Abstract
Each year there are more than 15,000 cases of human disease caused by infections with tick-borne viruses (TBVs). These illnesses occur worldwide and can range from very mild illness to severe encephalitis and hemorrhagic fever. Although TBVs are currently identified as neglected vector-borne pathogens and receive less attention than mosquito-borne viruses, TBVs are expanding into new regions and infection rates are increasing. Furthermore, effective vaccines, diagnostic tools and other countermeasures are limited. The application of contemporary technologies to TBV infections presents an excellent opportunity to develop improved, effective countermeasures. Experimental tick and mammal models of infection can be used to characterize determinants of infection, transmission and virulence and to test candidate countermeasures. The use of ex vivo tick cultures in TBV research provides a unique way to look at infection in specific tick organs. Mammal ex vivo organ slice and more recently, organoid, cultures are additional models that can be used to elucidate direct tissue-specific responses to infection. These ex vivo model systems are convenient for testing methods involving transcript knockdown and small molecules in tightly controlled conditions. They can also be combined with in vitro and in vivo studies to tease out possible host factors and potential vaccine or therapeutic candidates. In this brief perspective, we describe how ex vivo cultures can be combined with modern technologies to advance research on TBV infections.
Keywords: tick, virus, vaccine, therapeutic, organ, flavivirus
Graphical Abstract
Ex vivo organ cultures provide an avenue to identify potential countermeasures against tick-borne virus infection and/or transmission.

Tick-borne virus (TBV) infections are the cause of significant morbidity and mortality worldwide. TBVs are found in several different virus families, including Asfarviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae, Bunyaviridae and Flaviviridae1–2, however, the bulk of human disease can be attributed to tick-borne flaviviruses (TBFVs). These include Powassan virus (POWV), tick-borne encephalitis virus (TBEV), Omsk hemorrhagic fever virus (OHFV), Alkhurma hemorrhagic fever virus (AHFV) and Kyasanur forest disease virus (KFDV).
TBVs can cause a spectrum of disease symptoms. For example, TBFV disease manifestations range in severity from fever, headache and myalgia to more serious complications such as encephalitis and hemorrhagic fever. Effective commercial vaccines are available for TBEV and for KFDV2–4, but there are no other TBV-specific countermeasures available for prevention and/or treatment of infections. While commercial anti-tick vaccines exist, there are none specifically designed to prevent TBV transmission.
The natural vectors of TBV are predominantly ixodid (hard) ticks, although some argasid (soft) ticks have been implicated. These include species from Haemaphysalis, Hyalomma, Dermacentor, Ixodes and Ornithodoros genera of ticks1–2, 5. The range of several tick vector species is expanding and infections by viruses carried by these ticks are thought to be increasing. Specifically, an expanded distribution has been observed for Ixodes sp. ticks across the United States6 and an increase in POWV disease cases has also been observed7–10. Similarly, Ixodes ricinus tick populations have also been identified in new areas across Europe11 and this has been associated with an increase in TBEV disease cases12–13. Trends of vector expansion and disease increase may also be occurring with other tick species and other TBV diseases in other parts of the world.
Most TBVs must be handled at Biosafety Level (BSL) 3 or 4, and some are classified as Select Agents in the United States (https://www.selectagents.gov/selectagentsandtoxinslist.html). As a consequence, research must be done in facilities capable of meeting these requirements14–19. Studies on infected ticks also require specialized containment facilities. These factors constrain the opportunities to screen countermeasures, such as small molecule acaricide or antiviral candidates, on TBV-infected ticks. In addition, little is known regarding TBV spread in the tick, host responses and host proteins involved in TBV infection in specific tick organs. The application of ex vivo cultures provides a convenient avenue to explore TBV infection in ticks, especially within specific organs8.
In nature, ticks can become infected by both viremic and non-viremic transmission20–24. Viremic transmission occurs in the context of a tick taking a bloodmeal on a viremic mammal. Non-viremic transmission involves the transfer of virus from an infected to an uninfected tick when ticks are “cofeeding” in a localized area on the skin. The precise mechanism of non-viremic transmission is unclear, but may involve a combination of migrating immune cells and potential effects from tick saliva during tick feeding. Suitable mammal models exist to study both viremic and non-viremic transmission25–26; however, these studies may underestimate the complexity of in vivo infections and may not faithfully recapitulate disease in all potential mammal hosts. For example, the response to POWV infection in laboratory mice appears to mimic human disease syndromes but the reservoir species Peromyscus leucopus restricts the infection and does not suffer any obvious disease27–28. This highlights the complexity of TBV pathogenesis in hosts and the need to model infections in multiple mammal systems consisting of both susceptible and reservoir hosts. Such in vivo animal models can be costly and logistically challenging. On the other hand, simple in vitro cell culture fails to account for the unique characteristics of complex organ systems. As a result, ex vivo approaches such as organ slice cultures and, recently, organoid culture29–30 can bridge the gap between in vitro cell culture and in vivo mammal models in arbovirus studies. The characteristics of ex vivo tick and mammal systems abrogates the need for infecting intact lab animals and arthropods and may also simplify containment requirements needed for TBV-infected in vivo animals. The remainder of this perspective describes both ex vivo tick and mammal culture systems that exist and provides potential applications for research focused towards controlling TBV infection.
Ex Vivo Tick Culture Systems
Ticks are exposed to TBVs either by feeding on viremic mammal hosts or by the process of cofeeding as described in the previous section. Virus subsequently must replicate and successfully traverse several barriers in order to disseminate throughout the tick. The midgut is considered the initial or primary organ barrier to systemic infection31–32, and successful passage through the midgut allows access to hemocoel and other organs like the salivary glands, ovaries and the synganglion33. The salivary glands are generally considered the final organ barrier to virus transmission34–35, and virus is transferred in saliva as the tick feeds. However, the virus survives transstadially as the tick undergoes metamorphosis into the next life stage, and in some cases virus infects ovarian cells33, 36 and is passaged transovarially20.
A wealth of information about TBV research using ex vivo tick cultures has been compiled over the years. The Tick Cell Biobank of the Institute of Infection and Global Health at University of Liverpool provides an extensive bibliography on ex vivo tick culture research (https://www.liverpool.ac.uk/infection-and-global-health/research/tick-cell-biobank/bibliography/) and offers training opportunities on different ex vivo tick culture methods (https://www.liverpool.ac.uk/infection-and-global-health/research/tick-cell-biobank/services/). Additionally, “in-house” training from research groups on tick dissection procedures may provide other resource avenues. Currently, there are three types of ex vivo tick culture variations available for use in research as described in the following (strengths and weaknesses are listed in Table 1).
Table 1.
Strengths and weaknesses of ex vivo tick and mammal cultures in TBV research.
| Culture method | Strengths | Weaknesses |
|---|---|---|
| Backless tick | • Multiple tick organs involved in this type of culture • All organs intact in tick body cavity • Once in culture, easy to transfer or perform rinsing procedures |
• Only unfed ticks used for dissection • Autofluorescence in organs can be amplified because of multiple organs near each other |
| Metamorphosing tick | • Multiple tick organs involved in this type of culture • Focuses on the metamorphosing adult stage in between engorged nymph and mature adult stage • Cultured outside of tick body cavity |
• Wait time before dissecting metamorphosing adult stage out of the engorged nymph can be longer than the other methods • Autofluorescence can be amplified because of multiple organs near each other • Potential mechanical tear in tissues are possible during dissection |
| Tick organ | • Single organs can be cultured at a time • Organs from bloodfed and unfed adult ticks can be used for dissection • Can reduce down autofluorescence in organs • Transcript knockdown in single organs in culture is efficient • Small molecule assays can be efficient in this type of culture • Antigens can be screened in individual organs |
• Once in culture, difficult to transfer or perform rinsing procedures • Dissection of larval or nymphal organs may be challenging due to size limitations to use in culture • Potential mechanical tear in tissues are possible during dissection |
| Mammal organ slice | • Can be placed into culture immediately once sliced from organs • Maintains natural cell-to-cell connections within slice • Transcript knockdown in organ slice cultures is efficient • Organs from knockout mammals may be used in this type of culture • Small molecule assays can be efficient in this type of culture |
• Thickness of tissue is limited due to issues with necrosis • Prolonged culture can decrease viability |
| Mammal organoid | • Cytostructure fairly similar to natural organ • Can be generated from variety of cells or tissues • Single or multiple cell or tissue types together can be used in organoid cultures • Small molecule assays can be efficient in this type of culture • Overexpression or transcript knockdown is possible • CRISPR-Cas9 gene silencing is possible |
• Require longer growth period to obtain suitable organoid sizes • Issues with reproducibility • Lacks immune cell responses |
The backless tick method essentially utilizes multiple tick organs in situ (Figure 1). It involves excising the legs, mouthparts and the dorsal shield (scutum) of the tick, leaving the organs intact on the ventral half of the tick body cavity. This culture has been used with tick-borne bacteria and parasite infection studies in unfed adult ticks37–40. Although few virus studies involve this method, it is suitable for some studies because dissected organs from unfed ticks are viable in culture8 in which infection can be completed post dissection.
Figure 1. Ex vivo tick culture options.
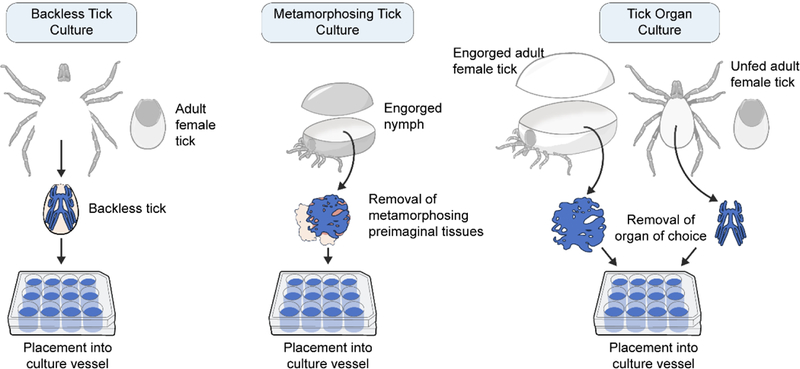
The backless tick culture involves removal of legs, mouthparts and the dorsal back of the tick before placing into culture. Metamorphosing tick culture focuses on the molting adult stage of the tick, which is removed from an engorged nymph before being placed into culture. Specific tick organs can be dissected from fed (engorged) or unfed ticks and placed directly into culture. These cultures can then be infected with virus in the culture vessel to provide infected cultures.
The most common ex vivo tick culture method involves dissecting and removing metamorphizing preimaginal ticks (developing adults) from engorged nymphs (Figure 1) to culture TBVs. This culture exposes multiple types of tick organ tissues to possible infection post dissection. Mechanical tearing of tissues during dissection using this method is a possibility, potentially allowing infection to occur in varying degrees on basal and luminal sides of organs. In addition, this focuses on the metamorphosis stage, a biological step implicated in transtadial transmission. TBVs such as Colorado Tick Fever virus, POWV and TBEV have been cultured from these types of ex vivo tick cultures41–45. Interestingly, Semliki Forest virus (SFV), a mosquito-borne alphavirus, grows in some cells of the Rhipicephalus evertsi tick cell line46–47, but not in the proposed midgut-derived cells of the cell population47. However, SFV is not considered to be transmitted by ticks. Recently, the metamorphosing tick culture has been used to show SFV growth in some R. evertsi organs, but not in the midgut47. This further suggested that SFV may not necessarily be transmitted by ticks since midgut cells were not infected in vitro or ex vivo (although confirmation with in vivo follow up studies would be beneficial). This later study provided a notable example of how ex vivo cultures can be a useful addition to vector competence studies with vector-borne viruses.
The third ex vivo tick culture method involves culturing dissected tick organs (Figure 1). This is a feasible option for tick-borne bacterium and TBV growth studies8, 45, 48. Possible mechanical tearing of tissues during dissection may occur using this method as well. Grabowski et al demonstrated the presence of TBFV infection and spread in I. scapularis organ cultures, providing numerous opportunities to study virus biology in specific tissues8. So far, we have looked at midgut, salivary glands and synganglion cultures. Future studies may also look at ovarian tissue. This will allow a focus on tissue related to transovarial transmission. Significant autofluoresence can occur when examining tick organs47, 49, but tick organ cultures can help reduce this “background noise” when organs are cultured separately and outside the body cavity. Using this method, we have looked at organs from unfed and bloodfed ticks and both provide suitable options.
Ex vivo cultures allow for tight control of many parameters. Some important parameters that can be precisely defined include using ticks of different sex or ticks of different life stages. However, one drawback of using the tick nymph stage (or even the larval stage) is size limitation, which may impact successful dissection. Additionally, the small sizes of nymphal or larval organs in culture can increase the difficulty in rinsing after virus adsorption and transferring organs once in culture due to possible misplacement of organs. Infection procedures can be varied as well. This may involve the use of different doses of virus to infect the cultures and different regimes of virus adsorption. Culture variations are possible48 when 1) culture medium is resupplied to maintain cultures over a longer time periods rather than using short-term cultures, 2) culture media components are varied, and/or 3) varied culture temperatures are used. The viability and/or deterioration of organs during culture may be affected by these culture variations, so it may be crucial to optimize before infection studies. Thus, ex vivo culture provides numerous opportunities and flexibility to tailor experimental setup precisely to address research questions efficiently.
Ex Vivo Mammal Culture Systems
Throughout mammalian species, the symptoms of TBV infection can range from no known medical significance to muscle aches, fevers, and arthralgia to severe encephalitis or hemorrhagic disease. As pathogenesis in mammals progresses, multiple organs become affected sequentially. Transmitted by the bite of a tick, the first mammal organ exposed to the virus is the skin. Hence, viruses spread to organs including the lymph nodes, lungs, liver, spleen, kidneys, and brain50. A long-held goal of researchers has been to examine aspects of viral infection in these very different organs.
Whole organ or organ slice cultures have been used since the 1950–1960s51. Slices from the brain, kidney, liver, and lungs, or whole organs (in the case of many embryonic organs), can be cultured in roller bottles or on membrane interfaces (Figure 2A). As with all experimental systems, there are advantages and drawbacks to using organ cultures (Table 1). Sections are generally limited in thickness to a few hundred microns because thicker slices are more prone to issues with gas exchange and tissue necrosis. These cultures can be maintained for hours to weeks, depending upon tissue type, but generally show thinning over longer incubations. Light microscope and electron microscope imaging are frequently employed. If the slices are collected serially from larger organs, the effect of different viruses, different virus doses or other treatments can be assessed. Importantly, cell organization, architecture and cell-to-cell connections remain intact within the slice.
Figure 2. Ex vivo mammal culture options.

(A) Sectioned organ slices are cultured on membrane inserts allowing efficient gas exchange and long-term culture maintenance. (B) Dissociated stem cells are allowed to aggregate within the culture vessel then extracellular matrix gel (blue) is added to support the developing organoids. Organoid culture is continued in tissue culture dishes or spinning bioreactors.
Virologists have employed these slice cultures to study the course of TBV replication and pathogenesis, most extensively with TBFVs. Some TBFV members have been investigated within cultures derived from spleen52, liver52, lymph nodes52, spinal cord52, brain52–53, pancreas54, and skin55 with regards to replication. Additionally, some insights and possible avenues for future TBFV-organ slice culture experiments can be made from looking at research with their mosquito-borne flavivirus (MBFV) counterparts. Both TBFVs and MBFVs have neurotropic members and most ex vivo MBFV work has focused on the central nervous system. Recently, embryonic mouse brain slices (E13, E15, and E19) were used to compare the neurotropism and developmental effects of several strains of Zika virus (ZIKV), a MBFV linked to microcephaly and fetal death. These studies elegantly demonstrated that infection impaired migration of neuronal precursors within the developing embryonic brain29. In addition to brain slice cultures, spinal cord ex vivo culture has been employed as a method to study West Nile virus (WNV)56–62. Many earlier experiments looked at replication and the spatial relationship between viral progeny and the myelin lamellae57–60. This work suggests that ex vivo organ slice cultures may be a very useful system for studying TBV pathogenesis.
Another method to model organs is the recently developed technique of stem cell organoids (strengths and weaknesses are listed in Table 1). Stem cell organoid cultures originate from pluripotent embryonic stem cells, induced pluripotent stem cells, or stem cells enriched from adult or fetal tissue. Stem cells are allowed to aggregate, incubated in specific growth factors that mimic organ niches, and then finally stabilized in an extracellular matrix. The cultures are then grown on non-adherent culture dishes or within bioreactors (Figure 2B). Organoids, unlike traditional cell culture lines, are developed from genetically normal cells but could also be developed from patient-derived cells to model disease. At this time they lack immune cells and surrounding cell-cell interactions present in in vivo organs and, due to the self-organizing nature of the cultures, have shown problems with culture variability63. Nevertheless, the organoid technique is versatile and has been used to develop organoids to model organs such as liver64–65, kidneys66–68, lungs69, and brain70–71, which are known tissue targets of TBVs.
Thus far, stem cell organoids have not been used for any TBVs but have recently been used for studies on microcephaly associated with ZIKV infection. Infection of organoid cultures with ZIKV disrupts neural development and decreases the number of neurons present72–73. Additional Zika-organoid work also suggests a role for toll-like receptor 3 activation in the development of microcephaly74. Because such limited viral work has exploited organoid cultures, this line of research is likely to increase, especially as modifications are made to the organoid process to improve the reproducibility75. In summary, both organ slice and organoid cultures provide beneficial systems to study TBV and host biology following virus infection.
Basic Science Applications Using Ex Vivo Tick and Mammal Cultures
Both tick and mammal ex vivo cultures can be used to study basic science questions and applied approaches. Most previous research have used these cultures simply to amplify virus, but a variety of additional studies can be envisioned. Other applications may include the following: (1) Vector competence in ex vivo tick cultures47, to identify whether certain tick organs can be infected and produce infectious virus. These studies provide substantive evidence to confirm particular tick species as potential vectors of TBVs when combined with tick-mammal transmission studies. (2) “Omic” analyses can identify associated transcriptome, proteome and metabolome with TBV infections in various ex vivo systems. (3) Functional analyses that involve CRISPR-Cas9 gene editing/knockout/RNA interference (RNAi) knockdown/overexpression or small molecule assays can be performed in ex vivo cultures. Ex vivo cultures can help identify (4) determinants of viral virulence (while looking at viral genome or protein changes)76 after culturing in different organs.
Given the increasing importance of TBV infections, determining host factors involved in TBV infection is a timely research direction and deserves more attention, especially because of the limited knowledge of TBV host (tick and mammal) factors. The sequenced, annotated and publicly available gene models of Ixodes scapularis tick77–79 provide a platform to work from in identifying genes that may be associated and possibly involved with virus infection80–83. For example, transfection of dsRNA specific to tick gene transcripts can be used to determine effect on TBV infection8 (Figure 3) in organ cultures. The application of CRISPR-Cas9 gene editing with ticks is in its infancy and the feasibility is currently unknown. Optimization efforts are most likely being put towards delivery of guide RNAs2. Using RNAi in ex vivo organ culture can be paralleled with mammal systems too53, where an active RNAi pathway is suggested in post-dissected organs and organ slices. Organs from knockout mammals can be directly used for TBV infection studies compared with proper controls. CRISPR-Cas9 gene editing is much further along in mammal systems and provides an attractive avenue if combined with ex vivo mammal cultures. Additionally, it is expected that some genes will be lethal knockouts. Hence, studies using ex vivo tick and mammal cultures can help identify cellular pathways and host factors associated and involved with TBV infection.
Figure 3. Standard experimental setup for dsRNA/siRNA/small molecule soaking and recombinant-live virus growth assays in ex vivo cultures.

Several approaches to use (A) small molecule and (B) siRNA/dsRNA-mediated RNA interference transcript knockdown assays with ex vivo tick or mammal cultures exist. Identifying viability of cultures can be completed alone with the small molecule/siRNA/dsRNA treatments. Depending on experimental setup, options to pretreatment or posttreatment with small molecule/siRNA/dsRNA before or after tick-borne virus (TBV) infection exist. In addition, (C) ex vivo cultures can be infected with recombinant TBVs that may serve as live-virus vaccine candidates. Virus-infected, ex vivo cultures can be analyzed at different timepoints post infection where multiple parameters of virus infection can be measured (genome replication, replication, antigen presence and genome presence).
The immune system is known to play a large role in TBV infections. Specifically, the skin interface where TBV-infected ticks attach and transmit TBVs has remained a research interest for over the last twenty years20, 55. The use of skin cultures to help confirm in vitro and in vivo observations may be useful for future experimental work in this area. While corresponding studies with TBFV have not yet been done, immune and inflammation responses in the neurological system implicated with mosquito-borne flavivirus infection have been studied with ex vivo mammal cultures. Clarke et al infected brain slice cultures from 3–4 day old mice with WNV, a neurotropic, mosquito-borne flavivirus56. These cultures were used to study the activation of caspase 8, the initiator caspase for death receptor signaling, without the need for peripheral immune response. More recently, the central nervous system immune responses during WNV infection and the role of anti-inflammatory responses have been investigated within spinal cord cultures61–62. These studies showed the power of utilizing ex vivo organ culture to tease out contributions of localized immune responses to virus infection and can be used similarly in TBV infection studies.
Using Ex Vivo Cultures in Research Focused on Controlling Tick-Borne Virus Infections and Transmission
Finally, ex vivo cultures may have a role in research to reduce TBV infection in natural population of ticks and/or disrupt TBV transmission by ticks. There are two specific aspects to consider. (1) The “cofeeding” aspect of ticks allows for efficient non-viremic transmission of TBFV infection to neighboring, uninfected ticks. (2) The rapid transmission of TBFVs, and hypothetically other TBVs, can occur within a few hours post-attachment to a mammal host7, 84–86. Thus, when designing vaccines or acaricides, it would it be important to reduce the amount of time or efficiency of tick feeding. Prevention of tick attachment or biting the mammal host, which would prevent the tick from releasing any saliva, would also be vital. Ex vivo cultures can be helpful in research efforts directed towards these goals, as described in the following content.
Determination of host factors involved in TBV infection is also important due to the relevance of finding targets for possible therapeutic candidates in the mammal system and vaccine candidates in the tick system. Studies working to identify potential mammal proteins for binding of inhibitory small molecules80, 87–89 can use ex vivo mammal cultures to focus on effects of TBV infection (Figure 3) in specific organs. TBV-specific antivirals90–95 can also be screened within specific mammal organs or organoids. Research has already begun to identify potential ZIKV inhibitors using high-throughput small molecule screens in ZIKV-infected organoids96. The effects on viability as well as caspase-3 cell death activity of the screened small-molecules was also considered.
Studies working to identify potential tick proteins for antigen development in anti-tick or transmission-blocking vaccine candidates can use ex vivo tick cultures to focus on the effects of TBV infection to specific organs, especially within salivary glands and midguts. Certain tick proteins may be more suitable for antigen development than others97–101. Screening of tick protein antigens in tick cell culture have been used before to identify the effect on pathogen infection102–103. This may be used in a similar fashion in tick organ culture in combination with the effect on TBV infection.
Infection studies in specific organ types (Figure 3) can provide valuable insight into developing live-virus vaccines to control TBV infection. Recombinant live-virus vaccine candidates have been grown before in cell lines and in in vivo ticks/mammals104 to determine replication efficiency. A reduced replication efficiency of these virus candidates can help confirm that suitable vaccine candidates have limited virus growth in both tick and mammal hosts.
Conclusions and Future Outlook
Ex vivo culture systems do not replace in vitro or in vivo systems (strengths and weaknesses of in vitro, ex vivo and in vivo systems are listed in Table 2); rather, they add a new dimension to the arsenal of research approaches to study TBV pathogenesis. Ex vivo tick and mammal cultures can provide safer options while working with BSL3/4 TBVs and can eliminate potential needle stick injuries with virus-contaminated inoculation and dissection apparati8. While ex vivo tick cultures may help identify cell types within tissues that are susceptible to virus infection, in vivo tick infection studies may provide confirmation. In vivo validation of dsRNA-mediated RNAi transcript knockdown in whole ticks is a crucial step in characterizing potential tick genes involved in TBV infection and/or TBV infection (Figure 4) and/or transmission to mammal hosts. However, widespread use of tick ex vivo culture work is limited due to the paucity of training resources available. Additionally, the potential for long-term ex vivo cultures to differentiate from the original state of the tissue after placement in culture, should be carefully heeded. Optimization to obtain minimal deterioration of tissue while in culture and to determine a relative endpoint for culture setup is highly suggested. Completion of high-throughput studies with ex vivo cultures may face some difficulties, especially if dissection is needed to obtain organs of interest, which is often the most time-consuming part of establishing the culture.
Table 2.
Strengths and weaknesses of using in vitro cell culture, ex vivo culture and in vivo tick/mammal work in research.
| Research parameter | In vitro cell culture | Ex vivo culture | In vivo tick/mammal work |
|---|---|---|---|
| Degree of reproducibility | High | Moderate to low | Moderate to low |
| Models natural route of infection | Poor | Moderate | Excellent |
| High-throughput potential | Excellent | Moderate to poor | Poor |
| Maintenance of tissue/organ cytostructure | Poor | Excellent to moderate | Excellent |
| Cost | Low | Moderate to low | High |
| Degree of institutional biosafety committee review | Low | Moderate to low | High |
| Degree of containment in research involving biosafety level 3 or 4 pathogens | Low | Low | High |
Figure 4. Use of dsRNA-mediated RNA interference assays in ex vivo tick cultures to help identify possible countermeasures.

Tick organ cultures can be used for dsRNA-mediated, RNA interference transcript knockdown studies in tick-borne virus (TBV)-infected tick organs to identify effect on virus infection. Organ viability can also be obtained post dsRNA-mediated transcript knockdown. dsRNA-mediated transcript knockdowns can also be completed in TBV-infected ticks to identify 1) biological phenotypes in ticks and 2) effect on virus infection/transmission. Select tick genes with transcript knockdowns resulting in decreased virus infection, and ideally virus transmission, may possibly be considered as candidate targets for antigen characterization and/or small molecule screening studies.
We have discussed ex vivo cultures available for TBV research use, listed strengths and weaknesses of each (Table 1), and provided examples of basic science and application-based uses of these cultures. We consider the strengths of ex vivo cultures to outweigh the negatives and we advocate for the use of ex vivo systems for TBV research. As previously mentioned, modern technologies are providing researchers the opportunity to harness techniques to identify properties and factors of TBV pathogenesis in both tick and mammal systems more quickly and efficiently. A consistent push for research geared toward controlling or reducing TBV infection and transmission is necessary, and “control plans” can help focus the TBV field towards this endeavor. Setting goals and timelines can eventually create therapeutic, vaccine and/or acaricide development options2, 105–108. While training in tick dissection approaches and ex vivo culture methods is limited, these established “control plans” can call for increased workshops to help the research field become more acquainted with these ex vivo techniques. TBV researchers should not only work towards understanding the basic virus biology, but should also translate this knowledge into experimental efforts aimed to control virus infection and transmission in real world setting.
Acknowledgements
We thank Ryan Kissinger and Anita Mora of the Visual and Medical Arts of the Research and Technologies Branch (NIAID/NIH, Hamilton, MT) for graphic art. Early review of the manuscript was provided by Cathryn L. Haigh (NIAID/NIH) and aid in literature search was provided by Taylor Robinson (NIAID/NIH).
JMG, DKO and MEB were supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Funding
JMG, DKO and MEB were supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Abbreviations
- TBVs
tick-borne viruses
- TBV
tick-borne virus
- TBFVs
tick-borne flaviviruses
- POWV
Powassan virus
- TBEV
tick-borne encephalitis virus
- OHFV
Omsk hemorrhagic fever virus
- AHFV
Alkhurma hemorrhagic fever virus
- KFDV
Kyasanur forest disease virus
- TBFV
tick-borne flavivirus
- BSL
biosafety level
- SFV
Semliki Forest virus
- MBFV
mosquito-borne flavivirus
- ZIKV
Zika virus
- WNV
West Nile virus
- RNAi
RNA interference
Footnotes
Conflict of Interest
The authors declare no financial competing interests or conflict of interests.
References
- 1.Labuda M; Nuttall PA, Tick-borne viruses. Parasitology 2004, 129 Suppl, S221–245. [DOI] [PubMed] [Google Scholar]
- 2.Grabowski JM; Hill CA, A Roadmap for Tick-Borne Flavivirus Research in the “Omics” Era. Front Cell Infect Microbiol 2017, 7, 519 10.3389/fcimb.2017.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook MR, Kyasanur forest disease. Antiviral Res 2012, 96 (3), 353–362. 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasabi GS; Murhekar MV; Sandhya VK; Raghunandan R; Kiran SK; Channabasappa GH; Mehendale SM, Coverage and effectiveness of Kyasanur forest disease (KFD) vaccine in Karnataka, South India, 2005–2010. PLoS Negl Trop Dis 2013, 7 (1), e2025 10.1371/journal.pntd.0002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobler G, Zoonotic tick-borne flaviviruses. Vet Microbiol 2010, 140 (3–4), 221–228. 10.1016/j.vetmic.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Eisen RJ; Eisen L; Beard CB, County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016, 53 (2), 349–386. 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebel GD, Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010, 55, 95–110. 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- 8.Grabowski JM; Tsetsarkin KA; Long D; Scott DP; Rosenke R; Schwan TG; Mlera L; Offerdahl DK; Pletnev AG; Bloom ME, Flavivirus Infection of Ixodes scapularis (Black-Legged Tick) Ex Vivo Organotypic Cultures and Applications for Disease Control. MBio 2017, 8 (4). 10.1128/mBio.01255-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinten SR; Beckett GA; Gensheimer KF; Pritchard E; Courtney TM; Sears SD; Woytowicz JM; Preston DG; Smith RP Jr.; Rand PW; Lacombe EH; Holman MS; Lubelczyk CB; Kelso PT; Beelen AP; Stobierski MG; Sotir MJ; Wong S; Ebel G; Kosoy O; Piesman J; Campbell GL; Marfin AA, Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis 2008, 8 (6), 733–740. 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- 10.National Academies of Sciences, E.; Medicine, Global Health Impacts of Vector-Borne Diseases: Workshop Summary The National Academies Press: Washington, DC, 2016; p 396 10.17226/21792. [DOI] [PubMed] [Google Scholar]
- 11.Medlock JM; Hansford KM; Bormane A; Derdakova M; Estrada-Pena A; George JC; Golovljova I; Jaenson TG; Jensen JK; Jensen PM; Kazimirova M; Oteo JA; Papa A; Pfister K; Plantard O; Randolph SE; Rizzoli A; Santos-Silva MM; Sprong H; Vial L; Hendrickx G; Zeller H; Van Bortel W, Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 2013, 6, 1 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenson TG; Hjertqvist M; Bergstrom T; Lundkvist A, Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors 2012, 5, 184 10.1186/1756-3305-5-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrer AT; Holbrook MR, Tick-borne Encephalitis Vaccines. J Bioterror Biodef 2011, 2011 (Suppl 1), 3 10.4172/2157-2526.S1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Committee of Medical, E.; American Society of Tropical, M.; Hygiene, Arthropod containment guidelines. A project of the American Committee of Medical Entomology and American Society of Tropical Medicine and Hygiene. Vector Borne Zoonotic Dis 2003, 3 (2), 61–98. 10.1089/153036603322163448. [DOI] [PubMed] [Google Scholar]
- 15.Thangamani S; Bente D, Establishing protocols for tick containment at Biosafety Level 4. Pathog Dis 2014, 71 (2), 282–285. 10.1111/2049-632X.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott TW, Containment of arthropod disease vectors. ILAR J 2005, 46 (1), 53–61. [DOI] [PubMed] [Google Scholar]
- 17.Tabachnick WJ, Laboratory containment practices for arthropod vectors of human and animal pathogens. Lab Anim (NY) 2006, 35 (3), 28–33. 10.1038/laban0306-28. [DOI] [PubMed] [Google Scholar]
- 18.S H, The Containment of Arthropod Vectors. In Biology of Disease Vectors, 2nd ed.; WC M, Ed. Elsevier Academic Press: 2005; pp 699–704. [Google Scholar]
- 19.Bouchard KR, W. S, Care, Maintenance, and Experimental Infestation of Ticks in the Laboratory Setting. In Biology of Disease Vectors, 2nd ed.; WC M, Ed. Elsevier Academic Press: 2005; pp 705–711. [Google Scholar]
- 20.Kazimirova M; Thangamani S; Bartikova P; Hermance M; Holikova V; Stibraniova I; Nuttall PA, Tick-Borne Viruses and Biological Processes at the Tick-Host-Virus Interface. Front Cell Infect Microbiol 2017, 7, 339 10.3389/fcimb.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labuda M; Kozuch O; Zuffova E; Eleckova E; Hails RS; Nuttall PA, Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology 1997, 235 (1), 138–143. 10.1006/viro.1997.8622. [DOI] [PubMed] [Google Scholar]
- 22.Havlikova S; Lickova M; Klempa B, Non-viraemic transmission of tick-borne viruses. Acta Virol 2013, 57 (2), 123–129. [DOI] [PubMed] [Google Scholar]
- 23.Labuda M; Jones LD; Williams T; Danielova V; Nuttall PA, Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J Med Entomol 1993, 30 (1), 295–299. [DOI] [PubMed] [Google Scholar]
- 24.Labuda M; Nuttall PA; Kozuch O; Eleckova E; Williams T; Zuffova E; Sabo A, Non-viraemic transmission of tick-borne encephalitis virus: a mechanism for arbovirus survival in nature. Experientia 1993, 49 (9), 802–805. [DOI] [PubMed] [Google Scholar]
- 25.Zivcec M; Safronetz D; Feldmann H, Animal models of tick-borne hemorrhagic Fever viruses. Pathogens 2013, 2 (2), 402–421. 10.3390/pathogens2020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzaran D; Bente DA, Animal models for viral haemorrhagic fever. Clin Microbiol Infect 2015. 10.1111/1469-0691.12630. [DOI] [PubMed]
- 27.Mlera L; Meade-White K; Saturday G; Scott D; Bloom ME, Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLoS Negl Trop Dis 2017, 11 (1), e0005346 10.1371/journal.pntd.0005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermance ME; Thangamani S, Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector borne and zoonotic diseases 2017, 17 (7), 453–462. 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld AB; Doobin DJ; Warren AL; Racaniello VR; Vallee RB, Replication of early and recent Zika virus isolates throughout mouse brain development. PNAS 2017. 10.1073/pnas.1714624114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta D; Clevers H, Organoid culture systems to study host-pathogen interactions. Curr Opin Immunol 2017, 48, 15–22. 10.1016/j.coi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de la Fuente J; Antunes S; Bonnet S; Cabezas-Cruz A; Domingos AG; Estrada-Pena A; Johnson N; Kocan KM; Mansfield KL; Nijhof AM; Papa A; Rudenko N; Villar M; Alberdi P; Torina A; Ayllon N; Vancova M; Golovchenko M; Grubhoffer L; Caracappa S; Fooks AR; Gortazar C; Rego ROM, Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front Cell Infect Microbiol 2017, 7, 114 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleiboeker SB; Scoles GA; Burrage TG; Sur J, African swine fever virus replication in the midgut epithelium is required for infection of Ornithodoros ticks. J Virol 1999, 73 (10), 8587–8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth TF; Davies CR; Jones LD; Staunton D; Nuttall PA, Anatomical basis of Thogoto virus infection in BHK cell culture and in the ixodid tick vector, Rhipicephalus appendiculatus. J Gen Virol 1989, 70 (Pt 5), 1093–1104. 10.1099/0022-1317-70-5-1093. [DOI] [PubMed] [Google Scholar]
- 34.Bowman AS; Ball A; Sauer JR, Tick salivary glands: the physiology of tick water balance and their role in pathogen trafficking and transmission. In Ticks: Biology, Disease and Control, Bowman AS; Nuttall P, Eds. Cambridge University Press: 2008; pp 73–91. [Google Scholar]
- 35.Valenzuela JG, Blood-Feeding Arthropod Salivary Glands and Saliva. In Biology of Disease Vectors, 2nd ed.; Marquardt WC, Ed. Elsevier Academic Press: 2005; pp 377–386. [Google Scholar]
- 36.Dickson DL; Turell MJ, Replication and tissue tropisms of Crimean-Congo hemorrhagic fever virus in experimentally infected adult Hyalomma truncatum (Acari: Ixodidae). J Med Entomol 1992, 29 (5), 767–773. [DOI] [PubMed] [Google Scholar]
- 37.Bell LJ, Organ culture of Rhipicephalus appendiculatus with maturation of Theileria parva in tick salivary glands in vitro. Acta Trop 1980, 37 (4), 319–325. [PubMed] [Google Scholar]
- 38.J R, Tick Culture: Applications in Biology. In Arthropod Cell Culture Systems, Maramorosch K, M. A, Ed. CRC Press, Inc: 1994; pp 69–70. [Google Scholar]
- 39.Sunyakumthorn P; Petchampai N; Grasperge BJ; Kearney MT; Sonenshine DE; Macaluso KR, Gene expression of tissue-specific molecules in ex vivo Dermacentor variabilis (Acari: Ixodidae) during rickettsial exposure. J Med Entomol 2013, 50 (5), 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunyakumthorn P; Petchampai N; Kearney MT; Sonenshine DE; Macaluso KR, Molecular characterization and tissue-specific gene expression of Dermacentor variabilis alpha-catenin in response to rickettsial infection. Insect Mol Biol 2012, 21 (2), 197–204. 10.1111/j.1365-2583.2011.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yunker CE; Cory J, Growth of Colorado tick fever (CTF) virus in primary tissue cultures of its vector, Dermacentor andersoni Stiles (Acarina: ixodidae), with notes on tick tissue culture. Exp Parasitol 1967, 20 (3), 267–277. [DOI] [PubMed] [Google Scholar]
- 42.Chunikhin SP; Kochetova GA; Stefutkina LF; Korolev MB, [Reproductive characteristics of the tick-borne encephalitis and Powassan viruses in Dermacentor silvarum imago explants removed from the nymphal cuticle]. Med Parazitol (Mosk) 1981, 50 (4), 61–64. [PubMed] [Google Scholar]
- 43.Chunikhin SP; Khozinskaia GA; Stefutkina LF; Korolev MB, [Mono- and mixed infection by the tick-borne encephalitis and Powassan viruses of tissue explants from ticks of the genus Hyalomma]. Parazitologiia 1984, 18 (2), 116–122. [PubMed] [Google Scholar]
- 44.Khozinskaya GA; Chunikhin SP; Khozinsky VV; Stefutkina LF, Variability of Powassan virus cultured in tissue explants and organism of Hyalomma anatolicum ticks. Acta Virol 1985, 29 (4), 305–311. [PubMed] [Google Scholar]
- 45.Yunker CE, Arthropod tissue culture in the study of arboviruses and rickettsiae: a review. Curr Top Microbiol Immunol 1971, 55, 113–126. [DOI] [PubMed] [Google Scholar]
- 46.Barry G; Alberdi P; Schnettler E; Weisheit S; Kohl A; Fazakerley JK; Bell-Sakyi L, Gene silencing in tick cell lines using small interfering or long double-stranded RNA. Exp Appl Acarol 2013, 59 (3), 319–338. 10.1007/s10493-012-9598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell-Sakyi L; Weisheit S; Rückert C; Barry G; Fazakerley J; Fragkoudis R, Microscopic Visualisation of Zoonotic Arbovirus Replication in Tick Cell and Organ Cultures Using Semliki Forest Virus Reporter Systems. Veterinary Sciences 2016, 3 (4), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.J R, Tick Tissue Culture and Arboviruses. In Invertebrate Tissue Culture: Applications in Medicine, Biology, and Agriculture, Kurstak E, M. K, Ed. Academic Press: 1976; pp 21–33. [Google Scholar]
- 49.Chernesky MA; McLean DM, Localization of Powassan virus in Dermacentor andersoni ticks by immunofluorescence. Can J Microbiol 1969, 15 (12), 1399–1408. [DOI] [PubMed] [Google Scholar]
- 50.Nuttall PA, Tick-borne viruses. In Biology of Ticks, Sonenshine DE; Roe RM, Eds. Oxford University Press: New York, New York, 2014; Vol. 2, pp 180–210. [Google Scholar]
- 51.Shamir ER; Ewald AJ, Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nature Reviews Molecular Cell Biology 2014, 15, 647–664. 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gresikova M; Sekeyova M, In vitro cultivation studies on the skalica strain of tick-borne encephalitis virus. Acta virologica 1980, 24 (6), 455–458. [PubMed] [Google Scholar]
- 53.Maffioli C; Grandgirard D; Leib SL; Engler O, siRNA inhibits replication of Langat virus, a member of the tick-borne encephalitis virus complex in organotypic rat brain slices. PLOS One 2012, 7 (9), e44703 10.1371/journal.one.0044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gresikova M; Tregerova V, Experimental infection of pancreatic organ cultures from suckling white mice with tick-borne encephalitis and sindbis viruses. Acta virologica 1982, 26, 405. [PubMed] [Google Scholar]
- 55.Labuda M; Austyn JM; Zuffova E; Kozuch O; Fuchsberger N; Lysy J; Nuttall PA, Importance of locailized skin infection in tick-borne encephalitis virus transmission. Virology 1996, 218, 357–366. 10.1006/viro.1996.0261. [DOI] [PubMed] [Google Scholar]
- 56.Clarke P; Leser JS; Quick ED; Dionne KR; Beckham JD; Tyler KL, Death receptor-mediated apoptotic signaling is activated in the brain following infection with West Nile Virus in the absence of a peripheral immune response. Journal of Virology 2014, 88 (2), 1080–1089. 10.1128/JVI.02944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahar A; Lustig S; Akov S; David Y; Schneider P; Friedmann A; Levin R, West Nile virions aligned along myelin lamellae in organotypic spinal cord cultures. Journal of Neuroscience Research 1990, 26, 495–500. [DOI] [PubMed] [Google Scholar]
- 58.Shahar A; Lustig S; Akov S; David Y; Schneider P; Levin R, Different pathogenicity of encephalitic togaviruses in organotypic cultures of spinal cord slices. Journal of Neuroscience Research 1990, 25, 345–352. [DOI] [PubMed] [Google Scholar]
- 59.Shahar A; Lustig S; David Y; Schneider P; Levin R, Spinal cord slices with attached dorsal root ganglia: a culture model for the study of pathogenicity of encephalitic viruses. Advances in Experimental Medicine and Biology 1991, 296, 111–119. [DOI] [PubMed] [Google Scholar]
- 60.Shahar A; Schupper H; Lustig S; Levin R; Friedmann A; Fuchs P, Neuronal cell cultures as a model for assessing neurotoxicity induced by encephalitic viruses. Neurotoxicology 1992, 13, 171–178. [PubMed] [Google Scholar]
- 61.Quick ED; Leser JS; Clarke P; Tyler KL, Activation of intrinsic immune responses and microglial phagocytosis in an ex vivo spinal cord slice culture model of West Nile virus infection. Journal of Virology 2014, 88 (22), 13005–13014. 10.1128/JVI.01994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quick ED; Seitz S; Clarke P; Tyler KL, Minocycline has anti-inflammatory effects and reduces cytotoxicity in an ex vivo spinal cord slice culture model of West Nile virus infection. Journal of Virology 2017. 10.1128/JVI.00569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xinaris C; Brizi V; Remuzzi G, Organoid Models and Applications in Biomedical Research. Nephron 2015, 130 (3), 191–199. 10.1159/000433566. [DOI] [PubMed] [Google Scholar]
- 64.Huch M; Dorrell C; Boj SF; van Es JH; Li VS; van de Wetering M; Sato T; Hamer K; Sasaki N; Finegold MJ; Haft A; Vries RG; Grompe M; Clevers H, In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494 (7436), 247–250. 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takebe T; Sekine K; Enomura M; Koike H; Kimura M; Ogaeri T; Zhang RR; Ueno Y; Zheng YW; Koike N; Aoyama S; Adachi Y; Taniguchi H, Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499 (7459), 481–484. 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 66.Xia Y; Nivet E; Sancho-Martinez I; Gallegos T; Suzuki K; Okamura D; Wu MZ; Dubova I; Esteban CR; Montserrat N; Campistol JM; Izpisua Belmonte JC, Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 2013, 15 (12), 1507–1515. 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 67.Taguchi A; Kaku Y; Ohmori T; Sharmin S; Ogawa M; Sasaki H; Nishinakamura R, Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2014, 14 (1), 53–67. 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Takasato M; Er PX; Becroft M; Vanslambrouck JM; Stanley EG; Elefanty AG; Little MH, Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 2014, 16 (1), 118–126. 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 69.Lee JH; Bhang DH; Beede A; Huang TL; Stripp BR; Bloch KD; Wagers AJ; Tseng YH; Ryeom S; Kim CF, Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014, 156 (3), 440–455. 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lancaster MA; Renner M; Martin C.-a.; Wenzel D; Bicknell LS; Hurles ME; Homfray T; Penninger JM; Jackson AP; Knoblich JA, Cerebral organoids model human brain development and microcephaly. Nature Cell Biology 2013, 501, 373–379. 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadoshima T; Sakaguchi H; Nakano T; Soen M; Ando S; Eiraku M; Sasai Y, Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 2013, 110 (50), 20284–20289. 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcez PP; Loiola EC; Madeiro da Costa R; Higa LM; Trindade P; Delvecchio R; Nascimento JM; Brindeiro R; Tanuri A; Rehen SK, Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352 (6287), 816–818. 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 73.Cugola FR; Fernandes IR; Russo FB; Freitas BC; Dias JL; Guimaraes KP; Benazzato C; Almeida N; Pignatari GC; Romero S; Polonio CM; Cunha I; Freitas CL; Brandao WN; Rossato C; Andrade DG; Faria Dde P; Garcez AT; Buchpigel CA; Braconi CT; Mendes E; Sall AA; Zanotto PM; Peron JP; Muotri AR; Beltrao-Braga PC, The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534 (7606), 267–271. 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dang J; Tiwari SK; Lichinchi G; Qin Y; Patil VS; Eroshkin AM; Rana TM, Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19 (2), 258–265. 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lancaster MA; Corsini NS; Wolfinger S; Gustafson EH; Phillips AW; Burkard TR; Otani T; Livesey FJ; Knoblich JA, Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 2017, 35 (7), 659–666. 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitzel DN; Best SM; Masnick MF; Porcella SF; Wolfinbarger JB; Bloom ME, Identification of genetic determinants of a tick-borne flavivirus associated with host-specific adaptation and pathogenicity. Virology 2008, 381 (2), 268–276. 10.1016/j.virol.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gulia-Nuss M; Nuss AB; Meyer JM; Sonenshine DE; Roe RM; Waterhouse RM; Sattelle DB; de la Fuente J; Ribeiro JM; Megy K; Thimmapuram J; Miller JR; Walenz BP; Koren S; Hostetler JB; Thiagarajan M; Joardar VS; Hannick LI; Bidwell S; Hammond MP; Young S; Zeng Q; Abrudan JL; Almeida FC; Ayllon N; Bhide K; Bissinger BW; Bonzon-Kulichenko E; Buckingham SD; Caffrey DR; Caimano MJ; Croset V; Driscoll T; Gilbert D; Gillespie JJ; Giraldo-Calderon GI; Grabowski JM; Jiang D; Khalil SM; Kim D; Kocan KM; Koci J; Kuhn RJ; Kurtti TJ; Lees K; Lang EG; Kennedy RC; Kwon H; Perera R; Qi Y; Radolf JD; Sakamoto JM; Sanchez-Gracia A; Severo MS; Silverman N; Simo L; Tojo M; Tornador C; Van Zee JP; Vazquez J; Vieira FG; Villar M; Wespiser AR; Yang Y; Zhu J; Arensburger P; Pietrantonio PV; Barker SC; Shao R; Zdobnov EM; Hauser F; Grimmelikhuijzen CJ; Park Y; Rozas J; Benton R; Pedra JH; Nelson DR; Unger MF; Tubio JM; Tu Z; Robertson HM; Shumway M; Sutton G; Wortman JR; Lawson D; Wikel SK; Nene VM; Fraser CM; Collins FH; Birren B; Nelson KE; Caler E; Hill CA, Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat Commun 2016, 7, 10507 10.1038/ncomms10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill CA; Wikel SK, The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends Parasitol 2005, 21 (4), 151–153. 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Pagel Van Zee J; Geraci NS; Guerrero FD; Wikel SK; Stuart JJ; Nene VM; Hill CA, Tick genomics: the Ixodes genome project and beyond. Int J Parasitol 2007, 37 (12), 1297–1305. 10.1016/j.ijpara.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 80.Grabowski JM; Gulia-Nuss M; Kuhn RJ; Hill CA, RNAi reveals proteins for metabolism and protein processing associated with Langat virus infection in Ixodes scapularis (black-legged tick) ISE6 cells. Parasit Vectors 2017, 10 (1), 24 10.1186/s13071-016-1944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weisheit S; Villar M; Tykalova H; Popara M; Loecherbach J; Watson M; Ruzek D; Grubhoffer L; de la Fuente J; Fazakerley JK; Bell-Sakyi L, Ixodes scapularis and Ixodes ricinus tick cell lines respond to infection with tick-borne encephalitis virus: transcriptomic and proteomic analysis. Parasit Vectors 2015, 8, 599 10.1186/s13071-015-1210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schnettler E; Tykalova H; Watson M; Sharma M; Sterken MG; Obbard DJ; Lewis SH; McFarlane M; Bell-Sakyi L; Barry G; Weisheit S; Best SM; Kuhn RJ; Pijlman GP; Chase-Topping ME; Gould EA; Grubhoffer L; Fazakerley JK; Kohl A, Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res 2014, 42 (14), 9436–9446. 10.1093/nar/gku657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ayllon N; Naranjo V; Hajdusek O; Villar M; Galindo RC; Kocan KM; Alberdi P; Sima R; Cabezas-Cruz A; Ruckert C; Bell-Sakyi L; Kazimirova M; Havlikova S; Klempa B; Kopacek P; de la Fuente J, Nuclease Tudor-SN Is Involved in Tick dsRNA-Mediated RNA Interference and Feeding but Not in Defense against Flaviviral or Anaplasma phagocytophilum Rickettsial Infection. PLoS One 2015, 10 (7), e0133038 10.1371/journal.pone.0133038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ebel GD; Kramer LD, Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am J Trop Med Hyg 2004, 71 (3), 268–271. [PubMed] [Google Scholar]
- 85.Costero A; Grayson MA, Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae). Am J Trop Med Hyg 1996, 55 (5), 536–546. [DOI] [PubMed] [Google Scholar]
- 86.Hermance ME; Thangamani S, Proinflammatory cytokines and chemokines at the skin interface during Powassan virus transmission. J Invest Dermatol 2014, 134 (8), 2280–2283. 10.1038/jid.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pastorino B; Nougairede A; Wurtz N; Gould E; de Lamballerie X, Role of host cell factors in flavivirus infection: Implications for pathogenesis and development of antiviral drugs. Antiviral research 2010, 87 (3), 281–294. 10.1016/j.antiviral.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Krishnan MN; Garcia-Blanco MA, Targeting host factors to treat West Nile and dengue viral infections. Viruses 2014, 6 (2), 683–708. 10.3390/v6020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang J; Schul W; Yip A; Xu X; Guo JT; Block TM, Competitive inhibitor of cellular alpha-glucosidases protects mice from lethal dengue virus infection. Antiviral research 2011, 92 (2), 369–371. 10.1016/j.antiviral.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eyer L; Zouharova D; Sirmarova J; Fojtikova M; Stefanik M; Haviernik J; Nencka R; de Clercq E; Ruzek D, Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antiviral research 2017, 142, 63–67. 10.1016/j.antiviral.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 91.Flint M; McMullan LK; Dodd KA; Bird BH; Khristova ML; Nichol ST; Spiropoulou CF, Inhibitors of the tick-borne, hemorrhagic fever-associated flaviviruses. Antimicrob Agents Chemother 2014, 58 (6), 3206–3216. 10.1128/AAC.02393-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lani R; Moghaddam E; Haghani A; Chang LY; AbuBakar S; Zandi K, Tick-borne viruses: a review from the perspective of therapeutic approaches. Ticks Tick Borne Dis 2014, 5 (5), 457–465. 10.1016/j.ttbdis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Lo MK; Shi PY; Chen YL; Flint M; Spiropoulou CF, In vitro antiviral activity of adenosine analog NITD008 against tick-borne flaviviruses. Antiviral research 2016, 130, 46–49. 10.1016/j.antiviral.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orlov AA; Drenichev MS; Oslovsky VE; Kurochkin NN; Solyev PN; Kozlovskaya LI; Palyulin VA; Karganova GG; Mikhailov SN; Osolodkin DI, New tools in nucleoside toolbox of tick-borne encephalitis virus reproduction inhibitors. Bioorg Med Chem Lett 2017, 27 (5), 1267–1273. 10.1016/j.bmcl.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 95.Osolodkin DI; Kozlovskaya LI; Dueva EV; Dotsenko VV; Rogova YV; Frolov KA; Krivokolysko SG; Romanova EG; Morozov AS; Karganova GG; Palyulin VA; Pentkovski VM; Zefirov NS, Inhibitors of tick-borne flavivirus reproduction from structure-based virtual screening. ACS Med Chem Lett 2013, 4 (9), 869–874. 10.1021/ml400226s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu M; Lee EM; Wen Z; Cheng Y; Huang WK; Qian X; Tcw J; Kouznetsova J; Ogden SC; Hammack C; Jacob F; Nguyen HN; Itkin M; Hanna C; Shinn P; Allen C; Michael SG; Simeonov A; Huang W; Christian KM; Goate A; Brennand KJ; Huang R; Xia M; Ming GL; Zheng W; Song H; Tang H, Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 2016, 22 (10), 1101–1107. 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de la Fuente J; Almazan C; Blouin EF; Naranjo V; Kocan KM, RNA interference screening in ticks for identification of protective antigens. Parasitol Res 2005, 96 (3), 137–141. 10.1007/s00436-005-1351-5. [DOI] [PubMed] [Google Scholar]
- 98.Nuttall PA; Trimnell AR; Kazimirova M; Labuda M, Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol 2006, 28 (4), 155–163. 10.1111/j.1365-3024.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 99.de la Fuente J; Merino O, Vaccinomics, the new road to tick vaccines. Vaccine 2013, 31 (50), 5923–5929. [DOI] [PubMed] [Google Scholar]
- 100.Merino O; Alberdi P; Perez de la Lastra JM; de la Fuente J, Tick vaccines and the control of tick-borne pathogens. Front Cell Infect Microbiol 2013, 3, 30 10.3389/fcimb.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villar M; Marina A; de la Fuente J, Applying proteomics to tick vaccine development: where are we? Expert Rev Proteomics 2017, 14 (3), 211–221. 10.1080/14789450.2017.1284590. [DOI] [PubMed] [Google Scholar]
- 102.Contreras M; Villar M; Alberdi P; de la Fuente J, Vaccinomics Approach to Tick Vaccine Development. Methods Mol Biol 2016, 1404, 275–286. 10.1007/978-1-4939-3389-1_19. [DOI] [PubMed] [Google Scholar]
- 103.Antunes S; Merino O; Mosqueda J; Moreno-Cid JA; Bell-Sakyi L; Fragkoudis R; Weisheit S; Perez de la Lastra JM; Alberdi P; Domingos A; de la Fuente J, Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasites & vectors 2014, 7, 42 10.1186/1756-3305-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsetsarkin KA; Liu G; Kenney H; Hermance M; Thangamani S; Pletnev AG, Concurrent micro-RNA mediated silencing of tick-borne flavivirus replication in tick vector and in the brain of vertebrate host. Sci Rep 2016, 6, 33088 10.1038/srep33088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brei B; Brownstein JS; George JE; Pound JM; Miller JA; Daniels TJ; Falco RC; Stafford KC 3rd; Schulze TL; Mather TN; Carroll JF; Fish D, Evaluation of the United States Department Of Agriculture Northeast Area-wide Tick Control Project by meta-analysis. Vector Borne Zoonotic Dis 2009, 9 (4), 423–430. 10.1089/vbz.2008.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dantas-Torres F; Chomel BB; Otranto D, Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol 2012, 28 (10), 437–446. 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Londono-Renteria B; Troupin A; Colpitts TM, Arbovirosis and potential transmission blocking vaccines. Parasites & vectors 2016, 9 (1), 516 10.1186/s13071-016-1802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sprong H; Trentelman J; Seemann I; Grubhoffer L; Rego RO; Hajdusek O; Kopacek P; Sima R; Nijhof AM; Anguita J; Winter P; Rotter B; Havlikova S; Klempa B; Schetters TP; Hovius JW, ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasit Vectors 2014, 7, 77 10.1186/1756-3305-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]


