Abstract
Uterine fibroids are the most common gynecological disorder, classically requiring surgery when symptomatic. Although attempts at finding a nonsurgical cure date back to centuries, it is only around the middle of the last century that serious attempts at a medical treatment were carried out. Initially, both progestins and estrogen–progestin combinations have been utilized, although proof of their usefulness is lacking. A major step forward was achieved when peptide analogs of the GnRH were introduced, first those with superagonist properties and subsequently those acting as antagonists. Initially, the latter produced side effects preventing their routine utilization; eventually, this problem was overcome following the synthesis of cetrorelix. Because both types of analogs produce hypoestrogenism, their use is limited to a maximum of 6 months and, for this reason, today they are utilized as an adjuvant treatment before surgery with overall good results. Over the last decade, new, nonpeptidic, orally active GnRH-receptor blockers have also been synthesized. One of them, Elagolix, is in the early stages of testing in women with fibroids. Another fundamental development has been the utilization of the so-called selective progesterone receptor modulators, sometimes referred to as “antiprogestins”. The first such compound to be applied to the long-term treatment of fibroids was Mifepristone; today, this compound is mostly used outside of Western Countries, where the substance of choice is Ulipristal acetate. Large clinical trials have proven the effectiveness of Ulipristal in the long-term medical therapy of fibroids, although some caution must be exercised because of the rare occurrence of liver complications. All selective progesterone receptor modulators produce unique endometrial changes that are today considered benign, reversible, and without negative consequences. In conclusion, long-term medical treatment of fibroids seems possible today, especially in premenopausal women.
Keywords: uterine myomas, progestin, gonadotropin-releasing hormone receptor blockers, selective progesterone receptor modulators, antiestrogens
Introduction
Uterine leiomyomata (myomas, fibroids) are the most common gynecological disorder; as such, it should not be surprising that, over the centuries, a whole series of sometimes very strange approaches have been attempted and unlikely methods proposed, mostly unsupported by any scientific evidence.1,2
Ever since the first hysterectomy was performed, treatment of symptomatic fibroids has been surgical. Different techniques have been employed, initially consisting of total abdominal hysterectomy or myomectomy. To decrease the impact of surgery, several mini-laparotomic techniques, including combined mini-laparotomy-assisted vaginal surgery, have also been utilized.
Major progress has been made with the introduction of laparoscopic techniques, as proven by randomized trials comparing the various approaches.3,4
Laparoscopy, however, posed the problem of removal of large fibroids from the abdominal cavity. To solve it, Steiner et al5 introduced in 1993 an electrical cutting device later coined “morcellator”. This technique has many advantages and has been widely utilized. Unfortunately, following reports of involuntary dissemination of portions of uterine sarcomas during morcellation,6,7 the US Food and Drug Administration (US-FDA) issued a warning against its use. To obviate the problem, it has been proposed to carry out morcellation of the uterus or myomas within an insufflated isolation bag.8 The decision by the FDA stimulated research on the true incidence and therefore on the risks associated with morcellation. A just published nationwide multicenter study in China involving 33,723 subjects calculated the prevalence of malignancy after morcellation: 62 cases (0.18%) were confirmed pathologically as malignant.9 At present, the situation can be summarized as follows: a small, but not irrelevant, risk of disseminating an occult sarcoma exists during tissue extraction.10 Containment systems are now available for both power and manual morcellation; tissue fragmentation, therefore, can take place within an enclosed specimen bag.11
In 1995, a French group introduced a novel procedure to treat myomas: uterine arteries embolization (UAE) with Ivalon particles. Symptoms resolved in 11 out of 16 patients and in 10, menstrual cycles returned to normal.12 In 1999, the results of a large trial including 305 patients was published, showing control of menorrhagia in 86% of the cases at 3 months and 92% at 12 months.13
Results with this procedure have been reviewed over the last years, concluding that there is strong evidence for both safety and effectiveness of the procedure, with symptom improvement and change in quality of life (QoL). A recent evaluation concluded that outcomes with UAE are similar to those obtained following myomectomy, with an intervention rate at 5 years of 20%–30%. It also concluded that in women wishing to become pregnant, myomectomy may be preferred when no previous surgery has been performed.14
A noninvasive technique, magnetic resonance imaging (MRI)-guided Focused Ultrasound Surgery has been available for some times and has been approved by the Medicine Agency of the European Union (EMA) in 2002 and by the US-FDA in 2004. It consists of a noninvasive thermoablative technique, combining anatomic details’ visualization through MRI, with the therapeutic potential of high-intensity-focused ultrasound waves capable of passing through the abdominal wall. It offers excellent three-dimensional anatomic resolution and real-time thermal monitoring, with an accuracy of 2°C. At the target site, tissue temperature increases rapidly up to 60°C or higher, inducing protein denaturation and resulting in coagulative necrosis, while the skin and overlying tissue layers outside the ablated area remain unaffected.6,15 In selected cases, the procedure offers the advantage of preserving fertility.
The technique can be used in a number of diseases, including liver tumor, hepatocellular carcinoma, pancreatic cancer, renal cell carcinoma, prostate cancer, breast cancer, fibroids, bone tumors, atrial fibrillation, glaucoma, Parkinson’s disease, essential tremor, and neuropathic pain. It seems that tissue destruction occurs due to direct heating within the lesion and to the mechanical effects of acoustic cavitation.16,17
To help both physicians and patients in choosing the best-suited treatment, Donnez et al18 have recently attempted an overall analysis of the advantages of both medical and surgical therapies and have proposed new overall guidelines for the management of fibroids, based on symptoms (bleeding, infertility) and the patient’s age. Irrespective of purely medical considerations, it is a fact that many women would prefer medical treatment over a surgery and, over more than half a century, serious attempts at a medical management of fibroids have been made with a varying degree of success.2
The present review aims at evaluating different options for medical treatment, including those combining medical and surgical approaches.
Medical treatment
Today, it is widely accepted that in subjects with asymptomatic uterine myomas who do not desire pregnancy, no special treatment is required and these patients need only periodic monitoring of their condition.2 For this reason, the most conservative options that minimize morbidity/risk and optimize outcomes should be selected.19
Evidence on medical treatments has been systematically analyzed in 201620 in a total of 75 randomized controlled trials (RCT), concluding that their overall quality was very low and that there was insufficient evidence to recommend any medical treatment in the management of fibroids. Interestingly, the same year another systematic review,21 after evaluating 52 studies, reached a different conclusion, namely that published information proves the efficacy of a number of agents, opening-up promising avenues for the development of medical alternatives to surgery.
Progestogens
The first attempts to treat with natural progesterone (P) women with uterine myomas were published in the 1940s;22,23 then, in 1966, Goldzieher et al24 utilized for the first time a synthetic progestin, megestrol acetate, with the aim of obtaining the so-called red degeneration observed occasionally in pregnancy. Within a few weeks, they observed the same histological modifications usually found toward the end of a pregnancy and claimed success. Unfortunately, their work has not been validated.
Today, guidelines usually specify that there is insufficient evidence of benefits from use of progestins and therefore they cannot be advocated as a medical therapy for uterine myomas.25,26
Recently, however, attention has been drawn again on the process of red degeneration: Nakai et al27 claimed that MRI can single out myomas undergoing this degenerative regression, including coagulative necrosis. Red degeneration has also been observed following GnRH analog (GnRHA) treatment.28
The situation is complex because it seems that P exerts a dual action on myomas: it stimulates their growth through upregulating expression of EGF and B-cell lymphoma 2 (Bcl-2) and it inhibits growth through downregulation of insulin-like growth factors (IGF) expression in the cells.29
Estrogen–progestin combinations (oral contraceptives)
Two different, but equally important issues must be considered when discussing the action of oral contraceptives (OC) on uterine myomas: on the one hand, whether OC use can act to prevent the appearance of fibroids; on the other, whether their use can decrease the size of already existing ones.
As per the first question, in spite of several large investigations, it remains basically unanswered: one trial30 found that prolonged use of OC decreased the risk of harboring fibroids; two other case–control investigations31,32 showed no effect.
The same uncertainty exists for the possibility that OC may have a beneficial effect on the size of already existing fibroids. In 1995, it was reported that the prolonged use of an OC produced a significant shrinkage in myomas’ size;33 however, immediately after, the journal published a formal retraction.34
Thus, at present, it is safe to state that whereas OC use does not carry any increased risk of developing a myoma, no valid data have been presented to support the concept that they can inhibit the growth or decrease the volume of existing fibroids.
Gonadotropin-releasing hormone receptor blockers
To block the action of the GnRH, two different routes have been followed. The first involved the synthesis of GnRHA with a superagonistic (GnRHSA) or antagonistic (GnRHAT) action. The second involved the synthesis of nonpeptidic substances with the ability of directly blocking the GnRH receptor (GnRHR).
GnRH analogs
Nowadays, two types of analogs are utilized: superagonists act through a prolonged activation of the GnRHR, leading to desensitization and consequently to suppressed gonadotropin secretion; the antagonists instead compete with GnRH for receptors on cell membranes, inhibiting the GnRH-induced signal transduction and consequently gonadotropin secretion.35
Superagonist analogs
A new era in the conservative treatment of fibroids was inaugurated by Filicori et al36 in 1983 with the publication of the case of a woman with a uterine fibroid where metrorrhagia was quickly stopped and the myoma size significantly reduced following “pituitary desensitization by a luteinizing hormone-releasing hormone analog (GnRHA)”.
This report was followed by a large number of trials and gave rise to great hope that a medical treatment of uterine myomas had been finally found and that medical therapy would avoid surgery altogether, at least in older women. Unfortunately, the specific features of this approach, as shown in Figure 1, indicate that, whereas GnRHSA do shrink most fibroids considerably, myoma’s regrowth and recurrence of symptoms invariably follows discontinuation of treatment.37–39 Not only the effect seems to vanish after discontinuing therapy, the use of the analog cannot be prolonged beyond 6 months, because of the untoward consequences of the hypoestrogenism caused by the GnRHA, first and foremost an increased risk of osteoporosis that cannot be counteracted by calcitonin.40
Figure 1.
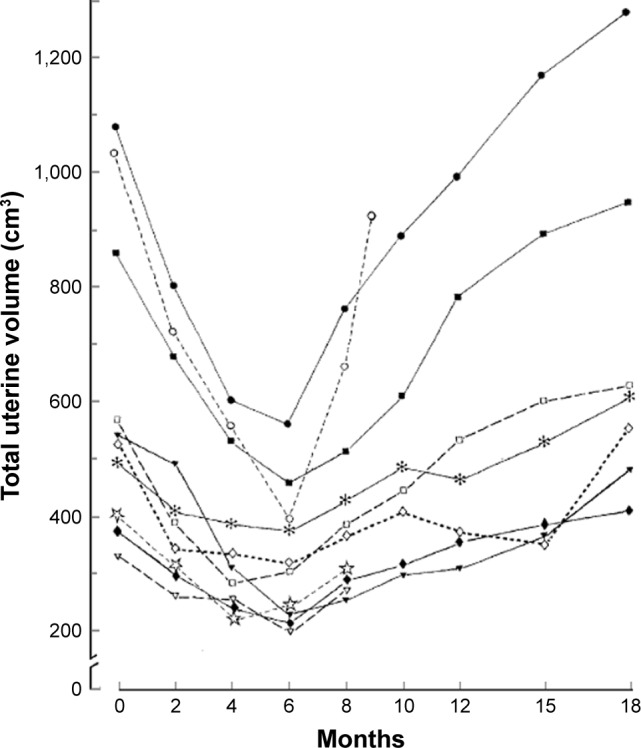
Reduction in uterine volume following administration of a GnRHSA and rebound following discontinuation of treatment.
Note: Reproduced with permission from Matta WHM, Shaw RW, Nye M. Long-term follow-up of patients with uterine fibroids after treatment with the LHRH agonist buserelin. Br J Obstet Gynaecol. Copyright © 2005, John Wiley and Sons.37
In terms of mechanism of action, beside the inhibition of estrogen synthesis, this treatment seems to cause DNA damage in myomas’ cells.41
Thus, it is recognized that GnRHSA can be useful in two main situations: as a pretreatment before surgery42,43 and in combination with the so-called add-back therapy.2
Pretreatment before surgery
The usefulness of a short (3-month) pretreatment with a GnRHSA has been extensively investigated in a variety of situations.
Adjuvant in classic surgery
Several factors may determine the success of pretreatment: first, a classic study by Stovall et al44 showed that volume shrinkage significantly increases the likelihood to proceed with a vaginal hysterectomy (81% vs 13%; P<0.05) entailing a shorter hospitalization and convalescence. Unfortunately, these advantages seem to disappear if the size of the uterus exceeds that of an 18-weeks gestation. Another study indicated that in laparotomic surgery, recurring to transverse rather than midline incision may be more feasible.43 Pretreatment may also be beneficial in specific instances, such as the presence of a large broad ligament or cervical fibroid.42
Pretreatment in patients with severe anemia offers three main advantages: stopping abnormal bleeding, improving hematological parameters, and reducing intraoperatory blood loss.38,45 An interesting observation was made in a three-prong multicenter trial in women suffering from myoma-induced chronic menorrhagia: whereas pretreatment with the association of a GnRHSA and iron supplementation did stop the bleeding altogether and restored satisfactory hemoglobin (Hb) red cell concentration, paradoxically, the group with iron-only supplementation fared better than the one receiving only GnRHSA46 (Figure 2). This might have been due to the chronic nature of excessive bleeding in these women.
Figure 2.
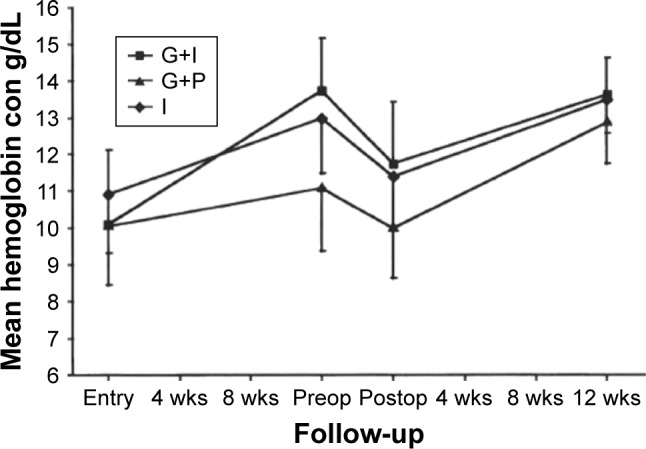
Hemoglobin levels during a 3-month pretreatment with a GnRHSA in three groups of patients with uterine myomas: G+I: GnRHSA plus Iron supplementation. G+P: GnRHSA+placebo. I: iron supplementation only.
Note: Reprinted from Fertility and Sterility, Vol 108(1), Benagiano G, Cronjé H, Kivinen ST, et al, Zoladex (goserelin acetate) and the anemic patient: results of a multicenter fibroid study, Pages 223–229, Copyright (1996), with permission from Elsevier.46
A systematic review published in 200243 indicated that pretreatment with a GnRHSA produces an increase in Hb and hematocrit, although its clinical relevance may be questionable. Two, although arbitrary, cutoff points have been described to identify patients who will benefit from pretreatment: Hb concentration <12.0 g/dL and red cell concentration <3×106/mL. Much will depend on whether menorrhagia had continued for a long period of time, something that, at least in the industrialized world, seems to become less and less frequent. Indeed, already 25 years ago, out of 171 patients with fibroids pretreated with a GnRHSA, 78.4% suffered from metro-menorrhagia, but only 54.5% had Hb concentrations <12.0 g/dL.47
Occasionally, the presence of large fibroids can be associated with a hematological disorder named erythrocytosis or isolated polycythemia.48 The pathogenesis of this phenomenon may be linked to an altered erythropoietin production, an anomaly observed in most patients with menorrhagia due to the presence of myomas. This may explain the relative rarity of cases of clinically relevant anemia despite the presence of menorrhagia.49
A clear rationale exists for advocating GnRHSA pretreatment with the aim of decreasing intraoperative blood loss, since it has been documented that they produce significant alterations in uterine arterial blood flow velocity waveforms, with a parallel increase in the resistance index. In a specific investigation, uterine arterial volume decreased from 656 to 386 mL and the uterine arterial resistance index increased from 0.52 to 0.92.50
Usefulness of pretreatment was documented in an RCT involving 71 hysterectomies where a significant blood loss reduction (on average 130 mL), a preoperative rise in Hb concentration, and relief of menstrual symptoms while waiting for surgery were observed following GnRHSA.51 Lethaby et al43 in their systematic review concluded that blood loss is lowered both at hysterectomy and myomectomy; however, transfusion requirements are not modified and the improvement in postoperative hematological parameters seems small.
Some 25 years ago, an evaluation of the advantages and disadvantages of GnRHSA treatment before myomectomy led to the conclusion that, in general, surgery becomes easier, although differences for several variables were small, and 3 months after surgery, there were no major ultrasonographic differences between the two groups.47
The abovementioned RCT evaluating efficacy of GnRHSA before myomectomy looked also at recurrence rates of myo-mas and myoma-related symptoms. After 27–38 months of follow-up, recurrence was not associated with presurgery medical therapy or preoperative uterine volume; however, it was greater when at least four myomas were resected.49
Adjuvant in laparoscopic myomectomy
Decreasing the size of the uterus and fibroids and intraoperative bleeding can be considered two important advantages of GnRHSA pretreatment in case of laparoscopic myomectomy. This will definitely be the case in the presence of a pedunculated or subserosal myoma; unfortunately, in the case of intramural tumors pretreatment may very well make cleavage more difficult and excision more problematic.
These advantages were recognized already in the early experience of pretreatment in 150 laparoscopic myomectomies by Mettler and Semm.52 Subsequently, the same group53 compared results obtained with leuprolide and triptorelin, observing at 3 months an identical reduction of myoma size (between 10% and 50%, in 88% of both groups).
Bearing in mind that laparoscopic, compared with laparotomic, myomectomy may per se carry a greater risk of recurrence within 5 years,54 the question arises whether fibroid shrinkage or, if small, their visual disappearance at the time of surgery may expose patients to a higher rate of recurrence. Unfortunately, two small trials addressing the issue came to opposite conclusions.55,56
Adjuvant in hysteroscopic myomectomy
In view of the increasing use of the hysteroscopic approach for myomectomy, the usefulness of GnRHSA has been carefully investigated. The combined approach has been pioneered by Donnez et al,57,58 who used Ng-YAG laser and made the interesting observation of a lower vascularity in the myometrium. They suggested that after pretreatment, a two-step procedure should be carried out. This would consist of resecting first the protruding part of the tumor, followed by the intramural portion that at this stage would become accessible to the laser beam. GnRHSA therapy would then be continued for another 8 weeks.
Among the advantages, it seems that pretreatment can facilitate hysteroscopic removal by substantially reducing bleeding, thinning the endometrium, and decreasing the volume of myomas. As always, disadvantages have also been identified, such as a more difficult procedure due to the “retreat” of the mass inside the myometrium.
Adjuvant in laparoscopic hysterectomy
In 2003, a randomized, prospective trial of total laparoscopic hysterectomy investigated the effect of pretreatment. The study found that in the presence of large uteri (380–680 mL volume), administering a GnRHSA over 3 months can decrease uterine size, facilitating surgery by reducing operating time and blood loss.59
Long-term use
Blocking ovarian function is an effective method of decreasing the size of myomas but has time limitation (usually 6 months) due to the hypoestrogenism that is invariably associated with the use of this class of compounds.
For this reason, in order to utilize GnRHSA for long periods of time, scientists had to first answer the question of how to continue treatment while at the same time avoid its short- and long-term negative consequences (respectively, hot flushes, insomnia, profuse sweating, and osteoporosis).
As it will be described below, today the availability of newer drugs has made the long-term use of GnRHSA unlikely and more of a historical than actual relevance.
Preventing adverse consequences of GnRH analog treatment
In the 1990s, several options have been evaluated, including the following:
Contemporary use of a progestin
In an early small-scale attempt, oral medroxyprogesterone acetate (MPA) was given either together or after treatment with the GnRHSA. This trial clearly indicated the superiority of the sequential approach, both in terms of reduction of uterine volume and lack of regrowth by 6 months.60 Unfortunately, an RCT by Carr et al61 found that, while MPA coadministration (20 mg/day) prevented, at least partially, bone mineral loss, it also blocked fibroid shrinkage and caused a reversal in the decrease in nonmyomatous tissue volume.
Contemporary use of a superagonist analog and raloxifene
An RCT involving 100 women tested the addition of the selective estrogen receptor (SERM) raloxifene to the analog over 6 months and, as expected, found that the drug produced a significant reduction in the volume of the myoma even in the no-SERM group. This effect, however, was significantly (P<0.05) more pronounced when both drugs were administered; in subjects treated with raloxifene, bone mineral density (BMD) and serum bone markers were unchanged.62,63
Use as an alternative to surgery: the add-back therapy
In the early days, it was hoped that a GnRHSA regimen could be developed that would enable women to avoid surgery altogether. In order to achieve this goal, it would be necessary to find ways to continue therapy indefinitely.
Several modalities were tested:
Lowering the dose of the agonist
Broekmans et al64 attempted to maintain the positive action of the GnRHSA while minimizing negative effects. With this in mind, after an initial treatment with a full GnRHSA dosage, they lowered the dose to allow some endogenous estrogen production. With this expedient, they were able to preserve the beneficial effects of the initial full dosage without apparently affecting BMD.
Using sequentially low-dose estrogen–progestin combinations
Another approach has been to prolong GnRHSA treatment by adding, once shrinkage has been obtained, a low-dosage estrogen–progestin combination. In 1990, Barbieri65 proposed a theory based on the existence of a “window” of estrogen concentration that would not allow myoma regrowth but would be sufficient to substantially reduce adverse effects (see Figure 3). When this theory was applied and small quantities of an estrogen followed by a progestin (as in hormonal replacement therapy) were given, GnRHSA treatment could be continued with minimal adverse effects and no regrowth.2,66
Figure 3.
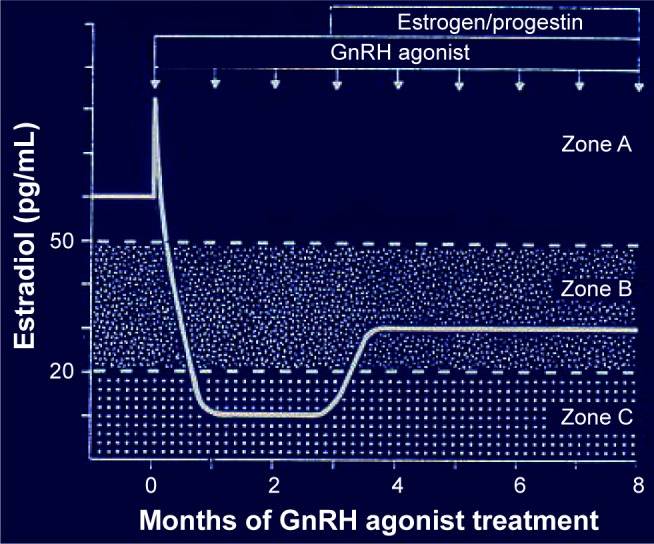
The hypothesis proposed by Barbieri in 1990.
Notes: It postulates the existence of three zones characterized by different levels of circulating estrogens following GnRHSA administration. Zone A, where high levels immediately follow administration of the analog. Zone C, where low levels are induced by the full action of the GnRHSA. Zone B, where some rebound exists following treatment with an add-back therapy. At these intermediate concentrations, only minimal regrowth occurs. Data from Barbieri et al, 1990.65
A review published in 2012 concluded that the use of add-back therapy has enabled wider application, longer duration of treatment, and an increase in compliance.67 The effectiveness of this approach at 1 year has been recently evaluated in a Cochrane review68 of 12 RCTs considered valid for the analysis, for a total of 622 subjects. With MPA, they found no evidence of an effect in relation to bone mass (BM), while there was some regrowth of the uterus. With tibolone, there was a decreased loss of BM and a better QoL was observed, although the true effect was doubtful. In addition, there might have been some increase in uterine volume and more uterine bleeding. With raloxifene, no evidence of an effect on QoL was found, while there was a beneficial action on BM, with no clear evidence of an effect on uterine volume, bleeding, or severity of vasomotor symptoms. With estriol, no studies reported on uterine size, bleeding, or vasomotor symptoms, but its use may entail a decreased BM loss. Use of ipriflavone was associated with decreased loss of BM in a single study and no evidence of an effect on vasomotor symptoms. Finally, one study suggested that adding conjugated estrogens to GnRHA resulted in a larger decrease in uterine volume in the placebo group.
Sequential use of high-dose progestin
Increased expression of progesterone receptor (PR) in myomas has been considered a consequence of overexpression of estrogen receptors (ER), resulting in increased end-organ sensitivity to estradiol (E2). Under these circumstances, the observation that analog therapy decreases ER concentration, whereas it increases PR expression, provides a rationale for the use of a progestin to block regrowth. Applying this paradigm, a comparative trial was carried out using high daily doses of MPA (200 mg orally for the first month, 100 mg for the second, 50 mg for the third and fourth, and 25 mg for the remaining 2 months) after the action of the analog had reduced the volume of the myomas. It was found that at the end of MPA treatment the size of the uterus was significantly increased, whereas significant myomas’ regrowth did not occur (from an average of 25.6±24.8 to 30.6±32.9 mL, P>0.3).69
Sequential use of danazol
Good results were also obtained when the administration of two long-acting GnRHSA for 6 months was followed by an additional 6-month treatment with 100 mg of danazol daily. Myomas’ regrowth was reduced and the increase in uterine volume was 31.2% lower than in the control group.70
Antagonist analogs
In theory, antagonists of the GnRH would offer the advantage over superagonists of an immediate effect, avoiding the “flare-up” observed with the latter. In practice, GnRHAT deprived of fastidious side effects were not available until the early 1990s. This is the reason why the results of the first trial in patients with uterine myomas treated with a GnRHAT (called Nal-Glu) appeared only in 1993. Results indicated a mean decrease in size of 52.8%±7.3%71 (see Figure 4).
Figure 4.
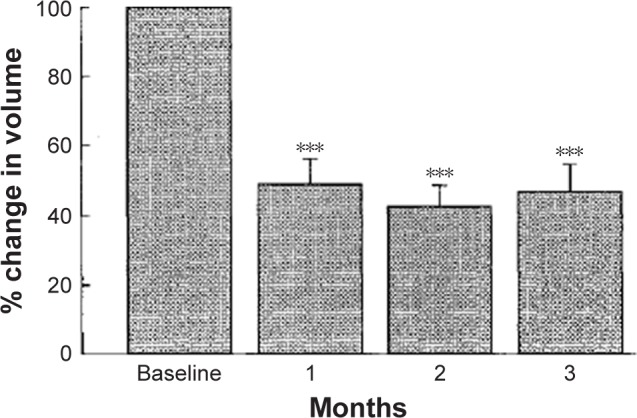
Effect of the administration of a GnRH antagonist on uterine size.
Notes: ***p<0.0001. Reprinted from Fertility and Sterility, Vol 108(1), Kettel LM, Murphy AA, Morales AJ, Rivier J, Vale W, Yen SS, Rapid regression of uterine leiomyomas in response to daily administration of gonadotropin-releasing hormone antagonist, Pages 642–646, Copyright (1993), with permission from Elsevier.71
Additional small trials followed; two utilized a pretreatment before myomectomy with a slow-releasing formulation of cetrorelix: one involved 18 subjects, and in 16 a progressive decrease in the size of the uterus (from a mean of 410.4±77.1 mL to 230±52.6 mL) was observed.72 The other included 20 patients, and in 16 there was a good response with the volume of the largest fibroid decreasing by 33.5%. The authors pointed out the expected rapidity of the myomas’ shrinkage with a mean reduction of 31.3% in only 14 days.73,74 These early attempts were followed by a randomized, double-blind RCT involving 109 premenopausal women, divided into four groups. Groups 1–3 received placebo, 5 and 10 mg of cetrorelix on days 1, 8, 15, and 22, respectively. Group 4 received 10 mg cetrorelix on days 1 and 15. Mean (±SD) reduction of uterine volume at 1 month (measured by MRI) with placebo was 5.1%±32.1%, rising to 15.6%±20.2% with 4×5 mg and to 15.4%±34.6% with 4×10 mg. In group 4 (cetrorelix 2×10 mg) there was no response (0.65%±30.6%). In conclusion, a significant response vs placebo (P<0.05) occurred in the group administered with cetrorelix 10 mg on days 1, 8, 15, and 22.75
In terms of mechanism of action, Chen et al76 demonstrated the presence of mRNAs encoding for GnRHR and EGF in cultured leiomyoma cells. When cultured with cetrorelix, the number of viable leiomyoma cells was significantly (P<0.01) decreased; therefore, cetrorelix downregulates proliferating cell nuclear antigen (PCNA) and EGF expression and upregulates apoptosis in association with enhanced poly(adenosine 5′-diphosphate-ribose) polymerase expression. In addition, Britten et al77 found that cetrorelix directly regulates type 1 collagen, fibronectin, and matrix proteoglycan production, thereby directly inhibiting extracellular matrix (ECM) production.
In 2005, the results of a small study in 19 subjects with another antagonist, ganirelix, were published.78 Decrease in fibroid size was very rapid: median duration of treatment to achieve maximal reduction was 19 days, with a range of 1–65 days. Reduction in fibroid volume at MRI was −29.2% (range −62.2% to 35.6%) and −42.7% (−77.0% to 14.1%) at ultrasound. Comparable uterine size reduction in uterus size was −25.2% (−63.6% to 28.9%) at MRI and −46.6% (−78.6% to −6.1%) at ultrasound.
Orally active antagonists of the gonadotropin-releasing hormone receptor
The perceived need for nonpeptidic, orally active, potent GnRHR blockers led over the last two decades to an active search for compounds capable of inhibiting the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and, through this mechanism, of reducing the levels of circulating estrogens in normal women.
The search identified a number of potential candidates, such as derivatives of quinolone-6-carboxamides, dimethylphenyl-tryptamine, mono- and di-amino pyrimidine, 2-phenylimidazol-pyrimidin, arylyrimidine, aminomethyl-imidazol-pyrimidone, arylmethyl-methyluracils, O-alkyl quinolone, benzimidazole-5-sulfonamides], piperazinyl- benzimidazoles, propargylated benzene core, and pyrimidinyl-phenyl-3-methoxyurea. A summary of the situation was presented in 2008.79
Additional compounds have been synthesized since, although only a few are being developed for use in subjects with fibroids. These will be described in detail.
Elagolix
In 2003, Zhu et al80 reported that a series of novel uracil derivatives [1-arylmethyl-3-(2-aminoethyl)–5-aryluracil] showed potent anti GnRHR activity. The group started a systematic evaluation of this class of compounds,81–86 and in 2008, Chen et al87 published the discovery of a potent and orally available nonpeptide antagonist of the GnRHR. Chemically, the compound is an uracil-phenyl-ethylamine bearing a butyric acid and was given the code name NBI-42902. It belongs to a class of compounds producing inhibition of CYP/CYP450 3A4 (CYP3A4), an enzyme member of the CYP/CYP450 superfamily of monooxygenases, encoded by a gene on chromosome 7q21.1 that catalyzes the synthesis of cholesterol, steroids, and other lipids. The group aimed at improving the CYP3A4 inhibition liability of these uracils, while maintaining their antagonistic action on GnRHR and focused on R-4-{2-[5-(2-fluoro-3-methoxyphenyl)–3-(2-fluoro-6-[trifluoromethyl]benzyl)–4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]–1-phenylethylamino} butyric acid sodium salt, later coined as “elagolix”. Its oral administration suppressed LH production in castrated macaques, making it suitable for potential use in humans.
In fact, even before the publication of the compound’s identification, Struthers et al88 reported on the safety, pharmacokinetics, and inhibitory effects on gonadotropin secretion of NBI-42902 in 56 postmenopausal women with FSH levels >40 IU/L, and a BM index within 20% of ideal values. Volunteers were administered orally 5, 10, 25, 50, 75, 100, 150, or 200 mg of the compound. They found that the medication was well tolerated, and it quickly reduced serum LH concentrations in a dose-dependent fashion. Suppression of FSH was less pronounced.
The following year, the group published a first in vitro and in vivo pharmacological characterization of the novel antagonist, reporting that titrated NBI-42902 binds with high affinity to a single class of binding sites and can be displaced by a range of peptide and nonpeptide GnRHR ligands. In vitro NBI-42902 acts as a potent functional competitive antagonist of GnRH-stimulated inositol phosphate accumulation, Ca(2+) flux, and extracellular signal-regulated kinases 1/2 activation. They concluded that the compound possessed all the pharmacological characteristics required for a nonpeptide GnRH antagonist to have clinical applications.89
Additional information on elagolix was published in 2009.90 Briefly, this investigation demonstrated that the compound was well tolerated and became rapidly bioavailable after oral administration, with median time of maximum plasma concentration (Tmax) values ranging from 0.5 to 1 hour, reaching peak plasma concentrations from 55.5±23.8 to 1,504±492 ng/mL in the 25- and 400-mg groups, respectively. It produced a rapid decline in circulating levels of gonadotropins, consistent with the rate of clearance of LH, suggesting an immediate blockade of the GnRHR. FSH levels followed a similar pattern, although they were suppressed to a lesser extent and the decline was slower. With doses of at least 50 mg/day, circulating levels of E2 also decreased and at the dosage of 50–200 mg/day or 50 mg twice daily, they remained low (17±3 to 68±46 pg/mL) in most subjects during late follicular phase. Importantly, effects were rapidly reversed after discontinuation. Elagolix was well tolerated at all doses; among side effects, worthy of mention are headache, abdominal pain, and hot flashes.
Because of the good results obtained with GnRHA in the treatment of endometriosis, the first clinical application of elagolix was in subjects with this condition, where the effects of the antagonist (150 mg every day, 75 mg twice a day) were compared to those obtained with subcutaneous injections of depot-medroxyprogesterone acetate (DMPA) over 24 weeks of treatment and 24 weeks of posttreatment follow-up.91 The study collected information on the effects of the medication on a number of parameters of interest for all subjects treated with elagolix. First, it was found that all treatments induced minimal mean changes in BMD at 24 weeks as follows: elagolix 150 mg, −0.11/−0.47%; elagolix 75 mg, −1.29–1.2%; and DMPA 0.99/−1.29% in the spine and total hip, respectively, with similar or lower changes at week 48 posttreatment. Circulating concentrations of N-telopeptide underwent minimal changes in all treatment groups. In terms of E2 circulating levels, they were measured pretreatment on days 2–7 after the onset of menses and were comparable in the two elagolix groups (41.1 and 39.1 pg/mL). During treatment (from weeks 4 to 24) median concentrations ranged from 36 to 63 pg/mL in the elagolix 150 mg/day group and from 23 to 31 pg/mL in the elagolix 75 mg twice a day group. During the posttreatment weeks, in both elagolix groups levels rapidly increased, consistent with a return of normal menstrual cycles. Interestingly, during the study, there were eight pregnancies among women treated with elagolix: three that occurred during the treatment period and five during the posttreatment period, ranging between 2 and 12 months after the last dose.
An important feature of this study was the safety assessment. As reported already, the most common adverse events in subjects treated with elagolix were headache, nausea, and nasopharyngitis. Only three patients in the elagolix 150 mg/day group and three in the elagolix 75 mg twice a day group experienced side effects that were considered serious. During the 1-month screening pretreatment period, one or more episodes of hot flash were experienced by 47.6% and 39.3% of the patients randomized to receive elagolix. During the 6-month treatment period, the percentage of elagolix-treated subjects experiencing one or more episodes of hot flash rose to 71.1% and 82.1%, respectively.
The good results obtained in this first large study led by Ezzati and Carr92 concluded that elagolix may become a valuable addition to the armamentarium of pharmacological agents to treat endometriosis-related pain and, indeed, two recently published double-blind, randomized, 6-month Phase III trials seem to confirm this conclusion. The two trials evaluated the effects of elagolix, 150 mg/day and 200 mg twice daily, compared with placebo in women with surgically diagnosed endometriosis and pain.93 Of interest is the observation that for all patients treated with elagolix, those who received it had higher rates of hot flushes (mostly mild or moderate), higher levels of serum lipids, and greater decreases from baseline in BMD than those who received placebo. Finally, these good results were confirmed in a very recently published long-term study of 569 patients with endometriosis. Treatment with elagolix produced a reduction in dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia.94 The pharmacokinetics and pharmacodynamics of elagolix have been further elucidated in a very recent report of a double-blind RCT with multiple ascending doses (150 mg once daily or 100, 200, 300, or 400 mg twice daily) or placebo administered for 21 days to 45 healthy premenopausal women.95 This investigation confirmed that elagolix is rapidly absorbed after ingestion, reaching maximum concentrations between 1 and 1.5 hours, with a half-life of 4–6 hours. Dose-dependent suppression of E2 was observed, with maximum suppression being achieved with 200 mg twice daily. Also, the suppression of FSH, LH, and E2 occurred within hours of the first day of administration. Dose-dependent suppression of FSH and LH was also observed, with maximal or near-maximal suppression achieved with the dose of 200 and 300 mg twice daily. Even at lower doses (≥100 mg twice daily), P concentrations remained at anovulatory levels throughout the 21 days. It seems, therefore, that elagolix allows for modulation of gonadotropin and ovarian hormone concentrations, with a partial suppression at lower doses and nearly full suppression at higher ones.
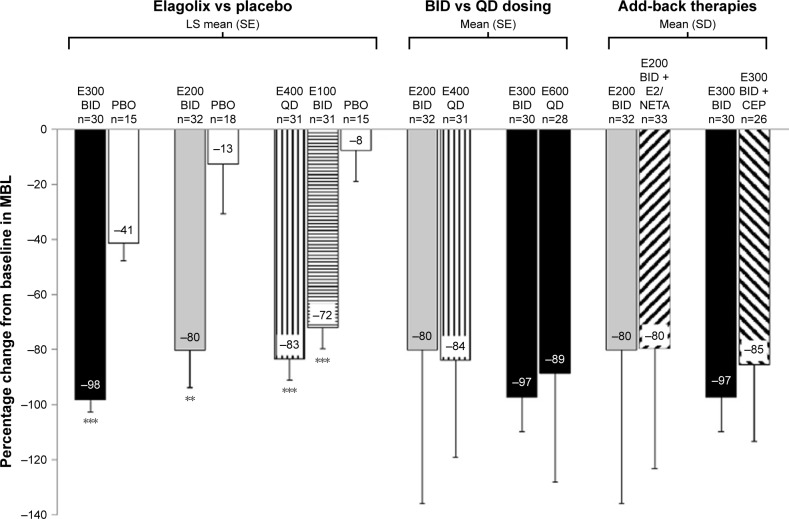
Following these encouraging results, elagolix has been now tested in premenopausal women with myomas and heavy menstrual bleeding (menstrual blood loss [MBL] >80 mL per cycle) in a three-prong trial: elagolix vs placebo and elagolix plus low-dose estrogen/progestogen add-back therapy.96 This Phase II proof-of-concept study has been conducted in 271 women with a mean age of 41.8 years. Of the randomized women (elagolix alone, n=160; placebo, n=50; elagolix with add-back therapy, n=61), 228 completed the 3-month treatment period. The elagolixalone arm tested the following dosages: 100, 200, 300, all twice a day and 400, 600 mg daily (all but the 600 mg group were placebo controlled). The third group received either elagolix 200 mg twice daily plus, as add-back therapy, continuous daily E2 0.5 mg/norethisterone acetate (NETA) 0.1 mg, or elagolix 300 mg twice daily plus E2 1 mg continuously and cyclical P 200 mg.
The least squares mean percentage change in MBL from baseline to day 28 was significantly greater with elagolix alone (range, −72% to −98%; dose-dependent reduction was highest with 300 mg twice daily) vs placebo (range, −8% to −41%); mean percentage changes with add-back regimens were −80% to −85%. Overall, adverse events were dose independent (elagolix alone, 70.0%–81.3%), but lower with placebo (56.0%) and add-back regimens (55.6%–70.6%). Hot flush was the most common side effect (elagolix alone, 45.5%–62.5%; placebo, 12.0%; add-back regimens, 18.5%–26.5%; Figure 5).
Figure 5.
Mean percentage change in menstrual blood loss during the last 28 days of treatment.
Notes: **P≤0.01; ***P≤0.001. P-values are for difference in LS mean change from baseline vs PBO. Reprinted from Fertility and Sterility, Vol 108(1), Archer DF, Stewart EA, Jain RI, et al, Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study, Pages 152–160, Copyright (2017), with permission from Elsevier.96
Abbreviations: BID, twice daily; CEP, E2 1 mg continuously and cyclical oral P 200 mg; E, elagolix (doses in mg); E2/NETA, E2 0.5 mg and norethisterone acetate 0.1 mg continuously; LS, least squares; PBO, placebo; QD, once daily; SE, standard error.
In conclusion, this preliminary study documented that Elagolix significantly reduced heavy menstrual bleeding in women with fibroids; in addition, low-dose add-back regimens substantially reduced flashing.
SKI-2670
This new, nonpeptidic, orally available GnRHR blocker97 (code-named SKI-2670) has been recently pharmacologically characterized both in vitro and in vivo through measurement of its binding affinity and antagonistic activity for GnRHR. In castrated monkeys at equivalent doses, a single administration lowered serum LH levels more significantly and with a longer duration compared with elagolix. In addition, in intact female monkeys, SKI-2670 suppressed serum levels of both gonadotropins and ovarian hormones, whereas elagolix suppressed only serum LH levels.
SKI-2496
Another orally available GnRHR blocker based on a uracil scaffold has recently been synthesized and characterized.98 It was given the code name of SK-2496 and it exhibits a highly selective antagonistic activity toward human GnRHR, as well as an inhibitory effect on GnRH-mediated signaling pathways. It has improved bioavailability and superior gonadotropic suppression activity compared with elagolix, thus representing a promising candidate for an orally available GnRHR blocker.
Relugolix (TAK-385)
The identification of a fourth orally active GnRHR blocker, relugolix (code named TAK-385) with high affinity and potent antagonistic activity for human and monkey GnRHR was reported by Miwa et al99 in 2011. In female knock-in mice, it induced constant diestrus phases within the first week, decreased the uterus weight to ovariectomized levels, and downregulated GnRHR mRNA in the pituitary after 4 weeks.100
OBE-2109
The human pharmacodynamics and safety of a fifth nonpeptide inhibitor (code-named OBE-2109) have just been published, both alone and in combination with E2 plus NETA add-back therapy. When given alone at the dose of 100 and 200 mg, within a month OBE-2109 reduced circulating E2 levels, respectively, to a median of 19.5 and 3.2 pg/mL and induced amenorrhea. On the other hand, given in combination with add-back therapy, it produced levels ranging between 25 and 40 pg/mL, but bleeding control was partially impaired.101
Selective progesterone receptor modulators (SPRMs)
Myomas’ growth is highly dependent on the presence of estrogens and, indeed, these tumors are exceptional before puberty and regress after the menopause.102
Progesterone plays also a role exerting a dual action: on the one hand it stimulates myoma growth through upregulating EGF and Bcl-2 expression; on the other, it inhibits growth through downregulation of IGF I expression in the cells.29 The beneficial effect on fibroid growth can be seen during pregnancy when often they remain unchanged and may even regress or undergo the so-called red degeneration.24
Evidence for more complex mechanisms came from studies with GnRHA where it was shown that the addition of a progestin at the beginning of treatment prevents fibroid shrinkage.60,61,103 Additional information came from morphological studies showing a higher mitotic index in myomas during the secretory phase.104
Today, it is widely accepted that a wide number of factors play a role in myoma growth, including EGF, IGF I and II, and growth hormone with P acting as a stimulant of these growth factor expressions.105
In view of this new evidence a call for rethinking the complex mechanism of fibroids’ growth and regression has been made106 and a rationale exists for using the new class of compounds called “antiprogestins”, today more correctly coined as “SPRM”. Several of them do not have “pure” antiprogestins activity, but it seems that they act through the suppression of the expression of the PR gene.107 A major synthetic program of these compounds was carried out in the last decade of the twentieth century by the Contraceptive Development Branch (CDB) of the Center for Population Research at the US National Institutes of Health (US-NIH).108 Among others, this led to the development of proellex, telapristone, and ulipristal.
Mifepristone (MFP)
MFP, initially code-named as RU486, represents the prototype SPRM, being the first to have been synthesized and utilized clinically.
The compound has been shrouded in a never-ending controversy because its first utilization has been for the medical interruption of gestation.109
A pioneering trial in 10 patients110 demonstrated that 50 mg of MFP produced a significant, but diverse reduction in myomas’ size (varying between 0% and 87%). Subsequently, the dosage was lowered to 25 mg daily with the same results.111 In an early comparative trial, MFP and leuprolide acetate were equally effective in decreasing uterine volume and blood flow to the uterus.112 Due to a series of circumstances, no progress was made for a decade and a search in 2004113 identified only six clinical trials with MFP for a total of 166 subjects, all with good results. Using low (5 mg) and ultralow (2.5 mg) daily MFP doses, an average reduction of 47% and 11% in uterine volume was obtained.114,115 With 10 mg, volume of the uterus, myomas, and largest fibroid declined by >25% and the amount of blood loss by almost 95%; Hb increased from 9.5 to 11.2 g/dL and dysmenorrhea was completely relieved in 80% of the subjects.116
Over the last decade, several additional studies have been carried out using daily dosages ranging from 50 to 10 mg, always reporting good results.117–121
A systematic Cochrane review of 2012122 found only three truly RCTs of MFP vs other medical therapies or placebo, involving 112 participants with different dosages, concluding that MFP use relieves heavy menstrual bleeding, although there was no conclusive evidence for an effect on the fibroid volume. Another metanalysis, published in the following year,123 included 11 RCTs (testing doses between 2.5 and 25 mg for 3–6 months and involving 780 subjects) and reached the opposite conclusion: MFP is effective in reducing uterine and fibroid volume, hypermenorrhea, MBL, pelvic pain, pelvic pressure, anemia, and dysmenorrhea. At this stage, a group in Spain concluded that a 5 mg dose was preferable to 10 mg.124,125
Recently, different administration schedules were evaluated in two Indian trials, using weekly or biweekly doses of 50 mg claiming good results.126,127 Using the vaginal route for the administration of 10 mg daily of MFP, treatment significantly reduced the volume of fibroids.128
In terms of mechanism of action, Chinese investigators have suggested that EGF mRNA levels in myomas seem controlled by P and MFP can inhibit EGF gene expression in myomas.129 Also, PR and its mRNA are overexpressed in fibroids and MFP may act by suppressing expression of the PR gene.130 The molecular basis of volume reduction in women treated with 50 mg MFP every other day was also investigated showing that the glutathione pathway was the most significantly altered with an overexpression of the glutathione-s transferase-Mu-1 (GSTM1) gene in good responders compared with nonresponders. Deletion of the GSTM1 in leiomyoma biopsies was found in 50% of the MFP-treated subject.131
Asoprisnil (ASP)
ASP, code-named J867, and its major metabolite (codenamed J912), represent a class of PR ligands with partial agonist and antagonist activities in vivo.132 Its early clinical application to the medical treatment of fibroids was reviewed by Chwalisz et al.133 The same group later published the results of a multicenter RCT using doses of 5, 10, and 25 mg daily. ASP significantly suppressed both the duration and intensity of uterine bleeding, inducing amenorrhea in a dose-dependent fashion (28%, 64%, and 83%), increasing Hb concentration, and significantly decreasing fibroid and total uterine volumes in the 25 mg group.134
Chen et al135 using an in vitro model produced evidence that ASP can decrease the number of myoma cells, the PCNA-positive rate, its protein expression, and selectively induce apoptosis in uterine leiomyoma cells without affecting normal myometrial cells. It can also increase the expression of PR-B, but not PR-A. In addition, in cultured myoma cells, ASP can selectively downregulate the expression of EGF, IGF I, transforming growth factor and their receptors;136 activate tumor necrosis factor-related apoptosis-inducing ligand-mediated signaling pathway; elicit stress-induced apoptosis137 and endoplasmic reticulum stress;138 selectively reduce collagen deposition in cultured leiomyoma cells, but not in normal myometrial cells through a decreased collagen synthesis.139 Finally, ASP does not induce proliferation of uterine tissues and does not suppress the tumor suppressor gene PTEN expression.140
Under the action of ASP, there is an alteration of uterine spiral arteries morphology leading to suppression of bleeding141 and a statistically significant reduction of genes in the IL-15 pathway, known to play a key role in uterine natural killer (uNK) development and function.142
In 2005, due to abnormal findings in endometrial biopsies of treated women (see section “Asoprisnil” under “Effects of SPRM on the endometrium” in this article), clinical trials with ASP were suspended by the manufacturer.
Proellex (telapristone)
In 2002, Attardi et al143 examined the in vitro properties of one of the compounds synthesized by the US-NIH (code name CDB-4124), later named as proellex, or telapristone, and its mono-de-methylated metabolite, code named CDB-4453. Both the SPRM bind with high affinity to rabbit uterine PR, do not exhibit agonist activity, and possess considerably lower antiglucocorticoid action than MFP. CDB-4124 selectively inhibits proliferation and induces apoptosis in myoma, but not in normal myometrial cells,144 although a subsequent investigation did not find any significant apoptosis in cultured fibroid cells, suggesting that apoptosis may not be the main pathway responsible for CDB-4124-induced fibroid shrinkage.145
At present, it does not seem likely that the manufacturer wishes to proceed further to apply Proellex to the medical treatment of myomas. In addition, during 2017, the US-FDA told the manufacturer that a new trial was needed to prove that using the oral route to administer the drug is safe. This prompted the manufacturer to consider refocusing on the vaginal route to deliver telapristone for the treatment of endometriosis.146
Ulipristal (UPA)
UPA, code-named CDB-2914 or VA-2914, is the most widely utilized and most promising SPRM available today. A practical method for its large-scale synthesis was published in 2000,147 starting a series of investigations on its pharmacological148 and clinical indications.149
UPA inhibits the proliferation of cultured leiomyoma cells by downregulating PCNA and Bcl-2 expression and by upregulating cleaved caspase-3 and poly (ADP-ribose) polymerase expression.150 It also downregulates VEGF, adrenomedullin and their receptor contents, and specifically modulates PR isoform contents in cultured leiomyoma cells.151,152
The induction of apoptosis by UPA in uterine fibroid cells has been confirmed in vivo in two groups of six and five subjects harboring leiomyomas, using daily doses of 5 and 10 mg UPA. Results were compared to those obtained in 17 women treated with a GnRHSA and 10 subjects with no hormonal treatment. It was found that apoptosis was present in a significantly higher proportion of patients treated with UPA compared with the GnRHSA and was absent in controls.153 In vitro, UPA decreases inhibin-βA subunits, follistatin, activin receptor type IIB and activin receptor-like kinase 4 mRNA expressions and blocks the activin A-induced increase in fibronectin or VEGF-A mRNA expression.154 It shows good oral bioavailability and a half-life allowing one single oral administration per day; being a steroid, UPA is a substrate for CYP3A4, although it is not an inducer or inhibitor of the CYP system.155
Starting in 2008, the selective action of UPA on fibroids was tested clinically; first, in a dose-finding study involving 22 subjects at the daily dose of 20 and 10 mg, obtaining a significant reduction in myoma’s volume of 36% and 21%, respectively.156 Further results were published 3 years later in 28 women treated for 3–6 months, with a second 3-month course also offered. In controls the total volume of fibroids increased by 7%, whereas it decreased by 24% and 17%, respectively, in the 20 and 10 mg groups. During the second 3-month period, fibroid volume further decreased by 11%. Amenorrhea occurred in 20/26 treated women and Hb concentration improved together with the subjects’ QoL.157
In the subsequent years, multicenter clinical investigations were carried out to prove the efficacy of UPA in the medical management of myomas. They were labeled “PEARL I–IV”.158 A first trial159 presented the results of a 13-week, three-prong investigation of subjects with anemia (Hb≤10.2 g dL) due to excessive blood loss. UPA was administered at the dose of either 5 or 10 mg/day to two groups of 96 and 98 patients, respectively; a third group of 48 women received placebo. Treatment with UPA resulted in a proper control of bleeding in 91% and 92% of the subjects vs 19% of those receiving placebo (P<0.001). Uterine volume decreased by 21% and 12%, respectively, and increased by 2% in controls.
A second, double-blind, noninferiority trial160 compared the action over 3 months of oral 5 or 10 mg UPA to that of a monthly injection of 3.75 mg leuprolide acetate in 307 subjects. Bleeding control was achieved in 90% of women receiving UPA 5 mg, 98% of those given UPA 10 mg and in 89% of patients injected with leuprolide acetate.
The third study tested the feasibility of long-term (up to 1 year) UPA treatment of symptomatic myomas in three groups totaling 209 patients.161 One group received UPA 10 mg daily, immediately followed by a 10-day double-blind administration of NETA or placebo. This resulted in myoma’s shrinkage by 63%, 67%, and 72% at 6, 9, and 12 months, respectively. The authors concluded that the use of NETA did not affect fibroid volume or endometrial histology.
Finally, the fourth trial dealt with the effectiveness and safety of 212-week cycles of 5 or 10 mg UPA daily in 451 women.162 They observed control of excessive bleeding in >80% of subjects, a mean reduction in fibroid volume of 54% and 58%, respectively, and an improvement in pain symptom and overall QoL.
A systematic metanalysis of the effects of UPA in women harboring fibroids was conducted in 2016;163 it included four RCTs (three comparing the drug with placebo and one comparing it with a GnRHA) for symptomatic relief. The trials reported improvement in excessive uterine bleeding as evidenced by the very significant attainment of amenorrhea (P<0.00001). An improved QoL parameters and reduction in fibroid size were noted in the UPA group.
A major advancement was made when an international group carried out a double-blind, RCT with on-and-off four 12-week courses of UPA (10 and 5 mg daily) for the long-term treatment of fibroids. Each treatment course was separated by a drug-free period of two spontaneous menstrual bleeds164 (see Figure 6). The study confirmed the efficacy of UPA in controlling pain and bleeding, in reducing fibroid volume, and in markedly improving QoL of the patients, even during the off-treatment intervals. No significant changes in laboratory parameters were observed, documenting the effectiveness of UPA as an alternative to surgery.
Figure 6.
Administration schedule for ulipristal in the treatment of uterine myomas.
Note: Reprinted from Fertility and Sterility, Vol 105(1), Donnez J, Donnez O, Matule D, et al, Long-term medical management of uterine fibroids with ulipristal acetate, Pages 165–173, Copyright (2016), with permission from Elsevier.164
Abbreviations: PA, ulipristal acetate; PBAC, pictorial blood assessment chart.
The abovementioned international group has now extended their study, using the 10 mg dosage, to assess long-term safety over ~4 years of extended repeated 3-month courses of UPA in 64 premenopausal women.165 No changes in the number and type of laboratory results outside the normal ranges were observed and, overall, incidence of adverse events with increasing treatment courses were reported in 16%, 19%, 14%, and 9% of the subjects, during treatment courses 5, 6, 7, and 8. The only frequently reported adverse events were headache and hot flushes.
Finally, the use of UPA can facilitate surgery or, following reduction of fibroid volume, improve the surgical approach and restore normal preoperative Hb.166 In view of the widespread use of UPA, now marketed as Esmya, and following reports of rare serious liver injury, including liver failure, the EMA Pharmacovigilance Risk Assessment Committee (PRAC) carried out a full evaluation and concluded that UPA may have contributed to the development of some of the reported cases. The PRAC, therefore, recommended measures to minimize liver injury, including contraindication when liver problems are known and liver tests before, during, and after stopping treatment.167
Notwithstanding the warning, on June 2018, EMA’s Committee for Medicinal Products for Human Use has recommended granting marketing authorization for UPA in the preoperative treatment of uterine myomas.168
Effects of SPRM on the endometrium
PR modulators induce unique effects on the endometrium that cannot be assessed using the criteria usually employed for dating it. To try to elucidate this phenomenon, a group of pathologists developed a “Dictionary of Endometrial Biopsy Diagnoses for Clinical Trials with SPRMs” to supplement existing conventional descriptive criteria.169 This situation prompted the US-NIH to sponsor a study170 to evaluate endometrial specimens from women receiving four different SPRM, concluding that under treatment endometrium usually appears as inactive or normally cycling, with no sign of premalignant lesions. At the same time, a singular picture consisting of an asymmetry of stromal and epithelial growth with cystic dilated glands and a mix of mitotic and secretory effects was observed in a subset of cases. One unique feature was “that the physiological separation between estrogen- and progesterone-induced changes, in which progesterone down-regulation of ERs prevent co-stimulation of both pathways, was perturbed in the PRM-exposed patients”. These changes were coined “PRM-associated endometrial changes” (PAEC) and may be identified and visualized at ultrasound.171
SPRM have different progestational and antiprogestational actions and therefore their endometrial effects must be considered separately.
Mifepristone
At the beginning of the twenty-first century work on MFP was resumed; initially conflicting results were reported: in one trial, no endometrial hyperplasia was noted in any participant,115 whereas in another,116 abnormalities described as “endometrial hyperplasia without atypia” were found. It was immediately pointed out that SPRM “do not cause endometrial hyperplasia, although they do produce endometrial appearances that are quite unusual”. For this reason, pathologists not familiar with these unique changes may mistake them as “hyperplasia”.172 A comparison of endometria from women treated with low-dose MFP and normal subjects evidenced the presence of nonsynchronous endometrium, large fluid-filled glands, and abnormal blood vessels, with cell injury and cell death in capillary endothelial cells,173 but no detectable effects on their mRNA expression for several markers.174
The already-mentioned Cochrane Review122 concluded that pooled data suggest that MFP induces an abnormal endometrial histology compared with placebo OR 31.65; 95% CI 4.83 to 207.35) but acknowledged that this appears different from the sometime premalignant endometrial hyperplasia associated with unopposed estrogen treatment. In addition, no significant differences were observed in the rate of atypical endometrial hyperplasia between the MFP-treated groups and controls.
Proellex (telapristone)
Although the development of this SPRM has not been pursued for the treatment of women harboring fibroids, its effect on the endometrium has been evaluated in samples from 58 pre-menopausal women treated with daily oral doses of 12.5, 25, or 50 mg.175 At 3 or 6 months, 103 out of 174 biopsies showed a number of histological changes similar to those observed with other compounds: the endometrium was generally inactive or atrophic with a superimposed formation of cystic dilated glands and secretory changes coexisting with mitoses and apoptotic bodies, but no hyperplasia. At ultrasound, the presence of an increased endometrial thickness could also be detected.
Asoprisnil
The already reported, early investigation of endometrial changes under the influence of ASP evidenced a unique pattern consisting of “partially developed secretory glandular appearances and stromal changes”.169 These consisted in low mitotic activity in glands and stroma, unusually thick-walled muscular arterioles, and prominent aggregations of thin-walled vessels in the stroma. The investigators concluded that such a nonphysiologic secretory effect was specific of ASP. A further double-blind, RCT attempted to elucidate the mechanism through which ASP suppresses endometrial bleeding; they identified a statistically significant reduction of genes in the so-called IL-15 pathway, involved in uNK function, with a major reduction in immunostaining of uNK cell marker CD56 (P<0.001). These findings are considered “a missing link in the complex interplay among endometrial stromal cells, uNK, and spiral arteries affecting physiologic and pathologic endometrial bleeding”.142
The action of ASP was also evaluated in vitro, providing novel evidence for its growth inhibitory effect, antiproliferative, proapoptotic, and antifibrotic actions on cultured leiomyoma cells in the absence of comparable effects on cultured normal myometrial cells.176 ASP modulated the ratio of PR isoforms A and B in cultured leiomyoma cells; decreased the cell viability; suppressed the expression of growth factors, angiogenic factors, and their receptors and induced apoptosis. Finally, it suppressed types I and III collagen synthesis.
Ulipristal acetate
Early investigations on the in vivo effects of UPA identified the presence of the endometrial anomalies that seem a peculiarity of all SPRM. As for other compounds, they were initially labeled “hyperplasia”. Interestingly, a preliminary investigation using 5- and 10-mg daily doses to 46 healthy subjects did not evidence any case of endometrial hyperplasia.177 A second study checked endometrial vascularization, fibrillar matrix, and VEGF-A expression and found that UPA does not alter any of these parameters.178 Further investigations found that during follicular phase, UPA causes a significant increase in the expression of a protein involved in chondrocyte differentiation (called Indian Hedgehog) and in genes involved in its signaling;179 in rhesus macaques, UPA administered intrauterus induces endometrial atrophy and amenorrhea, although some glandular cysts were found.180
In humans, UPA induces an inactive glandular epithelium with occasional subnuclear vacuolization, mitosis, and apoptosis. At the same time, glandular architecture is altered and extensive cystic dilatations are present, as well as abnormal stromal vessels. Hyperplasia was found in one case and in four subjects the formation of polyps was also observed; importantly, 6 months after treatment, the endometrial histology returned to normal in the vast majority of the patients.181
The already mentioned PEARL II trial measured a mean endometrial thickness of 9.4 mm with 5 mg and 10.7 mm with 10 mg, without any alteration that could be of clinical concern, with only one case of simple hyperplasia.160 The long-term study161 confirmed the presence of nonphysiological, benign endometrial histological alterations (both cyst formation, epithelial and vascular changes). These were observed at different lengths of treatment in 18.0%, 21.4% and 16.3% of biopsies; following discontinuation, changes persisted in 9.1% of biopsies.
The safety of this treatment regimen has now been summarized, concluding that published reports of RCTs have demonstrated that, whereas UPA induces endometrial changes, they are both benign and reversible.182
Recently, a Cochrane review tried to draw conclusions from 10 studies with a total of 1,450 participants’ existing data183 and made the following points:
All studies described the presence of PAEC in 41%– 78.8% of all patients.
After discontinuation, in three studies the percentage of PAEC decreased from 62% to 0%, 78.8% to 0%, and from 59% to 6%–7%.
In 0.4% of all subjects, endometrial hyperplasia was reported during or after UPA: simple hyperplasia (five cases) and simple atypical hyperplasia that resolved into benign secretory endometrium by the end of the treatment (one case). One endometrial adenocarcinoma was reported, but it was already present at the baseline biopsy.
Transvaginal ultrasound or MRI showed a transient increase of endometrial thickness during treatment, which returned to normal within a few weeks after discontinuation.
In conclusion, whereas the unique nature of endometrial changes produced by SPRMs on the endometrium is undeniable, its significance, possible long-term effects, and therefore regulatory aspects are still open to debate and to lack of agreement.
To minimize the occurrence of these changes one might try a mixed regimen using first a GnRHA and, after obtaining a proper volume reduction and an inactive endometrium, begin treatment with an SPRM.184
A look at the future
The consequences of the advent of this new class of compounds for the medical management of fibroids have been analyzed by several groups with regard to the most promising of them, UPA. Specifically, it has been pointed out that the choice of a given treatment is influenced by a number of factors, including the severity of symptoms, myomas’ characteristics, age, and a wish to preserve fertility. In this context, there is a real need for alternatives to surgical intervention, and today these exist through the long-term use of SPRM.
In spite of these advances, the time has also come to explore novel strategies for the management of uterine fibroids. One such new avenue has been recently evaluated by Islam et al185 who observed that ECM forms the bulk structure of fibroids, with the ECM-rich rigid structure possibly contributing to abnormal bleeding and pelvic pain. For this reason, a possible novel approach could be directly or indirectly targeting ECM. The presence of elevated levels of collagens, fibronectin, laminins, and proteoglycans can induce the so-called mechanotransduction process through which cells convert a mechanical stimulus into an electrochemical activity. This will result in an altered bidirectional signaling between myoma cells and the ECM. Although several antiproliferative agents are already available for the treatment of fibroids, in the future ECM should be considered as a crucial target.
Overall reviews
Two recent reviews dealt with the role of SPRM in the long-term medical treatment of hormone-dependent conditions like uterine fibroids, concluding that this class of compounds has emerged as a valuable option capable of offering women effective long-term medical management and avoid surgery altogether.
The first focused on pharmacological effects on the endometrium, their antiproliferative action, and their suppression of bleeding.186 The second is a Cochrane review of RCTs evaluating their effectiveness.187 The metanalysis involved a total of 11 studies comprising 1,021 participants: 685 received an SPRM (MFP, UPA, and ASP). Use of an SPRM vs placebo resulted in improvements in fibroid symptom severity (mean differences [MD] −20.04 points, 95% CI −26.63 to −13.46) and in health-related QoL (MD −22.52 points, 95% CI 12.87 to 32.17). Treatment resulted in reduced MBL, but the effect was considered small (standardized mean differences −1.11, 95% CI −1.38 to −0.83) and in higher rates of amenorrhoea (2.9% in the placebo group vs 23.7% to 96.1% in the SPRM group). No conclusion was reached for improvement in pelvic pain owing to variability in the estimates. No difference in effectiveness was found when an SPRM was compared to leuprolide acetate, except that endometrial changes were more common after SPRM therapy than after leuprolide treatment (OR 10.45, 95% CI 5.38 to 20.33).
Antiestrogens
In 2003, an RCT compared the ability to reduce fibroid growth before hysterectomy of the antiestrogen fulvestrant (as an intramuscular injection of 50, 125, or 250 mg) with that of the GnRHA goserelin (as a subcutaneous injection of 3.6 mg), or an injection-matched placebo, once every 4 weeks. A total of 307 women diagnosed with uterine fibroids requiring hysterectomy were enrolled over a period of 12 weeks.188
Goserelin significantly reduced fibroid growth and endometrial thickness compared with placebos, whereas fulvestrant did not significantly alter fibroid volume or endometrial thickness or change endpoints such as endometrial histology or vaginal bleeding.
It seems, therefore, that at dosages similar to those commonly utilized for treatment of breast cancer in postmenopausal women, fulvestrant does not significantly inhibit fibroid growth.
Overall considerations
The usefulness of the various treatment options on preoperative medical therapy before surgery for uterine fibroids up to 2017 has been the object of a recent Cochrane review by Lethaby and coll.189
Their search found 38 RCTs meeting their criteria and involving 3,623 subjects.
The following categories of trials were analyzed:
GnRHA vs no pretreatment (n=19)
GnRHA vs placebo (n=8)
GNRHA vs progestin, SPRM, SERM, dopamine agonists (n=7)
SPRM vs placebo
In general, studies were considered of low or moderate quality and often open to criticism.
Pretreatment with GnRHA vs no pretreatment or placebo was associated with reductions in both uterine (MD −175 mL, 95% CI −219.0 to −131.7) and fibroid volume (MD 5.7 mL to 155.4 mL) and increased preoperative Hb (MD 0.88 g/dL, 95% CI 0.7 to 1.1). Concomitantly, there was an increase of hot flushes (OR 7.68, 95% CI 4.6 to 13.0).
In the case of hysterectomy, pretreatment with GnRHA reduced duration of surgery (−9.59 minutes, 95% CI 15.9 to −3.28) and of blood loss (MD 25 mL to 148 mL), led to a lower utilization of blood transfusions (OR 0.54, 95% CI 0.3 to 1.0), and fewer postoperative complications (OR 0.54, 95% CI 0.3 to 0.9).
With myomectomy, preuse of GnRHA reduced intraoperative blood loss (MD 22 to 157 mL). There was no clear evidence of a difference in blood transfusions (OR 0.85, 95% CI 0.3 to 2.8) or postoperative complications (OR 1.07, 95% CI 0.43 to 2.64).
Preuse of GnRHA vs other medical therapies induced a greater reduction in uterine volume (−47 to −20 and −22% with 5 mg and 10 mg UPA), at the cost of increase in hot flashes (OR 12.3, 95% CI 4.04 to 37.48). There was no clear difference in bleeding (UPA 5 mg: OR 0.71, 95% CI 0.3 to 1.7; UPA 10 mg: OR 0.39, 95% CI 0.1 to 1.1), or Hb levels (MD −0.2, 95% CI −0.6 to 0.2). No clear difference was found in fibroid volume reduction when comparing GnRHA with cabergoline (MD −12.71 mL, 95% CI −5.9 to −31.3). Pretreatment with an SPRM (UPA, MFP, CDB-2914, and ASP) vs placebo was associated with greater reductions in uterine or fibroid volume than placebo and an increase in preoperative Hb levels (MD +0.93 g/dL, +0.5 to +1.4). UPA and ASP were also associated with greater reductions in bleeding before surgery (UPA 5 mg: OR 41.41, 95% CI 15.3 to 112.4; UPA 10 mg: OR 78.83, 95% CI 24.0 to 258.7; ASP: MD −166.9 mL; 95% CI −277.6 to −56.2).
This analysis led to the conclusion that there is good evidence that preoperative use of a GnRHA reduces uterine and fibroid volume and increases preoperative Hb levels. In the case of hysterectomy, blood loss, operation time, and complication rates were also reduced.
Conclusions
Attempts at a nonsurgical treatment of uterine leiomyomas probably began hundreds of years ago, but scientifically validated modalities became available only some 40 years ago. During this relatively short period of time, several regimens were introduced using different categories of drugs. Today, the most promising belong to two categories: PR modulators and orally active GnRHR blockers.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sutton C. Hysterectomy: a historical perspective. Baillieres Clin Obstet Gynaecol. 1997;11(1):1–22. doi: 10.1016/s0950-3552(97)80047-8. [DOI] [PubMed] [Google Scholar]
- 2.Maheux R. Treatment of uterine leiomyomata: past, present and future. In: Genazzani AR, Petraglia F, Volpe G, editors. Progress in Gynecology and Obstetrics. Carnforth: Parthenon Publishing Group; 1990. pp. 173–190. [Google Scholar]
- 3.Semm K. New methods of pelviscopy (gynecologic laparoscopy) for myomectomy, ovariectomy, tubectomy and adnectomy. Endoscopy. 1979;11(02):85–93. doi: 10.1055/s-0028-1098329. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AG, Magos AL. The development of laparoscopic surgery. Baillieres Clin Obstet Gynaecol. 1989;3(3):429–449. doi: 10.1016/s0950-3552(89)80003-3. [DOI] [PubMed] [Google Scholar]
- 5.Steiner RA, Wight E, Tadir Y, Haller U. Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet Gynecol. 1993;81:471–474. [PubMed] [Google Scholar]
- 6.della Badia C, Karini H. Endometrial stromal sarcoma diagnosed after uterine morcellation in laparoscopic supracervical hysterectomy. J Minim Invasive Gynecol. 2010;17(6):791–793. doi: 10.1016/j.jmig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Anupama R, Ahmad SZ, Kuriakose S, Vijaykumar DK, Pavithran K, Seethalekshmy NV. Disseminated peritoneal leiomyosarcomas after laparoscopic “myomectomy” and morcellation. J Minim Invasive Gynecol. 2011;18(3):386–389. doi: 10.1016/j.jmig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124(3):491–497. doi: 10.1097/AOG.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Xc L, Yao C, et al. Proportion of uterine malignant tumors in patients with laparoscopic myomectomy: a national multicenter study in China. Chin Med J. 2017;130:2661–2665. doi: 10.4103/0366-6999.218008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong M, de Wilde RL, Isaacson K. Reducing the spread of occult uterine sarcoma at the time of minimally invasive gynecologic surgery. Arch Gynecol Obstet. 2018;297(2):285–293. doi: 10.1007/s00404-017-4575-6. [DOI] [PubMed] [Google Scholar]
- 11.Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130(6):1251–1260. doi: 10.1097/AOG.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 12.Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346(8976):671–672. doi: 10.1016/s0140-6736(95)92282-2. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins FL, Jr, Worthington-Kirsch R, Berkowitz RP. Selective uterine artery embolization as primary treatment for symptomatic leiomyomata uteri. J Am Assoc Gynecol Laparosc. 1999;6(3):279–284. doi: 10.1016/s1074-3804(99)80061-9. [DOI] [PubMed] [Google Scholar]
- 14.Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59(1):93–102. doi: 10.1097/GRF.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 15.Jenne JW, Preusser T, Günther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Zeitschrift für Medizinische Physik. 2012;22(4):311–322. doi: 10.1016/j.zemedi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Brown MRD, Farquhar-Smith P, Williams JE, Ter Haar G, Desouza NM. The use of high-intensity focused ultrasound as a novel treatment for painful conditions – a description and narrative review of the literature. Br J Anaesth. 2015;115(4):520–530. doi: 10.1093/bja/aev302. [DOI] [PubMed] [Google Scholar]
- 17.She WH, Cheung TT, Jenkins CR, Irwin MG. Clinical applications of high-intensity focused ultrasound. Hong Kong Med J. 2016;22:382–392. doi: 10.12809/hkmj154755. [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Donnez O, Dolmans M-M. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice? Fertil Steril. 2014;102:640–648. doi: 10.1016/j.fertnstert.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 19.Singh SS, Belland L. Contemporary management of uterine fibroids: focus on emerging medical treatments. Curr Med Res Opin. 2015;31(1):1–12. doi: 10.1185/03007995.2014.982246. [DOI] [PubMed] [Google Scholar]
- 20.Gurusamy KS, Vaughan J, Fraser IS, Best LMJ, Richards T. Medical therapies for uterine fibroids-a systematic review and network meta-analysis of randomised controlled trials. PLoS One. 2016;11(2):e0149631art. doi: 10.1371/journal.pone.0149631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59(1):30–52. doi: 10.1097/GRF.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 22.Goodman AL. Progesterone therapy in uterine fibroma. J Clin Endocrinol Metab. 1946;6(5):402–408. doi: 10.1210/jcem-6-5-402. [DOI] [PubMed] [Google Scholar]
- 23.Segaloff A, Weed JC, Sternberg WH, et al. The progesterone therapy of human uterine leiomyomas. Endocrinol Metab. 1949;9(12):1273–1291. doi: 10.1210/jcem-9-12-1273. [DOI] [PubMed] [Google Scholar]
- 24.Goldzieher JW, Maqueo M, Ricaud L, Aguilar JA, Canales E. Induction of degenerative changes in uterine myomas by high-dosage progestin therapy. Am J Obstet Gynecol. 1966;96(8):1078–1087. doi: 10.1016/0002-9378(66)90516-3. [DOI] [PubMed] [Google Scholar]
- 25.Farquhar C, Arroll B, Ekeroma A, et al. The Working Party of the New Zealand Guidelines Group. An evidence-based guideline for the management of uterine fibroids. Aust N Z J Obstet Gynaecol. 2001;41:125–140. doi: 10.1111/j.1479-828x.2001.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre G, Vilos G, Allaire C, Jeffrey J. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003;25:396–405. [PubMed] [Google Scholar]
- 27.Nakai G, Yamada T, Hamada T, et al. Pathological findings of uterine tumors preoperatively diagnosed as red degeneration of leiomyoma by MRI. Abdom Radiol. 2017;42(7):1825–1831. doi: 10.1007/s00261-017-1126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hachiya K, Kato H, Kawaguchi S, et al. Red degeneration of a uterine fibroid following the administration of gonadotropin releasing hormone agonists. J Obstet Gynaecol. 2016;36(8):1018–1019. doi: 10.1080/01443615.2016.1234449. [DOI] [PubMed] [Google Scholar]
- 29.Xu Q, Ohara N, Liu J, et al. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14(3):181–191. doi: 10.1093/molehr/gan004. [DOI] [PubMed] [Google Scholar]
- 30.Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J. 1986;293(6543):359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parazzini F, La Vecchia C, Negri E, Cecchetti G, Fedele L. Epidemiological characteristics of women with uterine fibroids: a case-control study. Obstet Gynecol. 1988;72:853–857. doi: 10.1097/00006250-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Samadi AR, Lee NC, Flanders WD, Boring JR, 3rd, Parris EB. Risk factors for self-reported uterine fibroids: a case-control study. Am J Public Health. 1996;6:858–862. doi: 10.2105/ajph.86.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman AJ, Thomas PP. Does low-dose combination oral contraceptive use affect uterine size or menstrual flow in premenopausal women with leiomyomas? Obstet Gynecol. 1995;85(4):631–635. doi: 10.1016/0029-7844(95)00007-E. [DOI] [PubMed] [Google Scholar]
- 34.Pitkin RM. Does low-dose combination oral contraceptive use affect uterine size or menstrual flow in premenopausal women with leiomyomas [Retraction of Friedman and Thomas] Obstet Gynecol. 1995;86:728. [PubMed] [Google Scholar]
- 35.Ortmann O, Weiss JM, Diedrich K. Gonadotrophin-releasing hormone (GnRH) and GnRH agonists: mechanisms of action. Reprod Biomed Online. 2002;5(Suppl 1):1–7. doi: 10.1016/s1472-6483(11)60210-1. [DOI] [PubMed] [Google Scholar]
- 36.Filicori M, Hall DA, Loughlin JS, Rivier J, Vale W, Crowley WF., Jr A conservative approach to the management of uterine leiomyoma: pituitary desensitization by a luteinizing hormone-releasing hormone analogue. Am J Obstet Gynecol. 1983;147(6):726–727. doi: 10.1016/0002-9378(83)90463-5. [DOI] [PubMed] [Google Scholar]
- 37.Matta WHM, Shaw RW, Nye M. Long-term follow-up of patients with uterine fibroids after treatment with the LHRH agonist buserelin. Br J Obstet Gynaecol. 1989;96(2):200–206. doi: 10.1111/j.1471-0528.1989.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 38.Letteri GS, Shawker TH, Coddington CC, Shawker TH, Loriaux DL, Collins RL. Efficacy of gonadotropin releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951–956. doi: 10.1016/s0015-0282(16)60724-0. [DOI] [PubMed] [Google Scholar]
- 39.Benagiano G. Uterine fibroids: literature review and summary of posters. Horm Res. 1989;32(Suppl 1):120–124. doi: 10.1159/000181326. [DOI] [PubMed] [Google Scholar]
- 40.Roux C, Pelissier C, Listrat V, et al. Bone loss during gonadotropin releasing hormone agonist treatment and use of nasal calcitonin. Osteoporos Int. 1995;5(3):185–190. doi: 10.1007/BF02106098. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y-M, Chou C-Y, Huang S-C, Lin H-C. Oestrogen deficiency causes DNA damage in uterine leiomyoma cells: a possible mechanism for shrinkage of fibroids by GnRH agonists. Br J Obstet Gynaecol. 2001;108:95–102. doi: 10.1111/j.1471-0528.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 42.Lumsden MA, West CP, Baird DT. Goserelin therapy before surgery for uterine fibroids. Lancet. 1987;329(8523):36–37. doi: 10.1016/s0140-6736(87)90718-5. [DOI] [PubMed] [Google Scholar]
- 43.Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. Br J Obstet Gynaecol. 2002;109(10):1097–1108. doi: 10.1111/j.1471-0528.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 44.Stovall TG, Ling FW, Henry LC, Woodruff MR. A randomized trial evaluating leuprolide acetate before hysterectomy as treatment for leiomyomas. Am J Obstet Gynecol. 1991;164(6):1420–1425. doi: 10.1016/0002-9378(91)91419-w. [DOI] [PubMed] [Google Scholar]
- 45.Candiani GB, Vercellini P, Fedele L, et al. Use of goserelin depot, a gonadotropin releasing hormone agonist, for the treatment of menorrhagia and severe anemia in women with leiomyomata uteri. Acta Obstet Gynecol Scand. 1990;69(5):413–415. doi: 10.3109/00016349009013304. [DOI] [PubMed] [Google Scholar]
- 46.Benagiano G, Cronjé H, Kivinen ST, et al. Zoladex (goserelin acetate) and the anemic patient: results of a multicenter fibroid study. Fertil Steril. 1996;66:223–229. [PubMed] [Google Scholar]
- 47.Benagiano G, Morini A, Primiero FM. Fibroids: overview of current and future treatment options. Br J Obstet Gynaecol. 1992;99(s7):18–22. doi: 10.1111/j.1471-0528.1992.tb13534.x. [DOI] [PubMed] [Google Scholar]
- 48.Nedwich A, Frumin A, Meranze DR. Erythrocytosis associated with uterine myomas. Am J Obstet Gynecol. 1962;84(2):174–178. doi: 10.1016/0002-9378(62)90421-0. [DOI] [PubMed] [Google Scholar]
- 49.Levgur M, Dlevie M. The myomatous erythrocytosis syndrome: a review. Obstet Gynecol. 1995;86(6):1026–1030. doi: 10.1016/0029-7844(95)00292-y. [DOI] [PubMed] [Google Scholar]
- 50.Matta WHM, Stabile I, Shaw RW, Campbell S. Doppler assessment of uterine blood flow changes in patients with fibroids receiving the gonadotropin releasing hormone agonist buserelin. Fertil Steril. 1988;49:1083–1085. doi: 10.1016/s0015-0282(16)59966-x. [DOI] [PubMed] [Google Scholar]
- 51.Friedman AJ, Rein MS, Harrison-Atlas D, et al. A randomized, placebo-controlled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril. 1989;52:728–733. [PubMed] [Google Scholar]
- 52.Mettler L, Semm K. Laparoscopic approach of fibroid excision after treatment with GnRH analogues. In: Lunenfeld B, Carnforth IV, editors. Proceedings of the IIIrd International Symposium on GnRH Analogues in Cancer and Human Reproduction. UK: Parthenon Publ, London; 1993. pp. 95–98. [Google Scholar]
- 53.Mettler L, Alvarez-Rodas E, Semm K. Hormonal treatment and pelviscopic myomectomy. Diagn Ther Endosc. 1995;1(4):217–221. doi: 10.1155/DTE.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dodirot V, Dubuisson J-B, Chapron C, et al. Recurrence of leiomyomata after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2001;8:495–500. doi: 10.1016/s1074-3804(05)60610-x. [DOI] [PubMed] [Google Scholar]
- 55.Fedele L, Vercellini P, Bianchi S, Brioschi D, Dorta M. Treatment with GnRH agonists before myomectomy and the risk of short-term myoma recurrence. Br J Obstet Gynaecol. 1990;97(5):393–396. doi: 10.1111/j.1471-0528.1990.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 56.Friedman AJ, Daly M, Juneau-Norcross M, et al. Recurrence of myomas after myomectomy in women pretreated with leuprolide acetate depot or placebo. Fertil Steril. 1992;58:205–208. doi: 10.1016/s0015-0282(16)55164-4. [DOI] [PubMed] [Google Scholar]
- 57.Donnez J, Schrurs B, Gillerot S, Sandow J, Clerckx F. Treatment of uterine fibroids with implants of gonadotropin-releasing hormone agonist: assessment by hysterography. Fertil Steril. 1989;51(6):947–950. doi: 10.1016/s0015-0282(16)60723-9. [DOI] [PubMed] [Google Scholar]
- 58.Donnez J, Nisolle M, Grandjean P, Gillerot S, Clerckx F. The place of GnRH agonists in the treatment of endometriosis and fibroids by advanced endoscopic techniques. Br J Obstet Gynaecol. 1992;99(Suppl 7):31–33. doi: 10.1111/j.1471-0528.1992.tb13537.x. [DOI] [PubMed] [Google Scholar]
- 59.Seracchioli R, Venturoli S, Colombo FM, et al. GnRH agonist treatment before total laparoscopic hysterectomy for large uteri. J Am Assoc Gynecol Laparosc. 2003;10(3):316–319. doi: 10.1016/s1074-3804(05)60254-x. [DOI] [PubMed] [Google Scholar]
- 60.West CP, Lumsden MA, Hillier H, Sweeting V, Baird DT. Potential role for medroxyprogesterone acetate as an adjunct to goserelin (Zoladex) in the medical management of uterine fibroids. Hum Reprod. 1992;7(3):328–332. doi: 10.1093/oxfordjournals.humrep.a137643. [DOI] [PubMed] [Google Scholar]
- 61.Carr BR, Marshburn PB, Weatherall PT, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 1993;76:1217–1223. doi: 10.1210/jcem.76.5.8496313. [DOI] [PubMed] [Google Scholar]
- 62.Palomba S, Orio F, Jr, Russo T, et al. Long-term effectiveness and safety of GnRH agonist plus raloxifene administration in women with uterine leiomyomas. Hum Reprod. 2004;19(6):1308–1314. doi: 10.1093/humrep/deh296. [DOI] [PubMed] [Google Scholar]
- 63.Palomba S, Orio F, Jr, Morelli M, et al. Raloxifene administration in women treated with gonadotropin-releasing hormone agonist for uterine leiomyomas: effects on bone metabolism. J Clin Endocr Metab. 2002;87(10):4476–4481. doi: 10.1210/jc.2002-020780. [DOI] [PubMed] [Google Scholar]
- 64.Broekmans FJ, Hompes PGA, Heitbrink MA, et al. Two-step gonadotropin-releasing hormone agonist treatment of uterine leiomyomas: standard-dose therapy followed by reduced-dose therapy. Am J Obstet Gynecol. 1996;175(5):1208–1216. doi: 10.1016/s0002-9378(96)70030-3. [DOI] [PubMed] [Google Scholar]
- 65.Barbieri RL. Gonadotropin-releasing hormone agonists and estrogen-progestogen replacement therapy. Am J Obstet Gynecol. 1990;162(2):593–595. doi: 10.1016/0002-9378(90)90440-i. [DOI] [PubMed] [Google Scholar]
- 66.Friedman AJ. Treatment of leiomyomata uteri with short-term leuprolide followed by leuprolide plus estrogen–progestin hormone replacement therapy for 2 years: a pilot study. Fertil Steril. 1989;51(3):526–528. doi: 10.1016/s0015-0282(16)60568-x. [DOI] [PubMed] [Google Scholar]
- 67.Mclaren JS, Morris E, Rymer J. Gonadotrophin receptor hormone analogues in combination with add-back therapy: an update. Menopause Int. 2012;18(2):68–72. doi: 10.1258/mi.2012.012008. [DOI] [PubMed] [Google Scholar]
- 68.Moroni RM, Martins WP, Ferriani RA, et al. Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst Rev. 2015;20:CD010854. doi: 10.1002/14651858.CD010854.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benagiano G, Morini A, Aleandri V, et al. Sequential Gn-RH super-agonist and medroxyprogesterone acetate treatment of uterine leiomyomata. Int J Obstet Gynecol. 1990;33(4):333–343. doi: 10.1016/0020-7292(90)90520-u. [DOI] [PubMed] [Google Scholar]
- 70.Leo DV, Morgante G, Lanzetta D, et al. Danazol administration after gonadotropin-releasing hormone analogue reduces rebound of uterine myomas. Hum Reprod. 1997;12:357–360. doi: 10.1093/humrep/12.2.357. [DOI] [PubMed] [Google Scholar]
- 71.Kettel LM, Murphy AA, Morales AJ, Rivier J, Vale W, Yen SS. Rapid regression of uterine leiomyomas in response to daily administration of gonadotropin-releasing hormone antagonist. Fertil Steril. 1993;60(4):642–646. doi: 10.1016/s0015-0282(16)56214-1. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez-Barcena D, Alvarez RB, Ochoa EP, et al. Treatment of uterine leiomyomas with luteinizing hormone-releasing hormone antagonist Cetrorelix. Hum Reprod. 1997;12(9):2028–2035. doi: 10.1093/humrep/12.9.2028. [DOI] [PubMed] [Google Scholar]
- 73.Felberbaum RE, Germer U, Ludwig M, et al. Treatment of uterine fibroids with a slow-release formulation of the gonadotrophin releasing hormone antagonist Cetrorelix. Hum Reprod. 1998;13(6):1660–1668. doi: 10.1093/humrep/13.6.1660. [DOI] [PubMed] [Google Scholar]
- 74.Felberbaum RE, Ludwig M, Diedrich K. Medical treatment of uterine fibroids with the LHRH antagonist: Cetrorelix. Contracept Fertil Sex. 1999;27:701–709. [PubMed] [Google Scholar]
- 75.Engel JB, Audebert A, Frydman R, Zivny J, Diedrich K. Presurgical short term treatment of uterine fibroids with different doses of cetrorelix acetate: a double-blind, placebo-controlled multicenter study. Eur J Obstet Gynecol Reprod Biol. 2007;134(2):225–232. doi: 10.1016/j.ejogrb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 76.Chen W, Yoshida S, Ohara N, Matsuo H, Morizane M, Maruo T. Gonadotropin-releasing hormone antagonist cetrorelix down-regulates proliferating cell nuclear antigen and epidermal growth factor expression and up-regulates apoptosis in association with enhanced poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human leiomyoma cells. J Clin Endocrinol Metab. 2005;90(2):884–892. doi: 10.1210/jc.2004-1591. [DOI] [PubMed] [Google Scholar]
- 77.Britten JL, Malik M, Levy G, Mendoza M, Catherino WH. Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production. Fertil Steril. 2012;98(5):1299–1307. doi: 10.1016/j.fertnstert.2012.07.1123. [DOI] [PubMed] [Google Scholar]
- 78.Flierman PA, Oberyé JJ, Hulst VP, de Blok S. Rapid reduction of leiomyoma volume during treatment with the GnRH antagonist ganirelix. BJOG. 2005;112(5):638–642. doi: 10.1111/j.1471-0528.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 79.Betz SF, Zhu Y-F, Chen C, Struthers RS. Non-peptide gonadotropin-releasing hormone receptor antagonists. J Med Chem. 2008;51(12):3331–3348. doi: 10.1021/jm701249f. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y-F, Gross TD, Guo Z, et al. Identification of 1-arylmethyl-3-(2-aminoethyl)-5-aryluracil as novel gonadotropin-releasing hormone receptor antagonists. J Med Chem. 2003;46(11):2023–2026. doi: 10.1021/jm034041s. [DOI] [PubMed] [Google Scholar]
- 81.Guo Z, Zhu Y-F, Gross TD, et al. Synthesis and structure-activity relationships of 1-arylmethyl-5-aryl-6-methyluracils as potent gonadotropin-releasing hormone receptor antagonists. J Med Chem. 2004;47(5):1259–1271. doi: 10.1021/jm030472z. [DOI] [PubMed] [Google Scholar]
- 82.Rowbottom MW, Tucci FC, Zhu Y-F, et al. Synthesis and structure– activity relationships of (R)-1-alkyl-3-[2-(2-amino)phenethyl]-5-(2-fluorophenyl)-6-methyluracils as human GnRH receptor antagonists. Bioorg Med Chem Lett. 2004;14(9):2269–2274. doi: 10.1016/j.bmcl.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Tucci FC, Zhu Y-F, Struthers RS, et al. 3-[(2R)-Amino-2-phenylethyl]-1-(2,6-difluorobenzyl)-5-(2-fluoro-3-methoxyphenyl)-6-methylpyrim-idin-2,4-dione (NBI 42902) as a potent and orally active antagonist of the human gonadotropin-releasing hormone receptor. Design, synthesis, and in vitro and in vivo characterization. J Med Chem. 2005;48(4):1169–1178. doi: 10.1021/jm049218c. [DOI] [PubMed] [Google Scholar]
- 84.Zhao L, Guo Z, Chen Y, et al. 5-Aryluracils as potent GnRH antagonists – characterization of atropisomers. Bioorg Med Chem Lett. 2008;18(11):3344–3349. doi: 10.1016/j.bmcl.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 85.Chen C, Chen Y, Pontillo J, et al. Potent and orally bioavailable zwitterion GnRH antagonists with low CYP3A4 inhibitory activity. Bioorg Med Chem Lett. 2008;18(11):3301–3305. doi: 10.1016/j.bmcl.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 86.Regan CF, Guo Z, Chen Y, et al. Zwitterionic uracil derivatives as potent GnRH receptor antagonists with improved pharmaceutical properties. Bioorg Med Chem Lett. 2008;18(16):4503–4507. doi: 10.1016/j.bmcl.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 87.Chen C, Wu D, Guo Z, et al. Discovery of sodium R-(+)-4-{2-[5-(2-fluoro- 3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl -2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino} butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem. 2008;51(23):7478–7485. doi: 10.1021/jm8006454. [DOI] [PubMed] [Google Scholar]
- 88.Struthers RS, Chen T, Campbell B, et al. Suppression of serum luteinizing hormone in postmenopausal women by an orally administered nonpeptide antagonist of the gonadotropin-releasing hormone receptor (NBI-42902) J Clin Endocrinol Metab. 2006;91(10):3903–3907. doi: 10.1210/jc.2006-1110. [DOI] [PubMed] [Google Scholar]
- 89.Struthers RS, Xie Q, Sullivan SK, et al. Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin-releasing hormone receptor, NBI-42902. Endocrinology. 2007;148(2):857–867. doi: 10.1210/en.2006-1213. [DOI] [PubMed] [Google Scholar]
- 90.Struthers RS, Nicholls AJ, Grundy J, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab. 2009;94(2):545–551. doi: 10.1210/jc.2008-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carr B, Dmowski WP, O’Brien C, et al. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxy-progesterone acetate for the treatment of endometriosis: effects on bone mineral density. Reprod Sci. 2014;21(11):1341–1351. doi: 10.1177/1933719114549848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ezzati M, Carr BR. Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain. Womens Health. 2015;11(1):19–28. doi: 10.2217/whe.14.68. [DOI] [PubMed] [Google Scholar]
- 93.Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. doi: 10.1056/NEJMoa1700089. [DOI] [PubMed] [Google Scholar]
- 94.Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160. doi: 10.1097/AOG.0000000000002675. [DOI] [PubMed] [Google Scholar]
- 95.Ng J, Chwalisz K, Carter DC, Klein CE. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab. 2017;102(5):1683–1691. doi: 10.1210/jc.2016-3845. [DOI] [PubMed] [Google Scholar]
- 96.Archer DF, Stewart EA, Jain RI, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril. 2017;108(1):152–160. doi: 10.1016/j.fertnstert.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Kim SM, Yoo T, Lee SY, et al. Effect of SKI2670, a novel, orally active, non-peptide GnRH antagonist, on hypothalamic–pituitary– gonadal axis. Life Sci. 2015;139:166–174. doi: 10.1016/j.lfs.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 98.Kim S-M, Lee M, Lee SY, et al. Discovery of an orally bioavailable gonadotropin-releasing hormone receptor antagonist. J Med Chem. 2016;59(19):9150–9172. doi: 10.1021/acs.jmedchem.6b01071. [DOI] [PubMed] [Google Scholar]
- 99.Miwa K, Hitaka T, Imada T, et al. Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl] phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem. 2011;54(14):4998–5012. doi: 10.1021/jm200216q. [DOI] [PubMed] [Google Scholar]
- 100.Nakata D, Masaki T, Tanaka A, et al. Suppression of the hypothalamic– pituitary–gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Pharmacol. 2014;723:167–174. doi: 10.1016/j.ejphar.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Pohl O, Marchand L, Fawkes N, Gotteland J-P, Loumaye E. Gonadotropin-releasing hormone receptor antagonist mono- and combination therapy with estradiol/norethindrone acetate add-back: pharmacodynamics and safety of OBE2109. J Clin Endocrinol Metab. 2018;103(2):497–504. doi: 10.1210/jc.2017-01875. [DOI] [PubMed] [Google Scholar]
- 102.Zaloudek C, Norris HJ. In: Blaustein’s Pathology of the Female Genital Tract. 5th ed. Kurman RG, editor. New York: Springer-Verlag; 2002. pp. 373–408. [Google Scholar]
- 103.Friedman AJ, Barbieri RL, Doubilet PM. A randomized, double-blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404–409. doi: 10.1016/s0015-0282(16)59763-5. [DOI] [PubMed] [Google Scholar]
- 104.Kawaguchi K, Fujii S, Konishi I, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160(3):637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 105.Maruo T, Matsuo H, Shimomura Y, et al. Effects of progesterone on growth factor expression in human uterine leiomyoma. Steroids. 2003;68(10–13):817–824. doi: 10.1016/j.steroids.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 106.Smith SK. The regulation of fibroid growth: time for a re-think? Br J Obstet Gynaecol. 1993;100(11):977–978. doi: 10.1111/j.1471-0528.1993.tb15136.x. [DOI] [PubMed] [Google Scholar]
- 107.Wagenfeld A, Saunders PTK, Whitaker L, Critchley HOD. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets. 2016;20(9):1045–1054. doi: 10.1080/14728222.2016.1180368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Contraception and Reproductive Health Branch NICHD Report. 2004. [Accessed October 18, 2018]. Available from: https://www.nichd.nih.gov/publications/pubs/council_crhb_2004/index.
- 109.Herrmann W, Wyss R, Riondel A, et al. The effects of an antiprogesterone steroid in women: interruption of the menstrual cycle and of early pregnancy. C R Seances Acad Sci III. 1982;294(18):933–938. [PubMed] [Google Scholar]
- 110.Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76(2):513–517. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- 111.Yen SSC. Clinical use of RU 486 in the treatment of uterine fibroids. In: Donaldson MS, Dorflinger L, Brown SS, Benet LS, editors. Clinical Applications of Mifepristone (RU 486) and Other Antiprogestins. Washington, DC: National Academy Press; 1993. pp. 189–209. [PubMed] [Google Scholar]
- 112.Reinsch RC, Murphy AA, Morales AJ, Yen SS. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective, randomized study. Am J Obstet Gynecol. 1994;170(6):1623–1628. [PubMed] [Google Scholar]
- 113.Steinauer J, Pritts EA, Jackson R, Jacoby AF. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004;103(6):1331–1336. doi: 10.1097/01.AOG.0000127622.63269.8b. [DOI] [PubMed] [Google Scholar]
- 114.Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1381–1387. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- 115.Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra low-dose mifepristone for the treatment of uterine leiomyomata. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):215–218. doi: 10.1016/j.ejogrb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low-dose mifepristone in treatment of uterine leiomyoma: a randomized double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49(1):77–83. doi: 10.1111/j.1479-828X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 117.Engman M, Granberg S, Williams AR, et al. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24(8):1870–1879. doi: 10.1093/humrep/dep100. [DOI] [PubMed] [Google Scholar]
- 118.Mukherjee S, Chakraborty S. A study evaluating the effect of mifepristone (RU-486) for the treatment of leiomyomata uteri. Niger Med J. 2011;52(3):150–152. doi: 10.4103/0300-1652.86123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seth S, Goel N, Singh E, Mathur AS, Gupta G. Effect of mifepristone (25 mg) in treatment of uterine myoma in perimenopausal woman. J Midlife Health. 2013;4(1):22–26. doi: 10.4103/0976-7800.109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kulshrestha V, Kriplani A, Agarwal N, et al. Low dose mifepristone in medical management of uterine leiomyoma – an experience from a tertiary care hospital from north India. Indian J Med Res. 2013;137(6):1154–1162. [PMC free article] [PubMed] [Google Scholar]
- 121.Feng C, Meldrum S, Fiscella K. Improved quality of life is partly explained by fewer symptoms after treatment of fibroids with mifepristone. Int J Gynaecol Obstet. 2010;109(2):121–124. doi: 10.1016/j.ijgo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tristan M, Orozco LJ, Steed A, et al. Mifepristone for uterine fibroids. Cochrane Database Syst Rev. 2012;8:CD007687. doi: 10.1002/14651858.CD007687.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen Q, Hua Y, Jiang W, et al. Effects of mifepristone on uterine leiomyoma in premenopausal women: a meta-analysis. Fertil Steril. 2013;1006(6):1722–1726.e1–1722–1726.e10. doi: 10.1016/j.fertnstert.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 124.Carbonell JL, Acosta R, Pérez Y, et al. Safety and effectiveness of different dosage of mifepristone for the treatment of uterine fibroids: a double-blind randomized clinical trial. Int J Womens Health. 2013;5:115–124. doi: 10.2147/IJWH.S33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Esteve JL, Acosta R, Pérez Y, et al. Mifepristone versus placebo to treat uterine myoma: a double-blind, randomized clinical trial. Int J Womens Health. 2013;5:361–369. doi: 10.2147/IJWH.S42770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kapur A, Angomchanu R, Dey M. Efficacy of use of long-term, low-dose mifepristone for the treatment of fibroids. J Obstet Gynecol India. 2016;66(Suppl 1):494–498. doi: 10.1007/s13224-016-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arora D, Chawla J, Kochar SPS, Sharma JC. A randomized control trial to assess efficacy of mifepristone in medical management of uterine fibroid. Med J Armed Forces India. 2017;73(3):267–273. doi: 10.1016/j.mjafi.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yerushalmi GM, Gilboa Y, Jakobson-Setton A, et al. Vaginal mifepristone for the treatment of symptomatic uterine leiomyomata: an open-label study. Fertil Steril. 2014;101(2):496–500. doi: 10.1016/j.fertnstert.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 129.Yang Y, Zheng S, Zhang Z. Effects of mifepristone on gene expression of epidermal growth factor in human uterine leiomyoma. Zhonghua Fu Chan Ke Za Zhi. 1998;33(1):38–39. [PubMed] [Google Scholar]
- 130.Sun M, Zhu G, Zhou L. Effect of mifepristone on the expression of progesterone receptor messenger RNA and protein in uterine leiomyomata. Zhonghua Fu Chan Ke Za Zhi. 1998;33(4):227–231. [PubMed] [Google Scholar]
- 131.Engman M, Varghese S, Lagerstedt Robinson K, et al. GSTM1 gene expression correlates to leiomyoma volume regression in response to mifepristone treatment. PLoS One. 2013;8(12):e80114. doi: 10.1371/journal.pone.0080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Demanno D, Elger W, Garg R, et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68(10–13):1019–1032. doi: 10.1016/j.steroids.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]
- 134.Chwalisz K, Perez MC, Winkel C. Asoprisnil, a selective progesterone receptor modulator (SPRM), for the management of symptomatic leiomyomata. In: Brosens I, editor. Uterine Leiomyomata: Pathogenesis and Management. London and New York: Taylor & Francis; 2006. pp. 301–311. [Google Scholar]
- 135.Chen W, Ohara N, Wang J, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91(4):1296–1304. doi: 10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- 136.Wang J, Ohara N, Wang Z, et al. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- 137.Sasaki H, Ohara N, Xu Q, et al. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2007;92(2):616–623. doi: 10.1210/jc.2006-0898. [DOI] [PubMed] [Google Scholar]
- 138.Xu Q, Ohara N, Liu J, et al. Selective progesterone receptor modulator asoprisnil induces endoplasmic reticulum stress in cultured human uterine leiomyoma cells. Am J Physiol Endocrinol Metab. 2007;293(4):E1002–E1011. doi: 10.1152/ajpendo.00210.2007. [DOI] [PubMed] [Google Scholar]
- 139.Morikawa A, Ohara N, Qin X, et al. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23(4):944–951. doi: 10.1093/humrep/den025. [DOI] [PubMed] [Google Scholar]
- 140.Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Hum Reprod. 2009;24(5):1036–1044. doi: 10.1093/humrep/den494. [DOI] [PubMed] [Google Scholar]
- 141.Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93(12):4664–4671. doi: 10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]
- 142.Wilkens J, Male V, Ghazal P, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol. 2013;191(5):2226–2235. doi: 10.4049/jimmunol.1300958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188(1–2):111–123. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 144.Luo X, Yin P, Coon VJS, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93(8):2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roeder H, Jayes F, Feng L, Leppert PC. CDB-4124 does not cause apoptosis in cultured fibroid cells. Reprod Sci. 2011;18(9):850–857. doi: 10.1177/1933719111399929. [DOI] [PubMed] [Google Scholar]
- 146.Macdonald G. US FDA reaffirms hold on oral Proellex trials prompting Repros rethink (19-July-2017) [Accessed October 18, 2018]. https://www.in-pharmatechnologist.com/Article/2017/07/19/US-FDA-reaffirms-hold-on-oral-Proellex-trials-prompting-Repros-rethink.
- 147.Rao PN, Acosta CK, Bahr ML, et al. A practical large-scale synthesis of 17alpha-acetoxy-11beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione (CDB-2914) Steroids. 2000;65(7):395–400. doi: 10.1016/s0039-128x(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 148.Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. A promising candidate for use in contraception as well as treatment of uterine fibroids and endometriosis. Steroids. 2003;68:1005–1011. doi: 10.1016/s0039-128x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 149.Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68(10–13):1013–1017. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 150.Xu Q, Takekida S, Ohara N, et al. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab. 2005;90(2):953–961. doi: 10.1210/jc.2004-1569. [DOI] [PubMed] [Google Scholar]
- 151.Xu Q, Ohara N, Chen W, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21(9):2408–2416. doi: 10.1093/humrep/del159. [DOI] [PubMed] [Google Scholar]
- 152.Maruo T, Ohara N, Matsuo H, et al. Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception. 2007;75(6 Suppl):S99–S103. doi: 10.1016/j.contraception.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 153.Horak P, Mara M, Dundr P, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012:436174. doi: 10.1155/2012/436174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pohl O, Zobrist RH, Gotteland JP. The clinical pharmacology and pharmacokinetics of ulipristal acetate for the treatment of uterine fibroids. Reprod Sci. 2015;22(4):476–483. doi: 10.1177/1933719114549850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ciarmela P, Carrarelli P, Islam MS, et al. Ulipristal acetate modulates the expression and functions of activin A in leiomyoma cells. Reprod Sci. 2014;21(9):1120–1125. doi: 10.1177/1933719114542019. [DOI] [PubMed] [Google Scholar]
- 156.Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nieman LK, Blocker W, Nansel T, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril. 2011;95(2):767–772. doi: 10.1016/j.fertnstert.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Powell M, Dutta D. Esmya and the PEARL studies: a review. Womens Health. 2016;12:544–548. doi: 10.1177/1745505717692591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 160.Donnez J, Tomaszewski J, Vázquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 161.Donnez J, Vázquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565–1573. doi: 10.1016/j.fertnstert.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 162.Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103(2):519–527. doi: 10.1016/j.fertnstert.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 163.Kalampokas T, Kamath M, Boutas I, Kalampokas E. Ulipristal acetate for uterine fibroids: a systematic review and meta-analysis. Gynecol Endocrinol. 2016;32(2):91–96. doi: 10.3109/09513590.2015.1106471. [DOI] [PubMed] [Google Scholar]
- 164.Donnez J, Donnez O, Matule D, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril. 2016;105(1):165–173. doi: 10.1016/j.fertnstert.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 165.Fauser BC, Donnez J, Bouchard P, et al. Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLoS One. 2017;12(3):e0173523. doi: 10.1371/journal.pone.0173523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pourcelot AG, Capmas P, Fernandez H. Place of ulipristal acetate in the management of uterine fibroids: preoperative treatment or sequential treatment? J Gynecol Obstet Hum Reprod. 2017;46(3):249–254. doi: 10.1016/j.jogoh.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 167.European Medicine Agency Esmya: new measures to minimise risk of rare but serious liver injury. [Accessed May 13, 2018]. Available from: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Esmya/human_referral_prac_000070.jsp&mid=WC0b01ac05805c516f.
- 168. [Accessed on May 30, 2018];European Medicine Agency Committee for Medicinal Products for Human Use (CHMP) EMA/CHMP/417636/2018 Ulipristal Acetate Geden Richter. [Google Scholar]
- 169.Williams AR, Critchley HO, Osei J, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22(6):1696–1704. doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- 170.Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21(5):591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 171.Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21(4):318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- 172.Fraser IS. Low-dose mifepristone: effects on the endometrium. Aust N Z J Obstet Gynaecol. 2009;49:77–83. doi: 10.1111/j.1479-828X.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 173.Fiscella J, Bonfiglio T, Winters P, Eisinger SH, Fiscella K. Distinguishing features of endometrial pathology after exposure to the progesterone receptor modulator mifepristone. Hum Pathol. 2011;42(7):947–953. doi: 10.1016/j.humpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Helmestam M, Lindgren KE, Stavreus-Evers A, Olovsson M. Mifepristone-exposured human endometrial endothelial cells in vitro. Reprod Sci. 2014;21(3):408–414. doi: 10.1177/1933719113497284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ioffe OB, Zaino RJ, Mutter GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol. 2009;22(3):450–459. doi: 10.1038/modpathol.2008.204. [DOI] [PubMed] [Google Scholar]
- 176.Yoshida S, Ohara N, Xu Q, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28(3):260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- 177.Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P, VA2914 Study Group Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92(9):3582–3589. doi: 10.1210/jc.2006-2816. [DOI] [PubMed] [Google Scholar]
- 178.Ravet S, Munaut C, Blacher S, et al. Persistence of an intact endometrial matrix and vessels structure in women exposed to VA-2914, a selective progesterone receptor modulator. J Clin Endocrinol Metab. 2008;93(11):4525–4531. doi: 10.1210/jc.2008-0731. [DOI] [PubMed] [Google Scholar]
- 179.Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J Clin Endocrinol Metab. 2010;95(12):5330–5337. doi: 10.1210/jc.2010-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brenner RM, Slayden OD, Nath A, Tsong YY, Sitruk-Ware R. Intrauterine administration of CDB-2914 (Ulipristal) suppresses the endometrium of rhesus macaques. Contraception. 2010;81(4):336–342. doi: 10.1016/j.contraception.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;31(6):556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 182.Donnez J, Donnez O, Dolmans MM. Safety of treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Expert Opin Drug Saf. 2016;15(12):1679–1686. doi: 10.1080/14740338.2016.1248943. [DOI] [PubMed] [Google Scholar]
- 183.de Milliano I, van Hattum D, Ket JCF, Huirne JAF, Hehenkamp WJK. Endometrial changes during ulipristal acetate use: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;214:56–64. doi: 10.1016/j.ejogrb.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 184.Benagiano G, Primiero FM. The potential use of antiprogestins in gynaecological disorders. In: Motta M, Serio M, editors. Sex Hormones and Antihormones in Endocrine Dependent Pathology: Basic and Clinical Aspects. Amsterdam: Elsevier Science; 1994. pp. 391–399. [Google Scholar]
- 185.Islam MS, Protic O, Giannubilo SR, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013;98(3):921–934. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 186.Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets. 2016;20(9):1045–1054. doi: 10.1080/14728222.2016.1180368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev. 2017;4:CD010770. doi: 10.1002/14651858.CD010770.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Donnez J, Hervais Vivancos B, Kudela M, Audebert A, Jadoul P. A randomized, placebo-controlled, dose-ranging trial comparing fulvestrant with goserelin in premenopausal patients with uterine fibroids awaiting hysterectomy. Fertil Steril. 2003;79(6):1380–1389. doi: 10.1016/s0015-0282(03)00261-9. [DOI] [PubMed] [Google Scholar]
- 189.Lethaby A, Puscasiu L, Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. (art. no. CD000547).Cochrane Database Syst Rev. 2017;11:CD000547. doi: 10.1002/14651858.CD000547.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]