Abstract
Purpose
To characterize a biodegradable microsphere-hydrogel drug delivery system (DDS) for controlled and extended release of ranibizumab.
Methods
The degradable microsphere-hydrogel DDSs were fabricated by suspending ranibizumab-loaded or blank poly(lactic-co-glycolic acid) microspheres within a poly(ethylene glycol)-co-(L-lactic-acid) diacrylate/N-isopropylacrylamide (PEG-PLLA-DA/NIPAAm) hydrogel. The thermal responsive behavior of various DDS formulations was characterized in terms of volume phase transition temperature (VPTT) and swelling ratios changes from 22°C to 42°C. The mechanical properties were characterized using rheological methods. Degradability of hydrogels were also examined via wet weight loss. Finally, Iodine-125 was used to radiolabel ranibizumab for characterization of encapsulation efficiency and in vitro release.
Results
All DDS formulations investigated were injectable through a 28-gauge needle at room temperature. The VPTT increased with increase of cross-linker concentration. The swelling ratios decreased as temperature increased and were not influenced by presence of microspheres. Rheology data confirmed that increase of cross-linker concentration and microsphere loading made DDS stiffer. Increase of degradable cross-linker concentration facilitated hydrogel in vitro degradation. Controlled release of ranibizumab were achieved for investigated DDS formulations for 6 months; and increased degradable cross-linker concentration produced faster and more complete release.
Conclusions
The biodegradable DDSs are suitable for sustained release of ranibizumab. Considering ease of injection, degradability and release of ranibizumab, DDS with 3 mM cross-linker concentration and less than 20 mg/mL microsphere loadings is more favorable for future application.
Translational Relevance
The investigated DDS is promising for controlled and extended release of anti-VEGF therapeutics to achieve better treatment regimen in ocular neovascularizations.
Keywords: ocular drug delivery, anti-VEGF, AMD
Introduction
Since the identification of vascular endothelial growth factors (VEGFs) and their key role in ocular neovascularization at the end of last century,1,2 considerable progress has been made toward the treatment of neovascular eye diseases such as wet age-related macular degeneration (AMD), diabetic macular edema (DME), proliferative diabetic retinopathy (PDR), and retinal vein occlusion (RVO) with the development of anti-VEGF therapy. Several pivotal clinical trials, including ANCHOR and MARINA, demonstrated significant improvement in visual outcomes for all types of choroidal neovascularization (CNV) in wet AMD.3,4 Based on these studies, anti-VEGF therapy, namely bevacizumab (off-label, Avastin, Genentech, South San Francisco, CA), ranibizumab (Lucentis, Genentech), and aflibercept (Eylea, Regeneron, Tarrytown, NY) became the current standard of care for neovascular eye diseases.
Despite its great success in treatment of ocular neovascularization, a major problem for current anti-VEGF therapy is that the therapy must be performed monthly/bimonthly through intravitreal (IVT) injections due to fast clearance and short half-life of anti-VEGF drugs.5 These repeated IVT injections are associated with several complications including endophthalmitis, retinal detachment, IVT hemorrhage, cataract, and the nonoptimal pharmacokinetic profile of repeated bolus injection.6,7 Furthermore, a significant socioeconomic burden upon patients, family, and healthcare system cannot be ignored. Therefore, there is a great demand in developing a drug delivery system (DDS) for anti-VEGF that will result in a controlled and extended delivery and reduction in frequency of IVT injections.
Recent years have seen an increase in DDSs development for controlled and extended delivery of anti-VEGF drugs in the form of ocular implants, cell-based systems, injectable nano-/micro-particles, injectable hydrogels, and composite systems.8 Among these systems, injectable polymeric particles offer a good controlled and extended drug release (from months to years). Most of these particles are also fully degradable. Some disadvantages include particle migration, which can cause glaucoma and ocular inflammation, fast clearance by phagocytes, and large initial burst (IB) release.9–11 On the other hand, encapsulating protein drug directly into injectable hydrogels often resulted in a shorter release time of approximately 1 month due to hydrogels' inherent high water content causing fast diffusion of hydrophilic proteins.12,13 However, these challenges can be overcome by encapsulating injectable polymeric particles into injectable hydrogels. Since hydrogel provides localization of the particles to the injection site and acts as additional diffusion barrier to extend release time.8 Furthermore, the ability to control the amount and type of particles loaded within hydrogels can enhance the drug delivery potential.
Recently, a composite ocular DDS consisting of poly(lactic-co-glycolic acid) (PLGA) microspheres suspended within a thermoresponsive, injectable poly(ethylene glycol) diacrylate/N-isopropylacrylamide (PEG-DA/NIPAAm) hydrogel has been developed by our laboratory.14–16 It has been demonstrated that our system is capable of localized release of anti-VEGF drugs such as ranibizumab and aflibercept in a controlled manner for 6 months without long-term effects on the retinal function.15,17 Both in vitro bioactivity of released anti-VEGF and in vivo treatment efficacy in a rat laser CNV model by our DDS were recently demonstrated.14,15 However, the PEG-DA/NIPAAm hydrogel used in the previous study was nonbiodegradable and there was a large incomplete release of drugs. Using a hydrolytically biodegradable poly(ethylene glycol)-co-(L-lactic-acid) diacrylate/N-isopropylacrylamide (PEG-PLLA-DA/NIPAAm) hydrogel to make the microsphere-hydrogel DDS was hypothesized to be better and enhance the anti-VEGF release.
The objective of this study was to develop and characterize a biodegradable microsphere-hydrogel DDS for controlled and extended release of anti-VEGF drugs (ranibizumab) for 6 months. The effects of degradable cross-linker (PEG-PLLA-DA) concentrations on hydrogels' volume phase transition temperatures (VPTTs), swelling ratios, mechanical strength, and in vitro degradation were investigated. The effects of microsphere load amount on hydrogels swelling and mechanical strength, which could affect the injectability of the system, were also investigated. Finally, the feasibility of controlled and extended release of ranibizumab from the DDSs with varying PEG-PLLA-DA concentrations was demonstrated.
Materials and Methods
Fabrication of Microsphere-Hydrogel DDS
All subsequent chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Blank or drug-loaded PLGA 75:25 microspheres were fabricated using a modified double-emulsion, solvent evaporation technique described in detail elsewhere.16 Briefly, the primary water-in-oil emulsion (w1/o) was created by vortex; this first emulsion was immediately added to the outer aqueous phase (w2) containing polyvinyl alcohol to create a (water-in-oil)-in-water (w1/o/w2) double emulsion by vortex. Several excipients were used to stabilize and protect the ranibizumab during fabrication, storage, and release: bovine serum albumin (BSA), PEG (MW 8 kDa), and sucrose were added in the inner aqueous (w1) phase; Mg(OH)2 was added as a buffering salt in the oil phase (o). After solvent evaporation, microspheres were harvested by centrifugation, washed three times in deionized water, lyophilized to a dry powder, and stored at 4°C.
Degradable thermoresponsive hydrogels composed of poly(ethylene glycol)-co-(L-lactic acid) diacrylate (PEG-PLLA-DA) and N-isopropylacrylamide (NIPAAm) were synthesized by free radical polymerization method described in detail elsewhere.12 Briefly, hydrogels precursors were prepared by dissolving 350 mM NIPAAm, 50 mM N-tert-butylacrylamide, and 13 mM ammonium persulfate in pH 7.4 1 × phosphate-buffered saline (PBS). Different concentrations of PEG-PLLA-DA (1, 2, and 3 mM) were then added to synthesize hydrogels with different cross-linker densities. To create microsphere-hydrogel DDS with different microsphere load amount, 0, 10, and 20 mg/mL microspheres were suspended in the above hydrogel precursors. Polymerization of the hydrogel was initiated by mixing 168 mM N,N,N′,N′-tetramethylethylenediamine—pH adjusted to 7.4 with hydrochloric acid—into the hydrogel precursors; the reaction was allowed to proceed on ice for 30 minutes to form microsphere-hydrogel DDS. After polymerization, DDSs were collected and washed three times in DI water. All samples were made in the volume of 1 mL triplicate for the following experiments.
VPTT of Hydrogels
The effect of PEG-PLLA-DA concentrations on the VPTT of the hydrogels was determined by optical absorption spectroscopy using a temperature-controlled UV-vis plate reader (Molecular Devices SPECTRA Max Plus, Sunnyvale, CA). Briefly, hydrogels at various PEG-PLLA-DA concentrations were loaded into 96-well plates and an average absorbance of the loaded samples was measured at 560-nm wavelength. The temperature of the samples was increased from 25°C to 42°C at 0.5°C increments. The samples were allowed to equilibrate for at least 15 minutes at each temperature before absorbance measurements were taken. The absorbance of samples was normalized for determining VPTTs, and the VPTTs were defined as the temperature at which 50% of the maximum absorbance change occurred.
Temperature-Dependent Swelling Ratios
Hydrogel swelling ratios were measured at different temperatures—from 22°C to 42°C—to study hydrogels' thermoresponsive swelling behavior. Hydrogels prepared after free radical polymerization were initially incubated in de-ionized (DI) water bath at 22°C overnight to allow equilibration. The wet weights (Wwet) of hydrogels were measured at different temperatures using a temperature-controlled DI water bath. The temperature of incubation was increased from 22°C to 42°C at 4°C increments. At each temperature increment step the samples were allowed to equilibrate for at least 30 minutes before wet weight measurement. Each sample was then collected and dried using lyophilization to measure the dry weight (Wdry). The swelling ratio (Qswelling) was calculated using Equation 1:
 |
To investigate the effect of cross-linker concentration on hydrogel swelling ratios, three concentrations (1, 2, and 3 mM) of PEG-PLLA-DA were used to make blank hydrogels (without microspheres) for the above test. Additionally, three microsphere concentrations, 0 mg/mL (labeled as DDS-0), 10 mg/mL (DDS-10), and 20 mg/mL (DDS-20) were chosen to prepare 3 mM PEG-PLLA-DA/NIPAAm hydrogels for studying the effect of microsphere load amount on hydrogels' thermoresponsive swelling behavior.
Rheological Studies
The mechanical properties of hydrogels were studied using standard rheological methods. Blank hydrogels with different PEG-PLLA-DA concentrations (1, 2, and 3 mM) were used for studying the role of PEG-PLLA-DA cross-linker density on mechanical properties of PEG-PLLA-DA/NIPAAm hydrogels; and 3 mM PEG-PLLA-DA/NIPAAm hydrogels with different microsphere loadings (0, 10, and 20 mg/mL) were used for investigating effect of microsphere load amount on mechanical properties of hydrogels. The hydrogels were prepared as cylindrical disks of 1.5 mm thickness and 25 mm in diameter in small custom-designed metallic mold; they were then transferred and incubated in petri dishes filled with DI water overnight to reach equilibrium swollen state. The prepared samples were loaded into an MCR 301 rheometer (Anton Paar, Ashland, VA) with a gap between plates adjusted to 0.8 mm for complete coverage of plate surface area. Excess sample was trimmed from the edges of plates before any measurements were made. Frequency sweeps from 0.1 to 100 rad/s at a strain amplitude of 2.56 × 10−3 were used to determine both storage moduli (G′) and loss moduli (G″) of hydrogels for rheological analysis. Finally, a strain amplitude sweep from 0.1% to 100% was performed on 3mM PEG-PLLA-DA/NIPAAm hydrogels to investigate strain dependence.
In Vitro Degradation of Hydrogels
Three groups of PEG-PLLA-DA/NIPAAm hydrogels with different PEG-PLLA-DA concentrations (1, 2, and 3 mM) were investigated for in vitro degradation. Each hydrogel sample (1 mL in volume) was incubated in 5 mL pH 7.4 1 × PBS at 37°C; and the buffer was refreshed weekly. At predetermined time points, wet weight of hydrogels was measured. The weight loss (%) of each sample was calculated as weight loss (%) = (Wo − Wt)/Wo × 100, where Wt is the wet weight of hydrogel at corresponding time points and Wo is the initial wet weight before degradation.
Protein Radiolabeling and Encapsulation Efficiency
Ranibizumab (Lucentis, Genentech) was radiolabeled with iodine-125 using iodination beads (Pierce, Rockford, IL) and then dialyzed against DI water using a dialysis cassette (MWCO 2 kDa, Pierce) to remove unbound, free iodine. Radiolabeled proteins were lyophilized, weighed, and dissolved in pH 7.4 1 × PBS to create a stock solution of 10 mg/mL. The stock solution was then stored at −80°C for future use.
The encapsulation efficiency (E.E.) for microsphere was determined from the radioactivity measured using a gamma counter (Cobra-II, Auto-Gamma, Packard Instrument Co., Meriden, CT) before and after microsphere preparation. E.E. was defined as the percent-drug within the microspheres relative to the theoretical loading amount. Three batches of drug-loaded microsphere were made to obtain the average E.E. The above three batches of microspheres were pooled together for further characterization of E.E. of microspheres in hydrogels.
In Vitro Release of Ranibizumab
In vitro release profiles of radiolabeled ranibizumab from microsphere-hydrogel DDSs were determined using a separation method described in detail elsewhere.18 Briefly, 10 mg/mL ranibizumab-loaded microspheres were used to prepare microsphere-hydrogel DDS with different PEG-PLLA-DA concentrations (1, 2, and 3 mM) to study effect of degradable cross-linker concentrations on controlling drug release. Each DDS sample was prepared in 1 mL and incubated in 1.5 mL 1 × PBS at 37°C under mild agitation throughout the release. At predetermined time intervals, 1 mL supernatant was removed after a brief centrifugation and replaced with an equal volume of fresh buffer. Radioactivity of supernatants were measured using a gamma counter (Packard Instrument Co.) to determine amount of drug release. Cumulative release was calculated relative to E.E. of ranibizumab into each microsphere-hydrogel DDS. The IB release was also determined as percent drug released within the first 24 hours for different DDSs. The radioactivity counted by the gamma counter was normalized according to the half-life of iodine-125 (i.e., 60 days), and release was terminated when the detected radioactive signal decreased close to the background level of the gamma counter.
Statistical Analysis
All values are reported as mean ± standard error and in all graphs, error bars represent standard error. All statistical comparisons between different experimental groups were performed using Student's t-test, and a P value less than 0.05 was determined to be significantly different.
Results
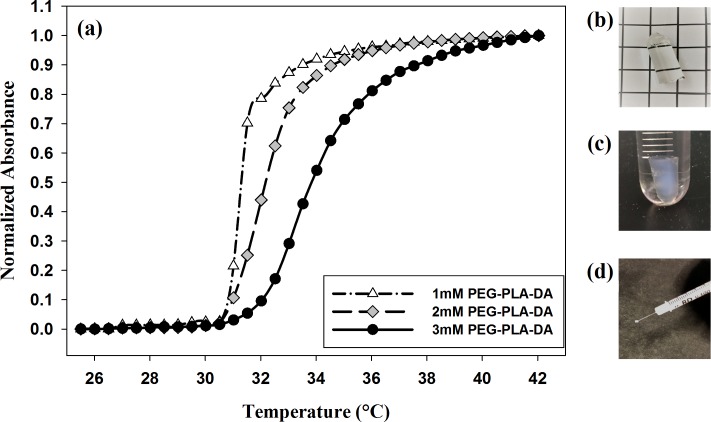
Blank and ranibizumab-loaded PLGA microspheres were successfully formed using modified double-emulsion, solvent evaporation technique. The average diameter of microspheres was 7.0 ± 1.3 μm by examining under microscope, which was consistent with our previous studies (7.5 ± 0.4 μm).15 Thermoresponsive PEG-PLLA-DA/NIPAAm hydrogels with different PEG-PLLA-DA concentrations (1, 2, and 3 mM) were also successfully formed and able to inject through a 28G needle at room temperature (23°C), which is of great importance for minimally invasive delivery. Additionally, suspending the microspheres (10 and 20 mg/mL) within hydrogels did not influence the injectability through a 28G needle at room temperature (Fig. 1d).
Figure 1.
Thermal transition of hydrogels. (a) Normalized absorbance of PEG-PLLA-DA/NIPAAm hydrogels as a function of temperature at different PEG-PLLA-DA cross-linker concentrations. (b) Hydrogel with 3 mM PEG-PLLA-DA at room temperature. (c) Hydrogel with 3 mM PEG-PLLA-DA at body temperature. (d) 3 mM hydrogel with 10 mg/mL microspheres injected through 28-gauge needle.
VPTT of Hydrogels
PEG-PLLA-DA/NIPAAm hydrogels were transparent and fluid-like at room temperature (Fig. 1b), but turned opaque and solidified after increasing temperature to body temperature (37°C) (Fig. 1c). This thermal transition behavior of hydrogels was depicted by hydrogels' optical absorbance changes as a function of temperature as shown in Figure 1a. The VPTTs of hydrogels were determined as the temperature at 50% of maximum change in normalized absorbance. The VPTTs for thermoresponsive hydrogels with different PEG-PLLA-DA concentrations were measured as below: 31.5°C, 32.5°C, and 33.5°C for hydrogels with 1, 2, and 3 mM PEG-PLLA-DA concentrations, respectively. It was also observed from Figure 1a that hydrogels with lower PEG-PLLA-DA cross-linker concentration exhibited a sharper thermal transition and a lower VPTT than those with higher PEG-PLLA-DA concentration.
Temperature-Dependent Swelling Ratios
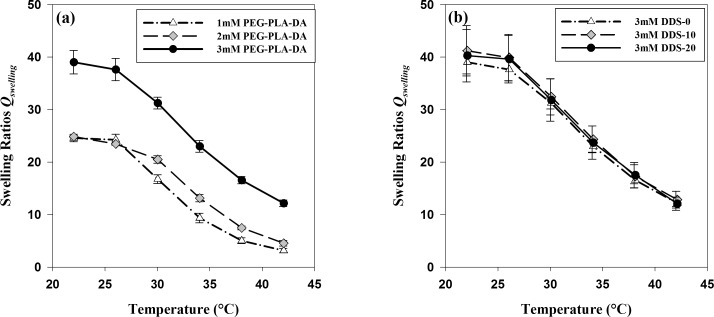
The thermoresponsive swelling behavior of hydrogels with and without microspheres is shown in Figure 2. Figure 2a depicted the effect of PEG-PLLA-DA cross-linker concentrations on hydrogels' swelling ratios at different temperatures, both below and above their VPTTs. At room temperature (22°C), hydrogels with different PEG-PLLA-DA concentrations were all highly swollen and hydrophilic; swelling ratios for hydrogels with PEG-PLLA-DA concentrations of 1, 2, and 3 mM were 24.61 ± 0.70, 24.87 ± 0.22, and 39.03 ± 2.23, respectively. With the temperature increased from 22°C to 42°C, the swelling ratios gradually lowered as hydrogels turned opaque with different PEG-PLLA-DA concentrations. Above body temperature (42°C), swelling ratios for hydrogels with 1, 2, and 3 mM PEG-PLLA-DA were 3.13 ± 0.43, 4.54 ± 0.52, and 12.15 ± 0.60, respectively. Comparing to room temperature, a decrease of 87.28%, 81.75%, and 68.87% in swelling ratios for hydrogels with 1, 2, and 3 mM PEG-PLLA-DA concentrations were seen, respectively. Over the above temperatures, hydrogels with higher PEG-PLLA-DA concentration exhibited higher swelling ratios than hydrogels with lower PEG-PLLA-DA concentration. The differences of swelling ratios between 3 and 2 mM hydrogels were more significant (P < 0.004) than that between 2 and 1 mM hydrogels. It was also found that presence and the amount of microspheres loaded in the hydrogels did not have an effect on hydrogels' swelling behavior. This was depicted in Figure 2b that 3 mM PEG-PLLA-DA/NIPAAm hydrogels with microspheres load amount of 0 mg/mL (DDS-0), 10 mg/mL (DDS-10), and 20 mg/mL (DDS-20) all exhibited similar swelling ratios at all investigated temperatures.
Figure 2.
Temperature-dependent swelling ratios of PEG-PLLA-DA/NIPAAm hydrogels with/without microspheres. (a) Effect of PEG-PLLA-DA concentrations on hydrogels' swelling ratios. (b) Effect of microsphere load amount on hydrogels' swelling ratios, where “DDS-0,” “DDS-10,” and “DDS-20” stands for hydrogels with 0, 10, and 20 mg/mL microsphere load amount, respectively. Error bars represent standard error (n = 3).
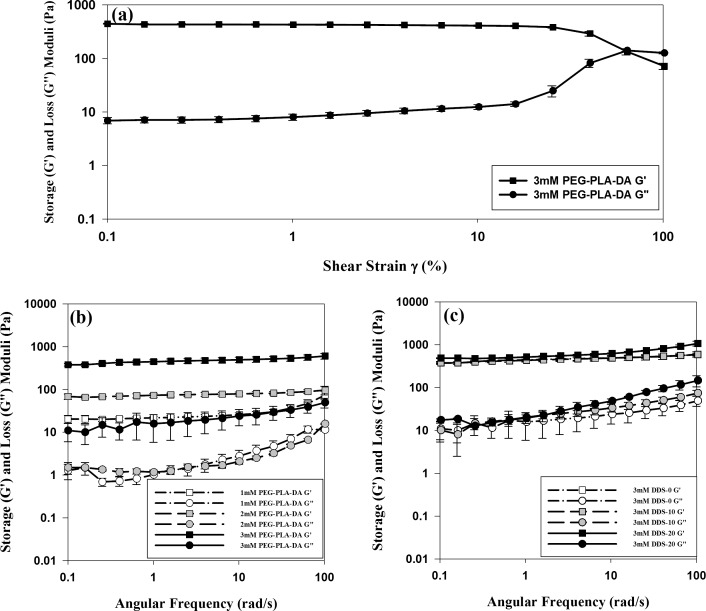
Rheological Studies
The strain dependence result of 3 mM hydrogels shown in Figure 3a indicated that the sample stayed well in the linear viscoelastic deformation range when a strain amplitude of 1% was applied. Therefore, this amplitude was applied in frequency sweep test for all hydrogel samples. Figure 3b shows frequency sweeps (at room temperature) for PEG-PLLA-DA/NIPAAm hydrogels with PEG-PLLA-DA concentrations of 1, 2, and 3 mM. For all investigated hydrogels, the storage modulus (G′) was higher than the loss modulus (G″) over the entire frequency range from 0.1 to 100 rad/s. The storage modulus increased with PEG-PLLA-DA concentrations, with more significant increase (P < 0.001) from 2 to 3 mM hydrogels than that from 1 to 2 mM hydrogels. However, there was no significant difference between loss moduli of 1 and 2 mM hydrogels (P > 0.10). In addition, both storage moduli (G′) and loss moduli (G″) increased with angular frequency. Figure 3c shows the effect of microsphere load amount—0 mg/mL (DDS-0), 10 mg/mL (DDS-10), and 20 mg/mL (DDS-20)—on 3 mM PEG-PLLA-DA/NIPAAm hydrogels' mechanical properties. Microsphere load amount at 10 mg/mL did not influence hydrogels' G′ and G″ comparing to blank hydrogels over the entire frequency range (P > 0.05). However, hydrogels with higher microsphere load amount at 20 mg/mL exhibited higher storage and loss moduli especially over the high frequency range (>10 rad/s).
Figure 3.
Rheological studies of PEG-PLLA-DA/NIPAAm hydrogels with/without microspheres. (a) Strain dependence of 3 mM PEG-PLLA-DA/NIPAAm hydrogels. (b) Effect of PEG-PLLA-DA concentrations on hydrogels' rheological properties. (c) Effect of microsphere load amount on hydrogels' rheological properties. Error bars represent standard error (n = 6).
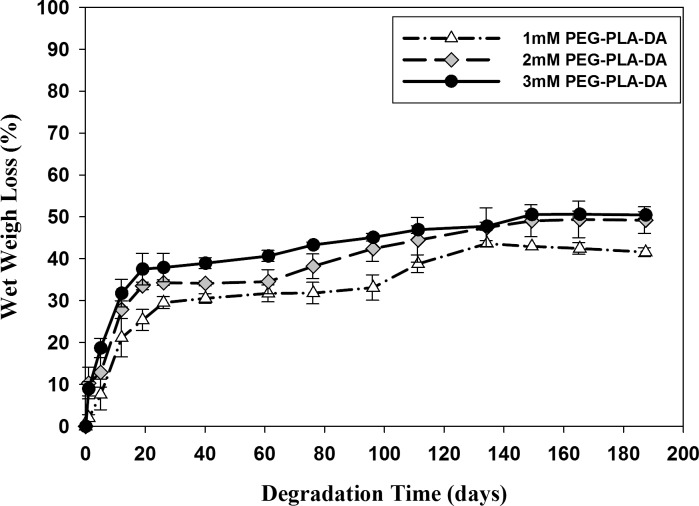
In Vitro Degradation of Hydrogels
PEG-PLLA-DA is a hydrolytically biodegradable polymer used to cross-link with NIPAAm to make the hydrogels degradable. The degradation of hydrogels with varying PEG-PLLA-DA concentrations were measured by wet weight loss (Fig. 4). The hydrogels with three varying PEG-PLLA-DA concentrations (1, 2, and 3 mM) all degraded faster during the first 3 weeks; then their wet weight loss (%) slowed down and finally did not change after 150 days. At the end of study (187 days), the wet weight loss for hydrogels with 1, 2, and 3 mM PEG-PLLA-DA concentrations were 41.56% ± 1.09%, 49.23% ± 3.18%, and 50.46% ± 0.78%, respectively. Hydrogels with higher amount of PEG-PLLA-DA cross-linker lost more wet weight than those with lower amount of PEG-PLLA-DA over the entire time of degradation. Although investigated hydrogels' wet weights did not change after 150 days, they started to lose their shape integrity and became amorphous, swollen, and loose after 190 days.
Figure 4.
Degradation of hydrogels with different PEG-PLLA-DA concentrations by wet weight. Error bars represent standard error (n = 3).
Ranibizumab E.E. and In Vitro Release
Ranibizumab was successfully encapsulated into PLGA microspheres, and the mean E.E. of ranibizumab in microsphere was 45.67% ± 2.09% (Table 1). The mean E.E. of microspheres in hydrogels with 1, 2, and 3 mM PEG-PLLA-DA concentrations were 60.93% ± 5.94%, 74.38% ± 6.45%, and 74.20% ± 6.49%, respectively. The E.E. for microsphere-hydrogel DDSs with 2 and 3 mM PEG-PLLA-DA concentrations (2 mM DDS-10 and 3 mM DDS-10) were not significantly different (P > 0.05), whereas E.E. for microsphere-hydrogel DDS with 1 mM PEG-PLLA-DA concentration (1 mM DDS-10) was ∼14% lower (P < 0.05).
Table 1.
E.E. of Microsphere-Hydrogel DDS
| DDS Formulation |
E.E. of Drug in Microsphere |
E.E. of Microsphere in Hydrogel |
| 1 mM DDS-10 | 45.67% ± 2.09% | 60.93% ± 5.94% |
| 2 mM DDS-10 | 74.38% ± 6.45% | |
| 3 mM DDS-10 | 74.20% ± 6.49% |
“1 mM,” “2 mM,” and “3 mM” stand for PEG-PLLA-DA concentrations for hydrogels; and number “10” after “DDS” refers to 10 mg/mL of microsphere load amount into hydrogels. Data presented as mean ± SEM (n = 3).
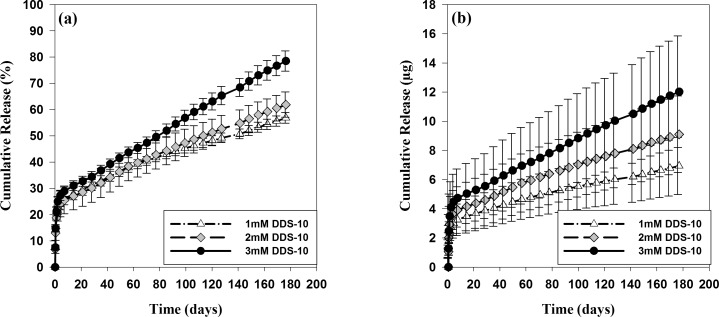
Figure 5 shows in vitro release profiles of ranibizumab from all three microsphere-hydrogel DDSs—1 mM DDS-10, 2 mM DDS-10, and 3 mM DDS-10—both in percent (Fig. 5a) and weight (Fig. 5b) at physiological temperature (37°C). As shown in the Figure, ranibizumab released rapidly from all investigated microsphere-hydrogel DDSs during the first week, then it released steadily in a linear manner. DDSs with higher PEG-PLLA-DA concentration had faster and more complete drug release at the end than those with lower PEG-PLLA-DA concentration. The release characteristics for DDSs with varying PEG-PLLA-DA concentrations are also summarized in Table 2. The radioactivity signals from released ranibizumab were detectable for all investigated DDSs up to 176 days. A total of 11.99 ± 2.92, 14.30 ± 5.20, and 15.78 ± 5.68 μg drug was loaded into DDSs with 1, 2, and 3 mM PEG-PLLA-DA concentrations, respectively. The IB release of ranibizumab from DDSs with 1, 2, and 3 mM PEG-PLLA-DA concentrations were 2.36 ± 0.75, 2.92 ± 1.24, and 3.49 ± 1.51 μg, respectively. No significant differences for IB release among DDSs with varying cross-linker concentrations were found. Beyond the first week of release, a controlled release rate of 0.02, 0.03, and 0.04 μg/day were estimated by linear regression analysis for DDSs with 1, 2, and 3 mM PEG-PLLA-DA concentrations, respectively. DDSs with higher PEG-PLLA-DA concentration had faster drug release rate than those with lower PEG-PLLA-DA concentration. Amount of drug entrapped in DDSs decreased with increase in PEG-PLLA-DA concentration at the end of release. Final percent drug release was 56.89% ± 2.01%, 61.90% ± 4.81%, and 78.52% ± 3.84% for DDSs with 1, 2, and 3 mM PEG-PLLA-DA concentrations, respectively.
Figure 5.
In vitro release of ranibizumab from degradable microsphere-hydrogel DDS with varying PEG-PLLA-DA concentrations and 10 mg/mL microsphere load amount. “1 mM,” “2 mM,” and “3 mM” in the legend represent PEG-PLLA-DA concentrations for hydrogels; “DDS-10” stands for 10 mg/mL microsphere load amount. (a) Percent cumulative release of ranibizumab from investigated DDS. (b) Cumulative release of ranibizumab in weight (μg). Error bars represent standard error (n = 3).
Table 2.
Characteristics of Ranibizumab Release
| DDS Formulation |
Drug Total Load Amount (μg) |
IB Release (%) |
Release Rate After 7 Days (μg/Day) |
Release Time (Days) |
Final Percent Release (%) |
| 1 mM DDS-10 | 11.99 ± 2.92 | 19.03 ± 1.38 | 0.02 (r2 = 0.99) | 176 | 56.89 ± 2.01 |
| 2 mM DDS-10 | 14.30 ± 5.20 | 18.84 ± 4.09 | 0.03 (r2 = 0.99) | 176 | 61.90 ± 4.81 |
| 3 mM DDS-10 | 15.78 ± 5.68 | 21.13 ± 1.64 | 0.04 (r2 = 0.99) | 176 | 78.52 ± 3.84 |
Daily release rates after 7 days were estimated using linear regression. Data presented as mean ± SEM (n = 3).
Discussion
To make thermoresponsive hydrogels suitable as a minimally invasive localized DDS, the VPTT of hydrogels should be well above room temperature for injection through small gauge needles. The VPTTs for PEG-PLLA-DA/NIPAAm hydrogels with varying PEG-PLLA-DA concentrations (1, 2, and 3 mM) investigated in this study ranged from 31.5°C to 33.5°C, and increased with PEG-PLLA-DA concentrations. All of hydrogels were injectable through a 28G needle at room temperature and transformed to a solidified collapsed material after temperature increased to physiological level. It is well understood that the VPTT behavior of NIPAAm-based hydrogels results from a balance between hydrophilic and hydrophobic moieties in the polymer chains.19,20 Hydrophilic moieties tend to increase the VPTT and hydrophobic moieties tend to lower the VPTT. The VPTT of PEG-PLLA-DA/NIPAAm hydrogels with lowest PEG-PLLA-DA concentration (1 mM) was similar to the VPTT of pNIPAAm alone, which is reported as ∼32°C.21 And increased VPTT in hydrogels with higher PEG-PLLA-DA concentration can be attributed to the hydrophilic character of PEG group added to the pNIPAAm chains.
The above thermal transition behavior of PEG-PLLA-DA/NIPAAm hydrogels was also evaluated by changes in swelling ratios at different temperatures below and above their VPTTs. The swelling ratio for polymeric hydrogels determines their water content and is positively related to the molecular weight of the polymer chains between cross-links, which scales with the mesh size of hydrogel network.22 Swelling ratios for PEG-PLLA-DA/NIPAAm hydrogels with varying PEG-PLLA-DA concentrations decreased as temperature increased above their VPTTs. This indicated that hydrogels started to lose water and collapsed due to more dominant hydrophobic polymer–polymer interaction at higher temperatures. Since the mesh size of hydrogels are well known to be proportional to the swelling ratios,23 this decrease in swelling ratios above VPTTs indicates a decrease of hydrogels' mesh size, which is favorable for sustained drug release. The decrease in swelling ratios and loss of water create convective flow and contribute to IB drug release. Interestingly, hydrogels with higher PEG-PLLA-DA concentration exhibited higher swelling ratios at investigated temperatures. This is opposite to the swelling of PEG-DA/NIPAAm hydrogels, where higher PEG-DA concentrations made hydrogel more rigid and less swelling.21 One possible explanation for this increased swelling at higher PEG-PLLA-DA cross-link concentration is that the higher MW of PEG-PLLA-DA (∼4288 g/mol) comparing to MW of PEG-DA (575 g/mol) produced longer polymer chains after copolymerization with pNIPAAm. This would result in larger space between cross-links allowing for more water exposure and enhanced hydrophilic character of PEG groups added to the pNIPAAm chains. Therefore, hydrogels became more swollen and less responsive to temperature changes at higher PEG-PLLA-DA concentration. In addition, we observed no effects of microsphere load amount on hydrogel's temperature-dependent swelling behavior. The 3 mM PEG-PLLA-DA/NIPAAm hydrogels with three different microsphere load amount (0, 10, and 20 mg/mL) exhibited similar swelling ratios with increasing of temperature. This indicated that presence of microspheres inside the hydrogels' network, at least at concentration up to 20 mg/mL, did not influence hydrogels' water content and thermoresponsive behavior, which is of great importance for microspheres' delivery and localization.
The mechanical properties of PEG-PLLA-DA/NIPAAm hydrogels with and without microspheres were characterized using small-amplitude oscillatory motion rheometry. The strain amplitude used for frequency sweeps test was 1%, and this was validated by results of strain dependence test in Figure 3a as the sample stayed well in the linear viscoelastic deformation range. From Figure 3b, which showed the effects of PEG-PLLA-DA cross-linker concentration on G′ and G″, we observed that increasing cross-linker concentration caused an increase in elastic moduli than in loss moduli, which means higher G″/G′ ratio at higher cross-linker concentration. The results suggested that increasing cross-linker concentration will make hydrogels more solid-like and difficult to inject through small gauge needles. This is in agreement with our preliminary studies, where we found that hydrogels with PEG-PLLA-DA concentrations beyond 3 mM are difficult to be injected through 28-gauge needles. All hydrogels investigated were elastic, demonstrated by higher storage moduli than loss moduli regardless of cross-linker concentration. In addition, the hydrogels in this study were designed to serve as injectable carriers for microspheres; therefore, it is important that the encapsulation of microspheres should not affect injectability of the system. We observed no differences in both G′ and G″ comparing 3 mM PEG-PLLA-DA/NIPAAm hydrogels with 10 mg/mL microspheres to those without microspheres. However, hydrogels with higher microsphere load (i.e., 20 mg/mL) presented increased G′ and G″—with more increase in G′ than G″— over the high frequency range (>10 rad/s). The current data suggest that microsphere load greater than 20 mg/mL can influence the mechanical properties and injectability. Considering the above results, 10 mg/mL was chosen as microsphere load amount for in vitro release study.
At the end of study (190 days), the shape of hydrogels started to disintegrate into amorphous polymers. We speculate that the hydrolytically degradable cross-linker PEG-PLLA-DA, which is important for maintaining hydrogels' shape integrity, has been hydrolyzed and the remaining PNIPAAm chains are still held together by physical cross-links formed through hydrophobic interactions. This hydrophobic interaction was confirmed when we lowered the temperature below hydrogels' VPTTs at the end of degradation (after 190 days of incubation), the remaining polymers became transparent and soluble. It is worth noting that the degradation of hydrogels was relatively faster during the first month, then became much slower before losing their shape integrity. This slow degradation was desirable for localizing the encapsulated microspheres since the complete degradation of microspheres take ∼154 days.16
Biocompatibility of the materials used in the DDSs is an important consideration. PLGA is a safe and highly biocompatible copolymer used in variety of FDA-approved therapeutic products. For example, Vivitrol, a naltrexone-loaded PLGA microsphere platform was developed for the treatment of alcohol dependence and received FDA-approval in 2006.24 And more recently, Durasert, a subconjunctival implant developed by EyePoint Pharmaceutics, also used PLGA as a biodegradable polymer to control release of fluocinolone acetonide for posterior uveitis. It was found that the system was safe and biocompatible, and is currently under FDA review.25 Although there are no products consisting of PEG-PLLA-DA or NIPAAm approved by FDA to date, various studies reported their excellent biocompatibility as tissue engineering scaffolds and drug delivery carriers.26,27 Our previous study also demonstrated that PEG-PLLA-DA and/or NIPAAm hydrogel was biocompatible in vitro and in vivo.12,17
To make ranibizumab-loaded PLGA microspheres, we used ranibizumab stock solution at clinical concentration of 10 mg/mL. An E.E. of 45.67% ± 2.09% was achieved for microspheres after washing, which was significantly lower than our previous study (91.3% ± 2.5%).15 The difference in E.E. may be due to the different methods for calculating E.E. In the previous study, the theoretical radioactivity of microspheres was calculated based on a theoretical ranibizumab to polymer ratio assuming 100% polymer and other excipients converted into microspheres. This assumption may not be true and result in higher calculated E.E than the actual value. The theoretical radioactivity of microspheres used for calculation of E.E in this study was directly measured before the double-emulsion, solvent evaporation process, which should be closer to the actual number. And another 26% to 40% of microspheres were lost after loading microspheres into hydrogels and washing.
A controlled and extended release of ranibizumab, with zero-order release after 1 week, was achieved for ∼176 days from microsphere-hydrogel DDSs with various degradable cross-linker concentrations. This duration of release timeframe was comparable to that of our previous nondegradable systems (196 days).15 It is worth noting that we terminated the release profiles at day 176 due to signal decaying of I-125 close to background level, thus, a longer and more complete release can be expected based on the slope of the curves in Figure 5. The degradability of hydrogels controlled by degradable cross-linker concentration were found to be correlated to the final percent release of ranibizumab. Microsphere-hydrogel DDS with highest degradable cross-linker concentration (3 mM) exhibited both highest degradation (50.46% ± 0.78% wet weight loss) and highest final percent ranibizumab release (78.52% ± 3.84%). The final percent release values from investigated degradable DDSs (ranging from 56.89% ± 2.01% to 78.52% ± 3.84% at 176 days) were all higher than that from nondegradable system (53.90% ± 4.92% at 196 days),15 which suggests that the degradation of hydrogels facilitated the complete release of ranibizumab. We believe that increase of degradable cross-linker concentration in hydrogels would further facilitate a complete drug release; however, the downside is a decreased injectability through a small gauge needle.
The microsphere-hydrogel DDSs with varied degradable cross-linker concentrations exhibited similar IB release of ∼20%, which is also comparable to IB release of ranibizumab from the nondegradable system (21.0% ± 2.0%).15 This IB release of encapsulated ranibizumab is most likely caused by convective mass transport of ranibizumab bound to the surface of microspheres, and free drugs in the hydrogels network, if any.29 This convective flow resulted from the quick loss of water during collapse of hydrogels' network as the temperature increased above the VPTTs, which was demonstrated by decrease in hydrogels' swelling ratios as shown in Figure 2. It is worth pointing out that the IB release of anti-VEGF could be favorable in neutralizing initial higher amount of VEGF at the beginning of treatment for ocular neovascularization.30 The release rate of ranibizumab after first week was found to be well controlled by degradable cross-linker concentration; DDSs with higher degradable cross-linker concentration presented faster daily release. The common triphasic release profiles from microspheres31,32 were not seen in these microsphere-hydrogel composite systems. In our system, steady zero-order release profiles were achieved for all cross-linker concentrations after 7 days, which supports our hypothesis that hydrogels act as a second barrier to further control and sustain the release of drug.
Clinical dosage of ranibizumab is 0.5 mg per IVT injection, with very high initial IVT drug concentration. The vitreous concentration of ranibizumab declined in a close to mono-exponential manner with a half-life of ∼3 days, and less than 0.1 μg/mL ranibizumab were still maintained in the vitreous humor after 1 month.33 DDSs in this work have an initial release of 2.28 to 3.31 μg within 24 hours and a steady state release of 0.02 to 0.04 μg/day. Given that ranibizumab exhibits strong binding affinity to VEGF at 2.4 ng/mL, and IC50 for human umbilical vein endothelial cells (HUVECs) was reported to be 54.72 ng/mL,34 we hypothesize that this sustained low dose release will have a therapeutic effect. In addition, the dosage of our DDSs can also be increased and adjusted by either initial drug stock solution concentration or microsphere load amount. Future in vivo efficacy of the DDSs is planned in appropriate animal models.
There are variety of DDSs for sustained anti-VEGF release currently under development. For example, refillable rigid port delivery system (RPDS) developed by Forsight Vision4 and Genentech is in clinical trials for evaluating safety and efficacy in treatment of wet AMD.35 RPDS is an IVT implant that is the size of a grain of rice. It consists of a subconjunctival refill port outside of the eye and an IVT reservoir preloaded with drug. One of the biggest advantages of RPDS is that on-demand refills can be performed in an in-office procedure using a customized exchange needle. However, since the drug reservoir is nonbiodegradable, both surgical implantation and removal are required, which may increase risks of complications. Comparing to RPDS, the major advantage of the microsphere-hydrogel DDS presented in this work is that no surgical implantation and removal are needed since the system can be injectable through a small gauge needle and is biodegradable. In addition, other advantages are adjustable release dosage from microsphere loading and reproducible and linear release with low IB. However, maintaining drug bioactivity over the long-term release for both systems needs to be addressed in future.
In conclusion, the current study characterized important biomaterial parameters of a degradable microsphere-hydrogel DDS for controlled and extended release of ranibizumab. The VPTTs of degradable thermoresponsive hydrogels were below physiological temperature and suitable for injection through 28G needles. This thermoresponsive behavior was also reflected by hydrogels' temperature-dependent swelling ratios, and the microspheres did not influence the hydrogels' swelling. The mechanical properties of hydrogels were found to be related to their cross-linker density; higher cross-linker density investigated in this study gave more solid-like behavior. Higher microsphere load amount (>20 mg/mL) could also potentially make DDS stiffer and more difficult to inject, which should be carefully considered before clinical applications. By controlling the degradable cross-linker (PEG-PLLA-DA) concentrations for hydrogels, we were able to control the release rate and final percent release of ranibizumab. Higher degradability of hydrogels produced faster drug release rate and prompted more complete release. Considering the ease of injectability and more complete release of ranibizumab, hydrogels with 3 mM PEG-PLLA-DA concentration and microsphere load amount (10 mg/mL) seemed more favorable for sustained delivery of ranibizumab. Our degradable DDS is promising for controlled and extended localized release of anti-VEGF therapeutics to achieve better treatment regimen in chronic ocular diseases such as AMD and other ocular neovascularizations.
Acknowledgments
This work was supported by the NIH/NEI (EY0254-34) research grant.
Disclosure: W. Liu, None; M.A. Borrell, None; D.C. Venerus, None; W.F. Mieler, None; J.J. Kang-Mieler, (P)
References
- 1.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 2.Mathews MK, Merges C, McLeod DS, Lutty GA. Vascular endothelial growth factor and vascular permeability changes in human diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:2729–2741. [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Sanharawi M, Kowalczuk L, Touchard E, Omri S, de Kozak Y, Behar-Cohen F. Protein delivery for retinal diseases: from basic considerations to clinical applications. Prog Retin Eye Res. 2010;29:443–465. doi: 10.1016/j.preteyeres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Exp Opin Drug Delivery. 2017;14:611–620. doi: 10.1080/17425247.2016.1227785. [DOI] [PubMed] [Google Scholar]
- 9.Elsaid N, Jackson TL, Elsaid Z, Alqathama A, Somavarapu S. PLGA microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol Pharm. 2016;13:2923–2940. doi: 10.1021/acs.molpharmaceut.6b00335. [DOI] [PubMed] [Google Scholar]
- 10.Adamson P, Wilde T, Dobrzynski E, et al. Single ocular injection of a sustained-release anti-VEGF delivers 6 months pharmacokinetics and efficacy in a primate laser CNV model. J Control Release. 2016;244:1–13. doi: 10.1016/j.jconrel.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moritera T, Ogura Y, Honda Y, Wada R, Hyon SH, Ikada Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest Ophthalmol Vis Sci. 1991;32:1785–1790. [PubMed] [Google Scholar]
- 12.Drapala PW, Jiang B, Chiu YC, et al. The effect of glutathione as chain transfer agent in PNIPAAm-based thermos-responsive hydrogels for controlled release of proteins. Pharm Res. 2014;31:742–753. doi: 10.1007/s11095-013-1195-0. [DOI] [PubMed] [Google Scholar]
- 13.Xie B, Jin L, Luo Z, et al. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin® to treat posterior segment disease. Int J Pharm. 2015;490:375–383. doi: 10.1016/j.ijpharm.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 14.Osswald CR, Guthrie MJ, Avila A, Valio JA, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017;42:1293–1301. doi: 10.1080/02713683.2017.1302590. [DOI] [PubMed] [Google Scholar]
- 15.Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res. 2016;41:1216–1222. doi: 10.3109/02713683.2015.1101140. [DOI] [PubMed] [Google Scholar]
- 16.Osswald CR, Kang-Mieler JJ. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann Biomed Eng. 2015;43:2609–2617. doi: 10.1007/s10439-015-1314-7. [DOI] [PubMed] [Google Scholar]
- 17.Turturro SB, Guthrie MJ, Appel AA, et al. The effects of cross-linked thermos-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32:3620–3626. doi: 10.1016/j.biomaterials.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 18.D'Souza. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014;2014:1–12. [Google Scholar]
- 19.Yoshida R, Sakai K, Okano T, Sakurai Y. Modulating the phase transition temperature and thermosensitivity in N-isopropylacrylamide copolymer gels. J Biomater Sci Polym Ed. 1994;6:585–598. doi: 10.1163/156856294x00536. [DOI] [PubMed] [Google Scholar]
- 20.Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Higginbotham CL. The synthesis, characterization, phase behavior and swelling of temperature sensitive physically crosslinked poly(1-vinyl-2-pyrrolidinone)/poly(N-isopropylacrylamide) hydrogels. Eur Polym J. 2006;42:69–80. [Google Scholar]
- 21.Drapala PW, Brey EM, Mieler WF, Venerus DC. Kang Derwent JJ, Perez-Luna VH. Role of thermoresponsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J Biomater Sci Polym Ed. 2011;22:59–75. doi: 10.1163/092050609X12578498952315. [DOI] [PubMed] [Google Scholar]
- 22.Li JY, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:1–12. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–1357. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks SL, Pinninti RR, Gill HS, et al. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs. J Pharm Sci. 2010;99:3072–3080. doi: 10.1002/jps.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. 2011;6:317–329. [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang B, Akar B, Waller TM, Larson JC, Appel AA, Brey EM. Design of a composite biomaterial system for tissue engineering applications. Acta Biomater. 2014;10:1177–1186. doi: 10.1016/j.actbio.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Lima LH, Morales Y, Cabral T. Ocular biocompatibility of poly-N-isopropylacrylamide (pNIPAM) J Ophthalmol. 2016;2016:5356371. doi: 10.1155/2016/5356371. Epub 2016 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloada L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur J Pharm Biopharm. 2015;97:338–349. doi: 10.1016/j.ejpb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic glycolic acid) microspheres. Pharm Res. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 30.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreas K, Zehbe R, Kazubek M, et al. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. Acta Biomaterialia. 2011;7:1485–1495. doi: 10.1016/j.actbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Thomson HA, Treharne AJ, Backholer LS, Cuda F, Grossel MC, Lotery AJ. Biodegradable poly(alpha-hydroxy ester) blended microspheres as suitable carriers for retinal pigment epithelium cell transplantation. J Biomed Mater Res A. 2010;95:1233–1243. doi: 10.1002/jbm.a.32940. [DOI] [PubMed] [Google Scholar]
- 33.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Identifier NCT02510794, Study of the efficacy and safety of the ranibizumab port delivery system (RPDS) for sustained delivery of ranibizumab in participants with subfoveal neovascular age-related macular degeneration (AMD) (LADDER) July 29, 2015 [cited October 16, 2018]; [1 page]. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02510794 Accessed October 16, 2018. [Google Scholar]