Abstract
Within in the liver, lymphatic vessels are found within the portal triad and their described function is to remove interstitial fluid from the liver to the lymph nodes where cellular debris and antigens can be surveyed. We were very interested in understanding how the lymphatic vasculature might be involved in inflammation and immune cell function within the liver. However, very little has been published establishing digestion protocols for the isolation of lymphatic endothelial cells (LEC) from the liver or specific markers that could be used to evaluated liver LECs on a per cell basis. Therefore, we optimized a method for the digestion and staining of the liver in order to evaluate the LEC population in the liver. We are confident that the method outlined here will be useful for the identification and isolation of LECs from the liver and will strengthen our understanding of how LECs respond to the liver microenvironment.
Keywords: Liver, lymphatic endothelial cells, flow cytometry, liver sinusoidal endothelial cells, collagenase IV
SUMMARY:
The goal of this protocol was to identify lymphatic endothelial cell populations within the liver using described markers. We utilized collagenase IV and DNase and gentle mincing of tissue combined with flow cytometry to identify a distinct population of lymphatic endothelial cells.
INTRODUCTION:
The role of lymphatic vessels and lymphatic endothelial cells (LEC) in the liver is not well understood. While lymphatic vessels are found within the portal triad of the liver1 and expand during disease2 very little is understood regarding the function and phenotype of lymphatic endothelial cells within the liver. With the discovery of markers that are found primarily on lymphatic endothelial cells the importance of these cells within different tissue niches in homeostasis and disease will fill a significant gap in our understanding. Lymphatic endothelial cells have a major role in maintaining peripheral tolerance in the lymph node and in metastatic tumors by interacting directly with T cells3–12 and LECs in the lymph node can promote protective immunity via their interactions with migratory dendritic cells13–15, thus there are multiple roles for LECs which may be specific to the tissues and interactions in which they are present. However, very little is understood about how LECs interact with immune cells in the tissue or how LECs function in different organ systems, thus evaluating LECs on a per cell basis within the liver or other organs may lead to advances in how LECs program tissue-specific immunity. While much of the literature focused on LECs in the liver used microscopy to visualize LECs using one or two markers and morphology16 very little has been done to specifically evaluate LECs on a cell by cell basis using flow cytometry, though one study did evaluate differences between liver sinusoidal endothelial cells (LSEC) and LECs17. Being able to analyze LEC populations in the liver by flow cytometry allows for the in depth study of LEC phenotype during normal homeostasis or disease.
To evaluate LECs by flow cytometry multiple surface markers are needed. Typically LECs are visualized by expression of prospero-related homeobox 1 (Prox-1), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) or vascular endothelial growth factor receptor 3 (VEGFR3) using microscopy. However, in the liver, the expression of these markers are not restricted to LECs. Prox-1 is widely expressed by hepatocytes during liver development, regeneration and injury18 and LYVE1 and VEGFR3 are expressed by the liver sinusoidal endothelial cells17. In the lymph node, LECs are identified using flow cytometry as cluster of differentiation (CD) CD45-, CD31+ and podoplanin + (PDPN)15. However, this approach is too minimal to isolate LECs in the liver since CD45- CD31+ cells will capture endothelial cells and the predominate population of vascular endothelial cells in the liver are liver sinusoidal endothelial cells (LSEC). Thus, other markers are needed to distinguish the rare LEC population from the abundant LSEC population. Both CD16/32, expressed by mature LSECs17, and CD146, a common vascular endothelial cell marker that is predominately expressed within the liver sinusoids by liver sinusoidal endothelial cells19 with little to no expression by lymphatic endothelial cells20, were candidate markers.
Therefore, we optimized a method for isolating and visualizing LECs in the liver using the above markers, CD45, CD31, CD146, CD16/32 and PDPN for flow cytometry. We describe the use of collagenase IV, DNase 1 and mechanical separation for liver tissue digestion into a single cell suspension. We also describe the use of OptiPrep for the isolation of non-parenchymal cells (NPC) and to eliminate cellular debris. Finally, we determined using multiple markers the optimal flow cytometry gating strategy to identify LECs from the liver with PDPN as the predominant marker.
PROTOCOL:
1.). Preparation of materials
Make up a 5mg/ml solution of DNase I in PBS
Make up digestion mixture by adding 5000U/ml of Collagenase IV into CLICKS/EHAA media
Warm digestion mixture at 37° for 30 minutes prior to use
Make up isolation buffer by adding 4.8% BSA and 2mM EDTA to HBSS
Make up red blood cell (RBC) lysis buffer by adding 100mM Ammonium Chloride, 10mM KHCO3 and 0.1mM EDTA to distilled H2O
2.). Preparation of single cell suspension from mouse liver
Euthanize mouse with CO2 and cervical dislocation. All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Colorado Anschutz Medical Campus.
Spray down mouse with 70% Ethanol to wet fur.
Pin mouse feet to dissection board. Using dissection scissors cut the skin about 1cm above the anus being careful to only cut the skin (about 1mm). Pull skin away from body with toothed forceps and insert scissors between skin and peritoneum. Open scissors to separate skin from peritoneum and then cut skin from incision to neck. Pin skin to dissection board using 1 pin under each arm and above each leg. Pull peritoneal sac up and cut up toward the neck. Grab the lobes of the liver and cut just below the sternum. Care should be taken if any of the liver will be used for IHC
Cut around the liver and remove the liver from the mouse and place in 4 ml of CLICKS/EHAA
Cut the liver using scalpels to ~1mm diameter pieces.
Add 500μl of the digestion mixture and 500μl of the DNase I (2mg/ml) to the liver.
Incubate the liver for 30 minutes at 37 °C. After 15 minutes, mix the liquid using a 5 ml pipette.
After 30 minutes of incubation transfer the digested sample through a 100um strainer into a 50 ml conical tube.
Gently push the remaining pieces through the filter with the back of a 1 ml syringe.
Wash the filter with 5 ml of isolation buffer and gently push the tissue through the strainer with the back of a plunger from a 1 ml syringe. Repeat this until you have washed the filter with 25 ml of isolation buffer.
Centrifuge the cells at 400xg for 5 minutes. Carefully aspirate off the liquid.
Resuspend the pellet with 4 ml of RBC lysis buffer. Incubate the cells at room temperature for 5 minutes.
Wash with 10 ml of isolation buffer and centrifuge at 400xg for 5 minutes
Resuspend cells in 5 ml of 20% OptiPrep, and layer with 1 ml of PBS
Centrifuge the cells at 300xg for 15 minutes without a brake.
Remove the layer between the PBS and the OptiPrep and place through a filter into a new 50 ml conical tube.
Wash the cells with 10 ml of isolation buffer and centrifuge at 400xg for 5 minutes.
Resuspend cells in 500 μl of phosphate buffered saline (PBS) with 2% fetal bovine serum (FBS).
3.). Flow cytometric analysis of single cells from liver
Aliquot approximately 5 million of the remaining non-parenchymal cells into a single well of a 96 well plate.
Centrifuge the cells at 400xg for 5 minutes
Resuspend the cells in 90 μl of PBS with 2% FBS.
Add in anti-CD45 (1:200), anti-CD146 (1:200), anti-CD31 (1:200), and anti-podoplanin (PDPN) (1:200) diluted in 10μl of 10× 2.4G2 or anti-CD16/32 (1:200) (note: NO FC block (2.4G2) was used when anti-CD16/32 labeled antibody was used.)
To determine where positive and negative gates should be set include a fluorescence minus one (FMO) stain for each color and an isotype control antibody.
To determine live versus dead cells, stain with a live/dead marker such as ghost red 780 (TONBO).
Incubate the cells at 4 °C for 30 minutes.
Wash the cells with 100 μl of PBS with 2% FBS.
Calibrate the flow cytometer with single stains.
Run the liver cells, making sure to collect all cells as the lymphatic endothelial population is very infrequent.
4.). Data analysis
Looking at Side Scatter-Area vs Forward Scatter-Area, gate on “live” cells based on size and granularity and a live/dead marker such as ghost red 780.
Next, using CD45 Brilliant Violet 510 and CD31 PerCp Cy5.5, gate on the CD45-CD31+ cells using the isotype controls and FMO to determine positive and negative populations.
Lastly, using CD146 v450 or CD16/32 FITC and PDPN APC, take the CD146- PDPN+ or CD16/32- PDPN+ cells again using isotype controls and FMO to determine positive and negative populations. These cells are the LECs.
REPRESENTATIVE RESULTS:
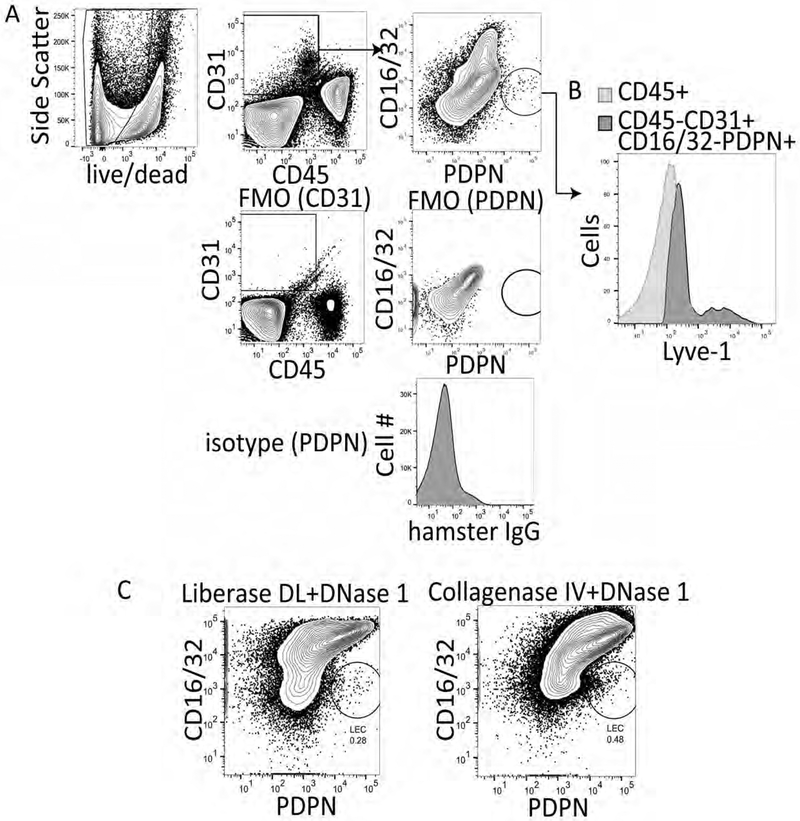
Studies analyzing liver lymphatics have primarily used immunohistochemistry to quantitate the frequency and diameter of lymphatic vessels in the liver. However, this method does not allow for the evaluation of LECs on a cell by cell basis or for expression of multiple markers, cytokines, chemokines or transcription factors. Therefore, we asked whether liver LECs could be isolated from the liver and evaluated using flow cytometry. Our previous work isolating lymph node LECs was performed using liberase DL and DNase 1 combined with mechanical separation of the lymph node tissue with needles13,15. Therefore, we performed the same digestion protocol on liver tissue or used collagenase IV and DNAse 1 as previously described for immune cell isolation from the liver21, and followed the digestion with a density gradient separation (OptiPrep) step to remove hepatocytes and increase the frequency of other cell types within the liver (Figure 1A). Following liver digestion and OptiPrep, the cells were stained with CD45, CD31, PDPN and CD16/32. We used CD16/32 as it has been described to be expressed by mature LSEC populations, but not LEC populations17. Thus, lymphatic endothelial cells were gated as CD45-, CD31+, CD16/32-, PDPN+ cells while LSECs were gated as CD45-, CD31+, CD16/32+ and PDPN- (Figure 1A). Gates were set on live cells (ghost red negative) and based on isotype controls and fluorescence minus one (FMO) staining (Figure 1A). We also confirmed expression of Lyve-1 APC on the LEC population (Figure 1B). Both methods allowed us to visualize the LEC population within the liver, however the staining profile of the cells was visually better using collagenase IV and DNAse 1 than liberase DL (Figure 1C). Therefore, all future manipulations were performed using collagenase IV and DNAse 1 as described in the protocol. On average we obtain approximately 1300 LECs per gram of liver tissue or 2200 LECs per naïve liver using this digestion method.
Figure 1. Representative flow cytometry analysis of liberase DL and collagenase IV digested murine liver tissue.
A. Gating strategy, fluorescence minus one staining and isotype controls for the procedure. Data were analyzed with FlowJo analysis software after acquisition on a BD FacsCanto II. B. Lyve-1 staining of CD16/32- PDPN+ cells. C. Final flow cytometry gate after digesting mouse livers with either liberase DL or Collagenase IV and evaluating CD16/32 PerCP X PDPN PE-Cy7 where LECs are CD16/32- and PDPN+.
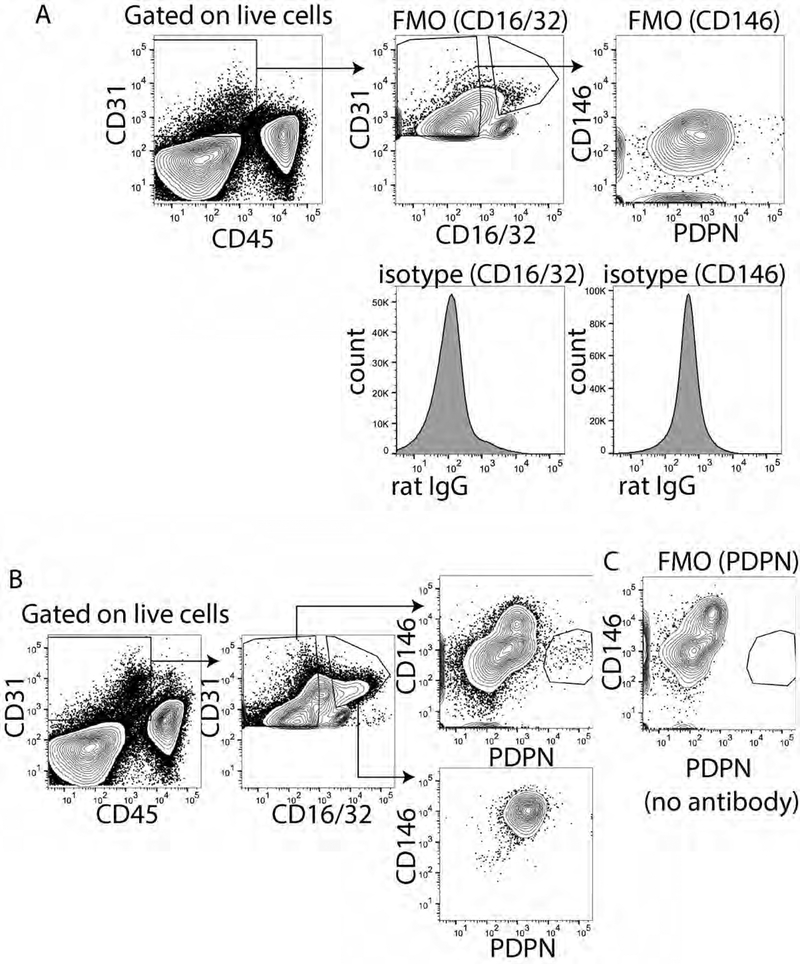
To optimize our gating strategy and combination of fluorphores and to eliminate contamination of liver sinusoidal endothelial cells from our population we used both CD16/32 and CD146. CD16/32 is FC gamma receptors II and III and is expressed on mature LSECs but not LECs17. We found CD16/32 could distinguish the PDPN+ CD16/32- cells from the PDPN- CD16/32+ cells, especially when using PDPN conjugated to APC or PE and CD16/32 conjugated to FITC (Figure 1A), but less well when PDPN was conjugated to PE-Cy7 (Figure 1C). Expression of CD16/32 is not found on LECs however, as CD16/32 prevented our use of Fc Block and immature LSECs may not express CD16/32 we also evaluated CD146 conjugated to V450, a vascular endothelial cell marker expressed highly by LSECs and other vascular endothelial cells19 with low to no expression by LECs22. We first optimized the staining of CD146 using FMO and isotype controls for both CD16/32 and CD146 (Figure 2A). We found that if we evaluated only the CD16/32+ cells or the CD16/32- cells that the CD16/32+ cells were all CD146+ and PDPN- while the CD16/32- cells were primarily CD146- and either PDPN- or PDPN+ (Figure 2B). To confirm this staining we using an FMO, removing PDPN from the stain and found that our population disappeared (Figure 2C). Interestingly, we did not find any CD146+ PDPN+ cells confirming that CD146 is not or very lowly expressed by PDPN+ LECs. Thus, we are confident that either of these markers can be used to gate out vascular endothelial cells in the liver.
Figure 2. Identification of CD146 and PDPN as appropriate markers for liver lymphatic endothelial cells.
A. Fluorescence minus one and isotype control for CD16/32 (left) and CD146 (right). B. Gated CD16/32 positive or negative cells determined from A. Shown is CD146XPDPN from both populations. C. Fluorescence minus one for PDPN to demonstrate that the staining is absent with the antibody is not added.
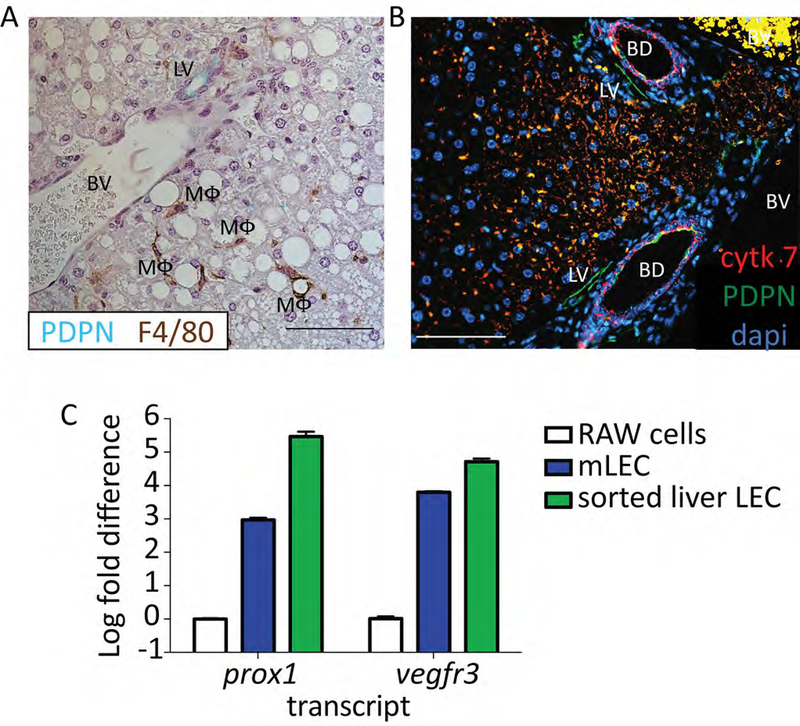
To further validate that PDPN was an appropriate marker for lymphatic endothelial cells we stained liver sections from mice with both PDPN (green) and F4/80 (brown) (Figure 3A). We found that neither the vascular endothelium nor macrophages expressed PDPN in the murine liver. We were also able to distinguish cholangiocytes, which can stain positive for PDPN, from lymphatic vessels based on the distinct nuclear structures of the bile ducts and by cytokeratin 7 staining (Figure 3B-red cytokeratin 7, green PDPN). Since we use multiple markers for flow cytometry, such as CD31 which is not expressed by cholangiocytes23, we are confident that we are removing these cells from our analysis. Thereby confirming that cells from the liver that are CD45-, CD31+, CD146lo/neg, CD16/32- and PDPN+ are LECs. Finally, to provide evidence that our stained cells are LECs we sorted this population of cells and used qRT-PCR to evaluate Vegfr3 and prox-1 expression. We evaluated both Vegfr3 and prox-1 as these transcripts are expressed by other cells in the liver-prox1 by hepatocytes and Vegfr3 by LSECS (among others)-but no other cells besides LECs, to our knowledge, express both. We found that expression of both these markers was significantly higher in our sorted population than in cultured murine macrophage cell line (RAW264.7) that does not normally express these markers, but similar in expression to cultured murine lymphatic endothelial cells (Figure 3C).
Figure 3.
A. Representative immunohistochemistry from a mouse liver stained with PDPN (blue/green) and F4/80 (brown). Lymphatic vessel (LV), blood vessel (BV) and macrophage (MΦ) are labeled. Scale bar is 100μm. Formalin Fixed paraffin embedded tissue was deparaffinized for 20 minutes in xylene. Hydrated to water through gradient of ethanol, and antigen retrieval was performed using perkin elmer pH6 antigen retrieval buffer in a pressure cooker for 15 minutes. Tissue was blocked using 0.1%BSA and stained using anti-mouse PDPN (8.1.1) 1:400 and anti-mouse F4/80 (MCA497) 1:100 for 1 hour at room temperature. Anti-hamster IgG HRP and anti-rabbit IgG HRP were used as secondary antibodies. DAB+ and Vina Green were used to detect the F4/80 and PDPN respectively. Tissue was counter stained with Hematoxylin and imaged on a Nikon eclipse Ti. B. As in A except PDPN in green, cytokeratin 7 in red and dapi in blue. Tissue was blocked using 5% donkey and 5% goat serum and stained using anti-mouse PDPN (8.1.1) 1:100 and anti-mouse cytokeratin 7 (ab181598) 1:200 for 1 hour at room temperature. Anti-hamster IgG AF647 and anti-rabbit IgG PE were used as secondary antibodies. Tissue was counter stained with DAPI and imaged. Scale bar is 100μm. Lymphatic vessel (LV), blood vessel (BV) and bile duct (BD) are labeled. C. Log fold change in Vegfr3 and prox-1 expression from sorted liver LECs based on staining in protocol (CD45-, CD31+, CD146lo/neg and PDPN+) compared to RAW cells or primary murine lymph node LECs. Sorted cells were passed through a QIAshredder column and RNA was extracted using a QIAgen RNeasy micro kit and cDNA was made using the Qiagen QuantiTect reverse transcription kit. Transcript abundance was normalized to the housekeeping gene, Gapdh for every sample. Primers for Vegfr3, prox-1 and Gapdh were obtained from Qiagen.
DISCUSSION:
The overall importance of lymphatic endothelial cells in immune homeostasis and regulation has recently come to light. Much of the published lymphatic literature focuses on skin and lymph node, however lymphatics are found throughout the body and thus our understanding of their importance in different organs is needed. Here we show a method in which LECs in the liver can be studied on a cell by cell basis to better understand their concurrent expression of different surface markers, cytokines, chemokines and intracellular proteins such as transcription factors. This method will be useful for future studies to assess the phenotype and function of lymphatic endothelial cells in the liver during health and disease.
One of the hurdles for the identification of LECs in the liver is their relatively low frequency compared to other cell types. Hepatocytes make up about 80% of the liver, and removing these cells using density gradient (OptiPrep) before running the liver on a flow cytometer requires less time and thus better viability. In order to distinguish the population of Lyve1+ LECs in the liver from Lyve1+ LSECs, we used the markers CD16/32 and CD146 found on LSECs and with low to no expression by LECs. This coupled with the lack or low expression of PDPN by any other endothelial cell in the liver allowed us to validate that the population we identified were LECs by flow cytometry. Indeed, downstream transcriptional analysis confirmed that this gating strategy produced LECs (Figure 3C).
The isolation of LECs from the lymph node is best done using Liberase DL, however we found that while this method does extract LECs from the liver, a liver digestion protocol using Collagenase IV provides better downstream analysis using flow cytometry. Using mechanical disruption of the liver allows the collagenase more surface area to better interact with the extracellular matrix associated cells, like LECs, and using CLICKS/EHAA media without FBS allows for the digestion of the liver to occur in only 30 minutes. This decreased time maintains LEC viability for downstream assays like flow cytometry or flow sorting. Indeed, we are able to recover enough viable cells to visualize LECs by flow cytometry and flow sorting for downstream transcriptional analysis.
Combined, the separation of hepatocytes from the non-parenchymal cells, the use of collagenase type IV, and the clarification and demonstration of markers specific to LECs in the liver and markers specific to other endothelial cell populations, such as CD16/32 and CD146, allowed us to properly identify LECs in the liver. These methods fill as significant gap in the literature about how LECs in the liver can be identified by flow cytometry-an organ with other cells that express markers known to be unique to LECs in the lymph node (prox1 and vegfr3), and will lead to downstream studies regarding liver LEC function. Additionally, this method could be modified for other tissues in order to better evaluate tissue specific LEC markers and subsets.
Supplementary Material
ACKNOWLEDGMENTS:
We would like to thank the GI and Liver Innate Immune Programs for monetary support of this project. BAT is also funded by R01 AI121209.
Footnotes
DISCLOSURES:
None
REFERENCES:
- 1.Tanaka M & Iwakiri Y Lymphatics in the liver. Curr Opin Immunol 53, 137–142, doi: 10.1016/j.coi.2018.04.028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollmar B, Wolf B, Siegmund S, Katsen AD & Menger MD Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol 151, 169–175 (1997). [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JN et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 207, 681–688, doi: 10.1084/jem.20092465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JN et al. Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PLoS One 9, e87740, doi: 10.1371/journal.pone.0087740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouhani SJ et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun 6, 6771, doi: 10.1038/ncomms7771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tewalt EF et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120, 4772–4782, doi: 10.1182/blood-2012-04-427013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubrot J et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med 211, 1153–1166, doi: 10.1084/jem.20132000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirosue S et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol 192, 5002–5011, doi: 10.4049/jimmunol.1302492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund AW et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 1, 191–199, doi:S2211–1247(12)00041–1[pii] 10.1016/j.celrep.2012.01.005 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Lund AW et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 126, 3389–3402, doi: 10.1172/JCI79434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz MA Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res 2, 701–707, doi: 10.1158/2326-6066.CIR-14-0115 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Dietrich T et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 184, 535–539, doi: 10.4049/jimmunol.0903180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedl R, Lindsay R, Finlon J, Lucas E, Friedman R, Tamburini BA. Migratory Dendritic Cells acquire archived antigen from Lymphatic Endothelial Cells for antigen presentation during lymph node contraction. Nature Communications 8, 2034, doi:DOI: 10.1038/s41467-017-02247-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedl RM & Tamburini BA Antigen archiving by lymph node stroma: A novel function for the lymphatic endothelium. Eur J Immunol 45, 2721–2729, doi: 10.1002/eji.201545739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamburini BA, Burchill MA & Kedl RM Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun 5, 3989, doi: 10.1038/ncomms4989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokomori H et al. Lymphatic marker podoplanin/D2–40 in human advanced cirrhotic liver--re-evaluations of microlymphatic abnormalities. BMC Gastroenterol 10, 131, doi: 10.1186/1471-230X-10-131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nonaka H, Tanaka M, Suzuki K & Miyajima A Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev Dyn 236, 2258–2267, doi: 10.1002/dvdy.21227 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Dudas J et al. Prospero-related homeobox 1 (Prox1) is a stable hepatocyte marker during liver development, injury and regeneration, and is absent from “oval cells”. Histochem Cell Biol 126, 549–562, doi: 10.1007/s00418-006-0191-4 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Schrage A et al. Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochem Cell Biol 129, 441–451, doi: 10.1007/s00418-008-0379-x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amatschek S et al. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood 109, 4777–4785, doi: 10.1182/blood-2006-10-053280 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Soldevila G, Leeker M, Flavell R & Crispe IN The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1, 741–749 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Shay T & Kang J Immunological Genome Project and systems immunology. Trends Immunol 34, 602–609, doi: 10.1016/j.it.2013.03.004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B et al. Adult Mouse Liver Contains Two Distinct Populations of Cholangiocytes. Stem Cell Reports 9, 478–489, doi: 10.1016/j.stemcr.2017.06.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.