Abstract
Introduction
Clinical observations and animal models suggest a critical role for the dynamic regulation of transmural pressure and peristaltic airway smooth muscle contractions for proper lung development. However, it is currently unclear how such mechanical signals are transduced into molecular and transcriptional changes at the cell level. To connect these physical findings to a mechanotransduction mechanism, we identified a known mechanosensor, TRPV4, as a component of this pathway.
Methods
Embryonic mouse lung explants were cultured on membranes and in submersion culture to modulate explant transmural pressure. Time-lapse imaging was used to capture active changes in lung biology, and whole-mount images were used to visualize the organization of the epithelial, smooth muscle, and vascular compartments. TRPV4 activity was modulated by pharmacological agonism and inhibition.
Results
TRPV4 expression is present in the murine lung with strong localization to the epithelium and major pulmonary blood vessels. TRPV4 agonism and inhibition resulted in hyper- and hypoplastic airway branching, smooth muscle differentiation, and lung growth, respectively. Smooth muscle contractions also doubled in frequency with agonism and were reduced by 60% with inhibition demonstrating a functional role consistent with levels of smooth muscle differentiation. Activation of TRPV4 increased the vascular capillary density around the distal airways, and inhibition resulted in a near complete loss of the vasculature.
Conclusions
These studies have identified TRPV4 as a potential mechanosensor involved in transducing mechanical forces on the airways to molecular and transcriptional events that regulate the morphogenesis of the three essential tissue compartments in the lung.
Electronic supplementary material
The online version of this article (10.1007/s12195-018-0538-7) contains supplementary material, which is available to authorized users.
Keywords: Lung morphogenesis, Mechanotransduction, Mechanics of morphogenesis, Airway smooth muscle, Lung reciprocal signaling
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease and the leading cause of perinatal mortality and morbidity, affecting over 30% of extremely preterm infants.8 The malformed nature of the pulmonary vasculature (PV)36,56 predisposes children suffering from BPD to pulmonary hypertension (PH), a severe complication with a mortality of 40–50% in their first year of life.11,15,36,57,64 The PH mortality rate has remained largely unchanged since the 1980s, a staggering realization for a disease that lacks an agreed-upon definition in the context of congenital defects and BPD.4,6,11,19,47 Whereas much of the etiology remains unclear, there is a recognized alteration in the PV, with a suggestion of both a decrease in pulmonary vasculature density early in the disease and compensatory dysmorphic PV at later disease stages.5 The decrease in pulmonary vessel density, or rarefication, is thought to be a precursor to development of PH.58,62 Although some advances have been made in symptomatic treatment of a specific subset of PH, pulmonary arterial hypertension,7,21,53 the ongoing mortality of PH underscores the need to identify novel regulators of the PV and overall lung growth.
It is well established that the development of the mammalian lung relies on reciprocal signaling: the complex interplay of soluble factors that are secreted and sensed between neighboring tissues to regulate morphogenesis. This cross-talk between tissue compartments guides the formation of new airway branches into the surrounding mesenchyme45,65 and ensures tight coordination of vascular morphogenesis around the airway tree.14,20,24,26,30,39,48,55 Whereas several signaling pathways important to vascular morphogenesis have been identified, notably HIF1α-mediated expression of VEGF in the airway epithelium,14,24,55 little is known about the upstream regulators of these pathways. As the PV develops in concert with the airways, it is likely both tissue compartments are influenced by shared regulators.
It has been long understood that in addition to the genetic and molecular regulators of lung development, physical forces also sculpt lung architecture. Clinical and in vivo observations going back decades have observed the build-up of fluid with the developing lung, resulting in a pressure of approximately 200–400 Pa during development.25,51,54 This differential pressure acting on the developing airways, termed transmural pressure, has been long suspected to be essential for lung growth. Increasing fluid retention or transmural pressure results in airway elaboration,3,50,63 and relieving fluid pressure results in reduced airway growth.3,18 This quasi-static pressure is coupled to active contractility at the cellular level.37,43,44 Most notably, airway smooth muscle (ASM), which wraps circumferentially around the epithelial lumen of the lung undergoes peristaltic contractions that have been shown to correlate with lung growth,31–35 possibly due to cyclic distension of the airway tips.38 Further, pharmacological activation or inhibition of ASM contractility leads to parallel acceleration or inhibition of lung branching.17,65 Additionally, in the nitrofen-induced rat model of congenital diaphragmatic hernia, a precursor of BPD, peristalsis is dysregulated and leads to disease progression through lung hypoplasia,16,35 suggesting that altered ASM contractions play a key role in the dysregulated mechanical environment present in BPD and PH.
Despite the long history demonstrating the profound importance of both quasi-static and dynamic mechanical forces on airway development, it remains to be elucidated how the cells of the tissue sense these forces. Whereas specific mechanisms remain unknown, a reasonable hypothesis is that there exists an unidentified mechanotransducer that regulates key signaling pathways, such as calcium (Ca2+) signaling. Ca2+ signaling has been shown to be essential for overall lung growth with signaling occurring in epithelium and smooth muscle compartments.12 As a prominent example of this, Ca2+ entry into the ASM is an essential part of peristalsis control,17 consistent with other smooth muscle tissues. For these reasons, we were interested in testing for the expression and function of a mechanosensitive Ca2+ channel as a putative mechanosensor molecule in lung development. One such candidate is TRPV4, a calcium permeable cation channel that has been previously implicated in stretch mechanotransduction in multiple cell and tissue systems.40,66 Due to its expression in the adult lung, TRPV4 has been implicated in mediating the mechanosensitive response to ventilator-induced barotrauma.22,23 While this response has been minimally investigated in late fetal lungs,49 TRPV4’s direct correlation to early lung development remains unstudied.
In the present work, we provide evidence that TRPV4 is present during fetal lung development and is a positive regulator of lung growth, airway smooth muscle activity, and early PV stability. Identification of TRPV4 as a positive regulator of lung growth reveals a novel signaling axis that offers new insight into morphogenesis and a potential therapeutic avenue for BPD and PH.
Methods
Explant Cultures
Briefly, timed pregnant CD-1 mice were euthanized at E13.5 by CO2 asphyxiation confirmed by cervical dislocation. Uteri were removed from timed pregnant females and temporarily stored in cold (4 °C) Phosphate Buffered Saline (PBS) with 1% penicillin–streptomycin (PS). Lungs were explanted from embryos in cold (4 °C) PBS with 1% PS. Tracheas of the explants were sutured closed, and then whole lungs were transferred to Matrigel (Corning) thin gels in the base of 24 well tissue culture plates. Transmural pressure was varied via the addition of different volumes (200 and 500 µL) of Dulbecco’s Modified Eagle Medium/Ham’s F12 nutrient mixture (DMEM/F12) with 5% Fetal Bovine Serum (FBS) and 1% PS. Lung explants were cultured for 24 and 48 h at 37 °C and 5% CO2. TRPV4 expression was pharmacologically activated with 100 nM GSK1016790A and inhibited with 10 μM GSK205 in DMEM/F12 with 5% FBS and 1% PS. Vehicle control was achieved by adding equivolume dimethyl sulfoxide (DMSO) to the culture medium.
ASM Contraction Quantification
Lungs were explanted and sutured shut as described above and then transferred to semipermeable membranes (Whatman Nuclepore Hydrophilic Membrane, 8.0 µm pore size, 25 mm circle) on raised PDMS pillars in 6 well culture dishes. DMEM/F12 with 5% FBS and 1% PS was filled under the membranes to support culture of the embryonic lungs. Pharmacological activation and inhibition of TRPV4 was achieved using the addition of 100 nM GSK1016790A or 10 μM GSK205 respectively, with DMSO acting as a vehicle control. Live imaging of explants was performed at 1 Hz for 72 h on a Zeiss Axio Observer with incubated stage (37 °C and 5% CO2). Peristaltic contractions were quantified by a masked observer.
Immunoflourescent Imaging and Vascular Morphometrics
Following culture, lungs were fixed in 4% paraformaldehyde/0.5% Triton-X in PBS for 90 min at 4 °C, permeabilized overnight in 0.5% Triton-X in PBS at 4 °C, blocked overnight with 1% BSA/0.2% cold-fish gelatin/0.1% Tween-20 in PBS at 4 °C, and then blocked for 72 h with Mouse on Mouse IgG Blocking Reagent (Vector Labs). Lungs were rinsed 3X in PBS then incubated overnight at 4 °C with rabbit anti-ECAD (1:500) (Cell Signaling) to label the airways, rat anti-PECAM (1:250) (Santa Cruz Biotechnology, Santa Cruz, CA) to label the vasculature, and mouse anti-α-Actin (1:250) (Santa Cruz Biotechnology) to label airway smooth muscle, or rabbit anti-TRPV4 (1:250) (Proteintech) in 1% BSA/0.2% cold-fish gelatin/0.1% Tween-20 in PBS. Specificity of the TRPV4 antibody was validated in fixed mouse lung epithelial cells (MLE12) using siRNA specific to TRPV4 (Supplemental Fig. 1). Lungs were then rinsed 3X for 20 min in 4 °C PBS and placed in appropriate secondary antibodies overnight at 4 °C and finally rinsed 3X in PBS. Lungs were initially imaged using a Zeiss Axio Observer. Lungs were then cleared via dehydration by rinsing 3X in 100% Methanol for 5 min and rehydrating 3X 15 min rinses with 1:1 Benzyl Alcohol: Benzyl Benzoate. For immunofluorescent staining of lung explant sections, lungs were harvest and fixed as described above. Fixed tissue was washed 3X in PBS for 5 min and placed in a 15%/sucrose PBS solution for overnight incubation at 4 °C. Over the next 3 days lung tissue was taken through a series of overnight incubations in 30% sucrose/PBS, 1:1 30%sucrose/PBS – TFM, and finally TFM. Lung samples were then placed in fresh TFM, oriented, and frozen using copper blocks chilled in liquid nitrogen. 6 μm sections were cut using a Leica CM3050S cryostat. Sections were blocked similar to whole lungs and then labelled with Anti-TRPV4 (Proteintech) at room temperature for 1 h. Following primary incubation, sections were washed 3X for 5 min in room temperature PBS prior to be incubated in appropriate secondary for 1 h at room temperature. DAPI was added to lung sections during the final 3X PBS washes before being mounted with gelvatol. High resolution images of lungs were then collected using a Zeiss LSM880 confocal microscope. For whole mount lungs, custom MATLAB scripts were used to segment confocal volumes into airway and mesenchyme, and validated against a masked observer. ASM intensity and PV density were normalized to controls.
Cellular Proliferation Assay
Adult mouse lung epithelial cells (MLE12) were routinely culture in HITES medium (as per ATCC protocol) with 1% PS. At confluence cells were seeded at 3500 cells/cm2 to 24 well culture plates where they were treated with TRPV4 inhibitor (10 μM GSK205), agonist (100 nM GSK1016790A,) vehicle control (DMSO), or standard culture media. Following 24 h of growth cells were isolated and run through a NovoCyte Flow Cytometer to quantify the total number of cells.
Western Blotting
Immediately after isolation, lungs were lysed in RIPA with 2X HALT protease and phosphatase inhibitors. As a positive control for TRPV4 expression, adult mouse lung epithelial cells (MLE12) (ATCC, Manassas, VA) were routinely cultured in HITES medium (as per formulation suggested by ATCC) with 1% PS, and similarly lysed. Protein was homogenized, quantified using a detergent compatible Lowry assay, and denatured at 95 °C in LDS sample buffer containing DTT (CBS Scientific). Protein was loaded at 20 μg per lane into a 4–12% TEO-SDS gel (CBS Scientific) and separated at 150 V for 1.5 h. Protein was transferred to Amersham™ Protran™ 0.2 μm NC membrane (GE Healthcare Life Sciences) using a G2 Fast Blotter (ThermoFisher Scientific). Membranes were blocked with 5% milk in Tris-buffered saline with 0.1% Tween 20 and probed with anti-TRPV4 (Alomone) and with anti-β-actin (Cell Signaling) as a loading control. Appropriate HRP-conjugated secondary antibodies were used and the membrane developed with Super Signal Femto ECL (ThermoFisher Scientific) and imaged with a ChemiDoc-IT2 bioimaging system (UVP).
Results and Discussion
The development of the lung is a tightly coordinated process that produces an airway and vascular architecture critical for survival. Clinical observations and animal models suggest a critical role for the dynamic regulation of physical forces including transmural pressure and peristaltic airway smooth muscle contractions for proper lung development. However, it is currently unclear how such mechanical signals are transduced into reciprocal signaling cascades between tissue compartments that regulate morphogenesis of the lung. To connect these physical findings to a mechanotransduction mechanism, we identified a known mechanosensor, TRPV4, and downstream Ca2+ signaling as a component of this pathway.
TRPV4 is Expressed in Embryonic Murine Lung During Pseudoglandular Development
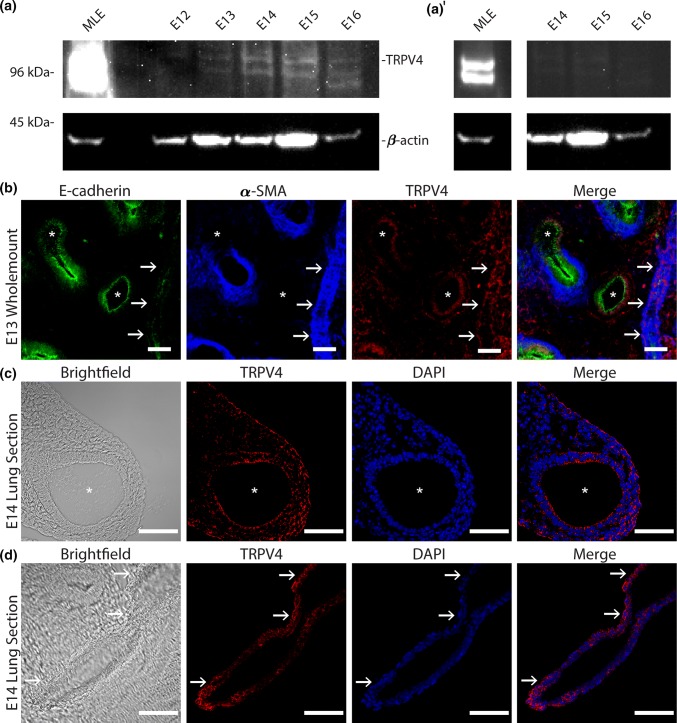
Given the known importance of Ca2+ signaling in ASM contractility,17,35 the mechanosensitive role of TRPV4 in other tissues, and its presence in the mature lung,22,23 we sought to determine if TRPV4 is expressed in the early embryonic lung. Immunoblots of lysates from lung explants at various gestational ages (E12-16) demonstrate the presence of TRPV4 in early lung development (Fig. 1a). Mature lung epithelial cells (MLES12s) serve as a positive control and we provide an adjusted contrast version of the blot (Fig. 1a′) to demonstrate the band morphology is conserved in the positive control. Similarly, whole-mount staining (Fig. 1b) and immunofluorescent imaging of cryo-sectioned E14 lung explants show that this mechanosensitive ion channel is localized to the apical and basal surfaces of airway epithelium, sub-epithelial mesenchyme, (Fig. 1c) and the major pulmonary vessels (Fig. 1d). Interestingly, these spatial patterns of TRPV4 correspond to key tissues that experience (epithelium) or generate (ASM and vascular smooth muscle) dynamic mechanical forces during embryonic lung development. The epithelium undergoes cyclic deformations as a result of ASM contraction-mediated fluid flows within the airway lumen. The presence of TRPV4 at the apical surface may serve to sense these deformations and regulate cellular behaviors necessary for the formation of new airways. This would be consistent with numerous studies that demonstrate increased airway branching with ASM contraction frequency and an arrest in airway branching with the absence of ASM contractions.16,31,50 Further, localization to the subepithelial mesenchyme and to the pulmonary vasculature, at an embryonic stage where the ASM and vasculature are rapidly assembling, suggests TRPV4 may regulate the differentiation, assembly, or function of these critical tissues.
Figure 1.
TRPV4 is expressed in embryonic murine lung during pseudoglandular development. (a) Mouse lung explants at embryonic day (E) 12–16 were homogenized, and TRPV4 expression was assessed via Western blotting. Positive expression at ~ 98 kDa was detected in E12–E16 lung lysates as well as in the positive control (MLE12 lysate). Blotting against β-actin was used as a loading control. Positions of molecular weight markers indicated to the left. (a′) The underexposed blot shows a conserved band morphology in the positive control as the lung lysate (E14 to E16). (b) E13 mouse lung was fixed and whole-mount stained for TRPV4 (red) along with counterstains for airway epithelium (E-cadherin, green) and smooth muscle (α-smooth muscle actin; αSMA, blue). Images are maximum projections of 286 × 286 × 15 µm confocal volume, median filtered for clarity. (c) Cryosections of E14 explants showing epithelial and (d) vascular structures were stained for TRPV4 and DAPI. TRPV4 primarily localizes to airway (white *) epithelium, the subepithelial mesenchyme, and major blood vessels (white arrows). Scale bars 50 µm.
TRPV4 Activity Regulates Murine Airway Morphogenesis
As a Ca2+ channel, TRPV4 is positioned to regulate a broad spectrum of cellular behaviors essential to airway morphogenesis. Ca2+ is a centralized second messenger signal molecule implicated in proliferation, differentiation, and contraction of cells.9,10 Further, at the tissue scale, gap junctions allow for cell–cell communication via propagation of intercellular Ca2+ waves.28,29 These waves propagate elevated levels of intracellular Ca2+ throughout cell populations, leading to coordinated proliferation, migration, and contraction of cells.17,41 Proliferation, migration, and cell–cell communication are all critical in the formation of airway branches.12,59 However, the molecular mechanism that propagates Ca2+ influx during airway development has yet to be identified. As we have demonstrated the expression of TRPV4 in the early lung, we further sought to determine if TRPV4 was a functional regulator in overall airway morphogenesis. We cultured the lung explants ex vivo at the air-medium interface using a floating membrane culture over 48 h. Lungs were treated with selective pharmacological modulators to activate (100 nM GSK1016790A; abbreviated GSK101)61 or inhibit (10 µM GSK205)52 TRVP4 (Fig. 2a), and the number of terminal airway branches were quantified at the start and end of culture. Activation of TRPV4 significantly increased airway branching with lung explants exhibiting a larger fold increase in the number of terminal branches over culture compared to controls (3.11 ± 0.22 vs. 2.49 ± 0.23; Fig. 2b). Conversely, antagonism of TRPV4 resulted in a smaller fold increase in terminal branches (1.96 ± 0.13); however, airway branching morphogenesis was not completely arrested. To determine if alterations in airway branching were the result of TRPV4-induced changes in epithelial proliferation we quantified MLE12 proliferation in culture in response to TRPV4 activation and inhibition (Fig. 2c). These studies demonstrate an approximately 60% decrease in proliferation over 24 h with TRPV4 inhibition and no appreciable change in proliferation with TRPV4 activation compared to untreated cells. These ex vivo and cell culture experiments provide compelling evidence that TRPV4 activity and the subsequent intracellular calcium signaling events play an active role in airway epithelial morphogenesis.
Figure 2.
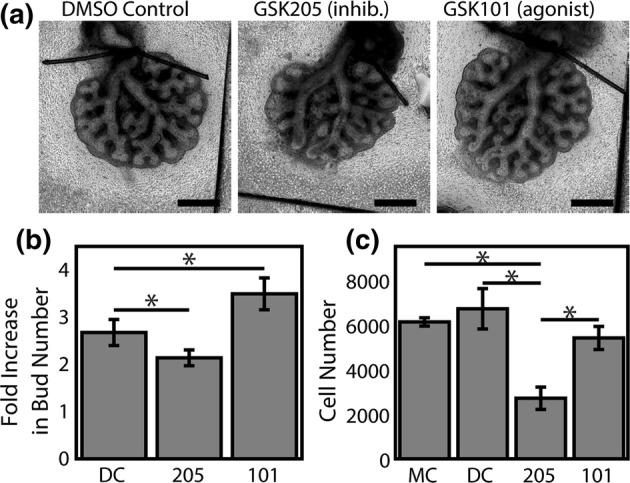
TRPV4 activity regulates murine lung branching morphogenesis. Embryonic mouse lungs were isolated at E12.5 and cultured on floating membranes for 48 h. (a) Lungs cultured with 10 µM GSK205, a TRPV4 inhibitor, were smaller with less elaborated airway structure than lungs treated with equivolume DMSO as a vehicle control. Conversely, lungs treated with 100 nM GSK1016790A (GSK101), a TRPV4 agonist, were larger and exhibited increased airway branching. Scale bars 500 µm. (b) Branch counts before and after culture by a masked observer demonstrate a significant effect of TRPV4 inhibition (205; 10 µM GSK205) and activation (101; 100 nM GSK1016790A). n = 11–12 (isolated from 6 uteri). (c) Proliferation of MLE12 cells following 24 h of culture show a significant decrease in cultured adult pulmonary epithelial cells with TRPV4 inhibition. * Indicates p < 0.05, Tukey’s HSD post hoc test after repeated measures ANOVA.
TRPV4 Regulates Active Contractility of the Developing Lung
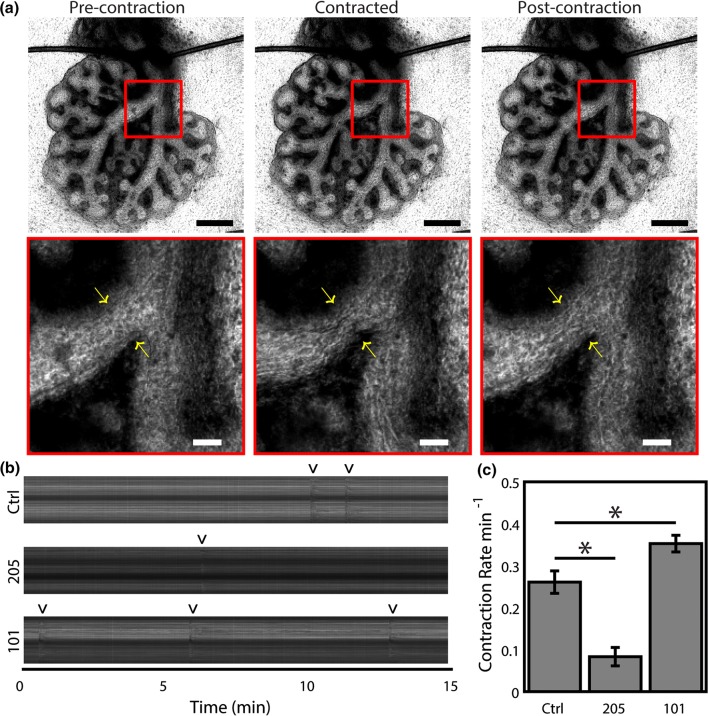
Building on the findings supporting the role of TRPV4 as a pressure sensitive Ca2+ channel, and our recent study demonstrating pressure-based regulation of airway contractility,50 we hypothesized that TRPV4 mediates the peristaltic ASM contractility of the lung. High-frequency timelapse imaging (1 Hz) was used to observe lung explants following 48 h of culture (Supplementary Movie 1). As ASM contraction events are irregular and vary spatially throughout the organ, the analysis of contractions was performed in the left and right mainstem bronchi across multiple lung explants (Fig. 3a). ASM contraction events were quantified with kymographs (Fig. 3b). TRPV4 agonism (GSK101) produced an increase in ASM contraction frequency, and inhibition (GSK205) resulted in sharply reduced contraction frequencies (Fig. 3c). These studies are consistent with the well-established role for Ca2+ signaling in ASM contractility in the developing lung and lend strong support to the hypothesis that TRPV4 is an important mechanosensor that regulates several phenotypes associated with overall lung growth.
Figure 3.
TRPV4 regulates active contractility of the developing lung. Embryonic mouse lungs were isolated at E12.5 and cultured on floating membranes for ~ 48 h before 1 Hz imaging. (a) Cultured lungs demonstrated active contraction. Contraction is visible throughout the lung as a decrease in brightness of the lumen as the airways contract down. Zoomed images (red boxes) show the contraction in more detail, with arrows indicating the location of the contraction. Scale bars 500 and 100 µm for the image and zooms, respectively. (b) Kymographs of contraction across the left and right bronchi. Contractions are visible as dark vertical streaks and indicated by ‘v’. Lungs cultured with 10 µM GSK205, a TRPV4 inhibitor, were less mechanically active than lungs treated with equivolume DMSO as a vehicle control. Conversely, lungs treated with 100 nM GSK1016790A (GSK101), a TRPV4 agonist, exhibited more frequent contractions. (c) Contraction rate measured by a masked observer over 1 h of culture demonstrate a significant effect of TRPV4 inhibition (205; 10 µM GSK205) and activation (101; 100 nM GSK1016790A). n = 6 (isolated from 3 uteri). * Indicates p < 0.05, Tukey’s HSD post hoc test after repeated measures ANOVA.
TRPV4 Regulates the Stability of the Pulmonary Vasculature
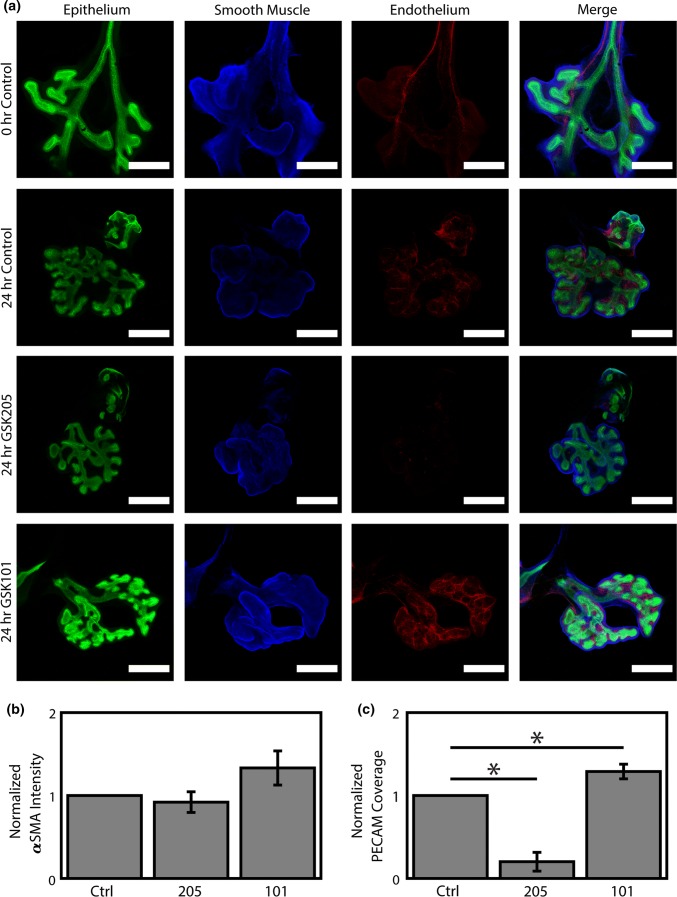
As multiple tissue compartments of the lung (epithelial, ASM, vascular) are known to extensively cross-regulate65 and immunostaining demonstrated TRPV4 localization to each of these areas, we proceeded to investigate the impact of TRPV4 modulation on ASM and PV morphogenesis. Ex vivo explant culture was conducted over 24 h in the presence of TRPV4 modulators. After culture, samples were fixed and stained for whole-mount confocal imaging (Fig. 4a). To determine the impact of TRPV4 modulation on the ASM and PV tissue compartments, we stained for αSMA and PECAM, respectively. For ASM, we observed a minimal correlation between αSMA staining intensity and TRPV4 activation; however, it did not rise to significance (Fig. 4b). These data suggest that the change in ASM contraction frequency we observed is likely due to a modulation of ASM function, rather than an increase or decrease of ASM coverage.
Figure 4.
TRPV4 regulates the expression of αSMA and PECAM in lung morphogenesis. Embryonic mouse lungs were isolated at E12.5 and cultured for ~ 24 h before fixation, staining, and whole mount imaging. (a) At isolation (0 h Control), lungs demonstrate an intricate vascular network (Endothelium; PECAM; red) and smooth muscle around the major airways (Smooth Muscle; αSMA; blue), which expand with airway (Epithelium; E-cadherin; green) elaboration over culture (24 h Control). However, TRPV4 inhibition (24 h GSK205; 10 µM) has minimal effect on smooth muscle labeling and dramatically ablates the existing vascular network, while TRPV4 activation (24 h GSK101; 100 nM GSK1016790A) increases the labeling of both smooth muscle and vascular compartments. Scale bars 250 µm. Images median filtered for clarity. (b) Quantification of average staining intensity of αSMA around the airways, normalized to control. (c) Quantification of vascular coverage based on a set threshold of PECAM intensity in the mesenchyme, normalized to control. n = 4. * Indicates p < 0.05 for a Fisher’s LSD following a significant 1-way ANOVA.
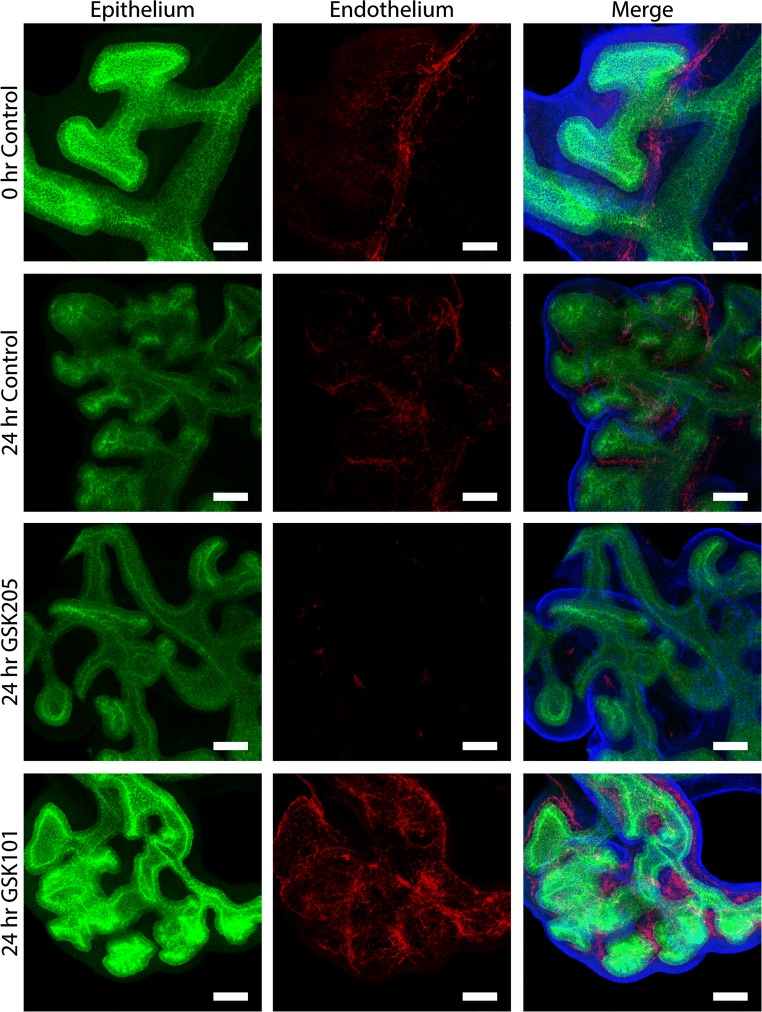
Surprisingly, in the PV, TRPV4 activation (GSK101) led to a consistent increase in vascular coverage, whereas inhibition (GSK205) led to the near complete ablation of the PV (Figs. 4a and 4c). An intricate, hierarchical vascular network can be observed in freshly explanted mouse lungs (Fig. 5) with an organization that is maintained over culture. TRPV4 agonism results in extensive new vascular formation and a similar hierarchical organization. Conversely, TRPV4 antagonism appears to destabilize the existing PV resulting in a loss of hierarchical organization and the widespread resorption of de novo angiogenesis in the lung. These data demonstrate that TRPV4 activity is an essential regulator of PV stability during early embryonic lung development. Further, given the known reciprocal signaling between the airway and PV during lung development,65 loss of TRPV4-mediated PV stability would likely impair overall lung growth.
Figure 5.
TRPV4 activity regulates the stability of PV during lung morphogenesis. Embryonic mouse lungs were isolated at E12.5 and cultured for ~ 24 h before fixation, staining, and whole mount imaging. At isolation (0 h Control), lungs demonstrate an intricate vascular network (Endothelium; PECAM; red), and this is maintained during routine culture (24 h Control). However, TRPV4 inhibition (24 h GSK205; 10 µM) nearly eliminates PECAM positive cells, while TRPV4 activation (24 h GSK101; 100 nM GSK1016790A) results in a more robust capillary network. Scale bars 50 µm.
Conclusion
In these studies, we have shown that the putative mechanosensor, TRPV4, is present in the embryonic murine lung and positively regulates its development. With pharmacological perturbation of TRPV4 we demonstrate that its regulation directly correlates with murine lung contractions and growth, providing further support for the hypothesis of contraction mediated growth.32,33,35 Importantly, we observed a marked decrease in PECAM expression after TRPV4 inhibition, when compared to both culture controls and freshly isolated lungs prior to culture. This demonstrates that TRPV4 activity is essential for stabilization of the PV during lung development. Whereas this is a completely novel finding in lung development, a similar role of TRPV4 has been shown in stabilizing vessels in tumors and after stroke,2,13,60 suggesting that TRVP4 may be a broad regulator of vessel behavior in tissues undergoing rapid angiogenesis. Importantly, through use of an ex vivo model, these studies have demonstrated that TRPV4 regulates multiple tissue compartments in an intact and growing organ. Future work will build on these whole organ studies by investigating the molecular mechanisms downstream of TRPV4-mediated Ca2+ signaling, for example, HIF-1α and its transcriptional target VEGF. Recent work identifying calcium signaling as an upstream regulator of HIF-1α,27,46 makes it a likely downstream target of TRPV4 activation. VEGF, a protein downstream of HIF-1α activation has been shown to be important in lung growth1,14,42,55 and is a known mediator of endothelial to epithelial intracellular growth signaling in lung. By identification of a novel mechanosensitive signaling modality in lung growth, this work provides a new avenue for the development of future therapies for BPD and PH, as well as providing novel directions for research into disease etiology.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1: TRPV4 antibody Specificity. Mouse lung epithelial (MLE12) cells were transfected with siRNA specific to TRPV4 or to a non-targeting control (siNT) using Lipofectamine RNAiMAX according to manufacturer instructions. After 72 h, the cells were fixed and stained for TRVP4 and counterstained with DAPI to identify the nuclei. There was a substantial decrease in TRPV4 staining intensity with siTRPV4 compared to siNT treated cells. Scale bars 50 µm. Supplementary material 1 (TIFF 29097 kb)
Movie S1: TRPV4 regulates active contractility of the developing lung. Embryonic mouse lungs were isolated at E12.5 and cultured on floating membranes for ~ 48 h before 1 Hz imaging. (A) Cultured lungs demonstrated active airway contraction, and this was influenced by TRVP4 modulation. This video spans 100 s, and the control lung visibly contracts at ~ 50 s, whereas 100 nM GSK1016790A (Activator) treated lung visibly contracts at ~ 36 and ~ 68 s, and the lung treat with 10 µM GSK205 (Inhibitor) does not visibly contract. Supplementary material 2 (MP4 1226 kb)
Acknowledgments
The authors would like to thank Mr. Peter Sariano and Ms. Julia Pelesko for their technical assistance. This work was supported in part by grants from the National Institutes of Health (R01HL133163, R21ES027962, P20GM103446, U54GM104941, S10OD016361), the National Science Foundation (1537256), the Oak Ridge Associated Universities Ralph E. Powe Junior Faculty Enhancement Award (J.P.G.) and the March of Dimes Basil O’Connor Award (5-FY16-33 to J.P.G).
Conflict of interest
Joshua T. Morgan, Wade G. Stewart, Robert A. McKee and Jason P. Gleghorn report no conflicts of interest.
Human and Animal Studies
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees at the University of Delaware.
Abbreviations
- BPD
Bronchopulmonary dysplasia
- TRPV4
Transient receptor potential cation channel subfamily V member 4
- Pa
Pascal
- PV
Pulmonary vasculature
- PH
Pulmonary hypertension
- HIF1α
Hypoxia-inducible factor 1 alpha
- VEGF
Vascular endothelial growth factor
- ASM
Airway smooth muscle
Footnotes
Jason P. Gleghorn is an Assistant Professor at the University of Delaware in the Department of Biomedical Engineering. Gleghorn received his Ph.D. from Cornell University under the mentorship of Lawrence Bonassar. He then completed postdoctoral fellowships at Princeton University with Celeste Nelson and Cornell University with Brian Kirby. During his postdoctoral training, Gleghorn applied microfluidic and microfabrication techniques to identify new physical mechanisms that regulate organ development and he created novel microfluidic systems to isolate rare circulating tumor cells from patient blood samples respectively. His lab, started in 2014 at the University of Delaware, develops and uses microfluidic and microfabrication technologies to determine how cells behave and communicate within multicellular populations to form complex 3D tissues and organs. The long-term goals of this research are to develop techniques to engineer physiologically relevant 3D culture systems with well-defined structure, flows, and cell-cell interactions to study tissue-scale biology and disease. These techniques in combination with what they learn in studies of the native cellular behaviors and interactions in the embryo are used to define new therapeutic approaches for regenerative medicine. Gleghorn’s honors include the ORAU Powe Junior Faculty Award, the March of Dimes Basil O’Connor Award, the UD Bernard Canavan Faculty Research Award, and the BMES CMBE Rising Star Award.
This article is part of the 2018 CMBE Young Innovators special issue.
Joshua T. Morgan and Wade G. Stewart have equally contributed to this work.
References
- 1.Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am. J. Respir. Cell Mol. Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- 2.Adapala RK, Thoppil RJ, Ghosh K, Cappelli HC, Dudley AC, Paruchuri S, Keshamouni V, Klagsbrun M, Meszaros JG, Chilian WM, Ingber DE, Thodeti CK. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J. Anat. 1977;123:649–660. [PMC free article] [PubMed] [Google Scholar]
- 4.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan. Med. J. 2013;60:A4688. [PubMed] [Google Scholar]
- 5.Alvira CM. Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Front. Med. 2016;3:21. doi: 10.3389/fmed.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi J-H, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ. J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 8.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 2013;37:124–131. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 11.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan SC, Finney BA, Lazarou M, Rosser AE, Scherf C, Adriaensen D, Kemp PJ, Riccardi D. Fetal calcium regulates branching morphogenesis in the developing human and mouse lung: involvement of voltage-gated calcium channels. PLoS ONE. 2013;8(11):e80294. doi: 10.1371/journal.pone.0080294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C-K, Hsu P-Y, Wang T-M, Miao Z-F, Lin R-T, Juo S-HH. TRPV4 activation contributes functional recovery from ischemic stroke via angiogenesis and neurogenesis. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0625-0. [DOI] [PubMed] [Google Scholar]
- 14.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 15.Evans NJ, Archer LN. Doppler assessment of pulmonary artery pressure during recovery from hyaline membrane disease. Arch. Dis. Child. 1991;66:802–804. doi: 10.1136/adc.66.7_Spec_No.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Featherstone NC, Connell MG, Fernig DG, Wray S, Burdyga TV, Losty PD, Jesudason EC. Airway smooth muscle dysfunction precedes teratogenic congenital diaphragmatic hernia and may contribute to hypoplastic lung morphogenesis. Am. J. Respir. Cell Mol. Biol. 2006;35:571–578. doi: 10.1165/rcmb.2006-0079OC. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am. J. Respir. Cell Mol. Biol. 2005;33:153–160. doi: 10.1165/rcmb.2005-0137OC. [DOI] [PubMed] [Google Scholar]
- 18.Fewell JE, Hislop AA, Kitterman JA, Johnson P. Effect of tracheostomy on lung development in fetal lambs. J. Appl. Physiol. 1983;55:1103–1108. doi: 10.1152/jappl.1983.55.4.1103. [DOI] [PubMed] [Google Scholar]
- 19.Fouron JC, Le Guennec JC, Villemant D, Perreault G, Davignon A. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatrics. 1980;65:529–535. [PubMed] [Google Scholar]
- 20.Galambos C, Ng Y-S, Ali A, Noguchi A, Lovejoy S, D’Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am. J. Respir. Cell Mol. Biol. 2002;27:194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil use in pulmonary arterial hypertension (SUPER) study group. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 22.Hamanaka K, Jian M-Y, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L353–L362. doi: 10.1152/ajplung.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamanaka K, Jian M-Y, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L923–L932. doi: 10.1152/ajplung.00221.2007. [DOI] [PubMed] [Google Scholar]
- 24.Hara A, Chapin CJ, Ertsey R, Kitterman JA. Changes in fetal lung distension alter expression of vascular endothelial growth factor and its isoforms in developing rat lung. Pediatr. Res. 2005;58:30–37. doi: 10.1203/01.PDR.0000163614.20031.C5. [DOI] [PubMed] [Google Scholar]
- 25.Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. Bethesda Md. 1996;1985(81):209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- 26.Hislop AA. Airway and blood vessel interaction during lung development. J. Anat. 2002;201:325–334. doi: 10.1046/j.1469-7580.2002.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF. Calcium signaling stimulates translation of HIF-alpha during hypoxia. FASEB J. 2006;20:466–475. doi: 10.1096/fj.05-5086com. [DOI] [PubMed] [Google Scholar]
- 28.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L221–L228. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- 29.Isakson BE, Seedorf GJ, Lubman RL, Evans WH, Boitano S. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;29:552–561. doi: 10.1165/rcmb.2002-0281OC. [DOI] [PubMed] [Google Scholar]
- 30.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 31.Jesudason EC. Small lungs and suspect smooth muscle: congenital diaphragmatic hernia and the smooth muscle hypothesis. J. Pediatr. Surg. 2006;41:431–435. doi: 10.1016/j.jpedsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Jesudason EC. Exploiting mechanical stimuli to rescue growth of the hypoplastic lung. Pediatr. Surg. Int. 2007;23:827–836. doi: 10.1007/s00383-007-1956-0. [DOI] [PubMed] [Google Scholar]
- 33.Jesudason EC. Airway smooth muscle: an architect of the lung? Thorax. 2009;64:541–545. doi: 10.1136/thx.2008.107094. [DOI] [PubMed] [Google Scholar]
- 34.Jesudason EC, Smith NP, Connell MG, Spiller DG, White MRH, Fernig DG, Losty PD. Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am. J. Respir. Cell Mol. Biol. 2005;32:118–127. doi: 10.1165/rcmb.2004-0304OC. [DOI] [PubMed] [Google Scholar]
- 35.Jesudason EC, Smith NP, Connell MG, Spiller DG, White MRH, Fernig DG, Losty PD. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L559–L565. doi: 10.1152/ajplung.00498.2005. [DOI] [PubMed] [Google Scholar]
- 36.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 37.Kim HY, Pang M-F, Varner VD, Kojima L, Miller E, Radisky DC, Nelson CM. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev. Cell. 2015;34:719–726. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Dev. Camb. Engl. 2013;140:3146–3155. doi: 10.1242/dev.093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Dev. Camb. Engl. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liedtke W. TRPV channels’ role in osmotransduction and mechanotransduction. Handb. Exp. Pharmacol. 2007 doi: 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- 41.Means AR. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 1994;347:1–4. doi: 10.1016/0014-5793(94)00492-7. [DOI] [PubMed] [Google Scholar]
- 42.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 43.Moore KA, Huang S, Kong Y, Sunday ME, Ingber DE. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J. Surg. Res. 2002;104:95–100. doi: 10.1006/jsre.2002.6418. [DOI] [PubMed] [Google Scholar]
- 44.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 45.Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mottet D, Michel G, Renard P, Ninane N, Raes M, Michiels C. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J. Cell. Physiol. 2003;194:30–44. doi: 10.1002/jcp.10176. [DOI] [PubMed] [Google Scholar]
- 47.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr. Opin. Pediatr. 2013;25:329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 48.Muratore CS, Nguyen HT, Ziegler MM, Wilson JM. Stretch-induced upregulation of VEGF gene expression in murine pulmonary culture: a role for angiogenesis in lung development. J. Pediatr. Surg. 2000;35:906–912. doi: 10.1053/jpsu.2000.6916. [DOI] [PubMed] [Google Scholar]
- 49.Nayak PS, Wang Y, Najrana T, Priolo LM, Rios M, Shaw SK, Sanchez-Esteban J. Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells. Respir. Res. 2015;16:60. doi: 10.1186/s12931-015-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson CM, Gleghorn JP, Pang M-F, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, Stone HA. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development. 2017;144:4328–4335. doi: 10.1242/dev.154823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olver RE, Walters DV, Wilson SM. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 2004;66:77–101. doi: 10.1146/annurev.physiol.66.071702.145229. [DOI] [PubMed] [Google Scholar]
- 52.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 54.Schittny JC, Miserocchi G, Sparrow MP. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am. J. Respir. Cell Mol. Biol. 2000;23:11–18. doi: 10.1165/ajrcmb.23.1.3926. [DOI] [PubMed] [Google Scholar]
- 55.Shinkai M, Shinkai T, Montedonico S, Puri P. Effect of VEGF on the branching morphogenesis of normal and nitrofen-induced hypoplastic fetal rat lung explants. J. Pediatr. Surg. 2006;41:781–786. doi: 10.1016/j.jpedsurg.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr. Crit. Care Med. 2010;11:S79–S84. doi: 10.1097/PCC.0b013e3181c76cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subhedar NV, Shaw NJ. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch. Dis. Child. Fetal Neonatal Ed. 2000;82:F243–F247. doi: 10.1136/fn.82.3.F243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taghizadeh A, Reynolds EO. Pathogenesis of bronchopulmonary dysplasia following hyaline membrane disease. Am. J. Pathol. 1976;82:241–264. [PMC free article] [PubMed] [Google Scholar]
- 59.Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342–345. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thoppil RJ, Cappelli HC, Adapala RK, Kanugula AK, Paruchuri S, Thodeti CK. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget. 2016;7:25849–25861. doi: 10.18632/oncotarget.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharmacol. Exp. Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 62.Tomashefski JF, Oppermann HC, Vawter GF, Reid LM. Bronchopulmonary dysplasia: a morphometric study with emphasis on the pulmonary vasculature. Pediatr. Pathol. 1984;2:469–487. doi: 10.3109/15513818409025895. [DOI] [PubMed] [Google Scholar]
- 63.Unbekandt M, del Moral P-M, Sala FG, Bellusci S, Warburton D, Fleury V. Tracheal occlusion increases the rate of epithelial branching of embryonic mouse lung via the FGF10-FGFR2b-Sprouty2 pathway. Mech. Dev. 2008;125:314–324. doi: 10.1016/j.mod.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walther FJ, Benders MJ, Leighton JO. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics. 1992;90:899–904. [PubMed] [Google Scholar]
- 65.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr. Top. Dev. Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: TRPV4 antibody Specificity. Mouse lung epithelial (MLE12) cells were transfected with siRNA specific to TRPV4 or to a non-targeting control (siNT) using Lipofectamine RNAiMAX according to manufacturer instructions. After 72 h, the cells were fixed and stained for TRVP4 and counterstained with DAPI to identify the nuclei. There was a substantial decrease in TRPV4 staining intensity with siTRPV4 compared to siNT treated cells. Scale bars 50 µm. Supplementary material 1 (TIFF 29097 kb)
Movie S1: TRPV4 regulates active contractility of the developing lung. Embryonic mouse lungs were isolated at E12.5 and cultured on floating membranes for ~ 48 h before 1 Hz imaging. (A) Cultured lungs demonstrated active airway contraction, and this was influenced by TRVP4 modulation. This video spans 100 s, and the control lung visibly contracts at ~ 50 s, whereas 100 nM GSK1016790A (Activator) treated lung visibly contracts at ~ 36 and ~ 68 s, and the lung treat with 10 µM GSK205 (Inhibitor) does not visibly contract. Supplementary material 2 (MP4 1226 kb)