.1% | 1549 (1074-2083) | 1694 (1170-2272) | 9.4% |
| Low back pain | 58 245 (39 934-78 139) | 83 063 (56 632-111 880) | 42.6% | 1099 (753-1474) | 1206 (822-1624) | 9.7% |
| Neck pain | 23 866 (16 535-33 105) | 33 640 (23 469-46 476) | 41.0% | 450 (312-624) | 488 (341-675) | 8.5% |
| Gout | 76 (48-112) | 114 (72-167) | 49.3% | 1 (1-2) | 2(1-2) | 14.9% |
| Other musculoskeletal disorders | 19 517 (16 148-22 127) | 28 226 (23 201-31 884) | 44.6% | 368 (305-417) | 410 (337-463) | 11.3% |
| Other non-communicable diseases | 66 478 (45 586-97 937) | 86 771 (59 561-128 605) | 30.5% | 1254 (860-1847) | 1259 (864-1867) | 0.4% |
| Congenital anomalies | 2620(2088-3333) | 3279 (2594-4167) | 25.2% | 49 (39-63) | 48 (38-60) | −3.7% |
| Neural tube defects | 569 (330-901) | 754 (439-1142) | 32.6% | 11 (6-17) | 11 (6-17) | 2.0% |
| Congenital heart anomalies | 498 (350-711) | 563 (388-804) | 13.0% | 9 (7-13) | 8(6-12) | −13.0% |
| Congenital heart anomalies | 189 (86-354) | 226 (103-425) | 19.1% | 4 (2-7) | 3 (1-6) | −8.3% |
| Heart failure due to congenital heart anomalies | 308 (203-442) | 337 (221-486) | 9.3% | 6 (4-8) | 5 (3-7) | −15.9% |
| Ccenter lip and ccenter palate | 259 (180-362) | 254 (181-346) | −1.7% | 5 (3-7) | 4(3-5) | −24.4% |
| Down's syndrome | 462 (306-664) | 627 (425-888) | 35.7% | 9(6-13) | 9(6-13) | 4.4% |
| Other chromosomal abnormalities | 191 (127-274) | 276 (182-392) | 44.1% | 4(2-5) | 4(3-6) | 10.8% |
| Turner syndrome | 3 (1-6) | 4(2-8) | 35.6% | <0.5 (0-0.5) | <0.5 (0-0.5) | 4.3% |
| Klinefelter syndrome | 5 (2-10) | 6 (3-14) | 38.7% | <0.5 (0-0.5) | <0.5 (0-0.5) | 6.8% |
| Chromosomal unbalanced rearrangements | 184 (123-261) | 265 (176-377) | 44.3% | 3(2-5) | 4 (3-5) | 11.1% |
| Other congenital anomalies | 642 (464-868) | 806 (596-1053) | 25.6% | 12 (9-16) | 12 (9-15) | −3.3% |
| Other congenital anomalies | 437 (325-580) | 595 (446-775) | 36.4% | 8 (6-11) | 9 (6-11) | 4.9% |
| Hearing loss due to other congenital anomalies | 225 (142-336) | 240 (152-363) | 6.6% | 4 (3-6) | 3 (2-5) | −17.9% |
| Skin and subcutaneous diseases | 26 273 (16 798-40 932) | 33 744 (21 503-52 280) | 28.4% | 496 (317-772) | 490 (312-759) | −1.2% |
| Eczema | 6890 (3508-10 872) | 8897 (4518-14 049) | 29.1% | 130 (66-205) | 129 (66-204) | −0.6% |
| Psoriasis | 742 (371-1179) | 1059 (528-1690) | 42.8% | 14 (7-22) | 15 (8-25) | 9.8% |
| Cellulitis | 302 (126-648) | 376 (163-831) | 24.5% | 6 (2-12) | 5(2-12) | −4.2% |
| Abscess, impetigo, and other bacterial skin diseases | 1038 (473-2016) | 1322 (599-2511) | 27.4% | 20(9-38) | 19 (9-36) | −1.9% |
| Impetigo | 871 (417-1624) | 1088 (514-2048) | 24.9% | 16 (8-31) | 16 (7-30) | −3.9% |
| Abscess and other bacterial skin diseases cases | 167 (54-386) | 235 (78-539) | 40.8% | 3 (1-7) | 3(1-8) | 8.3% |
| Scabies | 1881 (956-3384) | 1580 (807-2792) | −16.0% | 35 (18-64) | 23 (12-41) | −35.4% |
| Fungal skin diseases | 1618 (532-3754) | 2303 (740-5435) | 42.3% | 31 (10-71) | 33 (11-79) | 9.5% |
| Viral skin diseases | 2354(1058-4369) | 2731(1203-4941) | 16.0% | 44 (20-82) | 40 (17-72) | −10.7% |
| Molluscum contagiosum | 289 (88-702) | 270(85-645) | −6.6% | 5(2-13) | 4 (1-9) | −28.1% |
| Viral warts | 2065 (816-3960) | 2461 (984-4720) | 19.2% | 39 (15-75) | 36 (14-69) | −8.3% |
| Acne vulgaris | 3281 (1545-6205) | 4002 (1869-7575) | 22.0% | 62 (29-117) | 58 (27-110) | −6.2% |
| Alopecia areata | 1002 (313-1906) | 1352 (424-2567) | 35.0% | 19 (6-36) | 20 (6-37) | 3.9% |
| Pruritus | 1433 (682-2676) | 2086 (1004-3951) | 45.6% | 27 (13-50) | 30 (15-57) | 12.1% |
| Urticaria | 1968 (757-3431) | 2600 (980-4441) | 32.1% | 37 (14-65) | 38 (14-64) | 1.6% |
| Decubitus ulcer | 320 (165-524) | 476 (237-779) | 48.8% | 6 (3-10) | 7 (3-11) | 14.5% |
| Other skin and subcutaneous diseases | 3445 (1638-6437) | 4961 (2324-9239) | 44.0% | 65 (31-121) | 72 (34-134) | 10.8% |
| Sense organ diseases | 25 169 (18 140-35 220) | 34 733 (25 167-47 663) | 38.0% | 475 (342-664) | 504 (365-692) | 6.2% |
| Glaucoma | 443 (338-561) | 943 (725-1178) | 112.7% | 8(6-11) | 14 (11-17) | 63.7% |
| Cataracts | 4225 (3283-5364) | 4732 (3647-6010) | 12.0% | 80 (62-101) | 69 (53-87) | −13.8% |
| Macular degeneration | 513 (388-647) | 1329 (1026-1668) | 158.9% | 10 (7-12) | 19 (15-24) | 99.2% |
| Refraction and accommodation disorders | 3608 (2688-4762) | 5593 (4117-7468) | 55.0% | 68 (51-90) | 81 (60-108) | 19.3% |
| Other hearing loss | 12 211 (7258-19 495) | 15 761 (9455-25 210) | 29.1% | 230 (137-368) | 229 (137-366) | −0.7% |
| Other vision loss | 4069 (2171-7180) | 6240(3260-11208) | 53.4% | 77 (41-135) | 91(47-163) | 18.0% |
| Other sense organ diseases | 100 (34-231) | 136 (46-309) | 35.4% | 2 (1-4) | 2 (1-4) | 4.2% |
| Oral disorders | 12 417 (6824-20 984) | 15 015 (7795-26 482) | 20.9% | 234 (129-396) | 218 (113-384) | −7.0% |
| Dental caries | 3704 (1523-7150) | 4984(2086-9356) | 34.5% | 70 (29-135) | 72 (30-136) | 3.5% |
| Dental caries of baby teeth | 403 (164-774) | 425 (172-818) | 5.7% | 8 (3-15) | 6(3-12) | −18.7% |
| Dental caries of permanent teeth | 3302 (1347-6455) | 4559 (1907-8554) | 38.1% | 62 (25-122) | 66 (28-124) | 6.2% |
| Periodontal disease | 3440 (1310-7305) | 5410 (2051-11 286) | 57.3% | 65 (25-138) | 79 (30-164) | 21.0% |
| Edentulism | 5273 (3100-8127) | 4621 (2678-7296) | −12.4% | 99 (58-153) | 67 (39-106) | −32.6% |
| Injuries | 34 068 (24 209-47 034) | 47162 (32 958-66 050) | 38.4% | 643 (457-887) | 685 (478-959) | 6.5% |
| Transport injuries | 12062 (8524-16826) | 16 268 (11 304-22 717) | 34.9% | 228 (161-317) | 236 (164-330) | 3.8% |
| Road injury | 10 363 (7315-14 487) | 13 485 (9362-18 950) | 30.1% | 195 (138-273) | 196 (136-275) | 0.1% |
| Pedestrian injury by road vehicle | 3106 (2183-4360) | 4520 (3139-6367) | 45.6% | 59 (41-82) | 66(46-92) | 12.0% |
| Pedal cycle vehicle | 755 (536-1056) | 1025 (714-1436) | 35.7% | 14 (10-20) | 15 (10-21) | 4.4% |
| Motorised vehicle with two wheels | 1750 (1234-2435) | 2224 (1529-3133) | 27.1% | 33 (23-46) | 32 (22-45) | −2.2% |
| Motorised vehicle with three or more wheels | 4138 (2901-5793) | 5792 (4041-8114) | 40.0% | 78 (55-109) | 84 (59-118) | 7.7% |
| Road injury other | 1440 (1 013-2 020) | 1196 (824-1673) | −16.9% | 27 (19-38) | 17 (12-24) | −36.1% |
| Other transport injury | 1699 (1184-2386) | 2783 (1902-3872) | 63.8% | 32(22-45) | 40(28-56) | 26.0% |
| Unintentional injuries other than transport injuries | 19 036 (13 233-26 794) | 26 620 (18 472-37 641) | 39.8% | 359 (250-505) | 386 (268-546) | 7.6% |
| Falls | 13 324 (9110-18 725) | 19 459 (13 559-27 481) | 46.0% | 251(172-353) | 282 (197-399) | 12.4% |
| Drowning | 233 (161-326) | 281 (191-391) | 20.9% | 4 (3-6) | 4(3-6) | −7.0% |
| Fire, heat, and hot substances | 1010 (637-1575) | 1398 (857-2232) | 38.4% | 19 (12-30) | 20 (12-32) | 6.5% |
| Poisonings | 323(210-470) | 417(276-621) | 29.3% | 6 (4-9) | 6(4-9) | −0.5% |
| Exposure to mechanical forces | 922 (599-1387) | 1021 (662-1490) | 10.8% | 17 (11-26) | 15 (10-22) | −14.7% |
| Mechanical forces (firearm) | 526 (346-784) | 467 (305-676) | −11.2% | 10 (7-15) | 7(4-10) | −31.7% |
| Mechanical forces (other) | 710 (460-1074) | 910 (588-1336) | 28.2% | 13 (9-20) | 13(9-19) | −1.3% |
| Adverse effects of medical treatment | 585 (401-824) | 1088 (727-1537) | 85.9% | 11 (8-16) | 16 (11-22) | 43.1% |
| Animal contact | 437 (293-634) | 234 (154-329) | −46.4% | 8 (6-12) | 3(2-5) | −58.7% |
| Animal contact (venomous) | 355 (233-526) | 168 (110-242) | −52.5% | 7 (4-10) | 2 (2-4) | −63.4% |
| Animal contact (non-venomous) | 82 (55-119) | 66 (44-93) | −20.0% | 2(1-2) | 1 (1-1) | −38.4% |
| Unintentional injuries not classified elsewhere | 2202 (1484-3108) | 2720 (1866-3827) | 23.5% | 42 (28-59) | 39 (27-56) | −4.9% |
| Self-harm and interpersonal violence | 1571(1066-2188) | 1985 (1366-2726) | 26.4% | 30 (20-41) | 29 (20-40) | −2.8% |
| Self-harm | 308 (209-428) | 407 (278-577) | 32.3% | 6 (4-8) | 6 (4-8) | 1.8% |
| Interpersonal violence | 1263 (840-1775) | 1578 (1085-2180) | 24.9% | 24 (16-33) | 23(16-32) | −3.9% |
| Assault by firearm | 504 (336-714) | 587(404-808) | 16.5% | 10 (6-13) | 9(6-12) | −10.4% |
| Assault by sharp object | 368 (245-516) | 540 (369-743) | 46.8% | 7(5-10) | 8 (5-11) | 12.9% |
| Assault by other means | 543(362-758) | 678 (464-940) | 25.0% | 10 (7-14) | 10 (7-14) | −3.9% |
| Forces of nature, war, and legal intervention | 1399 (903-2080) | 2289 (1550-3341) | 63.6% | 26 (17-39) | 33 (22-48) | 25.9% |
| Exposure to forces of nature | 173 (110-269) | 2187 (1480-3210) | 1164.7% | 3(2-5) | 32 (21-47) | 873.1% |
| Collective violence and legal intervention | 1226 (779-1854) | 102 (66-153) | −91.7% | 23 (15-35) | 1 (1-2) | −93.6% |
Data are YLDs (95% uncertainty interval) or percentage change (%A). G6PD=glucose-6-phosphate dehydrogenase deficiency. Ecoli=Escherichia coli. H influenzae=Haemophilus influenzae. S pneumoniae=Streptococcus pneumoniae.
Mental and behavioural disorders accounted for 22·7% of all YLDs. YLDs for the category as a whole have increased by 37% from 1990 to 2010 from 129 million to 177 million and rates have also increased slightly by 5% over the two decades (from 2440 per 100 000 people to 2564 per 100 000 people). Within this category, six disorders or clusters of disorders accounted for more than 10 million YLDs each. The largest category was depressive disorders: major depressive disorder caused 63 million YLDs and dysthymia caused 11 million YLDs—together accounting for 9·6% of all YLDs. Schizophrenia, alcohol use disorders, drug use disorders, and bipolar disorder accounted for 12·9–16·4 million YLDs. Anxiety disorders were also a major global cause, contributing 3·5% of all YLDs. Another important category of diseases causing YLDs was diabetes, urogenital, blood, and endocrine diseases, which accounted for 56·9 million YLDs. Major causes included diabetes mellitus (20·8 million YLDs), benign prostatic hyperplasia (6·8 million YLDs), gynaecological disorders (10·0 million YLDs), and haemoglobinopathies and haemolytic anaemias (10·2 million YLDs). Together, musculoskeletal disorders caused 21·3% of all YLDs. The main contributors were low back pain (83·1 million YLDs), neck pain (33·6 million YLDs), osteoarthritis (17·1 million YLDs), and the other musculoskeletal category (28·2 million YLDs). Osteoarthritis of the knee accounted for 83% of the total osteoarthritis burden. We included the assessment of 13 separate skin diseases. Collectively they caused 33·7 million YLDs, with the largest cause being eczema followed by acne vulgaris. Many of the skin diseases have low disability weights but because of very high prevalences, they still accounted for a substantial number of YLDs. Oral disorders combined caused 15·0 million YLDs, with about equal shares caused by dental caries, periodontal disease, and edentulism. Injuries collectively caused 6·1% of global YLDs. Falls accounted for 41% of the total YLDs caused by injuries. The other major contributors were road injuries, causing 13·5 million YLDs.
Between 1990 and 2010, the total number of YLDs increased by 194 million—a 33·3% increase. We have decomposed this change into three components (table 3): growth in total population, ageing of the global population, and changes in the YLD rates. We have decomposed change both for YLDs from all causes and also for the three broad cause groups. For YLDs from all causes, population growth alone led to a 30·1% increase in YLDs and population ageing led to a further 10·9% increase in YLDs. Reductions in age-sex specific prevalence rates would have reduced YLDs by 7·7%, leading to an overall increase of about a third. Examination of change by the three broad groups shows distinct patterns. Age-specific and sex-specific YLD rates for communicable, maternal, neonatal, and nutritional disorders have decreased, and alone would have led to a 27·9% decrease in YLDs. Overall, YLDs from this cluster of causes increased, slightly, by 4·6% because of population growth, which increased more in the regions with the highest YLDs from these causes. For non-communicable diseases, the overall increase has been 40·3%, but this increase was driven by both population growth and population ageing, with very small decreases in prevalence rates. For injuries we saw a similar pattern, except that the decrease in age-sex specific rates would have caused a 9·1% decline.
Table 3:
Decomposition analysis of the change of global years lived with disability (thousands) by level 1 causes from 1990 to 2010 into total population growth, population ageing, and changes in age-specifi c, sex-specifi c, and cause-specifi c years lived with disability rates
| All causes | Communicable, maternal, neonatal, and nutritional disorders |
Non-communicable diseases |
Injuries | |
|---|---|---|---|---|
| 1990 YLDs (thousands) | 583393 | 113925 | 435400 | 34068 |
| YLDs expected with 2010 population, 1990 population age structure, and 1990 YLD rates (thousands) | 759024 | 158213 | 557726 | 43084 |
| YLDs expected with 2010 population, 2010 population age structure, and 1990 YLD rates (thousands) | 822452 | 150982 | 621220 | 50250 |
| 2010 YLDs (thousands) | 777401 | 119164 | 611076 | 47162 |
| Percentage change from 1990 due to population growth | 30.1% | 38.9% | 28.1% | 26.5% |
| Percentage change from 1990 due to population ageing | 10.9% | −6.3% | 14.6% | 21.0% |
| Percentage change from 1990 due to change in YLD rates | −7.7% | −27.9% | −2.3% | −9.1% |
| Percentage change from 1990 to 2010 | 33.3% | 4.6% | 40.3% | 38.4% |
YLD=years lived with disability.
For all causes of YLDs combined, the small decrease expected because of changes in age-specific and sex- specific YLDs per person of 7·7% shown in table 3 can also be seen in figure 3, which shows age-specific YLDs per person in 1990 and 2010 for both sexes. Values in figure 3 can be interpreted as the fraction of health lost to short-term and long-term disabling sequelae in each age group. As expected, the YLDs per person rose with age; YLDs per person aged 5 years were 5·4%, rising to28·6% (28·2% for women; 29·4% for men) for individuals aged 80 years. Female YLDs per person were higher than male YLDs for individuals aged 10–60 years at the global level; the difference is highest for individuals aged 30 years, when YLDs per woman were 1·4 percentage points higher than YLDs per man. The decrease in overall YLDs per person over the 20 year period (between 1990 and 2010) was much smaller than the approximate 20% decrease in mortality.68
Figure 3:
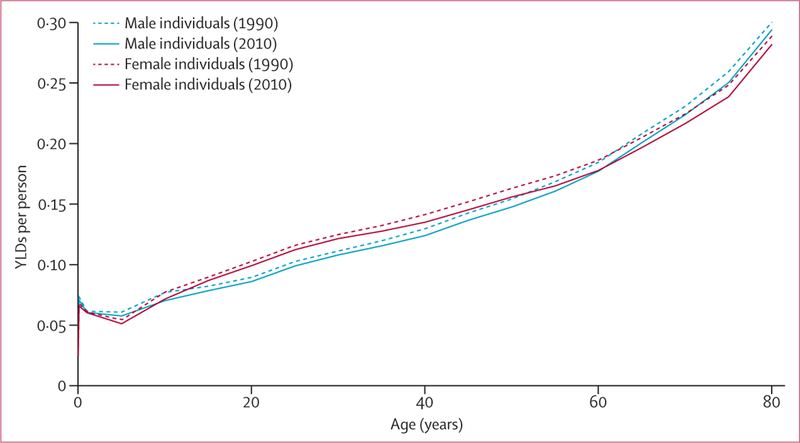
Global years lived with disability (YLDs) per person in 1990 and 2010 for all ages, by sex
Faster rates of increase in YLDs for non-communicable diseases led to their share of total YLDs increasing from 74·6% in 1990 to 78·6% in 2010. Causes are ordered by their mean rank across 1000 draws. The order based on the mean rank across draws is not the same as the order based on the mean value of YLDs. The 25 most common causes in 1990 and 2010 are shown in figure 4. Non-communicable diseases were the most common cause of YLDs (figure 4); 21 of the 25 leading causes are from non-communicable diseases, up from 19 of the 25 most common in 1990. The four leading causes in 2010 were also the four leading causes in 1990: low back pain, major depressive disorder, iron-deficiency anaemia, and neck pain. COPD increased from sixth to fifth, and anxiety and migraine retained the same ranking as in 1990 (figure 4). Other notable changes over the time period include the drop in the ranking of asthma, although the number of YLDs it caused increased by 28%. Road injury YLDs also increased but to a lesser extent than the increase in many of the non-communicable diseases, meaning that it also dropped in the rank list. We detected larger decreases in the rank of diarrhoea and tuberculosis than the other 25 most common causes in 1990.
Figure 4:
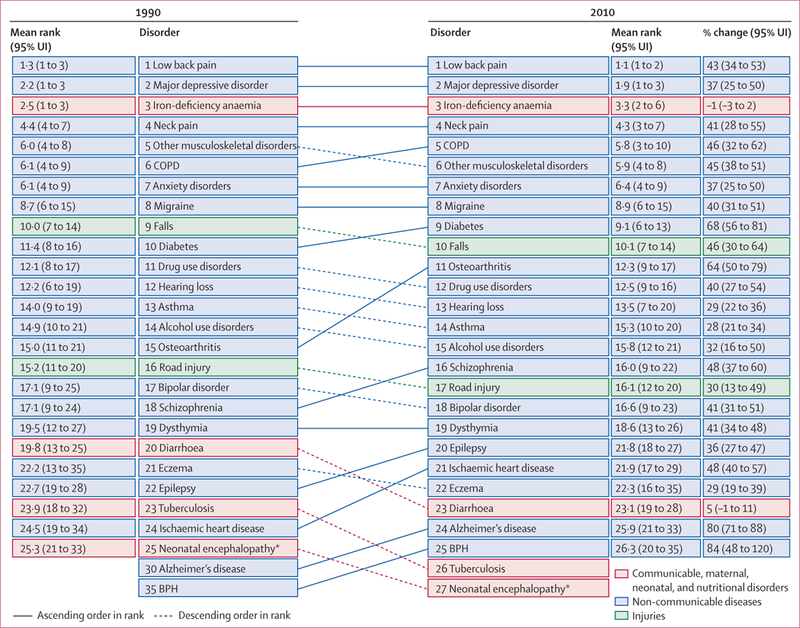
Global years lived with disability (YLDs) ranks with 95% uncertainty intervals (UI) for the 25 most common causes in 1990 and 2010. COPD=chronic obstructive pulmonary disease. BPH=benign prostatic hyperplasia. *Includes birth asphyxia/trauma. An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional.
The appendix (pp 280–88) shows YLDs per person by age and sex for the 21 GBD regions in 2010 and 1990. In general, in almost all age groups, the lowest YLDs per person were in the high-income Asia Pacific and east Asia regions. Western Europe and Australasia had the next lowest levels of YLDs per person, with rates of YLDs typically 10·15% lower than in high-income North America for most age groups. We estimate that the highest levels of YLDs per person were in the Caribbean, Oceania, and sub-Saharan Africa, particularly in the age groups affected by HIV in southern sub-Saharan Africa. The ratio of YLDs per person, comparing regions with the highest rates to the lowest rates, ranges from 9·71 in post-neonatal boys to 1·39 in men aged 80 years or older. This range is much smaller than we saw for YLLs across the same region-age-sex groups (the highest being 84·90 in male individuals aged 1–4 years and the lowest being 2·04 in male individuals aged 80 years or older).
Figure 5 shows how the broad composition of the causes of YLDs varied by region in 2010. At the 21 cause-group level, which is level 2 in the GBD cause hierarchy,29 we detected a clear association between the demographic and epidemiological transition. Mental and behavioural, musculoskeletal, other non-communicable, and chronic respiratory causes were consistently important in all regions. Some causes played a much more important part in regions that are less advanced in the demographic and epidemiological transition as measured by the mean age of death.86 HIV/AIDS and tuberculosis, neglected tropical diseases, and nutritional deficiencies stand out as being the most variable. For example, neglected tropical diseases and malaria ranged from 11·4% of total YLDs in western sub-Saharan Africa to less than 0·01% in high- income North America. Injuries have made a greater contribution to overall disability, in percentage terms, in those regions that are more advanced in the demographic and epidemiological transition. The contribution of stroke and diabetes, urogenital, blood, and endocrine diseases also increased with the demographic and epidemiological transition. Cardiovascular diseases did not contribute more than 5% of YLDs. The large fraction in the Caribbean attributable to war and disaster in 2010 is related to the Haiti earthquake.
Figure5:
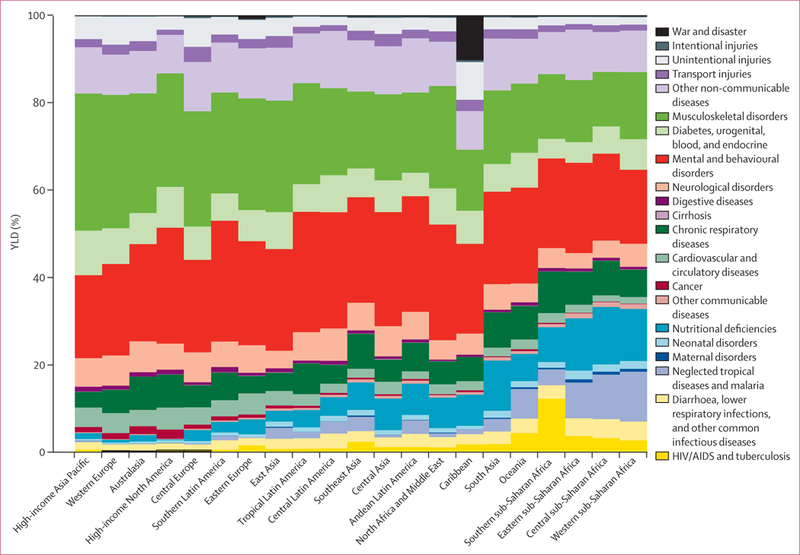
Percentage of years lived with disability (YLDs) by 21 major cause groupings and region for 2010 An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional.
Figure 6 shows how the leading causes of YLDs varied by region in 2010. Causes were included if they were in the 25 most common globally or in the 25 most common for any region. By contrast with a similar analysis for YLLs,68 we recorded much consistency in the ranking of causes of YLDs for the 15 most common causes, with the exception of iron-deficiency anaemia, which was the third most common cause globally. Iron-deficiency anaemia ranged from the most common cause in sub-Saharan Africa (western, eastern, and cent ral) to the 88th most common cause in high-income North America. Other causes that were highly variable across regions included malaria, cataracts, hookworm disease, sickle cell anaemia, thalassaemia, lymphatic filariasis, onchocerciasis, and schistosomiasis. The consistency of ranks for most major causes is related to the comparatively small variation in the prevalence of major mental and behavioural disorders and musculoskeletal disorders across different regions of the world.
Figure 6:
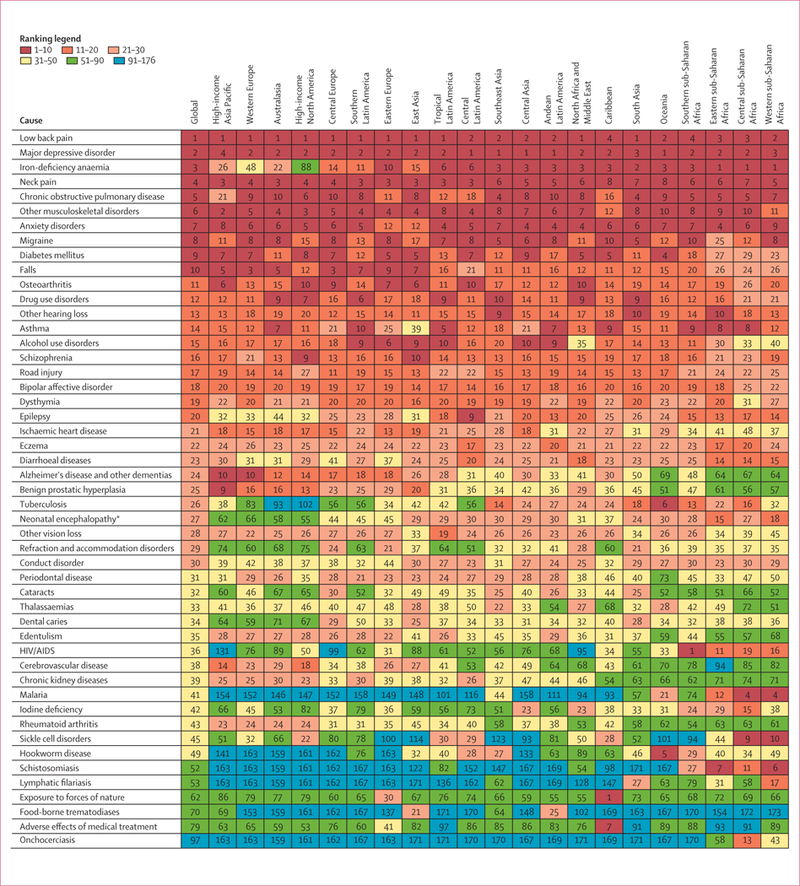
Variation in the leading causes of years lived with disability (YLDs), by region, in 2010 Causes in the figure are ordered according to global ranks for causes. The figure shows all causes that are in the 25 leading causes in at least one region. Ranks are also colour shaded to indicate rank intervals. *Includes birth asphyxia/trauma. An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional.
Injuries accounted for a total of 47·2 million YLDs in 2010, up from 34·1 million in 1990. Table 2 provides the results of YLDs for each external cause of injury (see appendix pp 37–270 for more detailed results by age and sex). In terms of external causes, falls and road injuries combined accounted for more than two-thirds (69·8%) of all YLDs due to injuries. YLDs from injuries stem from the nature of the injury rather than the external cause. Figure 7 shows the global breakdown of the nature of injury by age. In terms of the nature of injury that health services should address, 52·3% of YLDs were accounted for by the following: lacerations, multiple wounds, other dislocations, and eye injuries; fractures of the patella, fibula, tibia, or ankle; and moderate-to-severe traumatic brain injury. The number of YLDs from lacerations, multiple wounds, other dislocations, and eye injuries stemmed from the large numbers of people who had this type of injury and the evidence from follow-up studies that some individuals have long-term decreases in functioning. More severe injuries such as spinal cord injury are much less common according to the hospital and non-hospital data for external cause and nature of injury, even though they have more severe long-term consequences for individuals affected. The age pattern shows a slow rise by age of the fraction of the nature of injury due to fractures of the sternum, face, and pelvis. The percentage due to burns decreased with age as did moderate to severe brain trauma. (figure 7).
Figure 7:
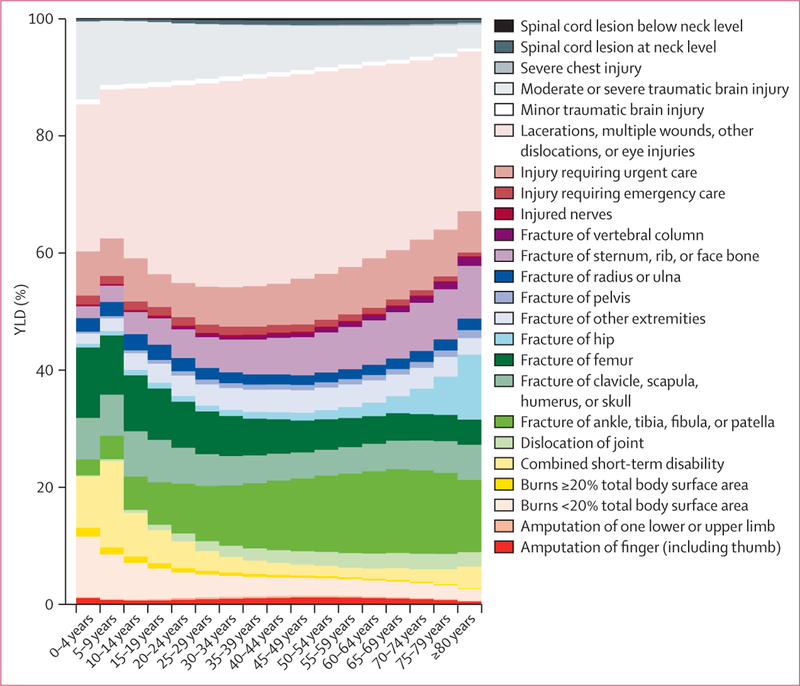
Global years lived with disability (YLDs) for injury in 2010, by type of injury and age
An important innovation in GBD 2010 was the assessment of selected impairments overall as well as their attribution by cause. The results of the impairment analysis are not easily discernible in table 2 because the burden is distributed across multiple disease or injury sequelae. Anaemia was perhaps the most important of these disorders in terms of its overall contribution to global YLDs. The burden of anaemia overall was large—68·2 million YLDs or almost a tenth (8·8%) of all YLDs worldwide, showing the high prevalence as well as the moderately severe disability weight especially for severe anaemia. By far the most important contributor to this health loss was iron-deficiency anaemia, which accounted for 62·2% of anaemia YLDs globally. However, our assessment of iron-deficiency anaemia was based on the results of iron supplementation trials which by their nature will capture both iron deficiency anaemia due to inadequate dietary intake but also some anaemia due to blood loss that is iron sensitive. The second leading specific cause of anaemia YLDs was thalassaemia (6·7% of total anaemia YLDs) followed by malaria (4·9%). Hookworm and sickle cell anaemia together account for a further 7·2%. Figure 8 shows the YLD rate per 100 000 individuals across regions; YLD rates varied from nearly 2300 in central sub-Saharan Africa to less than 300 in high-income North America. The cause composition of anaemia YLDs also varied across regions. In sub-Saharan Africa, higher anaemia rates were caused mainly by malaria, hookworm, schistosomiasis, sickle cell anaemia, and higher iron-deficiency anaemia. South Asia had the highest rates after sub-Saharan Africa, with the largest contributor being iron-deficiency anaemia. Although in absolute terms not a major cause of global anaemia, chronic kidney diseases accounted for a substantial proportion of anaemia burden in high-income regions.
Figure 8:
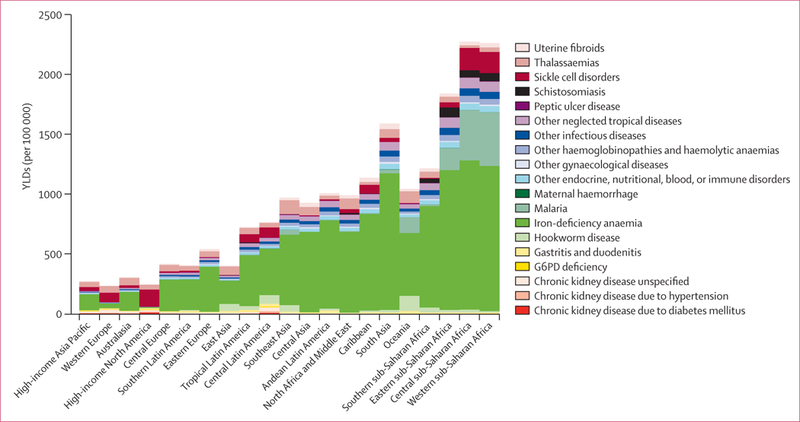
Years lived with disability (YLD) estimates for anaemia in 2010, by cause and region
Left-side and right-side heart failure was another impairment that was included in the GBD cause-sequelae list in many locations. Worldwide, we recorded an estimated 37·7 million cases of prevalent heart failure in 2010, leading to 4·2 million YLDs. This assessment of heart failure includes only symptomatic heart failure and does not include the large number of individuals with pre-symptomatic disease. For those with symptoms, the average disability weight was 0·12, although severity varies widely between individuals. Heart failure was distributed across 17 causes (figure 9). Slightly more than two-thirds (68·7%) of heart failure globally was due to four causes: ischaemic heart disease, COPD, hypertensive heart disease, and rheumatic heart disease. The pattern varied by region: ischaemic heart disease and COPD caused proportionally more YLDs in developed regions, whereas hypertensive heart disease, rheumatic heart disease, and cardiomyopathy and myocarditis made a larger contribution in some developing regions.
Figure 9:
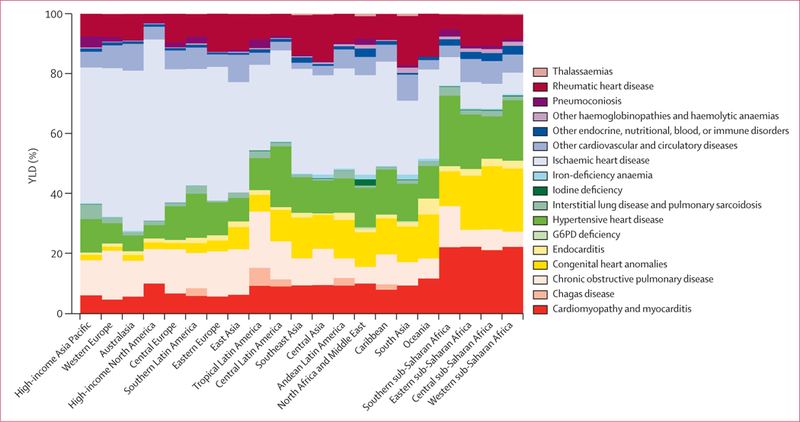
Years lived with disability (YLD) estimates for heart failure in 2010, by cause and region
Another important cause of global YLDs is blindness and low vision. Overall, visual impairment accounted for 21·1 million YLDs or 2·7% of the global total. Figure 10 shows the main causes of low vision and blindness. The largest global cause of YLDs from vision impairment globally was other vision loss (mainly from trauma, occupational exposures, and idiopathic disorders), which accounted for 29·5% of the total number of vision-loss YLDs. Uncorrected refractive error was the second most common cause and accounted for 26·5% of vision impairment. Cataracts were the third largest contributor (22·4% of vision-loss YLDs). Glaucoma and macular degeneration together accounted for a further 10·7%, with trachoma and onchocerciasis accounting for 2·1% of YLDs from vision loss in 2010. Most blindness and low vision YLDs were in individuals aged 45 years or older. We recorded a substantial increase in the absolute number of YLDs from low vision and blindness since 1990, primarily driven by changes in population age structure.The regional pattern shows that in sub-Saharan Africa, uncorrected refractive error, trachoma, onchocerciasis, and vitamin A deficiency play a much greater part than in other regions. As expected in more epidemiologically advanced regions, the composition of causes of blindness and low vision burden was shifted towards macular degeneration, glaucoma, diabetes, and other vision loss.
Figure 10:
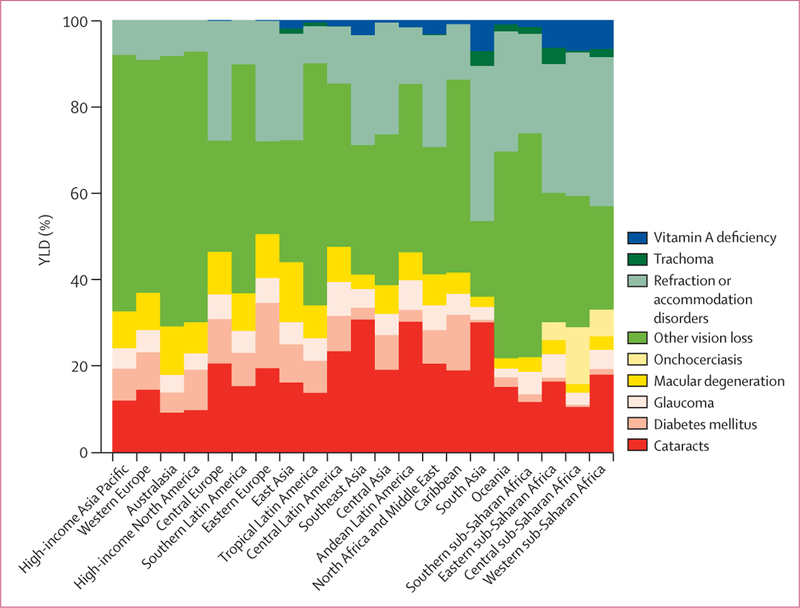
Years lived with disability (YLD) estimates for vision loss in 2010, by cause and region
Hearing impairment accounted for 19·9 million YLDs—2·6% of the total number of YLDs. Adult-onset hearing loss unrelated to a specific disease process accounted for 79·0% of the total YLDs due to hearingimpairment. Other major causes included otitis media, which caused 14·1% of hearing loss YLDs. Smaller causes included congenital hearing loss and meningitis. Of the 19·9 million YLDs due to hearing loss, mild-to-moderate severity accounted for 74·7%, whereas complete hearing loss accounted for only 3·7%. We detected a substantial increase in the number of YLDs due to hearing impairment since 1990, again driven by the ageing of populations.
Intellectual disability and borderline intellectual impairment accounted for 3·1 million YLDs. The prevalence of these disorders were quite low, ranging from 0·5% in high-income Asia Pacific to 2·2% in sub-Saharan Africa and south Asia, with disability weights from 0·003 for mild disorders to 0·149 for profound disorders. Prevalence varied across regions by about two-fold from east sub-Saharan Africa to high-income Asia Pacific. Figure 11 shows YLD rates per 100 000 people across regions by cause. Globally, the main causes of intellectual disability YLDs were idiopathic, Down’s syndrome, autism, preterm birth, and other congenital disorders. Some causes, however, were much more important in selected regions, such as meningitis in west and central sub-Saharan Africa and cretinism in south Asia. In terms of YLD rates, the largest variation across regions was from idiopathic causes.
Figure 11:
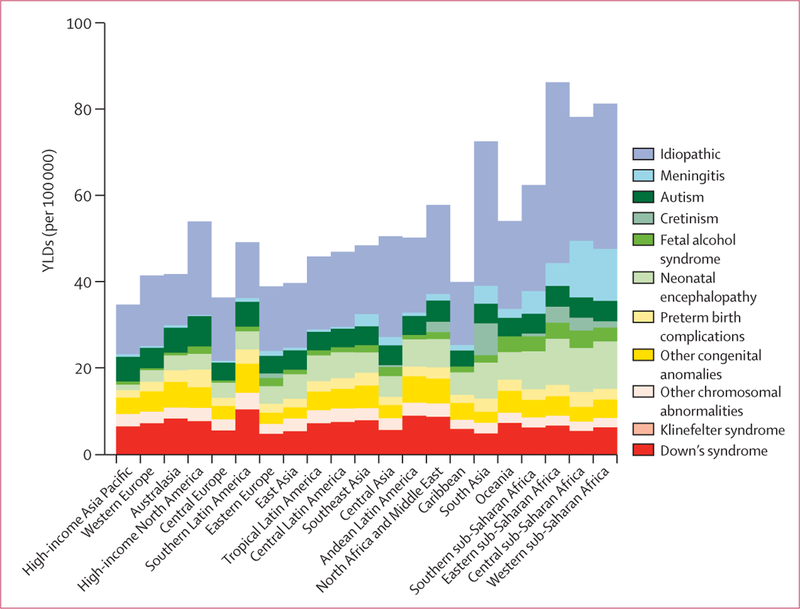
Years lived with disability (YLDs) estimates for intellectual disability in 2010, by cause and region
Discussion
We know of no other complete assessment of the prevalence of sequelae from diseases and injuries and their associated YLDs since GBD 1990. Prevalences of the 1160 sequelae ranged by more than a factor of 100 000 from the least to the most common. Taking into account severity, on average, every person in the world had an 11% reduction in their overall health in 2010 because of diseases and injuries. The prevalence of diseases and injuries and YLDs per person increased steadily with age in all regions. We have identified the main causes that contributed to YLDs as mental and behavioural disorders and musculoskeletal disorders. Neurological disorders, chronic respiratory diseases, some neglected tropical diseases, gynaecological disorders, and long-term disability from injuries were also important causes of YLDs. Compared with causes of mortality and years of life lost because of premature mortality, the main drivers of disability were much more consistent across regions. YLDs from non-communicable diseases ranged from 62·0% (central sub-Saharan Africa) to 92·6% (high-income North America) of the total. However, we detected large regional variation when assessing all disorders; the 25 most common disorders in any region included 49 different disorders globally.
There has been much debate in demographic, epidemiological, and gerontological studies about whether the prevalence of morbidity and disability increases or decreases with the epidemiological transition.87–93 Fries88 argued that with mortality reduction the onset of disabling chronic illness could be delayed, leading to individuals spending fewer years with morbidity—this hypothesis is known as the compression of morbidity hypothesis. Alternative views have stressed the effect of medical intervention in extending the lifespan of people with disabling disorders,92 which is commonly referred to as expansion of morbidity. Manton and colleagues94 argued using self-reported data that the prevalence of disability in elderly people in the USA was decreasing, providing support for the compression hypothesis. Demographic historians have noted the rise in reported morbidity as mortality decreases,87,89 which could be indicative of a real rise in disease pathology or a changing perception of the importance of lesser morbidities. The results reported here, constructed from multiple sources for nearly all major contributors to functional impairment, suggest that the prevalence of disability in nearly all regions of the world has been stable over the past two decades. In four regions (the Caribbean, western Europe, high-income North America, and southern sub-Saharan Africa), age- standardised YLDs per person increased, whereas in all other regions they decreased, although in all cases the changes were small. The implications of stable age-specific YLDs per person that steadily rise with age are important. As life expectancy increases, people can expect to spend a greater number ofyears living with reduced health because the added years are at older ages with increased rates of disability. If compression is defined as a decreasing number of years of life lived with disability, then our findings are not consistent with this hypothesis. Of course, the evidence for some causes of YLDs over time is scarce, which might mean that we did not identify important secular decreases in disability. However, for the leading causes of YLDs, such as major depressive disorder and most musculoskeletal disorders, much available evidence does not suggest clear trends in age-specific prevalences.
We detected a clear difference between patterns of selfrated health and the YLD rate per person estimated in the GBD 2010, which was constructed from a careful assessment of the evidence for 1160 disabling sequelae across regions. Analysis of the general health question in the World Health Survey,95 for example, suggests that levels of self-reported health are much lower in North America than they are in Africa. Yet we saw that YLDs per person are higher in Africa than they were in North America. The gap between these self-assessments and the results of the GBD derives from several key factors. First, many studies have been done on variations in the use of categorical responses across cultures;96–98 attempts to correct for this variation (eg, anchoring vignettes) have been proposed and implemented in various surveys.99–100 Second, in this study, we assumed the health loss, but not the welfare loss, associated with a sequela would be the same over time or across populations. Responses to general health questions could be confounded by other welfare or wellbeing considerations. In this analysis, however, we saw that self-rated functional health status measured using SF-12 or EQ5D survey instruments in cohort follow-up studies provided useful inputs into the assessment of long-term disability after an event and the distribution of severity within a disorder. Yet the same selfrated health data seem problematic when used to compare overall prevalence of functional impairment across linguistic or cultural groups. Our view of the gap is that substantial research will be needed to enhance the cross-population comparability of self-rated health instruments to the point at which they can be useful inputs for the assessment of the level of YLDs across populations.
The largest contributor to global YLDs were mental and behavioural disorders. In this study, the number of mental and behavioural disorders that we included increased from eight in GBD 1990 to 22 in GBD 2010. The present analysis used a much more extensive database than was used for GBD 1990, using data from multiple sources and survey programmes. Prevalence estimates for these disorders are based largely on self-reported symptoms with standardised screening instruments. In GBD 1990 and 2000, we included three specific anxiety disorders: post-traumatic stress disorder, panic disorder, and obsessive-compulsive disorder. On the basis of the high degree of comorbidity across anxiety disorders, we chose to assess the burden of all anxiety disorders but not to provide estimates for specific forms of anxiety disorders. Despite some claims to the contrary,101,102 our systematic analysis and meta-regression have not detected notable trends in the age-specific prevalences of these disorders overall; a notable exception is the rise in some regions in drug use disorders. The overall YLDs per person due to mental and behavioural disorders ranged from 2·0% in western sub-Saharan Africa to 3·3% in high-income North America. This narrow variation in the estimated YLD rates contrasts with some published analyses of variations in prevalence; the differences stem from both the data sources used and the methods applied for meta-regression.103 The findings of large and increasing YLDs due to mental and behavioural disorders draws attention to the urgent need for identification and implementation of effective and affordable strategies for this set of problems.
The second largest contributor to YLDs globally and in nearly all regions were musculoskeletal (MSK) disorders. Osteoarthritis (OA) of the knees and hips combined was the third most prevalent MSK disorder, and, because we did not include OA in other joints or the spine, is an underestimate of OA, although the burden of OA in other joints or the spine was captured under the categories of low back pain, neck pain, and other MSK. Low back pain stands out as the leading MSK disorder because of a combination of similarly high prevalence and a greater disability weight associated with this health state. Low back pain was one of the four most common disorders in all regions, and was the leading cause of YLDs in all developed countries; neck pain was also a major contributor in many regions. Low back and neck pain accounted for 70% of all YLDs from musculoskeletal disorders, and for every YLD due to neck pain there were 2· 5YLDs related to low back pain. The burden as estimated here is substantially higher than previously assessed in the GBD 1990 and GBD 2000 rounds of estimations. We believe the estimates presented here are more accurate because the empirical basis for prevalence generated through the systematic reviews and the analysis of survey data such as the World Health Survey is much stronger than in the past and a greater body of data was available for analysis. The increase in burden is also attributable to the higher disability weights that emerged from the disability weight surveys of the general population. Across all countries surveyed, respondents consistently recorded high levels of health loss caused by pain. These findings combined with the 33·3% increase in YLDs from 1990 to 2010 driven largely by population growth and ageing have important implications for health systems. Health systems will need to develop effective and affordable strategies to respond to this growing and nearly universal burden.
Intellectual disability (ID) accounted for 3·1 million YLDs, or 0·4% of the global total. This magnitude of ID is small compared with some claims about cognitive impairment in developing countries.102 There are several explanations for this discrepancy. First, the epidemiological data, especially those from low-income settings, are very scarce and our estimations consequently have large uncertainty intervals. Better data collection for ID would help in future revisions to narrow uncertainty intervals. Second, the disability weights selected by the general public for mild, moderate, severe, and profound intellectual disability ranged from 0·031 to 0·157, which were quite low. Some studies of anaemia and of helminth infections have reported evidence of irreversible cognitive deficits associated with these disorders.103–109 The reversible component of cognitive deficit associated with anaemia that is related to lethargy is captured in the disability weight for anaemia. The important issue, however, is the irreversible component of ID. In this analysis, this burden is classified as idiopathic intellectual disability. In the allocation of ID to different causes, 1·0 million YLDs were allocated to the idiopathic category in developing countries. If there are irreversible cognitive deficits associated with anaemia and helminth infections that lead to affected individuals being classified as disabled, we would capture this health loss in our estimates of intellectual disability. In sub-Saharan Africa and south Asia, the residual category of intellectual disability is larger than in other regions, which might be an indication of the effect of these other disorders. Nevertheless, the number of YLDs from idiopathic intellectual disability is not large enough to substantially change the ranks of the parasitic diseases or nutritional deficiencies presented here. Also, only IQs below 85 are assigned a disability weight so that if parasitic infections or nutritional deficiencies lowered IQ by two or three points in individuals but did not lower them below the threshold of 85, this effect would not be represented here. The disability weight, even for borderline ID (IQs of 70–84), is very small, suggesting that the general public does not consider small IQ reductions as a health loss; although such changes might have important effects on the general welfare of populations.
Hearing impairments accounted for less than 3% of all YLDs, which was a smaller contribution than that estimated in the GBD 2004 revision (4·5%).8 The main reason for this lower estimate is that disability weights for severe hearing loss are substantially lower in the current study than in the GBD 1990. As discussed by Salomon and colleagues,30 the main basis for estimation of disability weights comes from population-based surveys in which respondents make a series of paired comparisons between health states presented as brief lay descriptions. For hearing loss, the lay descriptions focused on the hearing impairment itself, excluding other possible outcomes that might accompany severe levels of hearing loss—eg, depression or learning disabilities. So far as these outcomes are part of the construct being measured in the Global Burden of Disease, their exclusion from the descriptions for hearing outcomes would be expected to lower the overall burden estimated for these causes. Furthermore, findings from some studies suggest that hearing loss can itself be a contributor to depression and other outcomes.110–112 To the extent that these relations are causal, the YLDs estimated in the present study for hearing loss do not capture these relations. These issues might also apply to the YLDs estimated for blindness or low vision.
A study of this magnitude with so many outcomes estimated for many different age-sex-country-years inevitably has many limitations. In view of the GBD philosophy that it is better to make estimates based on the best available evidence than not to make estimates, some YLD figures are based on a restricted database. The uncertainty intervals are meant to convey the strength of the evidence. Nevertheless, there are likely sources of uncertainty that have not been captured. In the GBD 2010 causes ofdeath analysis,68 we used out-of-sample predictive validity to more objectively quantify the validity of uncertainty intervals. We have not been able to apply this approach to the Bayesian meta-regression step in the YLD analysis for two reasons. First, the meta-regression step with DisMod-MR needed too much computing time to allow for repeated out-of-sample predictive validity testing. Future improvements in computational efficiency might allow such analysis, but at present it is not feasible. Second, data for many disorders are more scarce than they are for causes of death. Out-of-sample predictive validity testing is not very stable when data are very scarce. Another important limitation of the study is the disease and sequelae list itself. Although we included 1160 sequelae, there are many smaller sequelae of diseases and less common diseases that are only captured in the residual categories in the cause list. The estimates for these residual categories are, by their nature, very approximate. Compared with GBD 2000, the percentage of YLDs estimated in these residual categories has decreased from 9·0% to less than 2·0%. Future iterations of the GBD could add more disorders and reduce the uncertainty that stems from the residual categories.
Other limitations of this study include the restricted evidence-base for some disorders for crosswalking (adjusting data inputs based on less desirable study characteristics to the expected level of data inputs from optimally conducted studies) different case definitions or item recall periods such as 12-month versus 1-month recall. These crosswalks are estimated on the basis of a comparison of datapoints identified as having the desirable case definition, recall period, or other study quality characteristic with values with the less desirable attributes. This approach assumes that the relation between different study attributes is constant across age, sex, and region. In some cases, when such a relation does not exist, we estimated the crosswalks separately by age and sex using data collected with multiple definitions, such as for different decibel thresholds for hearing loss. The idea of using results of studies done with different definitions or diagnostic approaches in the final systematic analysis has substantially expanded the empirical basis available for assessing prevalence across age, sex, and regions. It does, however, draw attention to the importance of investigators publishing or making available data from existing studies using alternative case definitions or diagnostic approaches.
Another limitation of the study is that long-term follow- up data for injuries were available only from high-income countries. Long-term follow-up in developing countries could be different. Because of higher case-fatality rates in such countries, the average severity in surviving cases might be better than it is in high-income countries, if medical and surgical intervention extends the lifespan of those with more severe disabilities. Alternatively, the probability of long-term disability could be higher because of care that lowers mortality but does not restore function as effectively as does care in developed countries.
For the first time, we have adjusted GBD results for YLDs for comorbidity. The analysis of comorbidity, however, has several major limitations. Very few data are available that have been collected with a sufficiently large sample size and covering enough sequelae to estimate the correlation matrix for sequelae prevalence by age. National health information systems that capture detailed ICD-coded encounter data could provide a source of data for this type of analysis in the future. In general, if substantial dependent comorbidity (ie, one disease predisposes a person to be more or less likely to have another disease) exists, our estimates of YLDs might be slightly overestimated.83 The effect, however, is unlikely to be large because of the validation results seen in the 171 354 respondents in MEPS.77 In the microsimulation step for each country, age, sex, and year, we used 20 000 simulated individuals, then repeated the microsimulation 1000 times to capture uncertainty in the prevalences of all sequelae and disability weights. The effect of the microsimulation, especially for rare disorders, is to increase the estimated uncertainty in YLDs. For most disorders, this increase in uncertainty is small, but it can be quite substantial for rare disorders. The comorbidity process will tend to overestimate uncertainty in uncommon disorders. There are many potential uses of the comorbidity results of the GBD other than correction to YLD calculations. For instance, estimations of the expected number of individuals with multiple disorders might be useful for health planning purposes. We expect that comorbidity will be an important area for future burden research.
Consideration of comorbidity has put more emphasis on understanding the distribution of severity of disease. We directly model combinations of disorders and their effect on individual disability weights; to avoid double-counting, severity distributions for each disorder need to be estimated either controlling for comorbidity or in individuals without comorbidities, although the latter might be intractably affected by selection bias. In either case, the available data are limited. Datasets like the MEPS77 that collect repeated observations over time on functional health status and collect ICD-coded information on multiple conditions can be extremely useful for future assessments of severity. Other data collection strategies are possible but future burden of disease research needs to foster new data collection that provides direct assessments of severity distributions. Studies of severity need also to take into account that individuals might be asymptomatic for some time, and to quantify this as part of the protocol. In datasets in which clinical diagnoses can be verified, more routine collection of information using a standard self-reported functional health status instrument will enhance their utility.
In view of the fact that there is almost no relation between the prevalence of a sequela and the severity of the sequela as captured in the disability weights, recognition that our results depend on the validity of the disability weights themselves is crucial. Some disability weights have changed substantially compared with GBD 1990 weights, such as for blindness or profound hearing loss. Elsewhere, Salomon and colleagues30 describe the methods used to measure the GBD 2010 disability weights in multiple populations around the world. The shift to the use of samples of the general population, rather than small panels of health-care professionals as used for the GBD 1990 disability weights, we believe strengthens the findings presented here. Nevertheless, the crucial mechanism by which the general public can assess the level of health for different health states is through brief descriptions in lay language. Salomon and colleagues30 lay out a future research agenda to better understand how alternative lay descriptions of health states might affect the resulting disability weight.
One important function for health information systems should be to provide national decision makers with timely information about the burden of non-fatal health outcomes. The GBD 2010 analysis of YLDs provides important insights into which types of data can be informative for assessing non-fatal health outcomes. Not surprisingly, there is an important role for household surveys that involve interviews and the collection of blood and other functional tests (eg, hearing, vision, and lung function). Making sure a household survey collects data with a general functional health status instrument and collects information on a broad array of sequelae can make such data collection opportunities even more valuable. Building on the analysis of YLDs and YLLs as well, construction of a household interview and examination survey instrument that can capture the main sources of disease burden would be useful. The detail in the GBD 2010 now makes this approach feasible. Beyond household surveys, however, we have seen that ICD-coded hospital discharge and ambulatory care data, when individual records are available and other sociodemographic data have been collected, can be very valuable, especially if these datasets can be linked. Disease registries for cancer, renal disease, and congenital anomalies have also been an important resource, drawing attention to the importance of tracking individuals with chronic disorders over time who come into contact with health services.
Health priorities have, for much of the past 100 years or more, been largely driven by the imperative of improving the survival of populations, particularly child survival. This was justified, in view of the availability of technologies to treat and prevent childhood illness. However, societies also spend substantial resources on keeping people healthy, not only on keeping them alive into old age, so the availability of strategies to monitor their effectiveness in doing so is important. In this Article, we have shown that quantification of health loss in populations is possible, using comparable metrics that identify the leading causes of non-fatal illness in different regions, at different ages, and at different points in time. The principal findings, namely that mental health, musculoskeletal health, and the rising importance of diabetes need urgent policy responses, are well established. Monitoring progress in reducing the effect of these, and other major contributors to health loss, is as important for improving population health as monitoring progress against the leading causes of death. YLDs provide a convenient framework and metric to do so; ensuring the routine availability of data collection suitable for computation of these measures of health loss should be a key focus of national health information system strategies.
Panel:
Terminology used in the Global Burden of Disease study (GBD)
Disability
Disability refers to any short-term or long-term health loss. Many other definitions of disability are in use such as those in the WHO World Report on Disabilities.31 These definitions often stress moderate to severe health loss and the role of the environment in the loss of individuals’ wellbeing.
Sequelae
In the GBD 2010 cause list there are 291 diseases and injuries, of which 289 cause disability. In total, we have identified 1160 sequelae of these diseases and injuries. For example, diabetic neuropathy is a sequela of diabetes mellitus. To avoid double counting, a sequela can only be counted in the cause list once even if the same outcome might be caused by more than one disease.
Health state
Across the 1160 sequelae, we identified 220 unique health states. For example, both malaria and hookworm have mild anaemia as a sequela. Mild anaemia is a unique health state. The list of unique health states serves two purposes: to allow assessment of the total burden of some health states such as anaemia across various causes, and to simplify the task of measuring disability weights for sequelae.
Disability weights
A quantification of the severity of health loss associated with the 220 unique health states on a scale from 0 to 1, when 0 is commensurate with perfect health and 1 is commensurate with death. In the GBD 2010, disability weights for health states are measured based on survey respondents representing the general public.
Years lived with disability (YLDs)
For the GBD 2010, YLDs per person from a sequela are equal to the prevalence of the sequela multiplied by the disability weight for the health state associated with that sequela. YLDs for a disease or injury are the sum of the YLDs for each sequela associated with the disease or injury.
Impairments
In the GBD 2010 we estimated the prevalence and burden of several unique health states that are sequelae for multiple diseases including anaemia, heart failure, vision loss, seizures, hearing loss, infertility, and intellectual disability. These are referred to as impairments.
Acknowledgments
We would like to thank the countless individuals who have contributed to the Global Burden of Disease Study 2010 in various capacities. We would also like to specifically acknowledge the important contribution to this work from multiple staff members of the World Health Organization. We also wish to express our gratitude to the following organizations that hosted consultations during the final stages of the analytic process, providing valuable feedback about the results and the data to improve the study’s findings overall: Pan American Health Organization; Eastern Mediterranean Regional Office of WHO; UNAIDS; Ministry of Health, Brazil; China Centers for Disease Control; and the University of Zambia. We would like to thank Lori M Newman, Jordis Ott, Poul Erik Petersen, Shekhar Saxena, and Gretchen A Stevens for their collaboration and input into the analyses and estimates. Finally, we would like to acknowledge the extensive support from all staff members at the Institute for Health Metrics and Evaluation and specifically thank: James Bullard, Andrew Ernst, and Serkan Yalcin for their tireless support of the computational infrastructure required to produce the results; Linda A Ettinger for her expert administrative support in order to facilitate communication and coordination amongst the authors; Peter Speyer, Abigail McLain, Katherine Leach-Kemon, and Eden Stork for their persistent and valuable work to gain access to and catalog as much data as possible to inform the estimates; Erin C Mullany for her systematic efforts in organizing drafts of papers, formatting correspondence with expert groups, and preparing the final manuscript; Brad Bell for his review, critiques, and insights about the DisMod-MR software which helped to improve its performance and results; and Jiaji Du for his work developing the user interface for the DisMod-MR software so that multiple expert groups could use it and easily assess the results.
The following individuals would like to acknowledge various forms of institutional support. J P Abraham, B Bartels, and P Yeh recognize the support of the World Bank Global Road Safety Facility and Department of Global Health & Population, Harvard School of Public Health, and the World Health Organization Violence and Injury Prevention. B Bikbov acknowledges support from the Moscow State University of Medicine and Dentistry, Moscow, Russia; Academician V.I.Shumakov Federal Research Center of Transplantology and Artificial Organs, Moscow, Russia; International Society of Nephrology. R Bourne acknowledges the Vision & Eye Research Unit, Postgraduate Medical Institute, Anglia Ruskin University, Cambridge, UK. S Brooker is supported by a Wellcome Trust Senior Fellowship in Basic Biomedical Science (098045). T S Brugha received funding from the Department of Health London, for the National Health Service Information Centre, by the University of Leicester. R Buchbinder is partially funded by an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellowship, Monash University and Cabrini Health. P Burney and D Jarvis acknowledge that the Chronic Respiratory Disease Group received support from the BUPA Foundation, who had no role in study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript. C Cella, M Cortinovis, F Gaspari, V Miglioli, and N Perico on behalf of the entire Genitourinary Expert Group acknowledge the International Society of Nephrology (ISN). H Chen acknowledges that his participation in this study was in part supported by the intramural research program of the NIH, the National Institute of Environmental Health Sciences. L E Coffeng and W A Stolk received financial support from the Africa Programme for Onchocerciasis Control (WHO/APOC) for their work on onchocerciasis. B C Cowie received institutional support from the Victorian Infectious Diseases Reference Laboratory, Melbourne, Australia. M Cross and L March acknowledge the University of Sydney (USYD); Institute of Bone and Joint Research, University of Sydney, Department of Rheumatology, Royal North Shore Hospital, St Leonards NSW 2065 Australia. N Dahodwala was supported by NIH grant K23 AG034236 and the Parkinson Council while working on this project. L Degenhardt is supported by an Australian NHMRC Senior Research Fellowship and funding to support her work for illicit drug dependence was provided by the Australian National Drug and Alcohol Research Centre of the University of New South Wales, Australia. R Dellavalle was supported by the US Department of Veterans Affairs while contributing to this study. S Derrett acknowledges the Health Research Council of New Zealand and the University of Otago for their support. V Feigin and R Krishnamurthi were supported by the National Institute for Stroke and Applied Neurosciences, AUT University. E Fevre acknowledges the Wellcome Trust for grant 085308. W Hall was supported by an NHMRC Australia Fellowship. R Havmoeller was supported by a grant from the Swedish Research Council (#2011–1071). D Hoy was supported by the Bill and Melinda Gates Foundation and the Australian National Health and Medical Research Council. K H Jacobsen was supported by the World Health Organization for her work on hepatitis A. N Kawakami notes that the collection of data ultimately used in this study was supported by the following grants: The World Mental Health Japan (WMH-J) is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013) from the Japan Ministry of Health, Labour, and Welfare. He would like to thank staffmembers, field coordinators, and interviewers of the WMH Japan 2002–2004 Survey. L L Laslett is supported by an Australian Government Australian Postgraduate Award. She also notes that the TasOAC study, the results of which were used in this research, was supported by the National Health and Medical Research Council of Australia; Arthritis Foundation of Australia; Tasmanian Community Fund; Masonic Centenary Medical Research Foundation, Royal Hobart Hospital Research Foundation, and University of Tasmania Institutional Research Grants Scheme. R Malekzadeh funding from research grant of Tehran University of Medical Sciences to do the related studies. R Matzopoulos acknowledges the two institutions that support his research work: South African Medical Research Council Burden of Disease Research Unit; and the University of Cape Town School of Public Health and Family Medicine. T Merriman acknowledges the Health Research Council of New Zealand. K Naidoo was supported by the Brien Holden Vision Institute. P Nelson was supported by the National Drug and Alcohol Research Centre (UNSW, Australia). R G Nelson acknowledges his research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. C Olives was funded in part by the Biostatistics, Epidemiologic and Bioinformatic Training in Environmental Health Training Grant (ES015459). D Ozgediz acknowledges the staffand collaborators at the Mulago Hospital and Makerere University in Kampala, Uganda. K Pesudovs received institutional support from Flinders University. R Room’s position at the University of Melbourne and Turning Point Alcohol and Drug Centre is funded by the Foundation for Alcohol Research and Education and the Victorian Department of Health. J A Salomon received support from the Burke Global Health Fellowship while working on this study. U Sampson received funding support from the Harold Amos Medical Faculty Development Award of the Robert Wood Johnson Foundation and the Vanderbilt Clinical and Translational Scholars Award. L Sanchez-Riera acknowledges the Spanish Society of Rheumatology (Sociedad Española de Reumatología). M Segui-Gomez’s participation was partly supported by funds from the European Center for Injury Prevention, Universidad de Navarra. E Smith acknowledges the Department of Health and Ageing, Commonwealth Government of Australia, Institute of Bone and Joint Research (IBJR), University of Sydney (USYD). G D Thurston was supported in part by Center grant ES00260 from the National Institute of Environmental Health Sciences. D J Weatherall was supported by the Wellcome Trust UK, the Medical Research Council UK and the Anthony Cerami and Ann Dunne Research Trust.
Footnotes
Conflicts of interest
C E Canter has worked as an Optum Health consultant, Blue Cross Blue Shield consultant, and received Berlin Heart Honoraria and travel fees. E R Dorsey has received payments for consulting services from Lundbeck and Medtronic and research support from Lundbeck and Prana Biotechnology. T Driscoll was supported in part by funding from the National Occupational Health and Safety Commission (now Safework Australia). M Ezzati chaired a session and gave a talk at the World Cardiology Congress (WCC), with travel cost reimbursed by the World Heart Federation. At the WCC, he also gave a talk at a session organised by PepsiCo with no financial or other remuneration. F Guillemin did a study on osteoarthritis epidemiology in an institution that received grants from public sources: Assurance-Maladie (CNAMTS) InVS, Inserm, CHU de Nancy, CHU de Nice, Conseil Regional de Lorraine, Societe Francaise de Negma-Lerads, Pfizer, Pierre Fabre Medicaments, Sanofi-Aventis France. H J Hoffman is a US Federal Government employee of the National Institutes of Health (NIH). P J Hotez reports holding several positions: Dean, National School of Tropical Medicine, Baylor College of Medicine; Director, Sabin Vaccine Institute Texas Children’s Hospital Center for Vaccine Development; and President, Sabin Vaccine Institute. He also is an inventor on several patents: 5,527,937 “Hookworm Anticoagulant”; 5,753,787 “Nucleic Acids for Ancylostoma Secreted Proteins”; 7,303,752 B2 “Hookworm vaccine”; 12/492,734 “Human Hookworm Vaccine”; 61/077,256 “Multivalent Anthelminthic Vaccine”; and PCT-20100701/0.20.5.18 “Malaria Transmission blocking vaccine”. G A Mensah is a former employee of PepsiCo. F Perez-Ruiz was an advisor for Ardea, Menarini, Novartis, Metabolex; was a member of the Speaker’s Bureau for Menarini, Novartis; an advisor for educational issues for Savient; led investigation grants for the Spanish Health Ministry, Hospital de Cruces Rheumatology Association; and was principal investigator in clinical trials for Ardea. G V Polanczyk has served as a speaker or consultant to Eli-Lily, Novartis, Janssen-Cilag, and Shire Pharmaceuticals, developed educational material for Janssen-Cilag, and received an independent investigator grant from Novartis and from the National Council for Scientific and Technological Development (CNPq, Brazil). L Rushton received honorarium for board membership of the European Centre for Ecotoxicology and Toxicology of Chemicals and received research grants to Imperial College London (as PI) from the European Chemical Industry Council (CEFIC) and CONCAWE (Conservation of Clean Air and Water Europe). J A Singh has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Ardea, Regeneron, Allergan, URL pharmaceuticals, and Novartis. J A Singh is a member of the executive of OMERACT, an organisation that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology’s Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. J A Singh is supported by research grants from the National Institutes of Arthritis, Musculoskeletal and Skin Diseases (NIAMS), National Institute on Aging (NIA), National Cancer Institute (NCI) and the Agency for Health Quality and Research Center for Education and Research on Therapeutics (CERTs) and is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
Authors listed alphabetically
Joint senior authors
Contributor Information
Prof Theo Vos, School of Population Health.
Abraham D Flaxman, Institute for Health Metrics and Evaluation.
Mohsen Naghavi, Institute for Health Metrics and Evaluation.
Prof Rafael Lozano, Institute for Health Metrics and Evaluation.
Catherine Michaud, China Medical Board, Boston, MA, USA.
Prof Majid Ezzati, MRC-HPA Centre for Environment and Healthe, Department of Epidemiology and Biostatistics, School of Public Health.
Prof Kenji Shibuya, Department of Global Health Policy.
Prof Joshua A Salomon, School of Public Health.
Safa Abdalla, Sudanese Public Health Consultancy Group, Sudan.
Prof Victor Aboyans, Department of Cardiology, Dupuytren University Hospital, Limoges, France.
jerry Abraham, University of Texas, San Antonio, TX, USA.
Ilana Ackerman, University of Melbourne, Melbourne, VIC,Australia.
Prof Rakesh Aggarwal, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow,India.
Stephanie Y Ahn, Institute for Health Metrics and Evaluation.
Mohammed K Ali, Emory University, Atlanta, GA,USA.
Mohammad A AlMazroa, Ministry of Health, Riyadh, Saudi Arabia.
Miriam Alvarado, Institute for Health Metrics and Evaluation.
Prof H Ross Anderson, St George’s University of London, London, UK.
Laurie M Anderson, Department of Epidemiology, School of Public Health.
Kathryn G Andrews, Institute for Health Metrics and Evaluation.
Charles Atkinson, Institute for Health Metrics and Evaluation.
Prof Larry M Baddour, Mayo Clinic, Rochester, MN,USA.
Adil N Bahalim, Independent Consultant, Geneva, Switzerland.
Suzanne Barker-Collo, University of Auckland, Auckland, New Zealand.
Lope H Barrero, Department of Industrial Engineering, School of Engineering, Pontifi cia Universidad Javeriana, Bogota,Colombia.
David H Bartels, Harvard Medical School, Harvard University, Boston, MA, USA.
Prof Maria-Gloria Basáñez, Imperial College London, London, UK.
Amanda Baxter, Queensland Centre for Mental Health Research.
Prof Michelle L Bell, Yale University, New Haven, CT, USA.
Prof Emelia J Benjamin, Boston University, Boston, MA, USA.
Derrick Bennett, Clinical Trial Service Unit and Epidemiological Studies Unit.
Eduardo Bernabé, Dental Institute.
Kavi Bhalla, Harvard Medical School, Harvard University, Boston, MA, USA.
Bishal Bhandari, Queen Mary University of London, London, UK.
Boris Bikbov, Moscow State University of Medicine and Dentistry, Research Institute of Transplantology and Artifi cial Organs, Moscow, Russia.
Aref Bin Abdulhak, King Fahad Medical City, Riyadh, Saudi Arabia.
Prof Gretchen Birbeck, Michigan State University, East Lansing, MI, USA.
James A Black, MRC Epidemiology Unit, Cambridge,UK.
Hannah Blencowe, London School of Hygiene and Tropical Medicine, London, UK.
Jed D Blore, School of Population Health.
Fiona Blyth, University of Sydney, Sydney, NSW, Australia.
Ian Bolliger, Institute for Health Metrics and Evaluation.
Audrey Bonaventure, Inserm, Paris,France.
Soufiane Boufous, Transport and Road Safety Research.
Prof Rupert Bourne, Vision and Eye Research Unit, Anglia Ruskin University, Cambridge, UK.
Michel Boussinesq, Institut de Recherche pour le Développement, Martinique, France.
Tasanee Braithwaite, Moorfi elds Eye Hospital, London, UK.
Prof Carol Brayne, University of Cambridge, Cambridge, UK.
Lisa Bridgett, University of Sydney, Sydney, NSW, Australia.
Prof Simon Brooker, London School of Hygiene and Tropical Medicine, London, UK.
Peter Brooks, University of Melbourne, Melbourne, VIC,Australia.
Prof Traolach S Brugha, University of Leicester, Leicester, UK.
Claire Bryan-Hancock, Flinders University, Adelaide,SA, Australia.
Chiara Bucello, University of New South Wales, Sydney, NSW, Australia.
Prof Rachelle Buchbinder, Institute, Malvern, VIC, Australia.
Geoffrey Buckle, Bloomberg School of Public Health.
Christine M Budke, Texas A&M University, College Station, TX, USA.
Michael Burch, Great Ormond Street Hospital, London, UK.
Prof Peter Burney, Imperial College London, London, UK.
Roy Burstein, Institute for Health Metrics and Evaluation.
Bianca Calabria, National Drug and Alcohol Research Centre.
Benjamin Campbell, Institute for Health Metrics and Evaluation.
Prof Charles E Canter, Washington University, St Louis, MO, USA.
Prof Hélène Carabin, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Prof Jonathan Carapetis, Telethon Institute for Child Health Research, Centre for Child, Health Research.
Loreto Carmona, Universidad Camilo Jose Cela, Villanueva de la Cañada, Spain.
Claudia Cella, Mario Negri, Institute for Pharmacological, Research, Bergamo, Italy.
Fiona Charlson, School of Population Health.
Honglei Chen, National Institute of Environmental Health Sciences, Research, Triangle Park, NC, USA.
Prof Andrew Tai-Ann Cheng, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
David Chou, Institute for Health Metrics and Evaluation.
Prof Sumeet S Chugh, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Luc E Coffeng, Erasmus MC, University Medical Center, Rotterdam, Rotterdam, Netherlands.
Prof Steven D Colan, Boston Children’s Hospital.
Samantha Colquhoun, Menzies School of Health Research, Darwin, NT, Australia.
K Ellicott Colson, Institute for Health Metrics and Evaluation.
John Condon, Menzies School of Health, Research, Darwin, NT, Australia.
Myles D Connor, National Health Services, Fife, Edinburgh, Scotland, UK.
Prof Leslie T Cooper, Loyola University Medical School, Chicago, IL, USA.
Matthew Corriere, School of Public Health Sciences, Wake Forest University, Winston-Salem, NC, USA.
Monica Cortinovis, Mario Negri Institute for Pharmacological, Research, Bergamo, Italy.
Karen Courville de Vaccaro, Hospital Dr Gustavo N Collado, Puerto Chitre, Panama.
Prof William Couser, University of Washington, Seattle, WA, USA.
Benjamin C Cowie, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC, Australia.
Prof Michael H Criqui, University of California, San Diego, San Diego, CA, USA.
Marita Cross, University of Sydney, Sydney, NSW, Australia.
Kaustubh C Dabhadkar, Emory University, Atlanta, GA,USA.
Manu Dahiya, Institute of Dentistry.
Nabila Dahodwala, University of Pennsylvania, Philadelphia, PA, USA.
James Damsere-Derry, Building and Road Research Institute, Kumasi, Ghana.
Goodarz Danaei, School of Public Health.
Prof Adrian Davis, MRC Hearing and Communication Group, Manchester, UK.
Prof Diego De Leo, Griffi th University, Brisbane, QLD, Australia.
Prof Louisa Degenhardt, National Drug and Alcohol Research Centre; Centre for Health Policy, Programs and Economics.
Robert Dellavalle, Denver VA Medical Center, Denver, CO, USA.
Allyne Delossantos, Institute for Health Metrics and Evaluation.
Julie Denenberg, University of California, San Diego, San Diego, CA, USA.
Sarah Derrett, University of Otago, Dunedin, New Zealand.
Don CDes Jarlais, Beth Israel Medical Center, New York, City, NY, USA.
Prof Samath D Dharmaratne, Institute for Health Metrics and Evaluation.
Mukesh Dherani, The University of Liverpool, Liverpool, UK.
Cesar Diaz-Torne, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Prof Helen Dolk, University of Ulster, Ulster, UK.
E Ray Dorsey, Johns Hopkins University, Baltimore, MD, USA.
Tim Driscoll, Sydney School of Public Health.
Herbert Duber, University of Washington, Seattle, WA, USA.
Beth Ebel, University of Washington, Seattle, WA, USA.
Karen Edmond, London School of Hygiene and Tropical Medicine, London, UK.
Alexis Elbaz, Inserm, Paris,France.
Suad Eltahir Ali, Federal Ministry of Health, Khartoum, Sudan.
Holly Erskine, Queensland Centre for Mental Health Research.
Patricia J Erwin, Mayo Clinic, Rochester, MN,USA.
Patricia Espindola, Hospital Maciel, Montevideo, Uruguay.
Stalin E Ewoigbokhan, Emerald Public Health Consulting Services Ltd, Abuja, Nigeria.
Farshad Farzadfar, Tehran University of Medical Sciences, Tehran, Iran.
Prof Valery Feigin, National Institute for Stroke and Applied Neurosciences.
Prof David T Felson, School of Medicine.
Alize Ferrari, Queensland Centre for Mental Health Research.
Cleusa P Ferri, Federal University of São Paulo, São Paulo, Brazil.
Eric M Fèvre, University of Edinburgh, Edinburgh, Scotland, UK.
Mariel M Finucane, Department of Biostatistics.
Seth Flaxman, Carnegie Mellon University, Pittsburgh, PA, USA.
Louise Flood, Flinders University, Adelaide,SA, Australia.
Kyle Foreman, Imperial College London, London, UK.
Mohammad H Forouzanfar, Institute for Health Metrics and Evaluation.
Prof Francis Gerry R Fowkes, University of Edinburgh, Edinburgh, Scotland, UK.
Richard Franklin, Royal Life Saving Society, Sydney, NSW, Australia.
Marlene Fransen, Faculty of Health Sciences.
Michael K Freeman, Institute for Health Metrics and Evaluation.
Belinda J Gabbe, Department of Epidemiology and Preventive Medicine.
Prof Sherine E Gabriel, Mayo Clinic, Rochester, MN,USA.
Emmanuela Gakidou, Institute for Health Metrics and Evaluation.
Hammad A Ganatra, Emory University, Atlanta, GA,USA.
Bianca Garcia, University of Queensland, Brisbane, QLD, Australia.
Flavio Gaspari, Mario Negri Institute for Pharmacological, Research, Bergamo, Italy.
Prof Richard F Gillum, Howard University College of Medicine, Washington, DC, USA.
Prof Gerhard Gmel, Addiction Info Switzerland, Lausanne, Switzerland.
Prof Richard Gosselin, University of California, San Francisco, San Francisco, CA, USA.
Rebecca Grainger, University of Otago, Dunedin, New Zealand.
Justina Groeger, College of Medicine, SUNY Downstate Medicle Center, Brooklyn, NY, USA.
Prof Francis Guillemin, Université de Lorraine, Nancy, France.
Prof David Gunnell, University of Bristol, Bristol, UK.
Ramyani Gupta, St George’s University of London, London, UK.
Juanita Haagsma, Erasmus MC, University Medical Center, Rotterdam, Rotterdam, Netherlands.
Prof Holly Hagan, New York University, New York City, NY.
Yara A Halasa, Brandeis University, Waltham, MA, USA.
Prof Wayne Hall, University of Queensland, Brisbane, QLD, Australia.
Diana Haring, Institute for Health Metrics and Evaluation.
Josep Maria Haro, Parc Sanitari Sant Joan de Déu, CIBERSAM, Universitat de Barcelona, Sant, Boi de Llobregat, Spain.
Prof James E Harrison, Flinders University, Adelaide,SA, Australia.
Rasmus Havmoeller, Heart Institute; Karolinska University Hospital, Stockholm, Sweden.
Prof Roderick J Hay, Hospital NHS Trust.
Hideki Higashi, School of Population Health.
Catherine Hill, The Queen Elizabeth Hospital, Adelaide, SA, Australia.
Prof Bruno Hoen, Université de Franche-Comté, Besançon, France.
Howard Hoffman, National Institute on Deafness and Other Communication Disorders.
Prof Peter J Hotez, National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
Damian Hoy, Monash University, Melbourne, VIC, Australia.
John J Huang, Yale University, New Haven, CT, USA.
Sydney E Ibeanusi, University of Port Harcourt, Port Harcourt, Nigeria.
Kathryn H Jacobsen, George Mason University, Fairfax, VA, USA.
Spencer L James, Institute for Health Metrics and Evaluation.
Deborah Jarvis, Imperial College London, London, UK.
Rashmi Jasrasaria, Institute for Health Metrics and Evaluation.
Sudha Jayaraman, Brigham and Women’s Hospital.
Nicole Johns, Institute for Health Metrics and Evaluation.
Prof Jost B Jonas, Department of Ophthalmology, Medical Faculty Mannheim, Ruprecht Karls University, Heidelberg, Germany.
Ganesan Karthikeyan, All India Institute of Medical Sciences, New Delhi, India.
Nicholas Kassebaum, Department of Anesthesiology and Pain Medicine.
Prof Norito Kawakami, University of Tokyo, Tokyo,Japan.
Prof Andre Keren, Department of Cardiology, Hebrew University Hadassah Medical School, Jerusalem, Israel.
Jon-Paul Khoo, Queensland Centre for Mental Health Research.
Prof Charles H King, Case Western Reserve University, Cleveland, OH, USA.
Lisa Marie Knowlton, Harvard Humanitarian Initiative.
Olive Kobusingye, School of Public Health, Makerere University, Kampala, Uganda.
Adofo Koranteng, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Rita Krishnamurthi, Auckland Technical University, Auckland, New Zealand.
Prof Ratilal Lalloo, School of Dentistry and Oral Health.
Laura L Laslett, University of Tasmania, Tasmania, Australia.
Tim Lathlean, FlindersUniversity, Adelaide,SA, Australia.
Janet L Leasher, Nova Southeastern University, Fort, Lauderdale, FL, USA.
Yong Yi Lee, School of Population Health.
James Leigh, Sydney School of Public Health.
Stephen S Lim, Institute for Health Metrics and Evaluation.
Elizabeth Limb, St George’s University of London, London, UK.
John Kent Lin, School of Public Health.
Michael Lipnick, University of California, San Francisco, San Francisco, CA, USA.
Prof Steven E Lipshultz, Miller School of Medicine, University of Miami, Miami, FL, USA.
Wei Liu, Department of Epidemiology.
Maria Loane, University of Ulster, Ulster, UK.
Summer Lockett Ohno, Institute for Health Metrics and Evaluation.
Prof Ronan Lyons, Swansea University, Swansea, UK.
Jixiang Ma, National Center for Non-Communicable, Disease Control and Prevention, China Centers for Disease Control, Beijing, China.
Jacqueline Mabweijano, Mulago Hospital, Kampala, Uganda.
Michael F MacIntyre, Institute for Health Metrics and Evaluation.
Prof Reza Malekzadeh, Digestive Disease Research Center.
Leslie Mallinger, Institute for Health Metrics and Evaluation.
Sivabalan Manivannan, Bloomberg School of Public Health.
Prof Wagner Marcenes, Queen Mary University of London, London, UK.
Prof Lyn March, Institute of Bone and Joint Research.
Prof David J Margolis, University of Pennsylvania, Philadelphia, PA, USA.
Prof Guy B Marks, University of Sydney, Sydney, NSW, Australia.
Prof Robin Marks, University of Melbourne, Melbourne, VIC,Australia.
Akira Matsumori, Asian Pacific Society of Cardiology, Kyoto, Japan.
Richard Matzopoulos, Medical Research Council, Tygerberg, South Africa.
Prof Bongani M Mayosi, University of Cape Town, Cape Town, South Africa.
John H McAnulty, Legacy Health System, Portland, Oregon.
Prof Mary M McDermott, Northwestern University Feinberg School of Medicine, Evanston, IL, USA.
Neil McGill, University of Sydney, Sydney, NSW, Australia.
Prof John McGrath, Queensland Brain Institute.
Maria Elena Medina-Mora, National Institute on Psychiatry Ramón de la Fuente, Mexico City, Mexico.
Michele Meltzer, Thomas Jefferson University, Philadelphia, PA, USA.
Prof Ziad A Memish, Ministry of Health, Riyadh, Saudi Arabia.
Prof George A Mensah, Department of Medicine.
Tony R Merriman, University of Otago, Dunedin, New Zealand.
Ana-Claire Meyer, University of California, San Francisco, San Francisco, CA, USA.
Valeria Miglioli, Mario Negri Institute for Pharmacological Research, Bergamo, Italy.
Matthew Miller, School of Public Health.
Ted R Miller, Pacific Institute for Research and Evaluation, Calverton, MD, USA.
Prof Philip B Mitchell, University of New South Wales, Sydney, NSW, Australia.
Prof Ana Olga Mocumbi, National Institute of Health, Maputo, Mozambique.
Prof Terrie E Moffitt, Duke University, Durham, NC, USA.
Ali A Mokdad, Institute for Health Metrics and Evaluation.
Lorenzo Monasta, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy.
Marcella Montico, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy.
Maziar Moradi-Lakeh, Tehran University of Medical Sciences, Tehran, Iran.
Andrew Moran, Columbia University, New York City, NY, USA.
Prof Lidia Morawska, Queensland University of Technology, Brisbane, QLD, Australia.
Rintaro Mori, National Center for Child Health and Development, Tokyo, Japan.
Michele E Murdoch, Watford General Hospital, Watford, UK.
Michael K Mwaniki, Kemri-Wellcome Trust, Kilifi, Kenya.
Prof Kovin Naidoo, AVRI, University of KwaZulu-Natal, Durban, South Africa.
M Nathan Nair, Institute for Health Metrics and Evaluation, University of Washington, 2301 Fifth Avenue, Suite 600, Seattle, WA 98121, USA.
Luigi Naldi, Centro Studi GISED, Bergamo, Italy.
KM Venkat Narayan, Emory University, Atlanta, GA,USA.
Paul K Nelson, National Drug and Alcohol Research Centre.
Robert G Nelson, National Institute of Diabetes, and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Michael C Nevitt, Department of Epidemiology and Biostatistics.
Prof Charles R Newton, University of Oxford, Oxford, UK.
Sandra Nolte, Charité-Universitätsmedizin Berlin, Berlin, Germany.
Prof Paul Norman, University of Western Australia, Perth, WA, Australia.
Rosana Norman, School of Population Health.
Martin O’Donnell, HRB-Clinical Research Facility, National University of Ireland Galway, Galway, Ireland, UK.
Simon O’Hanlon, Imperial College London, London, UK.
Casey Olives, University of Washington, Seattle, WA, USA.
Saad B Omer, Schools of Public Health and Medicine.
Katrina Ortblad, Institute for Health Metrics and Evaluation, University of Washington, 2301 Fifth Avenue, Suite 600, Seattle, WA 98121, USA.
Prof Richard Osborne, Deakin University, Melbourne, VIC, Australia.
Doruk Ozgediz, Global Partners in Anesthesia and Surgery.
Andrew Page, School of Population Health.
Bishnu Pahari, B P Koirala Institute of Health Sciences, Dharan, Nepal.
Jeyaraj Durai Pandian, Betty Cowan Research and Innovation Center, Ludhiana, India.
Andrea Panozo Rivero, Hospital Juan XXIII, La Paz, Bolivia.
Prof Scott B Patten, University of Calgary, Calgary, AB, Canada.
Prof Neil Pearce, London School of Hygiene and Tropical Medicine, London, UK.
Rogelio Perez Padilla, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Fernando Perez-Ruiz, Hospital Universitario Cruces, Barakaldo, Spain.
Norberto Perico, Mario Negri Institute for Pharmacological Research, Bergamo, Italy.
Prof Konrad Pesudovs, Flinders University, Adelaide, SA, Australia.
David Phillips, Institute for Health Metrics and Evaluation.
Prof Michael R Phillips, Shanghai Mental Health Center, School of Medicine.
Kelsey Pierce, Institute for Health Metrics and Evaluation, University of Washington, 2301 Fifth Avenue, Suite 600, Seattle, WA 98121, USA.
Sébastien Pion, Institut de Recherche pour le Développement, Martinique, France.
Guilherme V Polanczyk, Medical School,Chicago, IL, USA.
Suzanne Polinder, Department of Public Health.
Prof C Arden Pope, III, Brigham Young University, Provo, UT, USA.
Svetlana Popova, Centre for Addiction and Mental Health, Toronto, ON, Canada.
Esteban Porrini, Hospital Universitario de Canarias, Tenerif, Spain.
Farshad Pourmalek, Faculty of Medicine, School of Population and Public Health, University of British Columbia, Vancouver, BC, Canada.
Prof Martin Prince, Psychiatry, King’s College London, London, UK.
Rachel L Pullan, London School of Hygiene and Tropical Medicine, London, UK.
Kapa D Ramaiah, Vector Control Research Centre, Pondicherry, India.
Dharani Ranganathan, Institute for Health Metrics and Evaluation, University of Washington, 2301 Fifth Avenue, Suite 600, Seattle, WA 98121, USA.
Homie Razavi, Center for Disease Analysis, Louisville, CO, USA.
Mathilda Regan, University of California, Berkeley, Berkeley, CA, USA.
Prof Jürgen T Rehm, Centre for Addiction and Mental Health, Toronto, ON, Canada.
David B Rein, NORC, University of Chicago, Chicago, IL, USA.
Prof Guiseppe Remuzzi, Mario Negri Institute for Pharmacological Research, Bergamo, Italy.
Kathryn Richardson, University of Cambridge, Cambridge, UK.
Prof Frederick P Rivara, University of Washington, Seattle, WA, USA.
Thomas Roberts, Institute for Health Metrics and Evaluation.
Carolyn Robinson, University of California, San Francisco, San Francisco, CA, USA.
Felipe Rodriguez De Leòn, Complejo Hospitalario Caja De Seguro Social, Panama City, Panama.
Luca Ronfani, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy.
Prof Robin Room, School of Population Health.
Lisa C Rosenfeld, Institute for Health Metrics and Evaluation.
Lesley Rushton, Imperial College London, London, UK.
Prof Ralph L Sacco, Miller School of Medicine, University of Miami, Miami, FL, USA.
Sukanta Saha, Queensland Centre for Mental Health Research.
Prof Uchechukwu Sampson, Vanderbilt University, Nashville, TN, USA.
Lidia Sanchez-Riera, Institute of Bone and Joint Research.
Ella Sanman, Institute for Health Metrics and Evaluation.
Prof David C Schwebel, University of Alabama at Birmingham, Birmingham, AL, USA.
James Graham Scott, Centre for Clinical Research.
Maria Segui-Gomez, Ministry of Interior, Madrid, Spain.
Saeid Shahraz, Brandeis University, Waltham, MA, USA.
Prof Donald S Shepard, Brandeis University, Waltham, MA, USA.
Hwashin Shin, Health Canada, Ottawa, ON, Canada.
Rupak Shivakoti, Johns Hopkins University, Baltimore, MD, USA.
Prof Donald Silberberg, Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
David Singh, Queens Medical Center, Honolulu, HI, USA.
Gitanjali M Singh, School of Public Health.
Jasvinder A Singh, University of Alabama at Birmingham, Birmingham, AL, USA.
Jessica Singleton, National Drug and Alcohol Research Centre.
David A Sleet, National Center for Injury Prevention and Control.
Prof Karen Sliwa, Hatter Institute.
Emma Smith, Department of Rheumatology, Northern Clinical School.
Jennifer L Smith, London School of Hygiene and Tropical Medicine, London, UK.
Nicolas J C Stapelberg, Griffith University, Brisbane, QLD, Australia.
Andrew Steer, Centre for International Child Health.
Prof Timothy Steiner, Norwegian University of Science and Technology, Trondheim, Norway.
Wilma A Stolk, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands.
Prof Lars Jacob Stovner, Department of Neuroscience.
Christopher Sudfeld, School of Public Health.
Sana Syed, Agwa Khan University, Karachi, Pakistan.
Giorgio Tamburlini, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy.
Mohammad Tavakkoli, School of Public Health.
Prof Hugh R Taylor, University of Melbourne, Melbourne, VIC,Australia.
Jennifer A Taylor, Drexel University School of Public Health, Philadelphia, PA, USA.
William J Taylor, University of Otago, Dunedin, NewZealand.
Bernadette Thomas, University of Washington, Seattle, WA, USA.
Prof W Murray Thomson, University of Otago, Dunedin, NewZealand.
Prof George D Thurston, NewYork University, NewYork City, NY.
Imad M Tleyjeh, King Fahad Medical City, Riyadh, Saudi Arabia.
Prof Marcello Tonelli, Alberta Kidney Disease Network, University of Alberta, Edmonton, AB, Canada.
Prof Jeffrey A Towbin, Cincinnati Children’s Hospital, Cincinnati, OH, USA.
Thomas Truelsen, Department of Neurology, Copenhagen University Hospital, Herlev, Denmark.
Prof Miltiadis K Tsilimbaris, University of Crete Medical School, Crete, Greece.
Clotilde Ubeda, Instituto Nacional de Epidemiología, ANLIS, Malbran, Argentina.
Eduardo A Undurraga, Brandeis University, Waltham, MA, USA.
Marieke J van der Werf, KNCV Tuberculosis Foundation, The Hague, Netherlands.
Prof Jim va nOs, Maastricht University Medical Centre, Maastricht, Netherlands.
Prof Monica S Vavilala, University of Washington, Seattle, WA, USA.
N Venketasubramanian, National University of Singapore, Singapore.
Mengru Wang, Institute for Health Metrics and Evaluation.
Prof Wenzhi Wang, Beijing Neurosurgical Institute, Capital Medical University, Beijing, China.
Kerrianne Watt, James Cook University, Townsville, QLD, Australia.
David J Weatherall, University of Oxford, Oxford, UK.
Prof Martin A Weinstock, Brown University, Providence, RI, USA.
Robert Weintraub, Department of Paediatrics.
Marc G Weisskopf, School of Public Health.
Prof Myrna M Weissman, Mailman School of Public Health.
Richard A White, School of Public Health.
Prof Harvey Whiteford, Queensland Centre for Mental Health Research.
Steven T Wiersma, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Prof James D Wilkinson, Miller School of Medicine, University of Miami, Miami, FL, USA.
Prof Hywel C Williams, University of Nottingham, Nottingham, UK.
Sean R M Williams, University of Western Sydney, Campbelltown, NSW, Australia.
Emma Witt, Auckland Technical University, Auckland, New Zealand.
Frederick Wolfe, Arthritis Research, Wichita, KS, USA.
Prof Anthony D Woolf, Royal Cornwall Hospital, Truro, UK.
Sarah Wulf, Institute for Health Metrics and Evaluation.
Pon-Hsiu Yeh, London School of Economics, London,UK.
Anita K M Zaidi, Agwa Khan University, Karachi, Pakistan.
Prof Zhi-Jie Zheng, School of Public Health, Shanghai Jiao Tong University, Shanghai, China.
David Zonies, Landstuhl Regional Medical Center, Landstuhl, Germany.
Prof Alan D Lopez, School of Population Health.
Prof Christopher J L Murray, Institute for Health Metrics and Evaluation.
References
- 1.Murray CJ, Lopez AD. Evidence-based health policy-lessons from the Global Burden of Disease Study. Science 1996; 274: 740–3. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Regional patterns of disability-free life expectancy and disability-adjusted life expectancy: Global Burden of Disease Study. Lancet 1997; 349: 1347–52. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The world health report 2000—Health systems: improving performance. 2010. http://www.who.int/whr/2000/en/whr00_en.pdf (accessed July 9, 2012).
- 4.World Health Organization. The world health report 2001—Mental Health: New Understanding, New Hope. 2001. http://www.who.int/whr/2001/en/whr01_en.pdf (accessed June 25, 2012).
- 5.World Health Organization. The world health report 2002— Reducing Risks, Promoting Healthy Life. 2002. http://www.who.int/whr/2002/en/whr02_en.pdf (accessed July 9, 2012).
- 6.World Health Organization. The world health report 2003—Shaping the future. 2003. http://www.who.int/whr/2003/en/index. html (accessed July 9, 2012).
- 7.World Health Organization. The world health report 2004— Changing history. 2004. http://www.who.int/whr/2002/en/whr02_en.pdf (accessed July 9, 2012).
- 8.Mathers C, Fat DM, Boerma JT. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization, 2008. [Google Scholar]
- 9.Begg SJ, Vos T, Barker B, Stanley L, Lopez AD. Burden of disease and injury in Australia in the new millennium: measuring health loss from diseases, injuries and risk factors. Med J Aust 2008;188:36–40. [DOI] [PubMed] [Google Scholar]
- 10.Michaud CM, McKenna MT, Begg S, et al. The burden of disease and injury in the United States 1996. Popul Health Metr 2006; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schopper D, Pereira J, Torres A, et al. Estimating the burden of disease in one Swiss canton: what do disability adjusted life years (DALY) tell us? Int J Epidemiol 2000; 29: 871–77 [DOI] [PubMed] [Google Scholar]
- 12.Kominski GF, Simon PA, Ho A, Luck J, Lim Y-W, Fielding JE. Assessing the burden of disease and injury in Los Angeles County using disability-adjusted life years. Public Health Rep 2002; 117: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S-C, Cai L, Wan C-H, Lv Y-L, Fang P-Q. Assessing the disease burden of Yi people by years of life lost in Shilin county of Yunnan province, China. BMC Public Health 2009; 9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman C, McKenna MT, Ahmed F, et al. Assessing the burden of disease among an employed population: implications for employer- sponsored prevention programs. J Occup Environ Med 2004; 46: 3–9. [DOI] [PubMed] [Google Scholar]
- 15.Hsairi M, Fekih H, Fakhfakh R, Kassis M, Achour N, Dammak J. Années de vie perdues et transition épidémiologique dans le gouvernorat de Sfax (Tunisie). Sante Publique 2003; 15: 25–37 [PubMed] [Google Scholar]
- 16.Dodhia H, Phillips K. Measuring burden of disease in two inner London boroughs using Disability Adjusted Life Years.J Public Health (Oxf) 2008; 30: 313–21. [DOI] [PubMed] [Google Scholar]
- 17.Soriano JB, Kiri VA, Maier WC, Strachan D. Increasing prevalence of asthma in UK primary care during the 1990s.Int J Tuberc Lung Dis 2003; 7: 415–21. [PubMed] [Google Scholar]
- 18.Goldman LS, Nielsen NH, Champion HC, and the Council on Scientific Affairs AMA. Awareness, diagnosis, and treatment of depression. J Gen Intern Med 1999; 14: 569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BMJ 2001; 322: 1079–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolf AD. The bone and joint decade 2000–2010. Ann Rheum Dis 2000; 59: 81–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen A, Kleinman A, Saraceno B. World mental health casebook: Social and Mental Health Programs in Low-Income Countries. Springer, 2002. [Google Scholar]
- 22.Brooks PM. Musculoskeletal medicine: the challenge of the Bone and Joint Decade. APLAR J Rheumatol 2004; 7: 272–77 [Google Scholar]
- 23.King CH, Bertino A-M. Asymmetries of poverty: why global burden of disease valuations underestimate the burden of neglected tropical diseases. PLoS Negl Trop Dis 2008; 2: e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooker S Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers—a review. Int J Parasitol 2010; 40: 1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 2007; 1: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases.J Clin Invest 2008; 118: 1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray CJL, Lopez AD. Global health statistics: a compendium of incidence, prevalence, and mortality estimates for over 200 conditions. Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank,1996. [Google Scholar]
- 28.Jamison DT, Breman JG, Measham AR, et al. , eds. Disease control priorities in developing countries, 2nd edn World Bank Publications, 2006. [PubMed] [Google Scholar]
- 29.Murray CJ, Ezzati M, Flaxman A, et al. The Global Burden of Disease Study 2010: design, definitions, and metrics. Lancet 2012; 380: 2063–66. [DOI] [PubMed] [Google Scholar]
- 30.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2129–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. World report on disability, 2011. http://whqlibdoc.who.int/publications/2011/9789240685215_eng.pdf (accessed Nov 26,2012).
- 32.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents, Vol. I Geneva: Union Internationale Contre le Cancer, 1966. [Google Scholar]
- 33.Doll R, Muir C, Waterhouse J. Cancer incidence in five continents, Vol. II Geneva: Union Internationale Contre le Cancer, 1970. [Google Scholar]
- 34.Waterhouse J, Muir C, Correa P, Powell J. Cancer incidence in five continents, Vol. III IARC Scientific Publications No. 15. Lyon: IARC, 1976. [Google Scholar]
- 35.Waterhouse J, Muir C, Shanmugaratnam K, Powell J. Cancer incidence in five continents, Vol. IV IARC Scientific Publications No. 42. Lyon: IARC, 1982. [Google Scholar]
- 36.Muir C, Waterhouse J, Mack T, Powell J, Whelan S. Cancer Incidence in Five Continents, Vol. V, IARC Scientific Publications No. 88. Lyon: IARC, 1987. [Google Scholar]
- 37.Parkin D, Muir C, Whelan S, Gao Y, Ferlay J, Powell J. Cancer incidence in five continents, Vol. VI IARC Scientific Publications No. 120. Lyon: IARC, 1992. [Google Scholar]
- 38.Parkin D, Whelan S, Ferlay J, Raymond L, Young J. Cancer incidence in five continents, Vol. VII IARC Scientific Publications No. 143. Lyon: IARC, 1997 [Google Scholar]
- 39.Parkin D, Whelan S, Ferlay J, Teppo L, Thomas D. Cancer Incidence in Five Continents, Vol. VIII IARC Scientific Publications No. 155. Lyon: IARC, 2002. [Google Scholar]
- 40.Curado M, Edwards B, Shin H, et al. Cancer incidence in five continents, Vol. IX IARC Scientific Publications No. 160. Lyon: IARC, 2007. [Google Scholar]
- 41.Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology 2003; 61: 1373–77 [DOI] [PubMed] [Google Scholar]
- 42.Strickland D, Bertoni JM. Parkinson’s prevalence estimated by a state registry. Mov Disord 2004; 19: 318–23. [DOI] [PubMed] [Google Scholar]
- 43.Bhidayasiri R, Wannachai N, Limpabandhu S, et al. A national registry to determine the distribution and prevalence of Parkinson’s disease in Thailand: implications of urbanization and pesticides as risk factors for Parkinson’s disease. Neuroepidemiology 2011; 37: 222–30. [DOI] [PubMed] [Google Scholar]
- 44.European Surveillance of Congenital Anomalies. http://www.eurocat-network.eu/ (accessed July 9, 2011).
- 45.Chen WQ, Zhao P, Rao KQ. [Strengthening cancer registration system in China]. Zhonghua Yu Fang Yi Xue Za Zhi 2010; 44: 374–75. [PubMed] [Google Scholar]
- 46.National Cancer Registry Programme. http://www.ncrpindia.org/ (accessed June 20, 2011).
- 47.Canadian Institute for Health Information. National Ambulatory Care Reporting System 2002–2009. 2011. [Google Scholar]
- 48.CDC National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey. 1992. [Google Scholar]
- 49.CDC National Center for Health Statistics. National Ambulatory Medical Care Survey. 1993. [Google Scholar]
- 50.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2005. http://www.rcgp.org.uk/pdf/ANNUAL%20REPORT%202005.pdf (accessed March 5, 2012).
- 51.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2006. http://www.rcgp.org.uk/pdf/bru_annual%20report%202006%20final.pdf (accessed March 5, 2012).
- 52.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2007. http://www.rcgp.org.uk/pdf/ANNUAL%20REPORT%202007%20FINAL%20COMPLETE.pdf (accessed March 5, 2012).
- 53.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2008. http://www.rcgp.org.uk/PDF/ANNUAL%20REPORT%202008%202%20FINAL.pdf (accessed March 5, 2012).
- 54.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2009. http://www.rcgp.org.uk/pdf/ANNUAL%20REPORT%202009%20FINAL.pdf (accessed March 5, 2012).
- 55.RCGP Research & Surveillance Centre. Weekly Returns Service Annual Report, 2010. http://www.rcgp.org.uk/pdf/ANNUAL%20REPORT%202010%20FINAL.pdf (accessed March 5, 2012).
- 56.Leonard H, Petterson B, Bower C, Sanders R. Prevalence of intellectual disability in Western Australia. Paediatr Perinat Epidemiol 2003; 17: 58–67 [DOI] [PubMed] [Google Scholar]
- 57.Hofman A, Rocca WA, Brayne C, et al. , and the Eurodem Prevalence Research Group. The prevalence of dementia in Europe: a collaborative study of 1980–1990 findings. Int J Epidemiol 1991; 20: 736–48. [DOI] [PubMed] [Google Scholar]
- 58.Jablensky A, McGrath J, Herrman H, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatry 2000; 34: 221–36. [DOI] [PubMed] [Google Scholar]
- 59.Hickman M, Taylor C, Chatterjee A, et al. Estimating the prevalence of problematic drug use: a review of methods and their application. Bull Narc 2002; 54: 15–32. [Google Scholar]
- 60.Imdad A, Jabeen A, Bhutta ZA. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: a meta-analysis of studies from developing countries. BMC Public Health 2011; 11 (suppl 3): S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health 2011; 11 (suppl 3): S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munos MK, Walker CLF, Black RE. The effect of rotavirus vaccine on diarrhoea mortality. Int J Epidemiol 2010; 39 (suppl 1): i56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health 2011; 11 (suppl 3): S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). Washington DC: American Psychiatric Association, 2000. [Google Scholar]
- 65.Murray CJL, Lopez AD. The global burden of disease:a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected in 2020. http://cdsweb.cern.ch/record/732931 (accessed June 25, 2012).
- 66.Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr 2003; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011; 378: 1461–84. [DOI] [PubMed] [Google Scholar]
- 68.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghys PD, Brown T, Grassly NC, et al. The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sex Transm Infect 2004; 80 (suppl 1): i5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.UNAIDS. Spectrum/EPP 2011. http://www.unaids.org/en/ataanalysis/tools/spectrumepp2011/ (accessed June 19, 2012).
- 71.Brooker S, Hay SI, Issae W, et al. Predicting the distribution of urinary schistosomiasis in Tanzania using satellite sensor data.Trop Med Int Health 2001; 6: 998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clements ACA, Firth S, Dembele R, et al. Use of Bayesian geostatistical prediction to estimate local variations in Schistosoma haematobium infection in western Africa. Bull World Health Organ 2009; 87: 921–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schur N, Hurlimann E, Stensgaard A-S, et al. Spatially explicit Schistosoma infection risk in eastern Africa using Bayesian geostatistical modelling. Acta Trop 2011; published online Oct 14. D0I: 10.1016/j.actatropica.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 74.New York Heart Association. Diseases of the heart and blood vessels: nomenclature and criteria for diagnosis. Boston: Little, Brown, 1964. [Google Scholar]
- 75.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. 2011. http://www.goldcopd.org/ (accessed July 6, 2012).
- 76.Kessler RC, Berglund P, Demler O, et al. , and the National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095–105. [DOI] [PubMed] [Google Scholar]
- 77.Agency for Healthcare Research and Quality. United States Medical Expenditure Panel Survey 2000–2009. Rockville, United States, Agency for Healthcare Research and Quality. [Google Scholar]
- 78.US National Institutes of Health National Institute on Alcohol Abuse and Alcoholism. National Epidemiologic Survey on Alcohol and Related Conditions Wave 1 and Wave 2. Bethesda, MD: National Institutes of Health. [Google Scholar]
- 79.Australian Bureau of Statistics. National Survey of Mental Health and Wellbeing of Adults 1997. Canberra: Australian Bureau of Statistics. [Google Scholar]
- 80.Polinder S, van Beeck EF, Essink-Bot ML, et al. Functional outcome at 2.5, 5, 9, and 24 months after injury in the Netherlands. J Trauma 2007; 62: 133–41. [DOI] [PubMed] [Google Scholar]
- 81.South Carolina Traumatic Brain Injury Follow-up Registry (SCTBIFR). http://www.musc.edu/sctbifr/ (accessed on July 9, 2012).
- 82.Mackenzie EJ, Rivara FP, Jurkovich GJ, et al. The National Study on Costs and Outcomes of Trauma. J Trauma 2007; 63: S54–67; [DOI] [PubMed] [Google Scholar]
- 83.Mathers CD, Iburg KM, Begg S. Adjusting for dependent comorbidity in the calculation of healthy life expectancy.Popul Health Metr 2006; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Baal PHM, Hoeymans N, Hoogenveen RT, de Wit GA,Westert GP. Disability weights for comorbidity and their influence on health-adjusted life expectancy. Popul Health Metr 2006; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Baal PHM, Hoogenveen RT, de Wit GA, Boshuizen HC. Estimating health-adjusted life expectancy conditional on risk factors: results for smoking and obesity. Popul Health Metr 2006; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Dwyer-Lindgren L, Lofgren KT, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2071–94. [DOI] [PubMed] [Google Scholar]
- 87.Alter G, Riley JC. Frailty, sickness, and death: models of morbidity and mortality in historical populations. Popul Stud (Camb) 1989;43: 25–5. [DOI] [PubMed] [Google Scholar]
- 88.Fries JF. Aging, natural death, and the compression of morbidity.N Engl J Med 1980; 303: 130–35. [DOI] [PubMed] [Google Scholar]
- 89.Johansson SR. The health transition: the cultural inflation of morbidity during the decline of mortality. Health Transit Rev 1991;1: 39–68. [PubMed] [Google Scholar]
- 90.Gruenberg EM. The failures of success.Milbank Mem Fund Q Health Soc 1977; 55: 3–24. [PubMed] [Google Scholar]
- 91.Verbrugge LM. Longer life but worsening health? Trends in health and mortality of middle-aged and older persons.Milbank Mem Fund Q Health Soc 1984; 62: 475–519. [PubMed] [Google Scholar]
- 92.Olshansky SJ, Rudberg MA, Carnes BA, Cassel CK, Brody JA. Trading off longer life for worsening health: the expansion of morbidity hypothesis. J Aging Health 1991; 3: 194–216. [Google Scholar]
- 93.Brody JA, Miles TP. Mortality postponed and the unmasking of age-dependent non-fatal conditions. Aging (Milano) 1990; 2: 283–89. [DOI] [PubMed] [Google Scholar]
- 94.Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the U.S. elderly population: experience from the 1982–2004 National Long-Term Care Survey.J Gerontol B Psychol Sci Soc Sci 2008; 63: s269–81. [DOI] [PubMed] [Google Scholar]
- 95.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370: 851–58. [DOI] [PubMed] [Google Scholar]
- 96.King G, Murray CJL, Salomon JA, Tandon A. Enhancing the validity and cross-cultural comparability of measurement in survey research. Am Polit Sci Rev 2004; 98: 191–207. [Google Scholar]
- 97.Salomon JA, Tandon A, Murray CJL. Comparability of self rated health: cross sectional multi-country survey using anchoring vignettes. BMJ 2004; 328: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwata N, Turner RJ, Lloyd DA. Race/ethnicity and depressive symptoms in community-dwelling young adults: a differential item functioning analysis. Psychiatry Res 2002; 110: 281–89. [DOI] [PubMed] [Google Scholar]
- 99.Kapteyn A, Smith JP, van Soest A. Vignettes and self-reports of work disability in the United States and the Netherlands.Am Econ Rev 2007; 97: 461–73. [Google Scholar]
- 100.Kristensen N, Johansson E. New evidence on cross-country differences in job satisfaction using anchoring vignettes.Labour Econ 2008; 15: 96–1177 [Google Scholar]
- 101.Kramer M The rising pandemic of mental disorders and associated chronic diseases and disabilities. Acta Psychiatr Scand 1980; 62: 382–977468297 [Google Scholar]
- 102.Eaton WW, Kalaydjian A, Scharfstein DO, Mezuk B, Ding Y. Prevalence and incidence of depressive disorder: the Baltimore ECA follow-up, 1981–2004. Acta Psychiatr Scand 2007; 116: 182–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative.World Psychiatry 2007; 6: 168–76. [PMC free article] [PubMed] [Google Scholar]
- 104.Eppig C, Fincher CL, Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci 2010;277: 3801–08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989; 84: 7–177 [PubMed] [Google Scholar]
- 106.Lozoff B, Wolf AW, Jimenez E Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr 1996; 129: 382–89. [DOI] [PubMed] [Google Scholar]
- 107.Watkins WE, Cruz JR, Pollitt E. The effects of deworming on indicators of school performance in Guatemala.Trans R Soc Trop Med Hyg 1996; 90: 156–61. [DOI] [PubMed] [Google Scholar]
- 108.Simeon DT, Grantham McGregor SM, Callender JEM, Robinson S, Wong MS. Treatment of mild to moderate trichuris trichiura infections in children: effects on school performance and growth— abstract. West Indian Med J 1995: 277 [Google Scholar]
- 109.Hotez PJ, Bundy DAP, Beegle K, et al. , eds. Helminth infections: soil-transmitted helminth infections and SchistosomiasisIn: Disease control priorities in developing countries, 2nd edn, World Bank Publications, 2006. [Google Scholar]
- 110.Wallhagen MI, Strawbridge WJ, Shema SJ. The relationship between hearing impairment and cognitive function: a 5-year longitudinal study. Res Gerontol Nurs 2008; 1: 80–86. [DOI] [PubMed] [Google Scholar]
- 111.Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc 2002;50: 482–88. [DOI] [PubMed] [Google Scholar]
- 112.Sugawara N, Sasaki A, Yasui-Furukori N, et al. Hearing impairment and cognitive function among a community-dwelling population in Japan. Ann Gen Psychiatry 2011; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]


