Abstract
Background
Thrombectomy has become established as a successful treatment strategy for ischemic stroke, and consequently, more patients are undergoing this procedure. Due to comorbid conditions, chronic disease states, and advanced age, many patients have anatomy which complicates revascularization, specifically difficult aortic arch anatomy, or tortuous common and internal artery anatomy, or both.
Methods
In the present study, these unfavorable anatomic parameters were analyzed for 53 patients undergoing acute thrombectomy for ischemic stroke. Statistical analysis was performed and the outcome TICI scores were compared. 26 of the patients analyzed had features of difficult femoral access.
Results
Difficult arch anatomy was associated with unsuccessful revascularization (p = 0.03, Fisher’s exact) with only 53% of patients with this feature having favorable TICI scores. Difficult common carotid access was also associated with unsuccessful revascularization (p = 0.004, Fisher’s exact) with 38% success. There was a trend toward significance for unsuccessful revascularization for difficult internal carotid artery access (p = 0.06, Fisher’s exact).
Conclusion
Any combination of the aforementioned anatomic parameters was associated with the decreased success of treatment which was an independent predictor in multivariate analysis (p = 0.009). As difficult access anatomy is commonly encountered in patients undergoing emergent thrombectomy, it is important for the treating physician to be prepared and to adapt access strategies to increase the likelihood of successful revascularization.
Keywords: Thrombectomy, ischemic stroke, difficult access, revascularization
INTRODUCTION
Thrombectomy for ischemic stroke has now been established as an effective treatment. Many stroke patients are elderly, have a history of cardiovascular disease, and their anatomy reflects these conditions. Frequently, they have tortuous anatomy of the common and internal carotid arteries and the aortic arch. When present, any one of these features complicates endovascular access; in combination, access to intracranial lesions is increasingly difficult via a femoral artery route. Prior studies have shown that difficult access to the carotid arteries increases complication rates for carotid stenting [1, 2]. The purpose of the present study was to evaluate the effect of unfavorable anatomic parameters on revascularization in the setting of emergent large vessel occlusion.
METHOD
Demographic and clinical data for patients undergoing emergent thrombectomy secondary to acute large vessel occlusion of the internal carotid or middle cerebral artery was collected to assess the success of revascularization between August 2013 and April 2017. Anatomic features of difficult femoral artery route access were collected for the primary analysis. This study was approved by the Institutional Human Subjects Protection Program.
Thrombectomy was performed via standard endovascular transfemoral technique with either stent-retrieval or distal aspiration system, at the discretion of the senior author. Relevant or potentially confounding demographic patient characteristics were retrospectively collected by the senior author from the medical record and imaging studies, including invasive and noninvasive angiography. The primary parameter for assessment was the anatomic features of difficult femoral artery access, which was defined as a combination of at least two of the following: (1) acute angle turn into the common carotid artery from its origin on the aorta such as seen with type III arch or bovine configuration of arch in left-sided procedures; (2) acute angle turn or loop of the common carotid artery; and (3) acute angle turn or loop of the internal carotid artery. Additional assessment parameters included patient age, distal thrombus (third segment of MCA, M3), and proximal occlusion of the internal carotid artery in cervical segments. The primary endpoint was revascularization of the large vessel occlusion, measured by TICI score [3] and considered to be a success with revascularization to TICI 2b or 3. Univariate and regression analyses were performed to compare revascularization for patients with or without difficult femoral artery access features.
RESULTS
53 patients met the criteria for this analysis. The relevant data are summarized in Table 1. Among the patients, the median age was 69 years (range 26–95) and 56% were women. The majority of cases had occlusion location of M1 (33% and 62%), followed in order of frequency by ICA (10% and 19%), M3 (6% and 11%), and M2 (4% and 8%). Distribution of occlusion laterality was relatively equal, with 28 (53%) on the right and 25 (47%) on the left. Revascularization was successful in 38 cases (72%). Failure of revascularization included four cases with the inability to place a guide catheter in a position to attempt thrombectomy.
Table 1. Case detail of 53 consecutive cases undergoing emergent thrombectomy.
| Case | Age | Occluded vessel | Laterality | Arch type | Bovine | Poor arch anatomy | Acute bend in CCA | ICA loop | Revascularization |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 91 | M1 | Right | 3 | 0 | 1 | 0 | 0 | 2b |
| 2 | 61 | M1 | Right | 1 | 0 | 0 | 0 | 0 | 2a |
| 3 | 81 | ICA | Right | 2 | 1 | 0 | 0 | 1 | 3 |
| 4 | 55 | M1 | Left | 2 | 1 | 1 | 1 | 0 | 0 |
| 5 | 58 | M1 | Right | 3 | 0 | 1 | 0 | 0 | 2b |
| 6 | 43 | M3 | Left | 1 | 0 | 0 | 0 | 0 | 2b |
| 7 | 54 | M1 | Right | 1 | 1 | 0 | 0 | 0 | 3 |
| 8 | 63 | M1 | Right | 3 | 0 | 1 | 1 | 0 | 2b |
| 9 | 78 | M1 | Left | 3 | 0 | 1 | 1 | 1 | 3 |
| 10 | 61 | M1 | Left | 3 | 1 | 1 | 0 | 0 | 3 |
| 11 | 49 | M3 | Left | 1 | 0 | 0 | 0 | 0 | 3 |
| 12 | 83 | M1 | Right | 3 | 0 | 1 | 1 | 0 | 0 |
| 13 | 94 | ICA | Left | 3 | 0 | 1 | 1 | 1 | 0 |
| 14 | 61 | M1 | Right | 3 | 0 | 1 | 1 | 1 | 0 |
| 15 | 95 | M1 | Right | 3 | 0 | 1 | 0 | 0 | 2b |
| 16 | 61 | M1 | Left | 1 | 0 | 0 | 0 | 0 | 2b |
| 17 | 82 | M1 | Left | 1 | 0 | 0 | 0 | 0 | 2a |
| 18 | 92 | ICA | Left | 3 | 0 | 1 | 0 | 1 | 2b |
| 19 | 85 | ICA/M1 | Left | 3 | 1 | 1 | 1 | 1 | 0 |
| 20 | 74 | M2 | Left | 1 | 1 | 0 | 0 | 0 | 0 |
| 21 | 63 | M1 | Right | 2 | 1 | 0 | 0 | 1 | 2b |
| 22 | 87 | M3 | Left | 2 | 0 | 0 | 0 | 0 | 0 |
| 23 | 52 | M1 | Right | 1 | 0 | 0 | 0 | 0 | 2b |
| 24 | 68 | M1 | Left | 3 | 0 | 1 | 0 | 0 | 2b |
| 25 | 80 | ICA | Right | 2 | 1 | 0 | 0 | 0 | 2b |
| 26 | 26 | M2 | Right | 1 | 0 | 0 | 0 | 0 | 2b |
| 27 | 73 | M1 | Left | 2 | 0 | 0 | 0 | 0 | 2b |
| 28 | 88 | ICA | Left | 1 | 0 | 0 | 1 | 1 | 0 |
| 29 | 80 | M1 | Left | 1 | 0 | 0 | 0 | 0 | 2b |
| 30 | 59 | ICA | Right | 1 | 0 | 0 | 1 | 0 | 3 |
| 31 | 89 | M1 | Right | 3 | 1 | 1 | 0 | 0 | 0 |
| 32 | 67 | M1 | Right | 2 | 0 | 0 | 0 | 0 | 2b |
| 33 | 54 | M2 | Right | 1 | 0 | 0 | 0 | 0 | 2b |
| 34 | 81 | M1 | Left | 2 | 0 | 0 | 0 | 0 | 3 |
| 35 | 71 | M1 | Left | 1 | 0 | 0 | 1 | 0 | 3 |
| 36 | 59 | M2 | Right | 1 | 0 | 0 | 0 | 0 | 2b |
| 37 | 87 | M1 | Left | 2 | 0 | 0 | 0 | 0 | 3 |
| 38 | 77 | M1 | Right | 3 | 0 | 1 | 1 | 0 | 2b |
| 39 | 69 | ICA | Right | 2 | 0 | 0 | 0 | 0 | 2b |
| 40 | 80 | M1 | Left | 2 | 1 | 1 | 0 | 0 | 3 |
| 41 | 33 | M1 | Right | 1 | 0 | 0 | 0 | 0 | 2b |
| 42 | 58 | M1 | Left | 1 | 0 | 0 | 0 | 0 | 2b |
| 43 | 69 | M3 | Left | 3 | 1 | 1 | 0 | 0 | 2a |
| 44 | 56 | M1 | Right | 2 | 0 | 0 | 0 | 0 | 2b |
| 45 | 64 | M3 | Right | 2 | 0 | 0 | 0 | 0 | 2b |
| 46 | 90 | M1 | Right | 1 | 0 | 0 | 0 | 0 | 3 |
| 47 | 69 | ICA | Left | 2 | 1 | 1 | 1 | 1 | 1 |
| 48 | 76 | M1 | Right | 1 | 0 | 0 | 0 | 1 | 2b |
| 49 | 69 | ICA | Left | 2 | 0 | 0 | 1 | 0 | 2a |
| 50 | 57 | M1 | Right | 2 | 0 | 0 | 0 | 0 | 3 |
| 51 | 81 | M1 | Left | 2 | 0 | 0 | 0 | 0 | 2b |
| 52 | 66 | M1 | Left | 2 | 0 | 0 | 0 | 0 | 3 |
| 53 | 83 | M3 | Right | 3 | 1 | 1 | 0 | 1 | 0 |
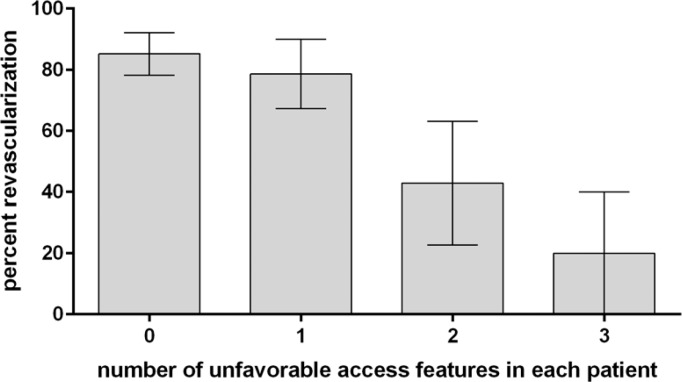
Difficult femoral artery access features were identified in 26 cases. These features included difficult arch access in 19 cases, common carotid loop or acute angle turn in 13 cases, and internal carotid loop or acute angle turn in 11 cases. Figures 2 and 3 show two demonstrative cases of difficult anatomical features. These features were associated with unsuccessful revascularization. Difficult arch access was associated with unsuccessful revascularization (p = 0.03, Fisher’s exact), with successful revascularization in 82% of patients with favorable arch anatomy compared to 53% of patients with difficult arch anatomy. Difficult common carotid artery access was associated with unsuccessful revascularization (p = 0.004, Fisher’s exact), with successful revascularization in only 5 of 13 such cases (38%), compared to 83% of cases without a loop or acute angle turn in the common carotid artery. Difficult internal carotid artery access was not statistically significant in regarding revascularization rates (p = 0.06, Fisher’s exact), though with revascularization of 5 of 11 such cases (45%) compared to 79% revascularization in cases with straightforward internal carotid artery configuration, there is a clear trend. It is likely that greater numbers will confirm this trend. Any combination of at least two difficult femoral artery access features was encountered in 12 cases, with successful revascularization in only 4 of these cases (33%). Multiple difficult access features correlated with unsuccessful revascularization (Figure 3). In a multivariate analysis, this was associated with the diminished revascularization independent of patient age, gender, and thrombus location (p = 0.009, Table 2).
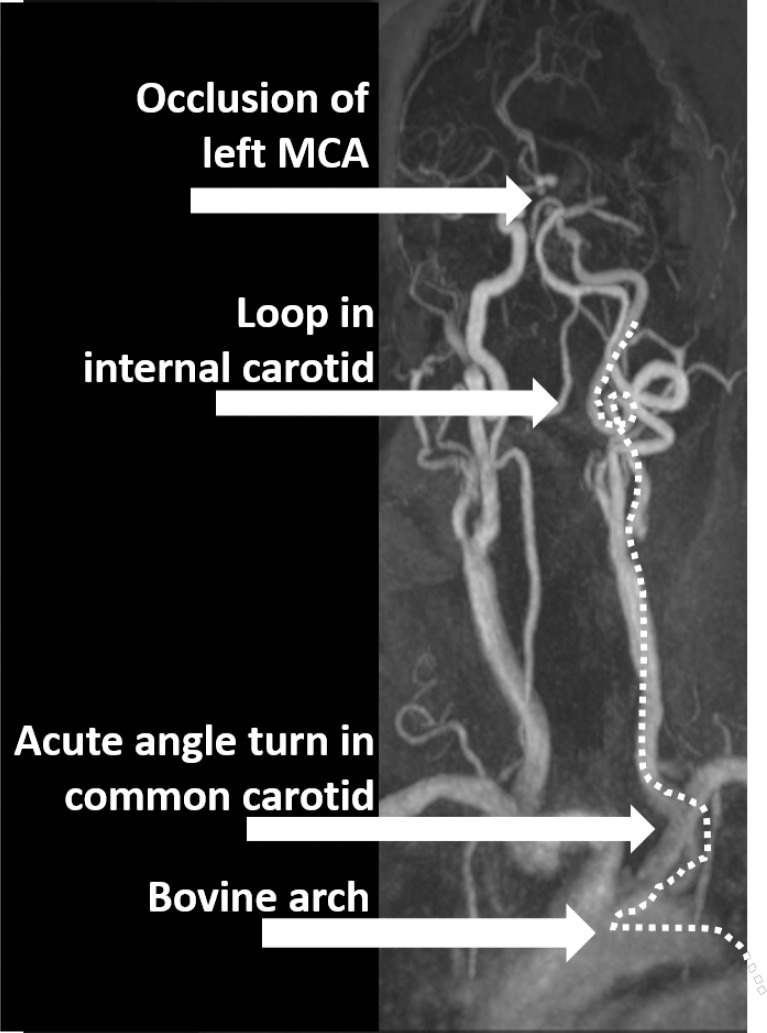
Figure 2. Case 47 presented with left middle cerebral artery occlusion. With the bovine arch configuration, acute angle turn in the common carotid artery, and 360° loop in the cervical internal carotid artery, insufficient access was obtained and a microcatheter was unable to be placed for stent-retriever or aspiration thrombectomy. The surgery was unsuccessful.
Figure 3. Two or more unfavorable vascular access features are associated with lower revascularization rates in emergent large vessel occlusion.
Table 2. Multivariate logistic regression analysis.
| Coefficient (B) | Standard error | Wald | p | Odds ratio | 95% C.I. for EXP(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Two or more unfavorable access features | –0.537 | 0.207 | 6.766 | 0.009 | 0.584 | 0.390 | 0.876 |
| M3 thrombus | –1.619 | 0.984 | 2.707 | 0.100 | 0.198 | 0.029 | 1.363 |
| Cervical occlusion | –1.768 | 1.474 | 1.438 | 0.231 | 0.171 | 0.009 | 3.070 |
| Age > 80 | –0.910 | 0.689 | 1.745 | 0.186 | 0.402 | 0.104 | 1.553 |
| Constant | –2.216 | 0.571 | 15.069 | 0.000 | 9.174 | ||
DISCUSSION
In this series, complicated anatomical features of vascular access were associated with diminished rates of successful revascularization in patients with large vessel occlusion who underwent emergent thrombectomy. Specifically, acute angle turns of the common carotid at its origin of the aortic arch and tortuous anatomy of the common carotid or cervical internal carotid artery alone or in combination were associated with lower revascularization rates. In many of these cases, the guide catheters were not positioned in a location permitting successful deployment of stent retrievers or aspiration catheters. These anatomical limitations translate to technical limitations which thereby limit the successful revascularization.
Revascularization in emergent large vessel occlusion is multifactorial; factors include the location and size of thrombus [4–6] and likely include thrombus composition [7–9]. It should be noted that several patients with straightforward arterial access did not have successful revascularization in this series. Although difficult access features are associated with lower revascularization rates, it is notable that several cases had successful revascularization despite their anatomic limitations.
Potentially, early identification of unfavorable access features would allow for alternate access techniques to be considered; carotid cutdown, for example, would subvert unfavorable aortic arch or common carotid features [10]. While potentially more morbid, this approach may be associated with better revascularization rates in patients with unfavorable arch or carotid anatomic features. As catheter technology evolves, the ability to place larger catheters in more distal positions of the intracranial vascular arbor will continue to improve [11, 12], and some aspects of technical difficulty in cases with unfavorable anatomy may be overcome via conventional femoral artery access.
This series is limited in size and contains patients treated by a single physician. Nevertheless, the correlation of unfavorable anatomic features of the aortic arch and carotid artery vascular tree with unsuccessful revascularization is strong and should not be discounted.
Figure 1. Case 9 presented with right M1 occlusion. Despite difficult access with Type III arch, common carotid acute angle turn, and internal carotid artery acute angle turn, successful thrombectomy was performed. This image represents postthrombectomy magnetic resonance angiography, displaying revascularized right middle cerebral artery.
Acknowledgements
None.
REFERENCES
- Dumont TM, et al. Understanding risk factors for perioperative ischemic events with carotid stenting: is patient age over 80 years or is unfavorable arch anatomy to blame? J Neurointerv Surg. 2014;6(3):219–224. doi: 10.1136/neurintsurg-2013-010721. [DOI] [PubMed] [Google Scholar]
- Fanous AA, et al. High-risk factors in symptomatic patients undergoing carotid artery stenting with distal protection: Buffalo Risk Assessment Scale (BRASS) Neurosurgery. 2015;77(4):531–542. doi: 10.1227/NEU.0000000000000871. ; discussion 542–533. [DOI] [PubMed] [Google Scholar]
- Higashida RT, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- Treurniet KM, et al. Clot burden score on baseline computerized tomographic angiography and intra-arterial treatment effect in acute ischemic stroke. Stroke. 2016;47(12):2972–2978. doi: 10.1161/STROKEAHA.116.014565. [DOI] [PubMed] [Google Scholar]
- Puetz V, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3(4):230–236. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- Mokin M, et al. Association of clot burden score with radiographic and clinical outcomes following Solitaire stent retriever thrombectomy: analysis of the SWIFT PRIME trial. J Neurointerv Surg. 2017;9(10):929–932. doi: 10.1136/neurintsurg-2016-012631. [DOI] [PubMed] [Google Scholar]
- Mokin M, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7(2):104–107. doi: 10.1136/neurintsurg-2013-011017. [DOI] [PubMed] [Google Scholar]
- Jagani M, et al. Correlation between clot density and recanalization success or stroke etiology in acute ischemic stroke patients. Interv Neuroradiol. 2017;23(3):274–278. doi: 10.1177/1591019917694478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Fernandez F, et al. Histopathological and bacteriological analysis of thrombus material extracted during mechanical thrombectomy in acute stroke patients. Cardiovasc Intervent Radiol. 2017;40(12):1851–1860. doi: 10.1007/s00270-017-1718-x. [DOI] [PubMed] [Google Scholar]
- Daou B, et al. Alternative access for endovascular treatment of cerebrovascular diseases. Clin Neurol Neurosurg. 2016;145:89–95. doi: 10.1016/j.clineuro.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Jindal G, et al. Navien catheter experience in neuroendovascular interventions. Interv Neuroradiol. 2017;23(5):551–555. doi: 10.1177/1591019917717575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, et al. Initial experience with SOFIA as an intermediate catheter in mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2016;9:1103–1106. doi: 10.1136/neurintsurg-2016-012750. [DOI] [PubMed] [Google Scholar]