Abstract
Introduction:
Health disparities in African-American (AA) kidney transplant recipients compared with non-AA recipients are well established. Cardiovascular disease (CVD) risk control is a significant mediator of this disparity.
Objective:
To assess the efficacy of improved medication safety, CVD risk control, and racial disparities in kidney transplant recipients.
Methods:
Prospective, pharmacist-led, technology-aided, 6-month interventional clinical trial. A total of 60 kidney recipients with diabetes and hypertension were enrolled. Patients had to be at least one-year post transplant with stable graft function. Primary outcome measured included hypertension, diabetes, and lipid control using intent-to-treat analyses, with differences assessed between AA and non-AA recipients.
Results:
The participants mean age was 59 years, with 42% being female and 68% being AA. Overall, patients demonstrated improvements in blood pressure <140/90 mmHg (baseline 50% vs. end of study 68%, p=0.054) and hemoglobin A1c <7% (baseline 33% vs. end of study 47%, p=0.061). AAs demonstrated a significant reduction from baseline in systolic blood pressure (−0.86 mmHg per month, p=0.026), which was not evident in non-AAs (−0.13 mmHg per month, p=0.865). Mean HgbA1c decreased from baseline in the overall group (−0.12% per month, p=0.003), which was similar within AAs (−0.11% per month, p=0.004) and non-AAs (−0.14% per month, p=0.029). There were no changes in low-density lipoproteins, triglycerides, or high-density lipoproteins over the course of the study. Medication errors were significantly reduced and self-reported medication adherence significantly improved over the course of the study.
Conclusion:
These results demonstrate the potential efficacy of a pharmacist-led, technology-aided, educational intervention in improving medication safety, diabetes, and hypertension and reducing racial disparities in AA kidney transplant recipients. (ClinicalTrials.gov NCT02763943)
Keywords: cardiovascular diseases, African Americans, kidney transplantation, medication errors, hypertension, healthcare disparities
Health disparities in African-American (AA) kidney transplant recipients remain a significant challenge to the transplant community. Based on recent studies, AAs historically had nearly a 50% higher risk of graft loss, as compared with Caucasians.(1, 2) Recently, changes to the organ allocation system and improvements in immunosuppression regimens have led to reduced disparities with regards to access to deceased donor organs and acute rejection rates; yet, long-term disparities in graft outcomes remain significant.(1, 2) These long-term disparities in AAs have traditionally been explained by higher immunologic risks leading to higher rejection rates,(3–6) socioeconomic status (SES) barriers,(7, 8) increased medication non-adherence,(9, 10) and a higher prevalence and progression of comorbidities.(11–13)
Previous racial disparities research in kidney transplantation has focused on understanding and mitigating immunologic and SES risks.(14, 15) Medication adherence has also been an area of focused research.(9, 10) Though acute rejection rate disparities have significantly decreased, graft loss differences between AAs and non-AAs have only marginally converged.(1, 16) In comparison, there are relatively few studies assessing the impact of cardiovascular risk factors and risk control on racial disparities in kidney transplantation.(17–19) Over the past five years, our research collaborative has set out to better understand the impact cardiovascular disease (CVD) risk factors and risk control has on racial disparities. Using both single-center and national cohort studies, we have previously demonstrated that CVD risk factors (diabetes, hypertension, and dyslipidemia) are more prevalent in post-transplant AA recipients.(20) Further, AA recipients have significantly reduced control of diabetes and hypertension after transplant, when compared with non-AA recipients. Through iterative multivariate modeling, we have demonstrated that control of CVD risk factors explains a significant portion of the increased risk of graft loss within AA kidney transplant recipients.(20)
Given this information, our goal for this clinical trial was to assess the preliminary efficacy of a pharmacist-led, technology-aided intervention on improving CVD risk factor control within kidney transplant recipients while also assessing if the potential improvements in CVD risk factor control differ by race.
Methods
Study Design and Patients
This was a prospective, interventional clinical trial assessing the potential efficacy of a pharmacist-led, technology-aided, education intervention for improving medication safety and cardiovascular risk factor control in adult solitary kidney transplant recipients, with a secondary aim of assessing if the impact of intervention varied by race. The full methods of this study have been previously published in detail.(21) In brief, all patients enrolled in the study received the intervention over a 6-month period. Outcomes were assessed at baseline and compared with end of study measures using intention to treat methodology. Six months was set as the intervention period as the authors felt this was sufficient to significantly influence the primary outcomes of CVD risk factor control. To be included in the study, patients must have been 18 years or older, received a kidney transplant, had an estimated glomerular filtration rate (eGFR) ≥20 mL/min/m2 (4-variable Modified Diet in Renal Disease (MDRD) equation), and be at least one-year post transplant. The patient must have had hypertension and diabetes mellitus, and were willing to comply with all study visits.
Interventions
The study intervention consisted of both a technology and health services component. The technology included providing Bluetooth enabled home monitoring devices for glucose and blood pressure (BP) to all study participants; this enabled the data to be automatically uploaded to a web-based portal using a third-party mobile health (mHealth) app (Test N’ Go, ForaCare®, Moorpark, CA). If a patient did not have a smartphone or Wi-Fi enabled portable electronic device to utilize the mHealth app, one was provided to them during the study. This allowed the patient and providers to easily see, aggregate, and review home-based monitoring for both BPs and glucose readings. At each follow-up visit, these data were reviewed with study participants and they were encouraged to share this information with their primary care providers as well. The technology component was coupled with monthly, face-to-face, pharmacist-led encounters designed to reduce patient-level factors creating barriers for CVD risk factor control, including medication non-adherence, medication errors, self-efficacy (an individual’s belief in his/her capacity to execute health-promoting behaviors), and lifestyle choices. The full description of the intervention has been previously described.(21) At each visit, different themes were addressed with patients. Visit 1 focused on training patients on the Bluetooth enabled glucometer and BP meter, as well as obtaining a full medication history. Visit 2 focused on medication education, medication expectations, drug indications, side effects, dosing, regimens, and medication self-efficacy. Visit 3 focused on medication adherence, including the importance of adherence and tools that can be used to improve adherence. Motivational interviewing techniques were used to facilitate these discussions.(22) Visit 4 focused on chronic disease state self-efficacy and self-care, while visit 5 focused on lifestyle choices, including diet and exercise. Finally, visit 6 was the close out visit that focused on ensuring patients have appropriate follow-up care with primary care providers.
Outcomes
The primary outcome of this study assessed CVD risk factor control including diabetes, hypertension, and dyslipidemia. Control was determined at baseline (study initiation), at each study visit for BP, and at visits 4 and 6 for diabetes and lipids, using the following definitions: hypertension control was set at thresholds of BP <130/80 mmHg; diabetes control was set at a threshold of hemoglobin A1c (HgbA1c) <7%; lipid control was defined as an low-density lipoproteins (LDL) of <100 mg/dL and triglycerides (TG) <150 mg/dL. Cholesterol was assessed by collecting a fasting lipid panel at baseline, month 4, and month 6 of the study, along with HgbA1c measures. BPs were assessed at monthly clinic visits by measuring three sitting and resting BPs and averaging the last two readings. Thresholds were also set at the following for exploratory analyses: BP <140/90 mmHg, HgbA1c <8% and LDL <70 mg/dL The change in mean values over the course of the study were also assessed.(23–25)
Secondary outcomes included acute rejections, hospitalizations, emergency department (ED) visits, graft loss, and death that occurred during the study period. These were assessed by conducting chart reviews to determine clinical events and interviewing participants at each monthly visit to assess clinical events (hospitalizations and ED visits) that occurred outside the study site. At each monthly visit, medication errors (patients taking medications in a method that was not intended or not accurate in the medical records) were also assessed using previously validated measures.(21) Medications used to manage CVD risk factors and immunosuppression regimens were also assessed during the study. Finally, patient-reported medication adherence was assessed at monthly visits using a validated survey instrument.(26–28)
Statistical Analysis
The study was powered based on improving post-transplant hypertension control to goal levels. Grounded on preliminary data, we expected approximately 30% of kidney recipients to meet optimal hypertension goals (<130/80).(20) Using this as the baseline rate of control, this study had 80% power to detect an 18% improvement in rate of optimal BP control (30% baseline, 48% at end of follow-up). Data for this clinical trial were analyzed in a paired intent-to-treat fashion, with each patient acting as their own control, and baseline data being compared with month 6 results (or the last follow-up values carried forward for patients that did not complete the study). Standard descriptive statistics were used to assess the baseline characteristics of the study group. Comparisons at baseline by race were done using Fisher’s exact test and Student’s T-test. CVD risk factor control analyses were conducted by comparing change in values (BPs, HgbA1Cs, and lipids) from baseline to end of study with percent of patients deemed at optimal control for each value at the beginning and end of study. Continuous variables were compared using the paired Student’s T-test, with categorical data compared using McNemar’s test. Analyses were stratified by race to assess if intervention effects differed between AA and non-AA patients. To assess changes in values overtime (repeated measures), generalized linear modeling was utilized, accounted for within patient correlation, with parameters estimated via GEE using Proc Genmod. A two-sided p-value <0.05 was considered statistically significant and all analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 177 patients were assessed for participation; 19 of who were screen failures and 98 declined to participate (38% consent rate [95% confidence interval (CI) 31–46%]). Primary reasons for declining included living too far from the study site to make monthly visits and not being interested in participating in clinical research. Of the 60 patients enrolled, 51 (85% completion rate, [95% CI 74–92%]) completed the study (see Supplemental Figure 1 for the Consort diagram). The participants’ mean age was 59 years, with 42% being female and 68% being AA; all had diabetes and hypertension, and most were overweight or obese with limited economic means (Table 1). AA participants had similar demographics, but significantly differed by socioeconomic indicators including education, salary, and health insurance. Most patients in the study had substantial cardiovascular comorbidities, including a history of cardiac surgery, acute myocardial infarction (AMI), heart catheterization, stroke, or peripheral vascular disease (PVD). AAs were less likely to have a history of cardiac catheterization, but were similar with regards to other cardiovascular comorbidities (Supplemental Table 1). Transplant characteristics and donor information are displayed in Supplemental Table 2. AAs were more likely to be sensitized, have HLA mismatches, and receive deceased donor transplants.
Table 1 –
Baseline Sociodemographics of the Study Cohort, Stratified and Compared Between AA and Non-AA Subjects
| Baseline Sociodemographics | Total (n=60) | non-AA (n=19) | AA (n=41) | p-value |
|---|---|---|---|---|
| Age (mean ± SD) | 59.4±11.1 | 62.7±11.1 | 57.9±10.9 | 0.121 |
| Female Gender | 41.7% | 31.6% | 43.3% | 0.281 |
| African-American | 68.3% | -- | -- | -- |
| Body Mass Index (kg/m2) | 32.8±4.3 | 34.1±4.4 | 32.2±4.1 | 0.135 |
| History of Smoking | 28.8% | 36.8% | 25.0% | 0.348 |
| Current Smoker | 3.4% | 5.3% | 2.5% | 0.274 |
| End Stage Renal Disease Etiology | ||||
| Diabetes | 50.9% | 47.4% | 52.6% | 0.294 |
| Hypertension | 38.6% | 31.6% | 42.1% | |
| Other | 10.5% | 21.0% | 5.3% | |
| Dialysis Prior to Transplant | 87.5% | 66.7% | 97.4% | 0.001 |
| Hemodialysis | 85.7% | 83.3% | 86.5% | 0.786 |
| Completed High School | 47.4% | 68.4% | 37.5% | 0.026 |
| Currently Married | 61.0% | 73.7% | 55.0% | 0.543 |
| Annual Salary ≥$50,000 | 18.5% | 47.4% | 5.0% | 0.001 |
| Medicare Insurance | 80.0% | 84.2% | 78.1% | 0.579 |
| Medicaid Insurance | 23.3% | 5.3% | 31.7% | 0.024 |
AA = African-American; SD = standard deviation.
With regards to the primary outcome, as compared with baseline values, after the 6-month intervention, the overall study group demonstrated clinically relevant improvements in the exploratory thresholds for BP <140/90 mmHg (50% vs. 68%, p=0.054) and primary threshold of HgbA1c <7% (33% vs. 47%, p=0.061). Thresholds cutoffs of BP <130/80 mmHg, HgbA1c <8%, LDL, and TG were similar at baseline and end of follow-up (top of Figure 1). A number of CVD risk factor control measurements improved more significantly in AAs, as compared with non-AAs (bottom of Figure 1). AAs were more likely to have significant improvements in BP <140/90 mmHg and HgbA1c <8%. Conversely, non-AAs were more likely to obtain tighter HgbA1c control (<7%), as compared with AAs. In the overall study group, reductions in mean SBP from baseline were not statistically significant (−0.63 mmHg per month, p=0.075). However, AAs demonstrated a significant reduction from baseline (−0.86 mmHg per month, p=0.026), which was not evident in non-AAs (−0.13 mmHg per month, p=0.865). A similar trend was noted for diastolic blood pressure (DBP) from baseline (AA −0.37 mmHg per month, p=0.073 vs. non-AA 0.28 mmHg per month, p=0.482, top of Figure 2). Mean HgbA1c decreased from baseline in the overall group (−0.12% per month, p=0.003), which was similar in magnitude within AAs (−0.11% per month, p=0.004) and non-AAs (−0.14% per month, p=0.029). There were no changes from baseline in LDL (−0.10 mg/dL per month, p=0.862), TGs (0.31 mg/dL per month, p=0.915) or high-density lipoproteins (HDL, 0.12 mg/dL per month, p=0.469) over the course of the study, which was similar in AAs and non-AAs (bottom of Figure 2 and Supplemental Figure 2).
Figure 1 –
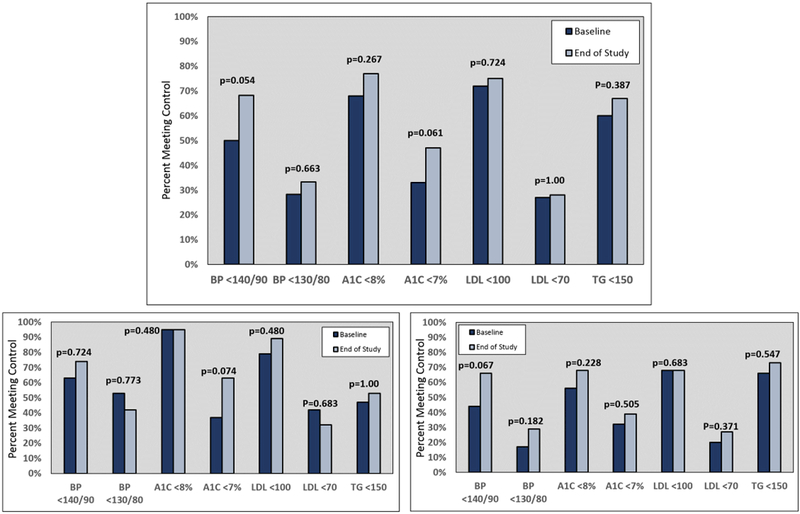
Cardiovascular risk factor control measures, compared at baseline and end of study for the entire cohort (top). The race-stratified results are in the bottom two charts, with non-AAs in the bottom left and AAs in the bottom right. AA = African-American; A1C = hemoglobin A1C; BP = blood pressure; LDL = low-density lipoproteins; TG = triglycerides
Figure 2 –
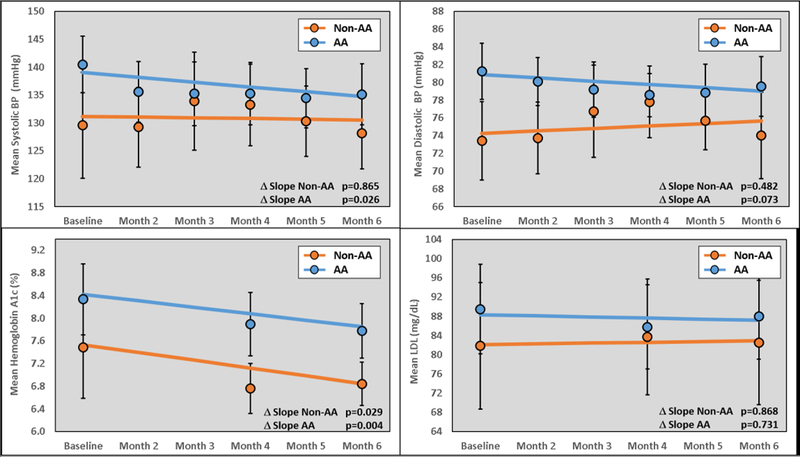
Change in cardiovascular risk parameters compared between non-AA and AA study participants. AA = African-American; A1C = hemoglobin A1C; BP = blood pressure; LDL = low-density lipoproteins; TG = triglycerides
Patient clinical outcomes, including acute rejections episodes, ED visits, hospitalizations, graft loss, and death are displayed in Table 2. During the study intervention, event rates were at or below expected rates for this population and were similar between AA and non-AA participants. In the entire study group, there was one acute rejection episode, one graft loss, and one death. The graft loss was due to severe rejection (Banff grade 2a) that was not responsive to anti-rejection therapies and the death was due to multifocal pneumonia and sepsis. Common reasons for ED visits and hospitalization included infections, cardiovascular events, diabetes complications, acute kidney injury, and gastrointestinal issues. New adverse events (AEs) are depicted in Supplemental Table 3. AEs occurred in 43% of patients, which was higher in non-AAs as compared with AAs (63.2% vs. 34.1%, p=0.035). The most common AEs in non-AAs included gastrointestinal issues, neurologic issues, and upper respiratory symptoms; within AAs, AEs were fairly equally spread across systems.
Table 2 –
– Clinical Outcomes for the Study Cohort, Stratified and Compared Between AA and Non-AA Subjects
| Clinical Outcomes | Total (n=60) | non-AA (n=19) | AA (n=41) | p-value |
|---|---|---|---|---|
| Biopsy Proven Acute Rejection | 1.7% | 0.0% | 2.4% | 1.000 |
| Emergency Room Visits | 28.3% | 15.8% | 34.1% | 0.219 |
| Hospitalizations | 20.0% | 15.8% | 22.0% | 0.735 |
| Graft Loss | 1.7% | 0.0% | 2.4% | 1.000 |
| Death | 1.7% | 0.0% | 2.4% | 1.000 |
AA = African-American.
Medication errors identified during each study visit occurred significantly less often over the course of the intervention (top of Figure 3). The mean number of medication errors reduced from roughly 3.0±2.7 per patient-visit at baseline to 0.14±0.44 per patient-visit at study end (−0.71 errors per month, p<0.001); these did not differ by race. The types of errors are displayed in Table 3. The majority of errors were related to discrepancies in what the patient was taking versus what was documented in the electronic medical record. Classes of medications that participants were taking throughout the study are displayed in Supplemental Figures 3 through 6. At baseline, 73% of patients were receiving statin therapy which increased during the study (78% in non-AAs and 91% in AAs at study end). At baseline, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) were utilized in 61% of patients (68% in non-AAs and 46% in AAs). At study end, this increased to 69% (72% in non-AAs and 61% in AAs). Aspirin use increased from 52% to 63% during the study. Other medications, including anti-hypertensives, anti-hyperglycemics, and immunosuppression regimens, remained consistent during the study intervention. None of these changes in medication use were statistically significant. Patient-reported medication adherence also improved throughout the study (bottom of Figure 3). At baseline, 59.6% of patients reported high medication adherence, which increased to 89.5% at study end. For each month in the study, patients had 34% higher odds of reporting high medication adherence (odds ratio 1.34, [95% CI 1.10–1.64], p=0.004).
Figure 3 –
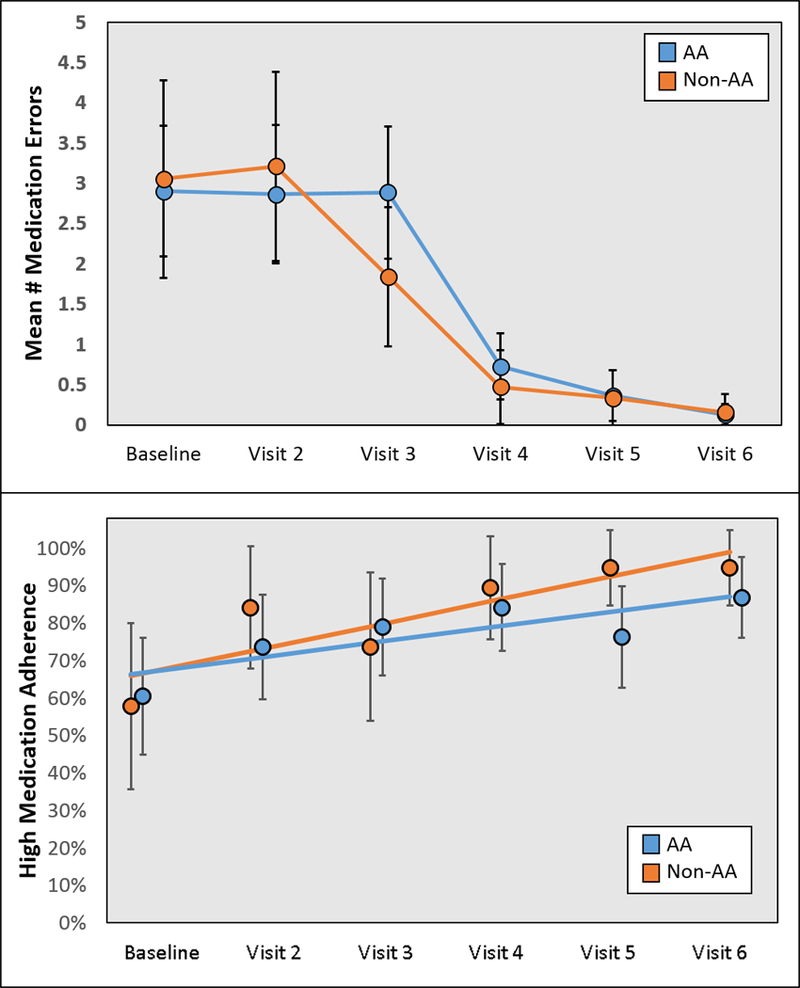
Medication errors and adherence at baseline and follow-up visits, stratified by participant race.
Table 3 –
Type and Frequency of Medication Errors Documented During the Study
| Type of Error | Frequency (%) |
|---|---|
| Drug dose too high | 19 (11.8%) |
| Drug dose too low | 22 (13.7%) |
| Inappropriate or suboptimal doses form | 1 (0.6%) |
| Medication discrepancy - medication in EMR patient not currently taking | 92 (57.1%) |
| Medication discrepancy - patient currently taking medication not in EMR | 27 (16.8%) |
| TOTAL | 161 |
EMR = electronic medical record.
Discussion
The results of this prospective clinical trial demonstrated improved medication safety, blood pressure, and glycemic control in kidney transplant recipients through a technology-assisted, pharmacist-led educational intervention. Further, these results established that some improvements varied by race, such that AA recipients had substantial improvements in BP control, mitigating disparities in this regard. These findings provide promising results that suggest focused educational interventions, aided through the use of technology, may help to abrogate racial disparities in kidney transplantation.
Hypertension is a predominant issue in racial disparities within chronic kidney disease and kidney transplant outcomes. Studies over the past 30 years have established that AAs have a higher prevalence and more rapid progression of hypertension; it occurs at an earlier age and is more commonly implicated in end-organ damage within AAs.(29, 30) Post-transplant, hypertension and poor BP control are major factors in disparate graft survival within AA recipients.(11, 31) In a study conducted by Cosio and colleagues, mean arterial pressures correlated with a shorter allograft half-life only in AA recipients (p=0.002) as compared with Caucasians (p=0.840).(11) Kidney transplant survival was eight times shorter in AAs with poor BP control as compared with Caucasians. In a more recent analysis using national data, our research group demonstrated that post-transplant BP control (<140/90 mmHg) was significantly less common in AAs as compared with non-AAs (60% vs. 69%, p<0.01). In multivariable modeling, adjusting for BP control significantly reduced the influence of race on graft loss.(20) Thus, it is likely that improved BP control is necessary to improve graft outcome disparities for AAs. The results of this clinical trial intervention provides preliminary evidence that educations efforts, focused on improving medication safety and self-efficacy coupled with technology, offer a promising mechanism to address this disparity. AAs entered the study with a substantial disparity in BP levels and control thresholds, and over the 6-month study, these differences were substantially mitigated.
Diabetes and glycemic control are also significant issues within AAs.(32–34) Post-transplant diabetes has a higher prevalence in AAs, as compared with Caucasians. Poor glycemic control is also more prevalent in AA kidney transplant recipients and this appears to influence racial disparities as well; albeit not to the same level as BP control.(35) The results of our clinical intervention demonstrated improved glycemic control in the entire study population, which did not appreciably differ by race. Thus, further studies are warranted to better understand how to best improve glycemic control in AA recipients. It may require multimodal interventions that not only utilize education and technology, but also employ different therapeutic modalities as well.(36) Lipid control did not differ across the study population, but given that the vast majority of patients were on medium to high intensity statin therapy at study initiation, this was to be expected. The most recent guidelines suggest that goal lipid levels are less important than maximizing pharmacotherapy based on patient risk.(37)
Medication errors and medication safety are major issues in transplant recipients, as the majority are receiving more than 10 medications and 20 doses per day; these agents are known to have substantial toxicities and numerous drug-interactions. Non-adherence, adverse drug events, and errors can have a major influence on clinical outcomes after transplant.(38–41) The results of this study demonstrated reduced medication errors (mainly in discrepancies within the electronic medical record) and improved patient-reported adherence, with low rates of newly reported adverse drug events. These findings offer promise that educational and technology interventions, driven by transplant pharmacists, may improve medication-related outcomes. Further studies are needed to determine the impact of such interventions on long-term clinical outcomes.
There are a number of limitations with this study. First, the study was not randomized and all patients received the intervention. This was intentionally designed to achieve maximum power in detecting differences while having a limited sample size. However, the study did not meet a priori power calculations, which was based on achieving BP control of 130/80 mmHg. This was chosen based on guidelines in place at the time of study design, but more recent guidelines allow for a less stringent goal of <140/90, a measurement that was clinically improved during the study.(25, 42) Another limitation was that there was a low consent rate of 38% (the goal was a priori set at 70% based on previous experience). This was largely due to patients’ lack of willingness to return to the clinic for monthly visits, as many lived more than 50 miles away. Transforming the study into a telehealth intervention would have largely solved this problem. We do feel we had a nice mix of both urban and rural participants, despite this geographic limitation. An additional limitation was the relative imbalance in participants by race, as 68.3% were AA. This was likely due to the fact that 55% of kidney transplant recipients at our center are AA, and the prevalence of hypertension and diabetes is significantly higher in AAs. However, under-representation of AAs within clinical trials is usually the norm and it is a promising sign that we were able to easily enroll AAs within a clinical interventional trial.(43) Finally, the relatively small sample size and short follow-up time period created barriers in finding statistically significant differences in clinical outcomes and larger studies with long-term follow-up are warranted to truly assess the impact of improved CVD risk factor control on graft outcomes in kidney transplantation.
Overall, these preliminary findings provide promising data that demonstrated the potential efficacy of a pharmacist-led, technology-aided, educational intervention on improved medication safety, glycemic control, and BP control in kidney transplant recipients. The improved BP control also demonstrated reduced racial disparities, as compared with baseline findings at study entry. Larger studies with long-term follow up are needed to truly assess the impact of such interventions on graft outcomes and racial disparities in kidney transplantation.
Supplementary Material
Baseline cardiovascular history for the entire study cohort, stratified and compared by race.
Baseline transplant characteristics for the entire study cohort, stratified and compared by race.
New adverse events that occurred during the study, stratified and compared by race.
Consort diagram depicting the formulation of the final study cohort.
Change in additional cardiovascular risk parameters and renal function compared between non-AA and AA study participants.
Cardiovascular risk factor medication use compared over the study period, stratified by race.
Diabetes medication use compared over the study period, stratified by race.
Hypertension medication use compared over the study period, stratified by race.
Immunosuppression medication use compared over the study period, stratified by race.
Acknowledgements and Sources of Funding:
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose as it relates to the content of this manuscript.
References
- 1.Purnell TS, Luo X, Kucirka LM, et al. Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J Am Soc Nephrol 2016;27(8):2511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taber DJ, Gebregziabher M, Hunt KJ, et al. Twenty years of evolving trends in racial disparities for adult kidney transplant recipients. Kidney Int 2016;90(4):878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciancio G, Burke GW, Suzart K, et al. The use of daclizumab, tacrolimus and mycophenolate mofetil in African‐American and Hispanic first renal transplant recipients. Am J Transplant 2003;3(8):1010–6. [DOI] [PubMed] [Google Scholar]
- 4.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation 1998;65(4):515–23. [DOI] [PubMed] [Google Scholar]
- 5.Podder H, Podbielski J, Hussein I, Katz S, Buren C, Kahan BD. Sirolimus improves the two‐year outcome of renal allografts in African‐American patients. Transplant Int 2005;14:135–42. [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Deng S, Arenas J, et al. Decreased rejection episodes in African-American renal transplant recipients receiving mycophenolate mofetil/tacrolimus therapy. Transplant Proc 1997;29:3669–70. [DOI] [PubMed] [Google Scholar]
- 7.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts: overriding effects of HLA matching and socioeconomic factors. N Engl J Med 1992;327:840–5. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JJ. Kidney transplantation: racial or socioeconomic disparities? Am J Kidney Dis 1999;34:756–8. [DOI] [PubMed] [Google Scholar]
- 9.Kalil R, Heim-Duthoy K, Kasiske B. Patients with a low income have reduced renal allograft survival. Am J Kid Dis 1992;20:63–9. [DOI] [PubMed] [Google Scholar]
- 10.Schweizer RT, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Noncompliance in organ transplant recipients. Transplantation 1990;49(2):374–7. [DOI] [PubMed] [Google Scholar]
- 11.Cosio FG, Dillon JJ, Falkenhain ME, et al. Racial differences in renal allograft survival: the role of systemic hypertension. Kidney Int 1995;47:1136–41. [DOI] [PubMed] [Google Scholar]
- 12.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 2002;62:1440–6. [DOI] [PubMed] [Google Scholar]
- 13.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant 2008;8:593–9. [DOI] [PubMed] [Google Scholar]
- 14.Young CJ, Kew C. Health disparities in transplantation: Focus on the complexity and challenge of renal transplantation in African Americans. Med Clin N Am 2005;89:1003–31. [DOI] [PubMed] [Google Scholar]
- 15.Gaston RS, Ayres I, Dooley LG, Diethelm AG. Racial equity in renal transplantation: the disparate impact of HLA-based allocation. J Am Med Assoc 1993;270:1352–6. [PubMed] [Google Scholar]
- 16.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MSA. African American kidney transplantation survival. Drugs 2009;69:2045–62. [DOI] [PubMed] [Google Scholar]
- 17.Taber DJ, Meadows HB, Pilch NA, Chavin KD, Baliga PK, Egede LE. The impact of diabetes on ethnic disparities seen in kidney transplantation. Ethn Dis 2013;23:238–44. [PubMed] [Google Scholar]
- 18.Taber DJ, Pilch NA, Meadows HB, et al. The impact of cardiovascular disease and risk factor treatment on ethnic disparities in kidney transplant. J Cardiovasc Pharmacol Ther 2013;18:243–50. [DOI] [PubMed] [Google Scholar]
- 19.Taber DJ, Douglass K, Srinivas T, et al. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol 2014;40:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taber DJ, Hunt KJ, Fominaya CE, et al. Impact of cardiovascular risk factors on graft outcome disparities in black kidney transplant recipients. Hypertension 2016;68:715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole AJ, Johnson II RW, Egede LE, Baliga PK, Taber DJ. Improving medication safety and cardiovascular risk factor control to mitigate disparities in African-American kidney transplant recipients: Design and methods. Contemp Clin Trials Commun 2018;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev 2009;29:283–93. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Clinical practice recommendations. 2012;35(suppl 1):S1–S113. [Google Scholar]
- 24.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, and the National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. J Am Med Assoc 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 26.Overhage JM, Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am J Health Syst Pharm 1999;56:2444–50. [DOI] [PubMed] [Google Scholar]
- 27.Musgrave C, Pilch N, Taber D, et al. Improving transplant patient safety through pharmacist discharge medication reconciliation. Am J Transplant 2013;13:796–801. [DOI] [PubMed] [Google Scholar]
- 28.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdinand KC, Saunders E. Hypertension-related morbidity and mortality in African Americans--why we need to do better. J Clin Hypertens 2006;8(1 Suppl 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferdinand KC. Management of high blood pressure in African Americans and the 2010 ISHIB consensus statement: meeting an unmet need. J Clin Hypertens 2010;12:237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taber DJ, Pilch NA, Meadows HB, et al. The impact of cardiovascular disease and risk factor treatment on ethnic disparities in kidney transplant. J Cardiovasc Pharmacol Ther 2013;18:243–50. [DOI] [PubMed] [Google Scholar]
- 32.Banerji MA. Diabetes in African Americans: unique pathophysiologic features. Curr Diabetes Rep 2004;4(3):219–23. [DOI] [PubMed] [Google Scholar]
- 33.Crook ED. Diabetic renal disease in African Americans. Am J Med Sci 2002;323:78–84. [DOI] [PubMed] [Google Scholar]
- 34.Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am 2005;89(5):949–75. [DOI] [PubMed] [Google Scholar]
- 35.Taber DJ, Meadows HB, Pilch NA, Egede LE. The impact of diabetes on ethnic disparities in kidney transplantation. Ethnic Dis 2013;23:238–44. [PubMed] [Google Scholar]
- 36.Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med 2006;166:675–81. [DOI] [PubMed] [Google Scholar]
- 37.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 38.Taber DJ, Pilch NA, Bratton CF, McGillicuddy JW, Chavin KD, Baliga PK. Medication errors and adverse drug events in kidney transplantation: incidence, risk factors, and clinical outcomes. Pharmacotherapy 2012;32:1053–60. [DOI] [PubMed] [Google Scholar]
- 39.Arms MA, Fleming J, Sangani DB, Nadig SN, McGillicuddy JW, Taber DJ. Incidence and impact of adverse drug events contributing to hospital readmissions in kidney transplant recipients. Surgery 2018;163:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vranian SC Jr, Covert KL, Mardis CR, et al. Assessment of risk factors for increased resource utilization in kidney transplantation. J Surg Res 2018;222:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Covert KL, Mardis CR, Fleming JN, et al. Development of a predictive model for drug‐related problems in kidney transplant recipients. Pharmacotherapy 2017;37:159–69. [DOI] [PubMed] [Google Scholar]
- 42.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Assoc 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 43.Shavers‐Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under‐represented in medical research studies? Impediments to participation. Ethn Health 1997;2:31–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline cardiovascular history for the entire study cohort, stratified and compared by race.
Baseline transplant characteristics for the entire study cohort, stratified and compared by race.
New adverse events that occurred during the study, stratified and compared by race.
Consort diagram depicting the formulation of the final study cohort.
Change in additional cardiovascular risk parameters and renal function compared between non-AA and AA study participants.
Cardiovascular risk factor medication use compared over the study period, stratified by race.
Diabetes medication use compared over the study period, stratified by race.
Hypertension medication use compared over the study period, stratified by race.
Immunosuppression medication use compared over the study period, stratified by race.


