Abstract
Aging has a profound impact on multiple facets of the immune system, culminating in aberrant functionality. The architectural disorganization of splenic white pulp is a hallmark of the aging spleen, yet the factors underlying these structural changes are unclear. Fibroblastic reticular cells comprise one stromal cell subset in the spleen that is important for maintenance of architectural organization, yet it remains to be determined how aging impacts these cells. In this study, we sought to determine how aging impacts splenic T cell zone reticular cell (TRC) numbers, morphology, and function. Using a mouse model of aging, we found that aged naive spleens have fewer TRCs than young spleens. This reduction in TRC number correlated with reduced CCL19 and CCL21 concentrations in aged spleens, which may contribute to impaired homing of T cells. CCL21 in both young and aged spleens localized with TRCs. Aged TRCs extended marginally into B cell follicles and may contribute to the blending of the T cell zone and B cell follicles in aged spleens. The described age-related changes in TRCs number and function may be an underlying factor contributing to impaired immune system function with age.
INTRODUCTION
Organization is a frequently overlooked component of an effective immune response (1). The likelihood of low-frequency, Ag-specific T cells interacting with the APC displaying their cognate Ag is amplified by multiple layers of organization, including secondary lymphoid organs (2–4). The spleen is one such organ located in the upper right portion of the abdomen (5). The spleen is attached to the vasculature (6) and is an important defense against bloodborne pathogens like encapsulated bacteria (7, 8). Blood enters into the spleen through blunt-ended central arterioles, which empty into the marginal sinus surrounding the splenic white pulp (6). From the marginal sinus, immune cells can use bridging channels to enter into the T cell zone (9). The T cell zone is surrounded by highly segregated B cell follicles in which germinal centers can develop and promote the production of high-affinity, classswitched Ab responses (10, 11).
Nonhematopoietic stromal cells provide the framework and directional cues to optimize the organization of immune cells in the splenic white pulp (12–14). In the spleen, there are multiple stromal cell subsets that localize to specific areas (15, 16). The splenic red pulp is populated with CXCL12-producing fibroblasts, which can attract CXCR4-expressing effector T cells and plasma cells away from the white pulp (17, 18). Red pulp fibroblasts also facilitate clearance of dying RBCs and direct blood flow (16). Blood vessels, including central arterioles, are lined with the stromal cell subset blood endothelial cells (BECs), which facilitate entry of cells and Ag into the splenic marginal zone (16). Fibroblastic reticular cells (FRCs) are the most abundant variety of stromal cell in the splenic white pulp (19). Multiple subsets of FRCs exist that localize to specific regions of the splenic white pulp and have distinct functional characteristics. FRCs can collectively be identified by their expression of the peptidoglycan podoplanin (PDPN), lack of expression of the vasculature marker CD31, and lack of hematopoietic lineage maker CD45 (16, 20). Marginal zone reticular cells are a MADCAM-1–expressing FRC subset that localize to the marginal zone. These cells produce CXCL13 and may facilitate trafficking of Ag into B cell follicles (21). T cell zone reticular cells (TRCs) form a lattice-like conduit network in the bridging channels and T cell zone upon which Ag, lymphocytes, and dendritic cells traffic (9, 14, 22). TRCs produce the homeostatic chemokines CCL19 and CCL21, which bind to CCR7-expressing cells to direct them into the T cell zone (12, 23, 24). A specialized subset for FRCs called follicular dendritic cells (FDCs) are located in the B cell follicles. FDCs produce the chemokine CXCL13, which interacts with CXCR5 on B cells and T follicular helper cells to direct them to the B cell follicle (25). FDCs also trap Agand provide cues to help B cells in the production of high-affinity Abs (26–28).
It is well established that aging leads to disorganized splenic architecture, characterized by merging of cells in the B cell follicles and T cell zone (29–33). Yet it is less clear how aging impacts the stromal cells underlying this organization (34). Although several studies have described age-related attrition of FDC function and morphology (35–38), it largely remains to be determined how aging impacts splenic TRCs. In this study, we sought to characterize how aging impacts the number, morphology, and function of splenic TRCs to further our understanding of the aging immune system.
Our results reveal that aged spleens have fewer TRCs and decreased T cell zone areas. Concomitant with the reduction of TRC number, homeostatic chemokine levels are reduced in aged spleens, correlating with impaired recruitment of young T cells. Interestingly, CCL21 largely colocalizes with TRCs and appears to extend slightly into the B cell follicles in aged but not young spleens. Reduction of TRC number and homeostatic chemokine concentrations may contribute to altered splenic architecture and impaired T cell homing found in aged spleens.
MATERIALS AND METHODS
Mice
Aged (18–20 mo) male C57BL/6J mice were obtained from the National Institute of Aging aging colony at Charles River Laboratories. Young male C57BL/6J mice (2–4 mo) were obtained from The Jackson Laboratory. CD45.1+ F5 RAG knockout (KO) mice expressing a transgenic TCR specific for a nucleoprotein (NP) peptide from H17 influenza were received as a generous gift from Dr. L. Cauley at the University of Connecticut School of Medicine and were used in transfer experiments at 2 mo of age (39). All mice were kept under specific pathogen-free conditions in sterilized, individually ventilated, high-efficiency particulate air–filtered cages. Mice were sacrificed by CO2 asphyxiation. The Institutional Animal Care and Use Committee at UConn Health reviewed and approved all experimental procedures using animals.
ELISA
Spleens were harvested, flash frozen, and stored at −80°C. Spleens were homogenized in 1 ml of T-PER (Thermo Fisher) with 5 μM EDTA (Invitrogen) and 1× Halt protease and phosphatase inhibitor mixture (Thermo Fisher). Homogenates were centrifuged at 1000 × g for 10 min, and supernatants were aliquoted and frozen at −80°C until analysis. CCL21 was measured using mouse CCL21/6Ckine DuoSet ELISA (R&D Systems). CCL19 was measured using mouse CCL19/MIP-3 beta DuoSet ELISA (R&D Systems). Chemokine concentrations were normalized to total protein concentration, determined using Pierce BCA Protein Assay (Thermo Fisher).
T cell transfer
CD8+ T cells were isolated from pooled spleens and lymph nodes of CD45.1+ F5 RAG KO mice using MojoSort Mouse CD8 T cell Isolation Kit (BioLegend) according to the manufacturer’s instructions. Before transfer, the purity of the isolated CD8+ T cells was confirmed at 85% or above. A total of 2.3 × 106 CD8+ T cells in 200 μl PBS were injected via the tail vein into each mouse. Mice were sacrificed exactly 30 min after transfer for analysis.
Immunofluorescence
Spleens were fixed overnight in medium containing 0.05 M phosphate buffer, 0.1 M L-lysine (pH 7.4), 2 mg/ml NaIO4, and 10 mg/ml paraformaldehyde and then dehydrated with 30% sucrose in phosphate buffer (33). Spleens were frozen in Tissue-Tek O.C.T. Compound (Sakura Finetek), and 20-μm sections were cut using a CM1850 Cryostat (Leica). Sections were blocked for 30 min with 2% goat serum and stained overnight at 4°C in a humidified chamber with the following primary Ab mixture: anti-PDPN clone 8.1.1 unconjugated (Origene) (1:300 dilution), anti-B220 PE-Dazzle AF594 clone RA3–6BP (BioLegend) (1:100 dilution), anti-CD31 AF647 clone MEC13.3 (BioLegend) (1:100 dilution), and anti-CD8α BV421 clone 53.6.7 (BioLegend) (1:100 dilution) in 2% goat serum. Sections were then washed in PBS and stained for 2 h at room temperature with goat anti-hamster AF488 (Invitrogen) (1:400 dilution). For detection of CCL21, sections were blocked as described and stained overnight with biotinylated anti-CCL21 Ab (R&D) (1:20 dilution) in 2% goat serum with 0.3% Triton X-100 (Sigma-Aldrich). After PBS washes, CCL21 signal was amplified using Alexa Fluor 594 Tyramide SuperBoost Kit and streptavidin (Thermo Fisher) according to manufacturer’s instructions. After staining, slides were mounted with Immu-Mount (Thermo Fisher) and coverslipped. Images were acquired using the Zeiss 780 Laser Scanning Microscope (air objective 20× Plan-Apochromat with numerical aperture 0.5 or water objective 10× C-Apochromat with numerical aperture 0.45; Carl Zeiss) using multichannel frame scans. The ZEN Black software (Carl Zeiss) was used for image acquisition. For full spleen images, the ZEN Black tile scan function was used to stitch individual 10× images together. PDPN, CD31, CD8α, and B220 areas were quantified by creating an isosurface of the staining of 20× images, and image processing was performed using Imaris 8.1 software (Bitplane).
Splenic stromal cell digestion/flow cytometry
To quantify the number of stromal cells in the spleen by flow cytometry, spleens were cut into 2.5-mm pieces and then enzymatically and mechanically digested as previously described (40). The chopped spleens were placed in 2 ml of enzyme mix composed of RPMI 1640 with 0.2 mg/ml of Collagenase P (Roche), 0.8 mg/ml Dispase II (Roche), and 0.1 mg/ml DNAse 1 (Sigma-Aldrich) and incubated for 20 min in a 37°C water bath, inverting every 5 min. The chopped spleens were gently pipetted up and down three times using a 1-ml pipette tip. The fragments were allowed to settle for 30 s, and the supernatant containing the cells released by digestion was collected into a tube containing 10 ml of ice-cold FACS buffer made from 2% FBS, 5 mM EDTA, and PBS and was centrifuged for 4 min at 300× g and 4°C. Two milliliters of freshly prepared enzyme mix was added to the pellet and incubated for an additional 10 min in the 37°C water bath. The spleens were then vigorously pipetted for 30 s using a 1-ml pipette tip. Fragments were again allowed to settle for 30 s, and the digested cells in the supernatant were added to 10 ml of FACS buffer and centrifuged. Two milliliters of new enzyme mix was added to the pellet and then incubated in the 37°C water bath. Every 5 min until the spleens were completely digested (no visible aggregates), the cells were vigorously pipetted for 30 s. On average, the aged spleens took ~5–10 min longer to digest than the young spleens. RBCs were lysed for 5 min at room temperature in ACK Lysing Buffer (Thermo Fisher Scientific). After digestion, cell number and viability were quantified using the Cellometer Auto 2000 (Nexcelom Biosciences) with acridine orange and propidium iodide. Five million viable splenocytes were stained for flow cytometric quantification of splenic stromal cell populations. Cells were stained in an Ab mixture of 100 μl in FACS buffer: anti-CD16/CD32 clone 93 Fc block unconjugated (Thermo Fisher), anti-PDPN Alexa Fluor 594 clone 8.1.1 (BioLegend), anti-CD45 PerCPcy5.5 clone 30F-11(BioLegend), anti-CD31 APC clone MEC13.3 (BioLegend), and Carboxylic Acid Succinimidyl Ester Alexa Fluor 350 (Life Technologies). Fluorescence minus one controls were created for each Ab and used to set gates for analysis. Without fixation, samples were acquired immediately on an LSRII flow cytometer (BD Biosciences). Flow cytometry data analysis was performed using Flow Jo software (Tree Star).
Statistical analysis
Data was tested for normality using the F test. For normally distributed data, an unpaired two-tailed t test was used to determine significance. For data with unequal variances, analysis was performed using the Mann–Whitney U test. Significance was set at p ≤ 0.05. Statistical analysis was performed using GraphPad Prism 6 software.
RESULTS
Reduction of FRC numbers in aged naive spleens
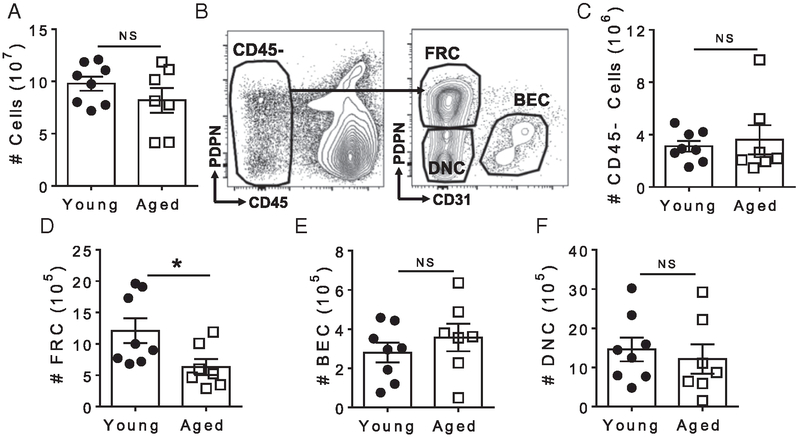
Despite the well-characterized disruption of splenic white pulp architecture with age, it is unclear how aging impacts the splenic stromal cell, which is critical for architectural organization. We first sought to quantify the number of stromal cells in both young and aged spleens. Using a rigorous mechanical and enzymatic digestion, stromal cells were isolated out of spleens and quantified using flow cytometry (40). No significant differences were found in either the total number of cells (Fig. 1A), CD45− stromal cells (Fig. 1C), BECs (Fig. 1E), or double-negative (CD31−PDPN−) splenic red pulp fibroblasts (double-negative cells [DNCs]) (Fig. 1F) in young and aged spleens. Interestingly, aged spleens had significantly fewer FRCs (Fig. 1D) as gated in Fig. 1B. The total number of CD45+ immune cells in young and aged spleens was also not significantly different (Supplemental Fig. 1).
FIGURE 1. Reduction of FRC number in aged spleens.
Spleens were mechanically and enzymatically digested to liberate stromal cells for flow cytometric quantification. (A) Total number of nucleated cells in young and aged spleens. (B) Samples were gated on live single cells that were CD45− (left). FRCs (PDPN+CD31−), BECs (PDPN−CD31+), and DNCs (PDPN− CD31−) were further gated as shown (right). (C) Numbers of CD45− stromal cells, (D) FRCs, (E) BECs, and (F) DNCs in young and aged spleens. Data are pooled from three independent experiments (n = 7–8 mice per group). Error bars = ±SEM. Statistical significance was determined by two-tailed t test. *p < 0.05.
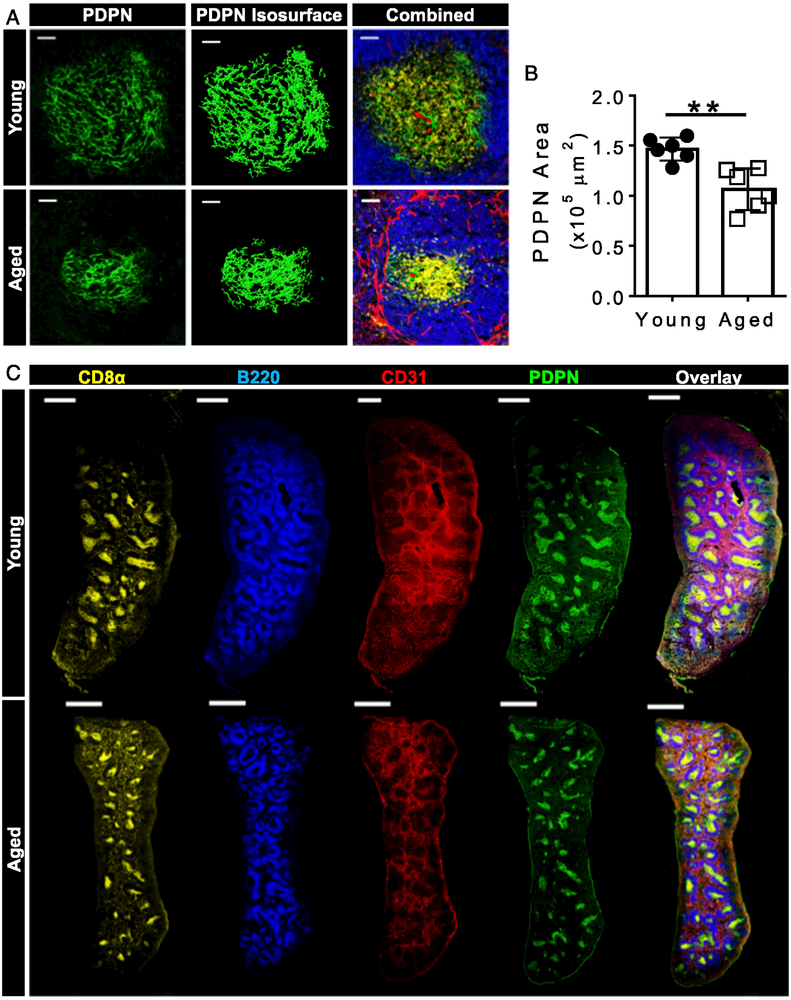
Although flow cytometry is a powerful tool, digestion of stromal cells from spleens is an imperfect method and may underrepresent thetruenumberofcells present. To further validate the reduction of FRC numbers and to determine if TRCs were reduced in aged spleens, quantitative confocal microscopy was used. T cell zone FRCs were identified by their expression of PDPN (Fig. 2A, left) and their localization under CD8+ T cells in the T cell zone (Fig. 2A, right). A digital isosurface rendering of the PDPN staining was created using Imaris imaging software (Fig. 2A, middle). Quantification of the PDPN isosurfaces revealed that the area of aged splenic TRCs in the white pulp were smaller than their young counterparts (Fig. 2B), confirming flow cytometric quantification (Fig. 1D). Full spleen images (Fig. 2C) provide further support to the reduction of TRC area in aged spleens. In agreement with the reduction in PDPN area in the splenic white pulp, there was also a reduction in the area of CD8a staining (Supplemental Fig. 2A, 2B), correlating loss of TRCs with reduced T cell zone area. Neither the area of the B cell zone (Supplemental Fig. 2A, 2C) or the area of BECs (Supplemental Fig. 2A, 2D) showed significant reduction in young compared with aged spleens. Full spleen images (Fig. 2C) provide further support to the reduction of the T cell zone in aged spleens. These results provide evidence that aged spleens have reduced numbers of TRCs but preserved numbers of other stromal cell subsets. We next sought to determine if TRC function was altered with age.
FIGURE 2. Smaller T cell zone FRC areas in aged spleens.
(A) Image analysis pipeline for splenic FRC quantification. Representative images from young (top) and aged (bottom) splenic white pulp. FRCs were identified by expression of PDPN (left). A digital rendering of the PDPN staining was created using Imaris isosurface function (middle). Combined image of CD8α (yellow), B220 (blue), CD31 (red), and PDPN (green) (right). Scale bar, 50 μm. (B) PDPN area quantification was accomplished by averaging the area of four different white pulp 20× z stack images (1-µm step size, four steps) per mouse (six mice per group) (C) Longitudinal images of entire spleens from naive young (top) and aged (bottom) mice. Scale bar, 200 µm. Data are representative of n = 6 mice per group. Error bars = ±SEM. Statistical significance was determined by two-tailed t test. **p < 0.01.
Attrition of aged splenic TRC function
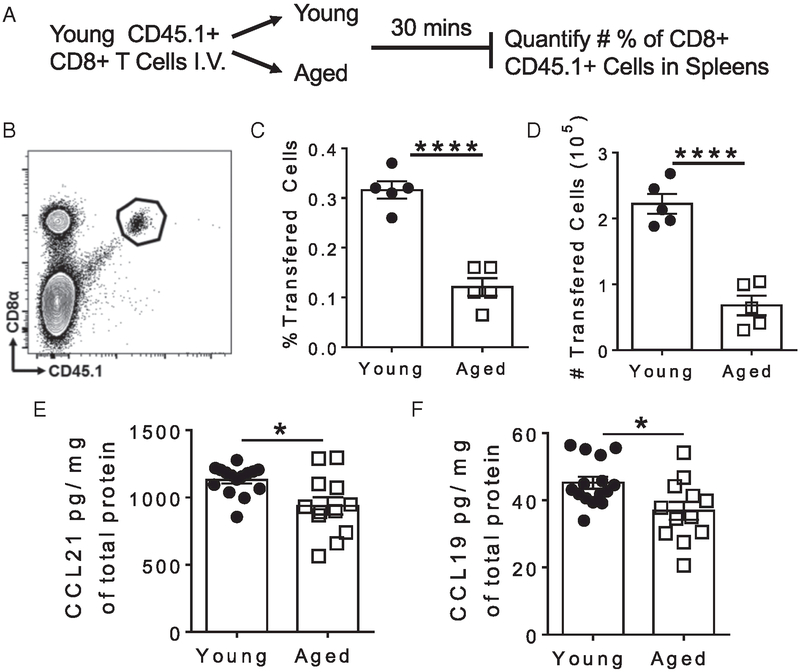
TRC-produced homeostatic chemokines CCL19 and CCL21 are important cues directing the localization and entry of immune cells into the T cell zone of the splenic white pulp (12, 23, 24). Mice deficient in CCL19 and CCL21 have severe deficiencies in the recruitment of T cells into the spleen (41). It has been previously reported that young CD4+ T cells have reduced recruitment into aged spleens compared with young spleens after Ag challenge (29, 42). To determine the impact of aging on CD8+ T cell recruitment, we transferred young CD8+ T cells into young and aged hosts and examined homing after 30 min, a time point where exit was unlikely (7) (Fig. 3A, 3B). In agreement with other studies, we found a similar reduction in the frequency (Fig. 3C) and number (Fig. 3D) of young transferred CD8+ T cells in aged spleens when compared with young spleens. Yet it remains unclear how aging impacts the concentrations and localization of homeostatic chemokines, which are important for recruitment of T cells into the spleen. Quantification of CCL21 (Fig. 3E) and CCL19 (Fig. 3F) protein levels from homogenized spleens revealed a reduction in the concentrations of both chemokines in aged spleens compared with young. Thus, the reduced homeostatic chemokine concentrations in aged spleens may contribute to the impaired recruitment of young CD8+ T cells from the vasculature into aged spleens.
FIGURE 3. Reduced homeostatic chemokines in aged spleens correlate with impaired recruitment of young T cells.
The ability of aged and young spleens to recruit young T cells was tested using the experimental design outlined in (A). Transferred young T cells were identified in young or aged spleens by gating on lymphocytes, single cells, viable cells, CD45+ cells, and (B) CD8α+CD45.1+ cells. (C) Frequency and (D) number of young transferred CD8+ T cells in young or aged spleens. (E) CCL21 and (F) CCL19 levels were quantified in homogenized naive spleens and normalized to total protein. (B–D) Data are one representative experiment of three performed independently (n = 4–5 mice per group). (E and F) Data are pooled from two independent experiments (n = 18–20 mice per group). Error bars = ±SEM. Statistical significance was determined by two-tailed t test. *p < 0.05, ****p < 0.0001.
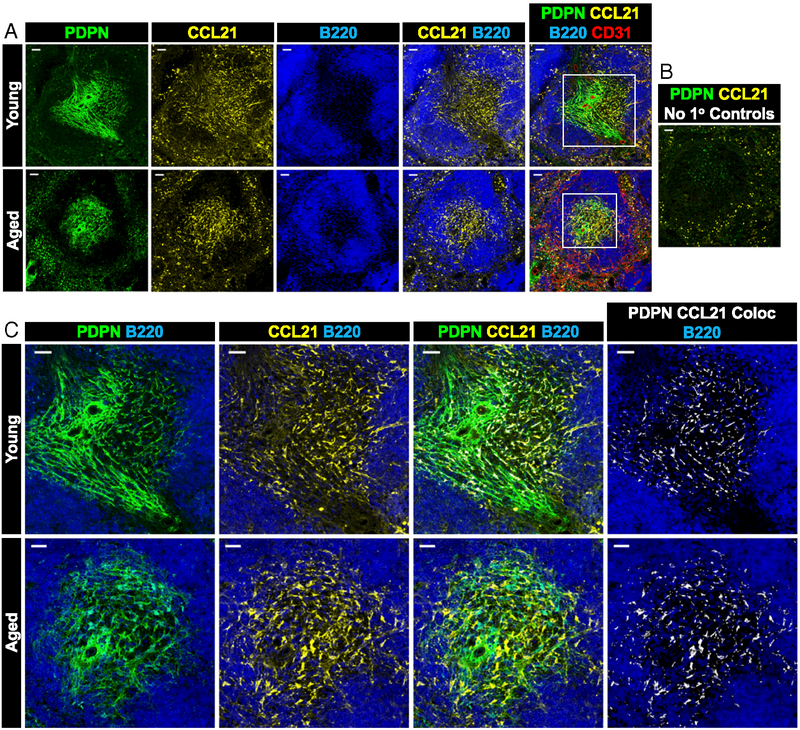
We have previously shown that following challenge with OVA protein and alum adjuvant, aged spleens exhibit altered CCL21 localization, spreading from the T cell zone into the B cell follicles (29). Because antigenic challenge has been shown to dramatically alter homeostatic chemokine concentration and localization (43), we next sought to determine if CCL21 localization in young and aged spleens at homeostasis was equally disrupted. Because of low concentrations, CCL19 was not detectable by microscopy in young or aged spleens, even with the use of amplification reagents (data not shown). CCL21 in both young and aged spleens localized with PDPN+ TRCs (Fig. 4A, 4C). Aged TRCs appeared to extend marginally into the B cell follicles, where there was less overlap in young spleens (Fig. 4A, 4C). Of note, despite blocking, some nonspecific punctate background stainingfor CCL21 was found in the splenic red pulp surrounding the B cell follicles (Fig. 4B). These data suggest that aging reduces TRC-produced homeostatic chemokine concentration, which localize near TRCs and may contribute to the reduced recruitment of young T cells into aged spleens.
FIGURE 4. CCL21 localizes to T cell zone FRCs in young and aged spleens.
(A) Representative images of young (top) and aged (bottom) splenic white pulp showing the localization of CCL21 to the T cell zone in which FRCs localize. (B) Negative controls for PDPN and CCL21 staining. Insets in (A) are magnified in (C), and colocalization of CCL21 and PDPN is shown in white (far right). Representative images from two independent experiments with n = 3 mice per group. Scale bar, 50 μm.
DISCUSSION
Aging is a multifactorial process that has profound effects on immune system function. Despite the extensive characterization of the aging immune system, many facets have yet to be rigorously cataloged and described. Several reports have implied that the environment of aged secondary lymphoid organs hinders the development of robust T cell responses (33, 42, 44, 45). We have previously reported that young OVA-specific CD4+ T cells transferred into aged hosts have delayed proliferation, reduced expansion, and impaired differentiation into T follicular helper cells compared with those transferred into young hosts following OVA immunization (29). In the aged hosts, the donor cells had impaired localization to the splenic T cell zones of the white pulp 18 h after immunization. This was in part due to altered localization of CCL21 (29). Although this report hinted at TRC dysfunction, it was not directly examined. We also found that the aged splenic environment is rich in active TGF-β and promotes the differentiation of young CD4+ T cells into regulatory T cells, which may contribute to impaired T cell and B cell immunity (33).
Studies have shown similar findings for young CD8+ T cells transferred into aged mice. Young influenza-specific clone 4 CD8+ T cells, when transferred into young or aged hosts that had been i.v. infected with influenza virus, had reduced expansion and decreased IFN-γ production in aged spleens (46). Another study found that alter i.v. Listeria infection, young CD8+ T cells transferred into aged mice had reduced expansion in the spleen. This study linked poor CD8+ T cell responses to reduced CD8α dendritic cell responses and did not consider the implications of the disorganized splenic architecture or stromal cell dysfunction (44),
In this current study, we sought to directly examine how aging impacts one component of the splenic microenvironment: stromal cells. The disorganized architecture of the splenic white pulp led us to hypothesize that aging may influence FRC number and function because of their important role in orchestration of splenic architecture (12, 19). We found that aged spleens had fewer TRCs, which results in smaller TRC area in the T cell zone of the splenic white pulp. We also described how aged spleens have decreased concentrations of homeostatic chemokines CCL19 and CCL21, which is corroborated by previous studies (29). We found that CCL21 localizes near FRCs, which extend marginally into the B cell follicles of the aged splenic white pulp and may contribute to architectural disruption. CCL21 localization in aged spleens at homeostasis did not appear to be as dramatically altered after antigenic challenge as previously reported, which may correlate with more dramatic changes in architecture after challenge (29). We also found that decreased homeostatic chemokine concentrations correlated with reduced homing of young T cells into aged compared with young spleens. Along with reductions in CCL19 and CCL21 concentrations, other alterations in aged spleens, like changes to splenic arteries, may contribute to T cell homing defects and remain to be examined.
FRCs are functionally important for the organization of the splenic white pulp but are most likely only one component contributing to the age-related changes in the spleen. Changes in chemokine receptor expression on immune cells and their ability to respond to chemotactic gradients has also been observed and may add to altered architecture (33, 47–51). The mechanism behind why there are fewer FRCs in aged spleens also remains to be determined. One possible explanation may be that there are interruptions to important maintenance signals via lymphotoxin β receptor (52–56). Whether aging impacts lymphotoxin β receptor expression on stromal cells or lymphotoxin β expression on immune cells remains to be determined.
Apart from their role in architectural organization, recent studies have elucidated roles for FRCs in the control (57, 58), survival (59, 60), and initiation (41) of T cell responses. It remains to be determined if aging impacts any of these functional attributes of FRCs. Whether lymph node stromal cells experience similar changes to splenic stromal cells also remains unclear (38, 61–63). On a broader scale, it also remains to be determined if splenic and lymph node FRCs are of similar origin and composition. Erosion of secondary lymphoid organ stromal cell function may have far-reaching effects on multiple facets of the aged immune system, and despite the advances made in this article, we are still in the infancy of understanding how aging impacts these cells.
Supplementary Material
Digested single cell suspensions from young and aged spleens were gated on alive, signal cells that are CD45+. Data are from one representative of three independent experiments. Data was analyzed for statistical significance using a two tailed T-test n=3–4 mice per group, error bars =+SEM.
A. Representative isosurfaces of CD8a (yellow), B220 (blue), CD31 (red) in splenic white pulp. Quantification of isosurface area for B. CD8a, C. B220, and D. CD31. Data are pooled from two independent experiments n=6 mice per group. Statistical significance was determined by students T-test, error bars =±SEM.
ACKNOWLEDGMENTS
We acknowledge Jenna Bartley, Erica Lorenzo, Jacob Hopkins, Spencer Keilich, Jacob Hopkins, Judith Kalinowski, and Sandra Jastrzebski for technical assistance with experiments. We also acknowledge Linda Cauley for supplying the F5 RAG KO mice.
This work was supported by National Institute on Aging Program Grant AG021600 and the UConn Health Center on Aging.
Abbreviations used in this article:
- BEC
blood endothelial cell
- DNC
double-negative cell
- FDC
follicular dendritic cell
- FRC
fibroblastic reticular cell
- KO
knockout
- PDPN
podoplanin
- TRC
T cell zone reticular cell
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Qi H, KastenmUller W, and Germain RN. 2014. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol 30: 141–167. [DOI] [PubMed] [Google Scholar]
- 2.Obar JJ, Khanna KM, and Lefrancois L. 2008. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity 28: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattman JN, Antia R, Sourdive DJD, Wang X, Kaech SM, Murali-Krishna K, Altman JD, and Ahmed R. 2002. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med 195: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu W, Jiang N, Ebert PJR, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, et al. 2015. Clonal deletion prunes but does not eliminate self-specific αβ CD8(+) T lymphocytes. Immunity 42: 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiniger B, and Barth P. 2000. Microanatomy and function of the spleen. Adv. Anat. Embryol. Cell Biol 151: III–IX, 1–101. [DOI] [PubMed] [Google Scholar]
- 6.Mebius RE, and Kraal G. 2005. Structure and function of the spleen. Nat. Rev. Immunol 5: 606–616. [DOI] [PubMed] [Google Scholar]
- 7.Perez OA, Yeung ST, Vera-Licona P, Romagnoli PA, Samji T, Ural BB, Maher L, Tanaka M, and Khanna KM. 2017. CD169+ macrophages orchestrate innate immune responses by regulating bacterial localization in the spleen. Sci. Immunol DOI: 10.1126/sciimmunol.aah5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deodhar HA, Marshall RJ, and Barnes JN. 1993. Increased risk of sepsis after splenectomy. BMJ 307: 1408–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajenoff M, Glaichenhaus N, and Germain RN. 2008. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J. Immunol 181: 3947–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen CDC, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, and Cyster JG. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol 5: 943–952. [DOI] [PubMed] [Google Scholar]
- 11.Allen CDC, Okada T, and Cyster JG. 2007. Germinal-center organization and cellular dynamics. Immunity 27: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luther SA, Tang HL, Hyman PL, Farr AG, and Cyster JG. 2000. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 97: 12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo VN, Cornall RJ, and Cyster JG. 2001. Splenic T zone development is B cell dependent. J. Exp. Med 194: 1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolte MA, Belien JAM, Schadee-Eestermans I, Jansen W, Unger WWJ, van Rooijen N, Kraal G, and Mebius RE. 2003. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med 198: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Haan JM, Mebius RE, and Kraal G. 2012. Stromal cells of the mouse spleen. Front. Immunol 3: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller SN, and Germain RN. 2009. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol 9: 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou Y-R, Littman DR, and Cyster JG. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med 194: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Vliet E, Melis M, Foidart JM, and Van Ewijk W. 1986. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem 34: 883–890. [DOI] [PubMed] [Google Scholar]
- 19.Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, et al. 2014. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol 15: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, and Aruffo A. 1992. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med 176: 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katakai T 2012. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front. Immunol 3: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worbs T, Mempel TR, Bolter J, von Andrian UH, and Forster R. 2007. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med 204: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, and Butcher EC. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol 6: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bromley SK, Thomas SY, and Luster AD. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol 6: 895–901. [DOI] [PubMed] [Google Scholar]
- 25.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, and Cyster JG. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406: 309–314. [DOI] [PubMed] [Google Scholar]
- 26.Nossal GJ, Ada GL, and Austin CM. 1965. Antigens in immunity. X. Induction of immunologic tolerance to Salmonella adelaide flagellin. J. Immunol 95: 665–672. [PubMed] [Google Scholar]
- 27.Papamichail M, Gutierrez C, Embling P, Johnson P, Holborow EJ, and Pepys MB. 1975. Complement dependence of localisation of aggregated IgG in germinal centres. Scand. J. Immunol 4: 343–347. [DOI] [PubMed] [Google Scholar]
- 28.Tew JG, and Mandel TE. 1979. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology 37: 69–76. [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, and Haynes L. 2012. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. AgingCell 11: 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birjandi SZ, Ippolito JA, Ramadorai AK, and Witte PL. 2011. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J. Immunol 186: 3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aw D, Hilliard L, Nishikawa Y, Cadman ET, Lawrence RA, and Palmer DB. 2016. Disorganization of the splenic microanatomy in ageing mice. Immunology 148: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wols HA, Johnson KM, Ippolito JA, Birjandi SZ, Su Y, Le PT, and Witte PL. 2010. Migration of immature and mature B cells in the aged microenvironment. Immunology 129: 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefebvre JS, Masters AR, Hopkins JW, and Haynes L. 2016. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep 6: 25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters AR, Haynes L, Su D-M, and Palmer DB. 2017. Immune senescence: significance of the stromal microenvironment. Clin. Exp. Immunol 187: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aydar Y, Balogh P, Tew JG, and Szakal AK. 2004. Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response. Ageing Res. Rev 3: 15–29. [DOI] [PubMed] [Google Scholar]
- 36.Aydar Y, Balogh P, Tew JG, and Szakal AK. 2002. Age-related depression of FDC accessory functions and CD21 ligand-mediated repair of co-stimulation. Eur. J. Immunol 32: 2817–2826. [DOI] [PubMed] [Google Scholar]
- 37.Aydar Y, Balogh P, Tew JG, and Szakal AK. 2003. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J. Immunol 171: 5975–5987. [DOI] [PubMed] [Google Scholar]
- 38.Turner VM, and Mabbott NA. 2017. Structural and functional changes to lymph nodes in ageing mice. Immunology 151: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mamalaki C, Elliott J, Norton T, Yannoutsos N, Townsend AR, Chandler P, Simpson E, and Kioussis D. 1993. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev. Immunol 3: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M, and Turley SJ. 2011. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori S, Nakano H, Aritomi K, Wang C-R, Gunn MD, and Kakiuchi T. 2001. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med 193: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richner JM, Gmyrek GB, Govero J, Tu Y, van der Windt GJW, Metcalf TU, Haddad EK, Textor J, Miller MJ, and Diamond MS. 2015. Age-dependent cell trafficking defects in draining lymph nodes impair adaptive immunity and control of west nile virus infection. PLoS Pathog. 11: e1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, and Matloubian M. 2007. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317: 670–674. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Smithey MJ, Rudd BD, and Nikolich-Zugich J. 2012. Age-associated alterations in CD8a+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging Cell 11: 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefebvre JS, and Haynes L. 2013. Vaccine strategies to enhance immune responses in the aged. Curr. Opin. Immunol 25: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang J, Fisher EM, and Murasko DM. 2011. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res. Rev 10: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, and Leng SX. 2008. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J. Am. Geriatr. Soc 56: 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, and Yung RL. 2003. T cell chemokine receptor expression in aging. J. Immunol 170: 895–904. [DOI] [PubMed] [Google Scholar]
- 49.Park J, Miyakawa T, Shiokawa A, Nakajima-Adachi H, Tanokura M, and Hachimura S. 2014. Attenuation of migration properties of CD4+ T cells from aged mice correlates with decrease in chemokine receptor expression, response to retinoic acid, and RALDH expression compared to young mice. Biosci. Biotechnol Biochem 78: 976–980. [DOI] [PubMed] [Google Scholar]
- 50.Yung R, Mo R, Grolleau-Julius A, and Hoeltzel M. 2007. The effect of aging and caloric restriction on murine CD8+ T cell chemokine receptor gene expression. Immun. Ageing 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yung RL, and Mo R. 2003. Aging is associated with increased human T cell CC chemokine receptor gene expression. J. Interferon Cytokine Res 23: 575–582. [DOI] [PubMed] [Google Scholar]
- 52.Kumar V, Dasoveanu DC, Chyou S, Tzeng T-C, Rozo C, Liang Y, Stohl W, Fu Y-X, Ruddle NH, and Lu TT. 2015. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity 42: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endres R, Alimzhanov MB, Plitz T, Fiitterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewsky K, and Pfeffer K. 1999. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J. Exp. Med 189: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, and Cyster JG. 1999. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med 189: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackay F, Majeau GR, Lawton P, Hochman PS, and Browning JL. 1997. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol 27: 2033–2042. [DOI] [PubMed] [Google Scholar]
- 56.Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C, Cupovic J, Danuser R, Sparwasser T, Luther SA, Thiel V, et al. 2013. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38: 1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan O, Headley M, Gerard A, Wei W, Liu L, and Krummel MF. 2011. Regulation of T cell priming by lymphoid stroma. PLoS One 6: e26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, and Turley SJ. 2011. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol 12: 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, and Luther SA. 2007. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol 8: 1255–1265. [DOI] [PubMed] [Google Scholar]
- 60.Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, et al. 2012. IL-7-producing stromal cells are critical for lymph node remodeling. Blood 120: 4675–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denton AE, Roberts EW, Linterman MA, and Fearon DT. 2014. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 111: 12139–12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies JS, Thompson HL, Pulko V, Torres JP, and Nikolich-Z ugich J. 2017. Role of cell-intrinsic and environmental age-related changes in altered maintenance of murine T cells in lymphoid organs. J. Gerontol. A Biol. Sci. Med. Sci DOI: 10.1093/gerona/glx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, Spasova DS, Sarkisyan G, LeRoy E, Tan JT, et al. 2016. The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci. Rep 6: 30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Digested single cell suspensions from young and aged spleens were gated on alive, signal cells that are CD45+. Data are from one representative of three independent experiments. Data was analyzed for statistical significance using a two tailed T-test n=3–4 mice per group, error bars =+SEM.
A. Representative isosurfaces of CD8a (yellow), B220 (blue), CD31 (red) in splenic white pulp. Quantification of isosurface area for B. CD8a, C. B220, and D. CD31. Data are pooled from two independent experiments n=6 mice per group. Statistical significance was determined by students T-test, error bars =±SEM.