Abstract
Malignant melanoma typically metastasizes to lymph nodes (LNs) as a primary or in-transit lesion before secondary metastasis occurs, and LN biopsy is a common procedure to diagnose melanoma progression. Since cancer metastasis is a complex process where various interactions between tumor cells and the stroma play key roles in establishing metastatic lesions, the exact mechanisms underlying melanoma metastasis to LNs remains unknown. It has been known that focal adhesion kinase (FAK) activity promotes the expression of proinflammatory vascular cell adhesion molecule-1 (VCAM-1). As VCAM-1 is a major receptor for α4 integrin and plays a key role in leukocyte recruitment, we reasoned that inhibition of FAK activity may reduce VCAM-1 expression within LNs and thus reduce metastasis of α4 integrin-expressing melanoma to LNs. First, we found that a pharmacological FAK inhibitor, PF-271, blocked tumor necrosis factor-α (TNF-α)-mediated VCAM-1 expression on human dermal lymphatic endothelial cells (HDLECs). In vitro, PF-271 significantly decreased B16F10 melanoma adhesion to and transmigration through HDLECs compared to TNF-α treated cells. Furthermore, in vivo FAK inhibition by oral PF-271 administration reduced VCAM-1 expression in inguinal, cervical, and popliteal LNs compared to vehicle treated mice. Finally, in a footpad metastasis model, B16BF10 melanoma cells were injected into the right footpad of C57BL/6 mice, and PF-271 (50 mg/kg, twice daily for 6 days) was orally administrated after 1 week of tumor transplantation. While untreated mice exhibited significant metastatic melanoma lesions in popliteal LNs, PF-271 treated mice showed only marginal melanoma metastasis. These results support the possibility that FAK inhibitors may be a novel preventative option in melanoma metastasis by blocking VCAM-1 expression in LNs.
Keywords: FAK, VCAM-1, α4 integrin, lymph node, melanoma, metastasis
Introduction
Tumor metastasis, as opposed to primary tumor growth, is a major cause of cancer mortality [1, 2]. Tumor metastasis is a complex, multi-step process by which tumor cells must first gain the ability to migrate away from the original tumor and enter either the bloodstream or the lymphatic system in a process known as intravasation [3]. Next, the cancer cell must be able to attach to blood or lymph vessel and enter the surrounding tissue, known as extravasation [3]. Only a few of these tumor cells that undergo intravasation can survive in circulation, and successfully colonize distant tissues [3]. Several components of the tumor stroma, such as modified extracellular matrix and cell adhesion molecules induced by local inflammation, play key roles in promoting cancer metastasis [4, 5].
The primary or in-transit site of metastasis for many cancers, including melanoma, are nearby sentinel lymph nodes (LNs) [6]. As such, sentinel LN biopsy is a common procedure to diagnose and stage melanoma progression [7, 8]. Sentinel LNs are often the primary site of metastasis due to tumor cell-stromal cell interactions that lead to increased expression of growth factors, such as vascular endothelial growth factor-C (VEGF-C) and VEGF-B, that promote lymphangiogensis to the primary tumor [9]. As a tissue with a high rate of immune cell trafficking, LNs express several cell adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecules (ICAMs), that slow down and arrest immune cells. However, the exact mechanism of melanoma metastasis to lymph nodes remains unknown.
Focal adhesion kinase (FAK) is a protein tyrosine kinase that is activated via integrins and growth factor receptors [10]. Activation of FAK plays a critical role in cellular migration and proliferation as well as tumor metastasis and angiogenesis [11, 12]. A FAK kinase-dead knockin mouse was found to be embryonic lethal at E9.5 due to loss of VCAM-1, which is critical for chorion-allantois fusion [13]. Recently, we found that pharmacological FAK inhibition also reduced proinflammatory VCAM-1 expression in human endothelial cells (ECs) [14]. Since LNs constantly express VCAM-1 [15] and most melanomas that express α4 integrin (a ligand for VCAM-1) showed greater metastatic potential to regional LNs [16, 17], we hypothesized that FAK inhibition could reduce VCAM-1 expression in LNs and thus block metastasis of α4 integrin-expressing melanoma to LNs.
In this study, we used the α4 integrin-expressing murine B16F10 melanoma to test pharmacological FAK inhibition (PF-271) on VCAM-1 expression and melanoma metastasis to LNs. Both in vitro and in vivo FAK inhibition reduced VCAM-1 expression in the lymphatic ECs. Furthermore, FAK inhibition reduced B16F10 adhesion to and migration through human dermal lymphatic ECs. Finally, using a mouse footpad metastasis model, we found that FAK inhibition effectively diminished B16F10 melanoma metastasis to LNs by reducing FAK activity and VCAM-1 expression in lymphatic vessels. Taken together, our data demonstrate that pharmacological FAK inhibitors may provide a potential treatment option for initial metastasis to sentinel LNs.
Methods
Cells
Murine B16F10 cells were obtained from ATCC and introduced with red fluorescent protein (RFP) via lentiviral transduction. Stable RFP-expressing B16F10 cells were created as previously described [18] and were sorted using fluorescence-activated cell sorting (FACS) in the University of South Alabama flow cytometry facility. B16F10-RFP cells were cultured in DMEM. Human dermal lymphatic endothelial cells (HDLECs) (Lonza) were cultured on 0.1% gelatin-coated dishes using EGM-2 MV media (Lonza).
Reagents
Reagents were purchased from the following vendors: Lentiviral pCDH-RFP (#CD512B-1) construct from Systembio; FAK inhibitor PF-562,271 (PF-271) from MedKoo; anti-FAK (#05–537 clone 4.47) and anti-GAPDH (#MAB374) from Millipore; anti-VCAM-1 (mAb6434) for blotting, anti-pY397 FAK (mAB4528) for staining, and TNF-α from R&D Systems; IL-1β from Miltenyi Biotec; anti-pY397 FAK (#44–624G) for blotting, Alexa Fluor 488/594, and FITC conjugated secondary antibodies for staining from Invitrogen; anti-mouse α4 integrin (Clone 9C10) and anti-VCAM-1 (BD550547) for staining from BD Biosciences; anti-LYVE-1 (H-156, sc-28190) from Santa Cruz Biotech.
Animal experiments
Animal experiments were approved by and performed in accordance with the guidelines of the University of South Alabama IACUC. Both C57BL/6 male and female mice (6- to 8-week old) were used for a mouse footpad metastasis. The footpad model was used as previously described [19]. Briefly, mice were injected in the right hind footpad with 200,000 RFP-expressing B16F10 cells in 50 μl PBS. After 8 days, mice were treated twice daily with either vehicle (30 % [2-Hydroxypropyl]-β-cyclodextrin, 3 % dextrose) or PF-271 (50 mg/kg) by oral gavage. At day 14, mice were euthanized, and tissues were collected for blotting and immunohistochemistry.
Flow cytometry analyses
B16F10 cells were stained with either control IgG or α4 integrin antibody for 30 min, and then stained with FITC conjugated secondary antibody for 30 min. Expression of α4 integrin levels was determined by FACS.
Cell adhesion assay
HDLECs were grown to confluency in 6-well culture dishes, and then treated with either DMSO or PF-271 (1 μM) for 1 h prior to stimulation with TNF-α (10 ng/ml) for 4 h. Then, cells were washed twice with PBS, and 5,000 B16F10-RFP cells were allowed to adhere for 30 min at 37 °C. Unattached cells were washed off with PBS three times and fixed with 4% paraformaldehyde. Adhered cells were visualized (Nikon TE 2000-E) and enumerated.
Transmigration assay
HDLECs were seeded onto Boyden chamber (8 μm pore size, Millipore) coated with collagen type 1 from rat tail (1 μg/ml, BD Biosciences). Confluent HDLECs were treated with either DMSO or PF-271 (1 μM) for 1 h prior to stimulation with TNF-α (10 ng/ml) for 4 h. HDLECs were washed twice with PBS and 1×105 B16F10-RFP cells were added and allowed to transmigrate for 2 h at 37 °C. B16F10-RFP cells that migrated through the chamber were enumerated.
Immunoblotting
Cell and tissue lysates were made using RIPA lysis buffer. Clarified lysates were run on SDS-PAGE and transferred to PVDF membranes for immunoblotting. Membranes were developed using ECL solution and imaged with Fujifilm imager.
Immunohistochemistry
Lymph nodes collected from mice were embedded in OCT compound (Tissue Tek), and frozen sections (7 μm) were made for immunostaining. Slides were fixed in 4% paraformaldehyde and subsequently incubated in blocking solution for 1 h. Sections were incubated with anti-VCAM-1 (1:100), anti-LYVE-1 (1:100), or anti-pY397 FAK (1:100) overnight at 4 °C. Images were visualized using Nikon A1R confocal microscope (Nikon). Co-localization and fluorescent intensity analyses were performed by using Image J software (ver1.51s).
Statistical Analysis
Statistical significance was evaluated by t-test or ANOVA, and p<0.05 was considered to be statistically significant. Analyses were performed using GraphPad Prism.
Results
FAK inhibition reduces VCAM-1 expression, B16F10 adhesion and transmigration on HDLECs
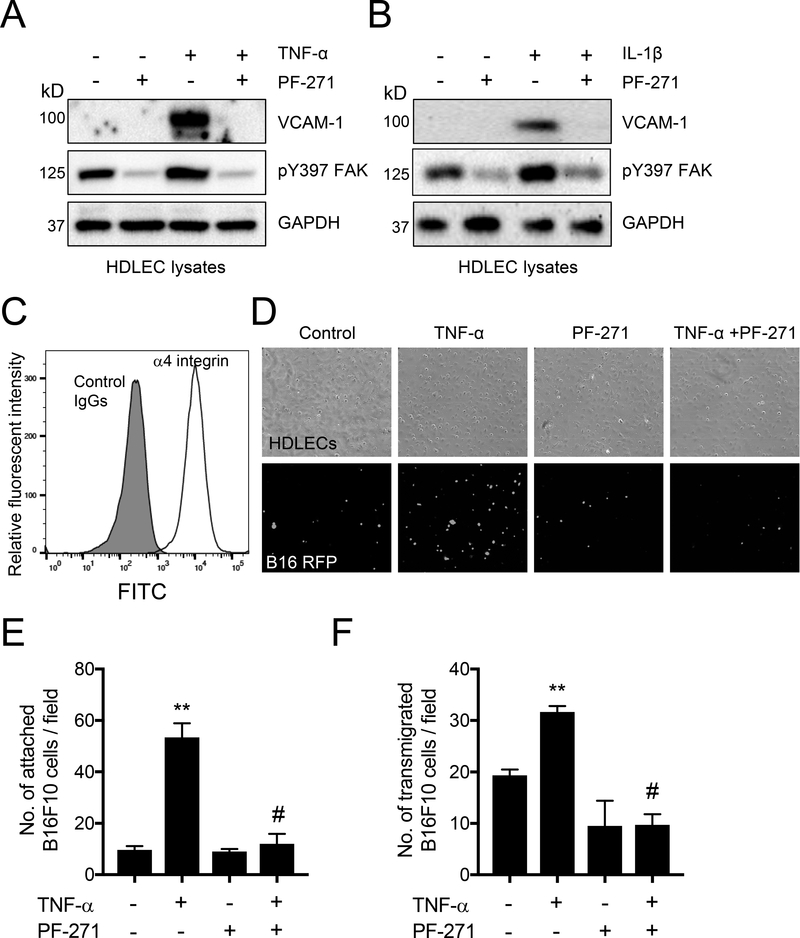
To determine if FAK activity contributes to the expression of VCAM-1 in HDLECs, VCAM-1 expression was induced by stimulation with either tumor necrosis factor-α (TNF-α) or interleukin-1β (IL-1β) (Fig. 1A and B). Pretreatment with a FAK inhibitor (PF-271) reduced FAK activity, as monitored by tyrosine 397 autophosphorylation (pY397), and blocked TNF-α- and IL1β-induced VCAM-1 expression (Fig. 1A and B). In addition, TNF-α and IL-1β increased FAK pY397 levels compared to a basal level (the first lanes in Fig. 1A and B). This suggests that FAK inhibition is an effective regulator of proinflammatory cytokine-stimulated VCAM-1 expression in HDLECs. Since it is known that expression of the α4 integrin on B16F1 cells promotes cell adhesion to lymphatic ECs via binding to VCAM-1 [17], we verified that our B16F10 cells expressed high levels of α4 integrin by FACS analysis (Fig. 1C). We next explored if FAK activity regulates B16F10 cell adhesion and transmigration to HDLECs using in vitro inflammation assays. To facilitate visualization of B16F10 cells, B16F10 cells were transduced to stably express RFP and sorted via FACS to get a homogeneous B16F10-RFP population. While B16F10-RFP adhesion in TNF-α treated HDLECs increased by 5-fold compared to control cells, pretreatment with PF-271 failed to increase the number of B16F10-RFP cell adhesion to HDLECs (Fig. 1D and 1E). Additionally, B16F10-RFP cells showed approximately 1.5-fold increase in transmigration through TNF-α stimulated HDLECs compared to control (Fig. 1F). However, HDLECs pretreated with PF-271 reduced the number of transmigrated B16F10RFP cells by approximately 2-fold, even when stimulated with TNF-α (Fig. 1F). Taken together, these results indicate that inhibition of FAK activity in HDLECs leads to decreased expression of VCAM-1, thus reducing B16F10 adhesion to and transmigration through HDLECs.
Figure 1. FAK inhibition reduces VCAM-1 expression, B16F10 adhesion and transmigration on HDLECs.
Confluent HDLECs were treated with PF-271 (1 μM) for 1 h prior to stimulation with either TNF-α (10 ng/ml) or IL-1α (20 ng/ml) for 4 h. (A and B) Whole cell lysates were immunoblotted as indicated (n=3). (C) α4 integrin expression of B16F10 was analyzed by FACS. Gray area; control IgG, white area; anti-α4 integrin staining. (D) B16F10-RFP were allowed to adhere to stimulated TNF-α stimulated HDLECs for 30 min. Top; bright field, bottom; RFP images. (E) Shown is the quantification of B16F10 adhesion on HDLECs per field (± S.D., n=3). (F) Enumeration of B16F10 that migrated through TNF-α stimulated HDLECs in a Boyden chamber after 2 h (± S.D., n=3). ** p<0.005 vs. control; # p<0.0005 vs. TNF-α. Scale bar, 200 μm.
PF-271 significantly reduces VCAM-1 expression in LNs in vivo
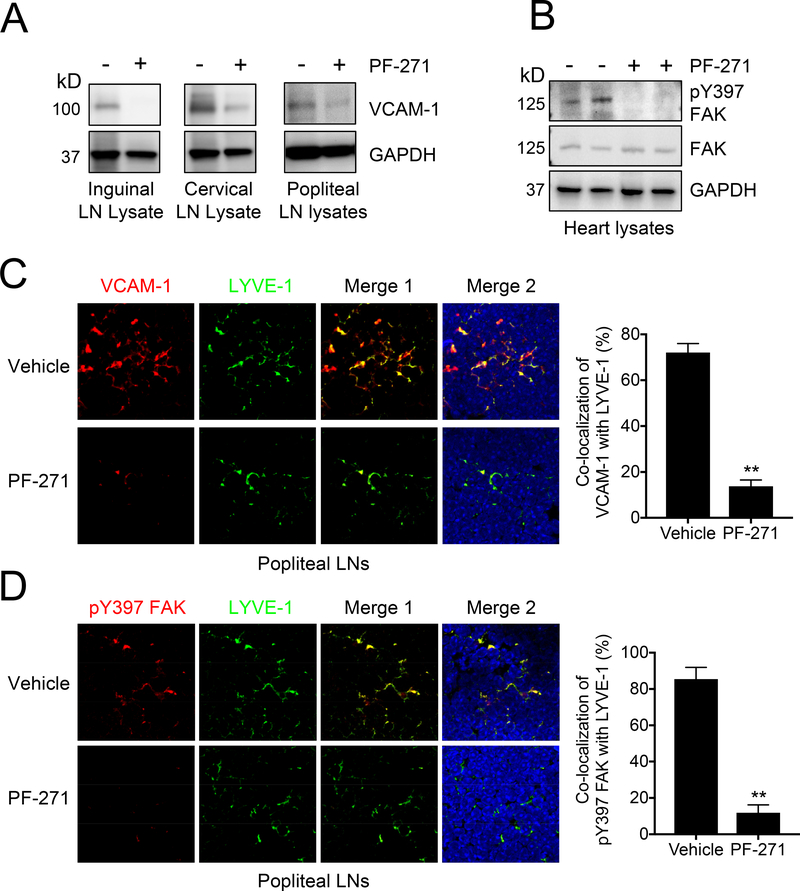
Before performing a melanoma metastasis assay, we first examined whether FAK inhibition could reduce VCAM-1 expression in LNs. Mice were treated with PF-271 (50 mg/kg, twice daily) via oral gavage for 2 days. Several different LNs were then collected for either immunoblotting or immunohistochemistry. Immunoblotting revealed that inguinal, cervical, and popliteal LNs (PLNs) express VCAM-1 under basal conditions, and that treatment with PF-271 significantly reduced VCAM-1 expression in these LNs (Fig. 2A). To verify efficacy of PF-271 treatment, we performed immunoblotting with LN lysates for pY397 FAK and total FAK. However, we were unable to detect pY397 FAK or total FAK in either control or treated mice. Instead, heart lysates were used to confirm that mice treated with PF-271 had low levels of pY397 FAK when compared to untreated mice (Fig. 2B). These data suggest that FAK may not be highly expressed in immune cells, which comprise a large majority of the lymph node tissues. To assess where VCAM-1 is expressed within LNs, we preformed immunostaining of PLNs (Fig. 2C). Co-staining of LYVE-1 (lymphatic vessel hyaluronan receptor-1, a lymphatic vessel marker) and VCAM-1 showed high co-localization within PLNs (Fig. 2C). In contrast, PLNs collected from mice treated with PF-271 showed reduced VCAM-1 staining and co-localization with LYVE-1 (Fig. 2C). As we were unable to detect pY397 FAK via immunoblotting of LN lysate, we performed pY397 FAK immunostaining of PLNs to see if FAK expression was limited to certain areas of the LNs. Interestingly, co-staining of pY397 FAK and LYVE-1 showed that FAK appears to be primarily limited to lymphatic ECs (Fig. 2D). This might explain the reason why we were unable to detect pY397 FAK and total FAK via immunoblotting. These results demonstrate that FAK activity is critical for VCAM-1 expression and PF-271 efficiently blocks VCAM-1 expression in lymphatic vessels in LNs.
Figure 2. Pharmacological FAK inhibition significantly reduces VCAM-1 expression in lymph nodes.
Mice were treated with either vehicle or PF-271 (50 mg/kg, twice daily) via oral gavage for 2 days prior to collection of either (A) LNs or (B) hearts. (A) LN and (B) heart lysates were immunoblotted as indicated (n=3). (C) Shown on left are immunostainings of popliteal LNs for VCAM-1 (red), LYVE-1 (green), and DAPI (blue). Merge 1; VCAM-1 and LYVE-1. Merge 2; VCAM-1, LYVE-1, and DAPI. Scale bar, 200 μm (n=4). Shown on the right is co-localization quantification of VCAM-1 and LYVE-1. ** p<0.005 (± S.D, n=4). (D) Shown in left are immunostainings of PLNs for pY397 FAK (red), LYVE-1 (green), and DAPI (blue). Merge 1; pY397 FAK and LYVE-1. Merge 2; pY397 FAK, LYVE-1, and DAPI. Scale bar, 200 μm (n=4). Shown on right is co-localization quantification of pY397 FAK and LYVE-1. ** p<0.005 (± S.D, n=4).
PF-271 treatment reduces metastasis of B16F10 cells to popliteal LN
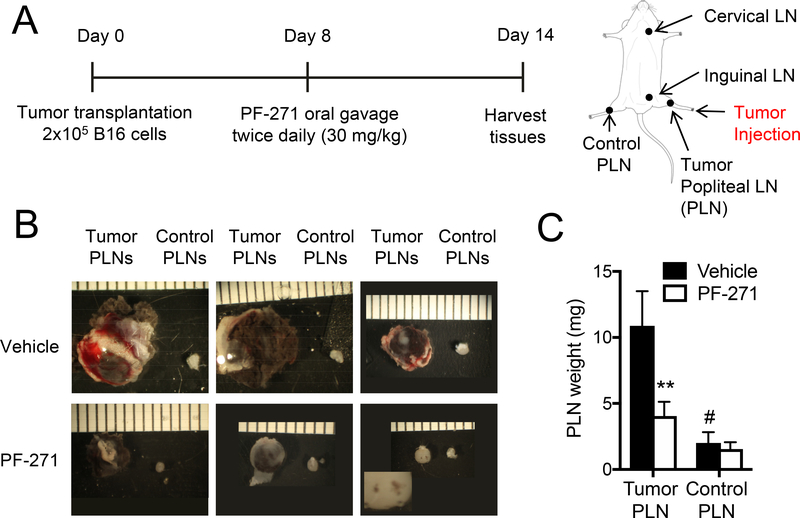
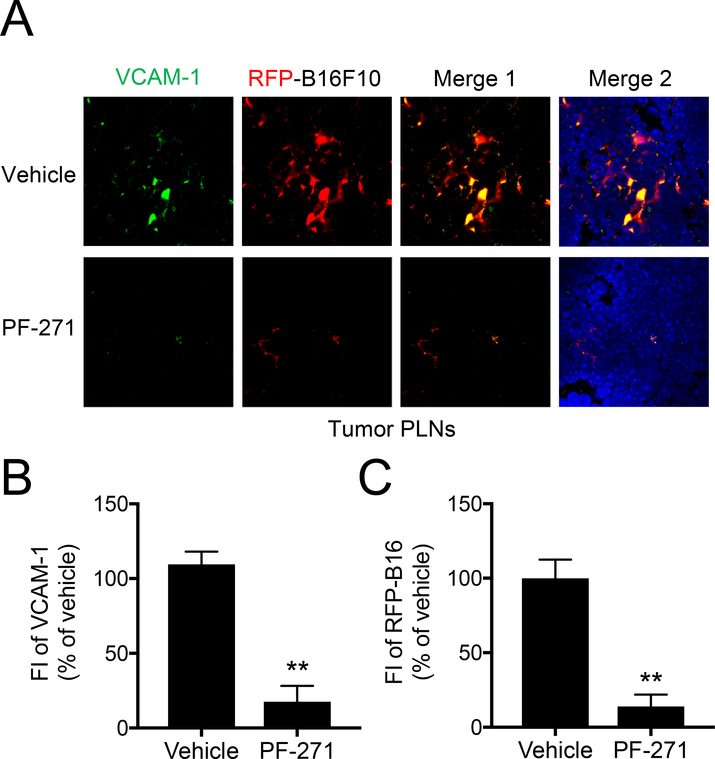
Our finding that VCAM-1 expression in LNs is reduced in PF-271 treated mice supported our hypothesis that VCAM-1 expression within LNs promotes melanoma metastasis. To explore this question, we used a footpad melanoma metastasis model whereby 2×105 B16F10-RFP cells were injected into the right footpads of wild-type C57BL/6 mice as previously described [19]. After 8 days, mice were then treated with either vehicle or PF-271 for 6 days (Fig. 3A). After 14 days, we observed significant metastasis of B16F10-RFP cells to the PLN closest to the injection site (tumor PLN), when compared to the control PLN in the opposite leg (Fig. 3B). Additionally, tumor PLNs showed a significant increase in both size and weight compared to controls (Fig. 3B and C). In contrast, melanoma metastasis to PLN and PLN size and weight were all significantly reduced in PF-271 treated groups (Fig. 3B and C). The results indicate that melanoma metastasis to PLNs can be efficiently inhibited by PF-271 treatment. To further verify B16F10-RFP cell metastasis to PLNs, we performed immunostaining on metastasized PLNs. Vehicle treated mice showed an abundance of B16F10-RFP cells colocalized with VCAM-1 expression within PLNs (Fig. 4A). In agreement with our hypothesis that VCAM-1 is important for metastasis, we observed that B16F10-RFP cells in the PLN were primarily located near VCAM-1 positive lymphatic vessels (Fig 4A). However, mice treated with PF-271 had a reduction in both VCAM-1 and B16F10-RFP cell signal within the PLN when compared to the vehicle treated mice (Fig. 4A and B). Taken together, these results provide a new role of FAK activity in melanoma metastasis to LNs and FAK inhibition may be a novel preventative option in melanoma metastasis by blocking VCAM-1 expression in lymphatic ECs of LNs.
Figure 3. PF-271 treatment reduces B16F10 metastasis to PLNs in a mouse footpad metastasis model.
(A) Experimental timeline of footpad metastasis model is depicted. Various LNs are indicated (right). Mice were treated with either vehicle or PF-271 (50 mg/kg, twice daily) via oral gavage starting on day 8. PLN: Popliteal lymph nodes. (B) Shown are representative images of PLNs collected at day 14. Tumor PLN: PLNs of tumor injected foot, Control PLN: PLNs of no tumor injected foot. The white dashed box shows the magnified image of black melanoma cells in the PLN. Scale bar, 5 mm. (C) Weight of PLNs were measured (± S.D., n=4). ** p<0.0005 vs vehicle; # p<0.0001 vs tumor PLN.
Figure 4. PF-271 treatment reduces VCAM-1 expression in PLNs and B16F10 metastatic potential to PLNs.
Mice were injected with B16F10-RFP cells and then treated with either vehicle or PF-271 (50 mg/kg, twice daily) via oral gavage starting on day 8. Tumor PLNs were collected on day 14. (A) Shown are immunostaining of tumor PLNs for VCAM-1 (green), B16F10-RFP (red), and DAPI. Merge 1; VCAM-1 and B16F10-RFP. Merge 2; VCAM-1, B16F10-RFP, and DAPI. Scale bar, 200 μm (n=4). (B and C) Fluorescent intensity (FI) of VCAM-1 (green, B) and FI of RFP (red, C) was measured using Image J. Scale bar, 200 μm. (± S.D., n=9). ** p<0.005.
Discussion
The process by which tumor cells metastasize from a primary lesion to a distant tissue is a complex process that requires several successful steps: 1) intravasation, 2) adherence to the endothelium and subsequent extravasation, and 3) creating a new niche at the site of metastasis [20]. Although 90% of cancer deaths are due to metastasis [21], the molecular mechanisms of metastasis are still not fully defined.
Here, we showed that inhibition of FAK activity both in vitro and in vivo decreased VCAM-1 expression in lymphatic ECs (Figs. 1 and 2). In vitro, FAK inhibition reduced B16F10-RFP cell adhesion and transmigration on HDLECs upon TNF-α stimulation (Fig. 1). Furthermore, FAK activity is critical for constitutive VCAM-1 expression in the lymphatic vessels of LNs (Fig. 2). Additionally, treatment with PF-271 reduced B16F10-RFP cell metastasis to popliteal LNs (Figs. 3 and 4). While most trials for FAK inhibitors in clinical studies are focused on tumor cell proliferation and augmenting tumor cell apoptosis [22, 23], our current study has shed new insights into the correlation between FAK activity-mediated changes in potential metastatic sites and tumor metastasis. Most notably, FAK activity regulating VCAM-1 expression in LNs raises the possibility that small molecule FAK inhibitors may be a novel preventative option for metastatic cancers that express α4 integrin, but not limited to melanoma [24, 25].
As adherence to the endothelium and subsequent extravasation into the surrounding tissue are key steps in metastasis, several studies have investigated the role cell adhesion molecules play in this process. Expression of cell adhesion molecules, such as ICAM-1 and E-selectin, in certain tissues seem to play an important role in their prevalence as sites of metastasis. ICAM-1 plays a role in promoting metastasis to the liver [26], and ICAM-1 knockdown reduced B16F10 metastasis to the liver in mice [27]. FAK activity was shown to regulate E-selectin expression, which was important for metastasis to the lungs [28]. It will be intriguing to test if FAK activity not only regulates expression of these different cell adhesion molecules in LNs, but also in tissues with a high rate of metastasis.
Over 50% of all melanomas have activating BRAF mutations [29] and inhibition of the Ras/Raf/MEK/ERK signaling pathways is one of the most promising treatments for malignant melanoma [30]. However studies using BRAF inhibitors have identified various feedback mechanisms to activate BRAF pathway in melanoma with the BRAF V600E mutation, which comprises over 90% of all melanoma BRAF mutations [29, 31]. BRAF inhibitor resistance is not limited to melanoma, as another study showed that BRAF inhibitors increased FAK activation and the downstream Wnt/GSK-3β/β-catenin signaling pathway to promote cell proliferation in BRAF V600E colorectal cancer cells [32]. In addition to FAK inhibitors reducing melanoma metastasis through reduction of VCAM-1 in LNs, FAK inhibitors could be used in combination with BRAF inhibitors to target BRAF V600E bearing melanomas.
Cells that comprise the tumor microenvironment also play key roles in promoting metastasis [33]. Interestingly, it was shown that increased lymphangiogensis occurs prior to subsequent melanoma metastasis to LNs [19], and that this process may be driven my tumor associated macrophages. Macrophages, when cultured with B16 melanoma cells, showed increased expression of TNF-α and IL-1β, and these cytokines increased the expression of VEGF-C, an important growth factor in lymphangiogensis [9]. Cancer cells, through expression of CC chemokine receptor-7 (CCR7) then make use of the new lymphatic vessels to metastasize to LNs [34]. Overexpression CCR7 in B16 melanoma cells enhanced metastasis to LNs and antibody neutralization of CCL21, the ligand for CCR7, reduced B16 metastasis to LNs [34]. Recent evidence has also demonstrated that FAK can alter the immune cell population of the tumor microenvironment through regulation of chemokine expression in skin cancer [35]. While we did not investigate the expression of growth factors, chemokines, or cytokines in our footpad model, we hypothesize that melanoma burdened LNs in vehicle treated mice are more inflammatory compared to the PF-271 treated group, as seen by increased size and weight of the melanoma burdened LNs and increased expression of VCAM-1 (Fig. 3 and 4). Future studies are needed to better understand the other tumor-promoting roles of FAK in the tumor microenvironment.
In summary, our study demonstrated a role for FAK activity in regulating tumor metastasis to LNs, and that FAK inhibition may be a novel preventative option in melanoma metastasis by blocking VCAM-1 expression in LNs.
Highlights.
Focal adhesion kinase (FAK) activity regulates proinflammatory VCAM-1 expression in lymphatic endothelial cells.
FAK inhibition reduces a4 integrin-expressing B16F10 melanoma adhesion to and transmigration through lymphatic endothelial cells.
Pharmacological FAK inhibition reduces VCAM-1 expression in lymph nodes (LNs) and prevents B16F10 melanoma metastasis to LNs.
Small molecule FAK inhibitors in clinical trials could be used to treat melanoma metastasis to LNs.
Acknowledgments
Funding
This work was supported by Mitchell Cancer Grant 2013–2015 (to ST Lim), American Heart Association Scientist Development Grant 12SDG10970000 (to ST Lim), National Institutes of Health R01HL136432 (to ST Lim), and R01CA190688 (to EY Ahn).
Abbreviations
- FAK
focal adhesion kinase
- HDLEC
human dermal lymphatic endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- IL-1β
interleukin-1β
- LN
lymph node
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor-1
- pY397 FAK
autophosphorylation at tyrosine 397 of FAK
- RFP
red fluorescent protein
- TNF-α
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial cell growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steeg PS, Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med 12 (2006) 895–904, 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC, Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2 (2002) 563–572, 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DX, Bos PD, Massague J, Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer 9 (2009) 274–284, 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 4.Horimoto Y, Polanska UM, Takahashi Y et al. , Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adh Migr 6 (2012) 193–202, 10.4161/cam.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdick MM, McCarty OJ, Jadhav S et al. , Cell-cell interactions in inflammation and cancer metastasis. IEEE Eng Med Biol Mag 20 (2001) 86–91, [DOI] [PubMed] [Google Scholar]
- 6.Jones D, Pereira ER, Padera TP, Growth and immune evasion of lymph node metastasis. Front Oncol 8 (2018) 36, 10.3389/fonc.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balch CM, The role of elective lymph node dissection in melanoma: Rationale, results, and controversies. J Clin Oncol 6 (1988) 163–172, 10.1200/JCO.1988.6.1.163. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Buzaid AC, Soong SJ et al. , New TNM melanoma staging system: Linking biology and natural history to clinical outcomes. Semin Surg Oncol 21 (2003) 43–52, 10.1002/ssu.10020. [DOI] [PubMed] [Google Scholar]
- 9.Peppicelli S, Bianchini F, Calorini L, Inflammatory cytokines induce vascular endothelial growth factor-c expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett 8 (2014) 1133–1138, 10.3892/ol.2014.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra SK, Hanson DA, Schlaepfer DD, Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol 6 (2005) 56–68, 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 11.Frame MC V -src informs integrin signalling. Nat Rev Mol Cell Biol 14 (2013) 548, 10.1038/nrm3647. [DOI] [PubMed] [Google Scholar]
- 12.Sulzmaier FJ, Jean C, Schlaepfer DD, Fak in cancer: Mechanistic findings and clinical applications. Nat Rev Cancer 14 (2014) 598–610, 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim ST, Chen XL, Tomar A et al. , Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem 285 (2010) 21526–21536, 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim ST, Miller NL, Chen XL et al. , Nuclear-localized focal adhesion kinase regulates inflammatory vcam-1 expression. J Cell Biol 197 (2012) 907–919, 10.1083/jcb.201109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boscacci RT, Pfeiffer F, Gollmer K et al. , Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for icam-1, icam-2, and vcam-1 in lymphocyte homing. Blood 116 (2010) 915–925, 10.1182/blood-2009-11-254334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemke M, Weschenfelder T, Konstandin MH et al. , High affinity interaction of integrin alpha4beta1 (vla-4) and vascular cell adhesion molecule 1 (vcam-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol 212 (2007) 368–374, 10.1002/jcp.21029. [DOI] [PubMed] [Google Scholar]
- 17.Rebhun RB, Cheng H, Gershenwald JE et al. , Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the b16 murine melanoma. Neoplasia 12 (2010) 173–182, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiscornia G, Singer O, Verma IM, Production and purification of lentiviral vectors. Nat Protoc 1 (2006) 241–245, 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 19.Harrell MI, Iritani BM, Ruddell A, Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 170 (2007) 774–786, 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang AC, Massague J, Molecular basis of metastasis. N Engl J Med 359 (2008) 28142823, 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlen P, Puisieux A, Metastasis: A question of life or death. Nature reviews Cancer 6 (2006) 449–458, 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 22.Tanjoni I, Walsh C, Uryu S et al. , PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther 9 (2010) 764–777, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes JB, Adair SJ, Slack-Davis JK et al. , Inhibition of focal adhesion kinase by PF562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther 10 (2011) 2135–2145, 10.1158/1535-7163.MCT-11-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slack-Davis JK, Atkins KA, Harrer C et al. , Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res 69 (2009) 1469–1476, 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 25.Young SA, McCabe KE, Bartakova A et al. , Integrin alpha4 enhances metastasis and may be associated with poor prognosis in mycn-low neuroblastoma. PLoS One 10 (2015) e0120815, 10.1371/journal.pone.0120815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benedicto A, Romayor I, Arteta B, Role of liver icam-1 in metastasis. Oncol Lett 14 (2017) 3883–3892, 10.3892/ol.2017.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfeil SA, Häfele V, Dietsch B et al. , Hepatic endothelial notch activation protects against liver metastasis by regulating endothelial-tumor cell adhesion independent of angiocrine signaling. Cancer Research (2018) canres.1752.2018, 10.1158/0008-5472.can-18-1752. [DOI] [PubMed] [Google Scholar]
- 28.Hiratsuka S, Goel S, Kamoun WS et al. , Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A 108 (2011) 3725–3730, 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ascierto PA, Kirkwood JM, Grob JJ et al. , The role of BRAF V600 mutation in melanoma. J Transl Med 10 (2012) 85, 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray-Schopfer V, Wellbrock C, Marais R, Melanoma biology and new targeted therapy. Nature 445 (2007) 851–857, 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 31.Chan XY, Singh A, Osman N et al. , Role played by signalling pathways in overcoming braf inhibitor resistance in melanoma. Int J Mol Sci 18 (2017), 10.3390/ijms18071527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Gao C, Gao X et al. , Wnt/beta-catenin pathway activation mediates adaptive resistance to BRAF inhibition in colorectal cancer. Mol Cancer Ther 17 (2018) 806–813, 10.1158/1535-7163.MCT-17-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkwill FR, Capasso M, Hagemann T, The tumor microenvironment at a glance. J Cell Sci 125 (2012) 5591–5596, 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 34.Wiley HE, Gonzalez EB, Maki W et al. , Expression of CC chemokine receptor-7 and regional lymph node metastasis of b16 murine melanoma. J Natl Cancer Inst 93 (2001) 1638–1643, [DOI] [PubMed] [Google Scholar]
- 35.Serrels A, Lund T, Serrels B et al. , Nuclear FAK controls chemokine transcription, tregs, and evasion of anti-tumor immunity. Cell 163 (2015) 160–173, 10.1016/j.cell.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]