Abstract
Purpose:
Bevacizumab (BV) monotherapy leads to compensatory upregulation of multiple signaling pathways, resulting in mTOR activation. We evaluated combining BV and everolimus (EV), an mTOR kinase inhibitor, to circumvent BV-resistance in women with recurrent or persistent ovarian, fallopian tube or primary peritoneal cancer (OC).
Patients and Methods:
Eligible OC patients had measurable (RECIST1.1) or detectable disease, 1–3 prior regimens, performance status (PS) 0–2, and no prior m-TOR inhibitor. All patients received BV 10 mg/kg IV every 2wks. Patients were randomized (1:1) to oral EV (10 mg daily) or placebo stratified by platinum-free interval (PFI), measurable disease and prior BV. Primary endpoint was progression-free survival (PFS); secondary endpoints included safety and response.
Results:
150 patients were randomized to BV with (n=75) and without (n=75) EV. Arms were well-balanced for age (median 63: range 28–92), PS (0: 73%, 1–2: 27%), prior regimens (1: 37%, 2: 47%, 3: 16%), prior BV (11%), PFI (<6mos: 65%) and measurable disease (81%). The BV+EV vs BV median PFS was 5.9 vs 4.5 months (hazard ratio [HR] 0.95 (95% CI, 0.66–1.37, p=0.39)). Median OS was 16.6 vs 17.3 months (HR 1.16 (95% CI, 0.72~1.87, p=0.55). Objective measurable responses were higher with BV+EV (22% vs 12%). Study removal due to toxicity was higher with BV+EV (29% vs 12%). Toxicity (≥grade 3) from BV+EV were “other GI (mucositis)” (23 vs 1%) and “metabolic/nutrition” (19 vs. 7%); common >= grade 2 toxicities with BV+EV were cytopenia, nausea, fatigue and rash.
Conclusion:
The combination regimen (BV+EV) did not significantly reduce the hazard of progression or death relative to BV and was associated with higher rates of adverse events and study discontinuation when compared to BV alone.
Keywords: Ovarian Cancer, Bevacizumab, Everolimus, Targeted Therapy
INTRODUCTION
Anti-vascular endothelial growth factor (VEGF) treatments have been shown to be an effective strategy for controlling tumor growth in ovarian cancer (OC) via the angiogenic and other growth pathways.1–3 Bevacizumab (BV), a recombinant humanized monoclonal antibody against VEGF, has clinical activity as both a single agent and in combination with cytotoxic chemtherapy.4–12 Based on improved progression-free survival (PFS) and overall survival (OS) in certain populations, bevacizumab is indicated in both platinum-resistant and platium-sensitive recurrent ovarian cancer and in conjunction with platinum-based therapy in upfront treatment.
Everolimus, a rapalog-type inhibitor of mammalian target of rapamycin (mTOR) complex 1 (mTORC1), attenuates up regulation of HIF-1α levels, a resistance mechanism for antiangiongenics, and targets the PI3-Kinase/AKT/mTOR axis, commonly abberant in OC.13–16 Everolimus (Afinitor, Novartis) has indications in advanced renal cell carcinoma (RCC), well-differentiated advanced neuroendocrine tumors (NET), advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane, and renal angiomyolipoma and tuberous sclerosis complex.17
The Ovarian Carcinoma Cancer Genome Atlas Research Network identified PI3K/RAS pathway deregulation in 45% ovarian cases.18 Single agent rapalog trials in recurrent ovarian cancer have yielded modest results. The Gynecologic Oncology Group (GOG) performed a study of temsirolimus show ing a response rate (RR) of 9.3% (5/54 patients), with 24.1% of patients progression-free at 6 months.19 A similar study, performed by the AGO study group (AGO-GYN8) yielded a response rate of 4.5% (1/22 patients).20
Targreted therapy combinations that include blocking VEGF and other cancer growth pathways may circumvent resistance to angiogenesis inhibition and be more tolerable and effective compared to traditional cytotoxic combinations. Everolimus has been safely combined with bevacizumab and studied in a non-randomized phase 2 trial in advanced RCC.21–22 A phase 1 trial of bevacizumab and temsirolimus was performed in gynecologic malignancies showing safe delivery of full doses of both agents and a response rate of 17% (7/41 patients).23
The objective of this study was to assess PFS in a randomized phase II study of bevacizumab alone (with an oral placebo) versus the combination of bevacizumab and oral everolimus among women with recurrent epithelial ovarian cancer.
Patients and Methods:
The study was designed as a double-blind, placebo-controlled prospective randomized phase II trial of intravenous (IV) bevacizumab every 2 weeks in combination with either oral everolimus or an oral placebo (Gynecologic Oncology Group protocol 186-G; ClinicalTrials.gov. Identifier: NCT00886691).
Eligible patients included women older than 18 years of age with a GOG performance status of 0–2. All patients were required to have measurable (per RECIST 1.1) or detectable disease from persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Detectable disease required at least one of the following conditions: baseline values of cancer antigen (CA)-125 at least 2 × upper-limit-of normal (ULN), ascites and/or pleural effusion attributed to tumor, or solid and/or cystic abnormalities on radiographic imaging that do not meet RECIST 1.1 definitions for target lesions. Patients must have had one prior platinum-based chemotherapeutic regimen for management of primary disease and were allowed to receive up to two additional cytotoxic regimens for management of recurrent or persistent disease, with no more than 1 non-platinum, non-taxane regimen. Patients who have had one prior treatment must have had a GOG Performance Status of 0, 1, or 2; patients who have had two or three prior treatments must have had a GOG Performance Status of 0 or 1. Patients were allowed to receive biologic (non-cytotoxic) therapy, including VEGF blockade, as part of their primary treatment regimen, but not for recurrent or persistent disease. Patients with either platinum-sensitive (platinum-free interval (PFI) >182 days) or platinum-resistant (PFI ≤182 days) disease were eligible. Patients who received only one prior cytotoxic regimen must have had a PFI ≤365 days or had progressed during platinum-based therapy, or had persistent disease after a platinum-based therapy.
Adequate laboratory studies included 1- bone marrow function defined as absolute neutrophil count (ANC) ≥1,500/mcl, platelets ≥100,000/mcl; 2- renal function defined as creatinine ≤1.5 × institutional ULN; 3- hepatic function defined as bilirubin ≤1.5 × ULN, SGOT (AST) ≤3 × ULN and alkaline phosphatase ≤2.5 × ULN; 4- blood coagulation parameters with PT such that international normalized ratio (INR) was ≤1.5 (or an in-range INR, usually between 2 and 3, if a patient was on a stable dose of therapeutic warfarin for management of deep vein thrombosis including pulmonary embolism) and a PTT ≤1.5 times the upper limit of normal; 5- fasting serum cholesterol ≤300 mg/dL OR ≤7.75 mmol/L AND fasting triglycerides ≤300 mg/dL or 3.42 mmol/L; 6- Urine protein creatinine (UPC) ratio must have been <1.0 gm. If UPC ratio was ≥1, collection of 24-hour urine measurement of urine protein was recommended. UPC ratio of spot urine is an estimation of the 24-hour urine protein excretion - a UPC ratio of 1 is roughly equivalent to a 24-hour urine protein of 1 gm.
Patients were excluded if they had significant comorbifties including acute hepatitis, active infection requiring parenteral antibiotics, active bleeding (or bleeding disorder), CNS disease (including uncontrolled seizures, brain metastasis and history of cerebrovascular accident or bleed within six months of study treatment), cardiovascular disease (uncontrolled hypertension, defined as systolic >150 mm Hg or diastolic >90 mm Hg, myocardial infarction or unstable angina within six months prior to registration, New York Heart Association (NYHA) Class II or greater congestive heart failure, serious cardiac arrhythmia requiring medication, prior treatment with an anthracycline and had an ejection fraction <50%, or CTCAE Grade 2 or greater peripheral vascular disease), signs of gastrointestinal obstruction (including patients who require parenteral hydration and/or nutrition), serious wounds, abscess, or bowel perforation. Patients with prior recent invasive malignancies and those not recovered from the effects of recent surgery, radiotherapy, or chemotherapy were excluded. Patients of childbearing potential were required to have a negative serum pregnancy test before entering the study and were required to practice an effective form of contraception.
Any other prior therapy directed at the malignant tumor, including biological and immunologic agents, had to be discontinued at least three weeks prior to registration (including small molecules and murine monoclonal antibodies). Chimeric, human, or humanized monoclonal antibodies (including bevacizumab) or VEGF receptor fusion protein (including VEGF Trap, aflibercept) had to be discontinued for at least 12 weeks prior to registration. No investigational therapy within 30 days prior to the first date of study treatment was allowed and patients who had received prior everolimus or any other mTOR inhibitor were excluded.
All patients signed an approved informed consent and authorization permitting the release of personal health information.
Blinding
Blinding was employed to help preserve the integrity of the PFS and OS endpoints by eliminating biases in disease assessment monitoring, the declaration of disease progression and the institution/selection of future therapies. Therefore, it was understood that investigators, patients and research personnel would not know if patients have received everolimus or placebo.
Drug Administration and Supportive Care
Bevacizumab was administered at 10 mg/kg as a continuous intravenous (IV) infusion once every two weeks. The dose was based on the patient’s actual body weight and only recalculated if there was a weight change of >10% from baseline. The initial dose was administered over 90 minutes. If no adverse reactions occurred after the initial dose, the second dose was administered over 60 minutes. If no adverse reactions occurred after the second dose, all subsequent doses were administered over 30 minutes. If infusion-related adverse reactions occurred at any time, subsequent doses of bevacizumab were administered over the shortest period that was well tolerated. Routine premedication was not required for the first dose of bevacizumab. If infusion reactions occurred, diphenhydramine, dexamethasone and an H1 blocker were administered as premedication. IV bevacizumab was administered before the oral dose of everolimus or placebo.
Everolimus or placebo was dispensed as tablets at the beginning of each treatment cycle (day 1, every 4 weeks). All dosages were prescribed and dispensed to the patient and all dose changes during the study were recorded. Everolimus or placebo was administered orally as a once-daily dose of 10 mg (two 5 mg tablets) continuously from study day 1 until progression of disease or unacceptable toxicity. Patients were instructed to take everolimus or placebo in the morning, at the same time each day. Everolimus or placebo was taken by the patient in a fasting state or with no more than a light fat-free meal. Dietary habits around the time of tablet intake were kept as consistent as possible throughout the study and if applicable, no attempt to replace the vomited dose.
Prior to each IV treatment, special attention was given to known bevacizumab effects: blood pressure, proteinuria, bleeding, cardiovascular events and bowel perforation. Type of blood pressure medications was administered at the discretion of the investigator.
For the oral everolimus or placebo, nausea and vomiting were managed at the investigator discretion with guidelines given for interacting medications. Diarrhea was managed with loperamide. Mild mucositis treated with conservative measures such as non-alcoholic and/or salt water (0.9%) mouth wash; more severe mucositis required topical analgesic and/or topical corticosteroid mouth treatments. Significant fasting hypercholesterolemia (>300 mg/dL or 7.75 mmol/L) or hypertriglyceridemia (>300 mg/dL or >3.42 mmol/L) were treated with a statin or appropriate lipid-lowering medication in addition to diet. Patients with diabetes were instructed to monitor glucose levels closely and may alter insulin and/or oral hypoglycemic therapy requirements. To avoid drug interactions, patients were instructed not to take any additional medications (including over-the-counter products) during the course of the study without prior consultation with the investigator. Concurrent administration of everolimus and strong or moderate CYP3A4 inhibitors, strong CYP3A4 inducers and strong and moderate inhibitors of P-glycoprotein (PgP) were avoided.
Dose Modifications
Dose reductions were not allowed for bevacizumab. Interruption of bevacizumab was mandated for certain adverse events (grade 2 symptomatic hypertension, grade 3 thromboembolic events or hypertension, >3.5gm proteinuria over 24 hours, grade 3 hemorrhage) with intervention guidelines and parameters to restart. Bevacizumab was discontinued permanently for grade ≥2 arterial thromboembolic events or intracranial/pulmonary hemorrhage; grade >3 AE, including allergic infusion reactions and wound complications; any grade ≥4 including symptomatic venous thromboembolic events, hypertension, and hemorrhage. Any grade of posterior reversible encephalopathy syndrome (PRES), wound dehiscence, perforation, fistula or nephrotic syndrome (or persistent proteinuria >3.5 gm) required discontinuation of bevacizumab. If bevacizumab was interrupted for toxicity, everolimus or placebo could continue. Patients were removed from the study if bevacizumab was not given for greater than 8 weeks. If everolimus or placebo was interrupted for any reason for >3 weeks, oral therapy was discontinued; however patients could continue on the study with bevacizumab alone.
Dose reductions were allowed with everolimus or placebo (initial dose 10mg; level 1 dose reduction at 5 mg daily; level 2 dose reduction at 5 mg every other day). Persistent (˃24 hours) grade 3 non-hematologic toxicity in spite of optimal medical management required reduction of one dose level and delay in subsequent therapy for a maximum of three weeks until recovered to grade 1. Grade 2 (symptomatic) and grade 3 pneumonitis required corticosteroid administration. Everolimus or placebo was discontinued for any grade 4 AE, treatment hold for >3 weeks, or related adverse events requiring dose modification despite two previous dose reductions.
Study Endpoints
Tumor measurements using (computed tomography or magnetic resonance imaging) were made once during every other 4-week cycle according to RECIST 1.1 for the first six months and then every 3 cycles thereafter; and at any other time if clinically indicated based on symptoms or physical signs suggestive of progression of disease or rising serum CA-125 tumor markers. For those with measurable disease, progression (PD) was defined as at least a 20% increase in the sum of the diameters of the target lesions, taking as reference the smallest sum on the study. In addition to the relative increase of at least 20%, the sum must also demonstrate an absolute increase of at least 5mm. The appearance of one or more new lesions or unequivocal progression of non-target lesions was also considered PD. Partial response was defined as at least a 30% decrease in the sum of the diameters of target lesions from the baseline sum. Complete response was defined as the disappearance of all target and non-target lesions and no new lesions. Stable disease was any condition not meeting the above criteria. Responses required confirmation on repeat imaging at greater than or equal to 4 weeks from initial documentation.
For those with detectable but non-measurable disease, assessment was based on CA-125, malignant fluid (i.e. ascites or pleural effusion), and/or evaluation of indeterminate solid or cystic abnormalities. CA-125 responses, regardless of measurable status, were assessed with Rustin criteria. 27 Initial values within two weeks of starting therapy had to be 2x ULN to be considered evaluable. At least a 50% reduction in CA-125 levels from the baseline value was maintained for at least 28 days represented a partial response. A full response was defined as normalization (less than ULN) of CA-125 levels that maintained normal for at least 28 days. The date of progression by CA-125 level was determined by values ˃ 2x maximum (ULN, nadir) that was confirmed at least eight days later. If the date of progression was within eight weeks, PD was entered. If patients had evaluations more than eight weeks from study entry without PD, then their response was at least stable disease. Patients were evaluated by using best overall response while receiving study therapy.
Statistical Design
The primary objective of this study was to estimate the PFS hazard ratio (HR) of the combination of oral everolimus and bevacizumab to oral placebo and bevacizumab. The study was powered to detect a true 37.5% reduction in the hazard ratio of PFS by using the Cox proportional hazards model. The target enrollment was 150 patients (75 per arm) with 123 events to achieve 90% power with a 10% level of significance when conducted with an interim analysis (at approximately the 62nd event) using the method provided by Weiand et al. that rejected the study drug when the HR>1.24 There was no suspension of study accrual during the interim analysis. The final analysis involving efficacy used the intent to treat principle and occurred at approximately the 123rd event (taken from both arms).25 Secondary objectives included 1) to estimate and compare the nature and degree of toxicity of oral everolimus (or placebo) plus bevacizumab, 2) to characterize and compare PFS and OS in patients with measurable disease (RECIST criteria) and patients with detectable (non-measurable) disease, 3) to estimate the proportion of patients with measurable disease who have objective tumor responses by treatment, and 4) to provide descriptive information about CA-125 responses by regimen and where possible by objective tumor responses. Estimates of the differences between measurable versus non-measurable disease status on PFS and OS was proposed with plots of survival curves, estimates of quartiles, and hazard ratios where feasible. For everolimus or placebo randomization, patients were stratified according to their platinum-free interval (≤182 days versus >182 days), disease status (measurable versus detectable), and prior use of bevacizumab/aflibercept therapy (no use versus prior use).
RESULTS
The study opened on 12/28/2010, was suspended on 3/9/2011 due to problems with unblinding, reopened on 7/18/2011 and was closed to patient entry on 8/14/2012 after the enrollment of 150 patients. During the first 3-month suspension period, there were only 2 patients enrolled. However, a second unblinding occurred later where at least 52 patients in total were potentially unblinded. The group quickly obtained another distributor of the regimens to avoid further errors. The only ordinary indication for un-blinding was a serious adverse event in which it is determined by the Study Chair that un-blinding would improve patient safety.
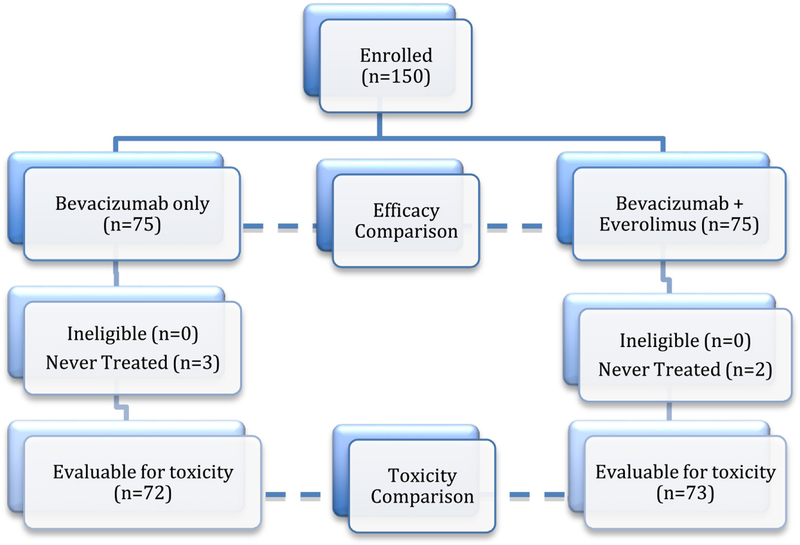
The primary outcome data was frozen on 8/26/2013 for the final analysis. All randomly assigned patients were included in the analysis of efficacy (intention to treat). Five patients were never treated (2 in the everolimus experimental arm and 3 in the bevacizumab only arm) and thus, 145 patients were evaluable for toxicity (Figure 1). Patient demographic characteristics are outlined in Table 1 and the two treatment arms were well balanced for age (median 63: range 28–92), PS (0: 73%, 1–2: 27%), prior regimens (1: 37%, 2: 47%, 3: 16%), prior BV (11%), PFI (<6mos: 65%) and measurable disease (81%).
Figure 1:
Study enrollment diagram
Table 1:
Demographics and Baseline Characteristics
| Bevacizumab alone | Bevacizumab + Everolimus | TOTAL | |||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Characteristics | |||||
| Age Groups, years | |||||
| 20–29 | 1 | 1.33 | 0 | 0 | 1 |
| 30–39 | 1 | 1.33 | 1 | 1.33 | 2 |
| 40–49 | 9 | 12.00 | 8 | 10.67 | 17 |
| 50–59 | 16 | 21.33 | 21 | 28.00 | 37 |
| 60–69 | 33 | 44.00 | 28 | 37.33 | 61 |
| 70–79 | 12 | 16.00 | 13 | 17.33 | 25 |
| >= 80 | 3 | 4.00 | 4 | 5.33 | 7 |
| Ethnicity | |||||
| Hispanic | 1 | 1.33 | 2 | 2.67 | 3 |
| Non-Hispanic | 65 | 86.67 | 69 | 92.00 | 134 |
| Unknown | 9 | 12.00 | 4 | 5.33 | 13 |
| Race | |||||
| Asian | 2 | 2.67 | 3 | 4.00 | 5 |
| African American | 6 | 8.00 | 3 | 4.00 | 9 |
| White | 65 | 86.67 | 69 | 92.00 | 134 |
| Unknown | 2 | 2.67 | 0 | 0 | 2 |
| Performance Status | |||||
| 0 | 58 | 77.33 | 52 | 69.33 | 110 |
| 1 | 17 | 24.00 | 21 | 28.00 | 38 |
| 2 | 0 | 2.67 | 2 | 2.67 | 2 |
| Cell Type | |||||
| Endometrioid | 4 | 5.33 | 3 | 4.00 | 7 |
| Serous | 59 | 78.67 | 54 | 72.00 | 113 |
| Clear cell | 7 | 9.33 | 5 | 6.67 | 12 |
| Mixed epithelial | 3 | 4.00 | 4 | 5.33 | 7 |
| Undifferentiated | 0 | 0.00 | 2 | 2.67 | 2 |
| Adenocarcinoma, NOS | 2 | 2.67 | 7 | 9.33 | 2 |
| No. of prior regimens | |||||
| 1 | 23 | 32.00 | 32 | 42.67 | 55 |
| 2 | 39 | 54.67 | 31 | 41.33 | 70 |
| 3 | 13 | 21.33 | 12 | 16.00 | 25 |
| Prior Radiation | |||||
| No | 74 | 98.67 | 73 | 97.33 | 147 |
| Yes | 1 | 1.33 | 2 | 2.67 | 3 |
| Prior Immunotherapy | |||||
| No | 73 | 97.33 | 74 | 98.67 | 147 |
| Yes | 2 | 2.67 | 1 | 1.33 | 3 |
| Prior Surgery | |||||
| No | 5 | 6.67 | 1 | 1.33 | 6 |
| Yes | 70 | 93.33 | 74 | 98.67 | 144 |
| Prior bevacizumab | |||||
| No | 66 | 88.00 | 68 | 90.67 | 134 |
| Yes | 9 | 12.00 | 7 | 9.33 | 16 |
| Measurable disease | |||||
| No | 12 | 16.00 | 17 | 22.67 | 29 |
| Yes | 63 | 84.00 | 58 | 77.33 | 121 |
| Platinum interval | |||||
| Platinum resistant (<6mo PFI) | 47 | 62.67 | 51 | 68.00 | 98 |
| Platinum sensitive (6 < PFI <12mo) | 28 | 37.33 | 54 | 32.00 | 52 |
| TOTAL (n=150) | 75 | 100% | 75 | 100% | 150 |
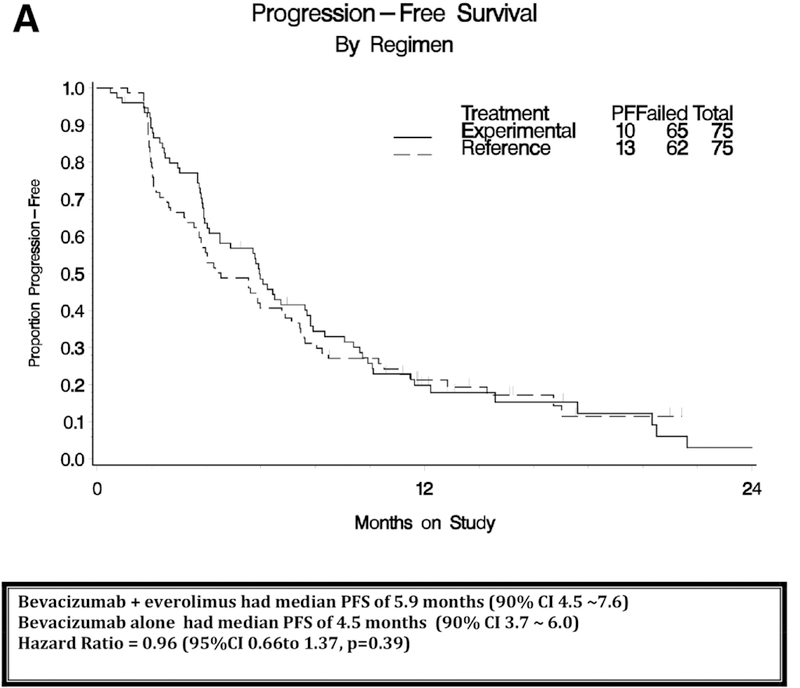
The interim analysis was triggered after 62 PFS events and the final analysis after 127 PFS events. Adding everolimus to bevacizumab did not significantly prolong PFS compared to bevacizumab alone (median PFS 4.5 months for bevacizumab and 5.9 months for bevacizumab plus everolimus; HR, 0.95; with 95% CI, 0.66 to 1.37; one-sided P=0.39; Figure 2A). At the time of this data analysis, 73 had died and median follow-up on the bevacizumab alone arm was 15 months and 15.7 months on the everolimus arm. There was no significant difference in OS (median OS 17.3 months for bevacizumab and 16.6 months for bevacizumab plus everolimus; HR, 1.16; with 95% CI, 0.72 to 1.87; two-sided P=0.55; Figure 2B).
Figure 2A:
Progression-free survival (PFS) analysis by intent to treat.
Figure 2B:
Overall survival (OS) analysis by intent to treat.
Among patients with measurable disease, the objective response was about 2x higher with bevacizumab plus everolimus (22% vs 12%; relative probability of responding was 1.84, 95% CI 0.80 to 4.24). Among patients with CA-125 evaluable disease, the CA125 response was about 2x higher response with bevacizumab plus everolimus (27% vs 15%). The odds ratio for radiographic response (experimental to reference) was 2.08 (95% Exact CI 0.71 to 6.60), so although the data favored the experimental regimen through point estimates, the interval estimates were not suggestive of the experimental regimen being superior. The frequency of study removal due to toxicity or patient refusing further treatment was high with the combination regimen (41%) compared to bevacizumab alone (17%). (Table 2) There was no significant association between treatment related death and study regimen. The two patients (one on each treatment arm) who had grade 5 adverse events related to study treatment had a thromboembolism and a hematoma. Sixty-five patients died from progressive disease. Treatment related adverse events are listed in Table 3. The addition of everolimus to bevacizumab was associated with higher toxicities including neutropenia, anemia, thrombocytopenia, nausea, oral mucositis, fatigue, metabolic abnormalities, dyspnea and rash. There were three events of a grade 3–4 gastrointestinal perforation (2%).
Table 2:
Response to Therapy and Patient Outcomes
| Bevacizumab alone | Bevacizumab + Everolimus | ||||
|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | Total |
| Response | |||||
| Complete response | 0 | 0 | 1 | 1 | 1 |
| Partial response | 7 | 9 | 13 | 17 | 20 |
| Stable disease | 30 | 40 | 32 | 43 | 62 |
| Progressive disease | 16 | 21 | 11 | 15 | 27 |
| Indeterminate | 5 | 7 | 6 | 8 | 11 |
| Non-measurable | 17 | 23 | 12 | 16 | 29 |
| Alive/cause of death | |||||
| Dead from disease | 31 | 41 | 34 | 45 | 65 (43%) |
| Dead, drug related | 0 | 0 | 1 | 1 | 1 (1%) |
| Dead, drug-related and disease | 1 | 1 | 1 | 1 | 2 (1%) |
| Dead, undetermined cause | 3 | 3 | 2 | 3 | 5 (3%) |
| Alive | 40 | 53 | 37 | 49 | 77(51%) |
| Reason off study | |||||
| Disease progression | 49 | 65 | 37 | 49 | 86 (57%) |
| Toxicity per protocol | 9 | 12 | 22 | 29 | 31 (21%) |
| Refused further treatment | 4 | 5 | 9 | 12 | 13 (9%) |
| Other disease | 1 | 1 | 2 | 3 | 3 (2%) |
| Other, unknown, or on study | 12 | 16 | 5 | 7 | 17 (12%) |
Note: The relative probability of response on the everolimus + bevacizumab arm compared with the bevacizumab alone arm (among patients with measurable disease) was 1.84 (95% CI 0.80–4.24). The odds ratio was 2.08 (95% CI, 0.71–6.60).
Table 3:
Treatment Adverse Events (no. of events)
| Bevacizumab alone (n=72) | Bevacizumab + Everolimus (n=73) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Blood/Lymphatics | ||||||||||
| Neutropenia1 | 5 | 1 | 0 | 0 | 0 | 11 | 15 | 5 | 0 | 0 |
| Anemia1 | 18 | 5 | 0 | 0 | 0 | 24 | 22 | 1 | 0 | 0 |
| Thrombocytopenia1 | 6 | 2 | 0 | 0 | 0 | 28 | 10 | 1 | 0 | 0 |
| Cardiac | 1 | 0 | 1 | 0 | 0 | 5 | 1 | 0 | 1 | 0 |
| Ear/labyrinth | 2 | 0 | 0 | 0 | 0 | 2 | 20 | 0 | 0 | 0 |
| Gastrointestinal | ||||||||||
| Nausea1 | 19 | 3 | 4 | 0 | 0 | 13 | 12 | 6 | 0 | 0 |
| Vomiting | 5 | 4 | 2 | 0 | 0 | 13 | 7 | 5 | 0 | 0 |
| Other GI1,2 | 34 | 8 | 1 | 0 | 0 | 23 | 22 | 13 | 1 | 0 |
| General/ | 43 | 9 | 1 | 0 | 0 | 34 | 20 | 7 | 0 | 0 |
| administrative site1 | ||||||||||
| Hepatobiliary | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Immune system | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Infections | 1 | 6 | 2 | 0 | 0 | 2 | 7 | 6 | 1 | 0 |
| Metabolism/nutrition1,2 | 18 | 4 | 4 | 1 | 0 | 15 | 14 | 13 | 1 | 0 |
| Musculoskeletal/ connective tissue | 24 | 6 | 1 | 0 | 0 | 12 | 8 | 4 | 0 | 0 |
| Nervous system | 21 | 6 | 2 | 0 | 0 | 28 | 9 | 3 | 0 | 0 |
| Peripheral neuropathy | 10 | 1 | 0 | 0 | 0 | 12 | 1 | 1 | 0 | 0 |
| Other investigations | 18 | 4 | 2 | 0 | 0 | 18 | 20 | 2 | 0 | 0 |
| Psychiatric | 5 | 1 | 0 | 0 | 0 | 8 | 4 | 0 | 0 | 0 |
| Renal/urinary | 7 | 0 | 2 | 0 | 0 | 8 | 5 | 1 | 0 | 0 |
| Respiratory/thoracic1 | 26 | 3 | 0 | 0 | 0 | 26 | 12 | 4 | 1 | 0 |
| Skin/subcutaneous1 | 16 | 2 | 0 | 0 | 0 | 24 | 10 | 2 | 0 | 0 |
| Vascular disorders | 7 | 11 | 10 | 2 | 1 | 4 | 7 | 12 | 1 | 1 |
Toxicities are significantly associated with treatment at the 5% level when classified as mild (Grade <=1), moderate (grade =2) and severe (Grade >=3) (highlighted as a light shadow in table)
Toxicities are significantly associated with treatment at the 5% level when classified as mild to moderate and severe (grade 3–5 non-hematologic and grade 4–5 hematologic events) (highlighted as a dark shadow in table)
DISCUSSION
Bevacizumab has clinical activity as both a single agent and in combination with cytotoxic chemtherapy. Based on the AURELIA trial (AURELIA: A Study of Avastin (bevacizumab) Added to Chemotherapy in Patients with Platinum-resistant Ovarian Cancer), bevacizumab first received approval in the United States in November 2014. By combining chemotherapy – weekly paclitaxel, liposomal doxorubicin or topotecan – with bevacizumab, the median PFS was improved by 3.3 months (range, 3.4 months to 6.7 months; HR, 0.48; 95% CI, 0.38 to 0.6) and patients reported improvement in disease-realated symptoms and quality of life.7–8 However, chemotherapy-related toxicties was significant. The GOG created a randomized phase II trial platform (GOG-186 series) to compare non-cytotoxic combinations to single agent targeted agents with the objective to improve the therapeutic ratio of emerging agents in ovarian cancer. A recent study on this GOG-186 series showed promising response and tolerability when combinging two antivascular agents (bevacizuamb plus flubretulin) compared to bevacizumab alone.26
Unfortunately, as demonstarted in our trial, everolimus when combined with bevacizumab was an active combination but did not reach the goal of improving PFS or tolerability. This randomized phase II trial comparing bevacizumab alone (plus oral placebo) to bevacizumab plus everolimus did not significantly prolong PFS compared to bevacizumab alone (median PFS 4.5 months for bevacizumab and 5.9 months for bevacizumab plus everolimus; HR, 0.95; with 95% CI, 0.66 to 1.37; one-sided P=0.39). However, the regimen was active particularly in women with measurable disease; the objective response (complete or partial response) was two-fold higher with bevacizumab plus everolimus (22% vs 12%). The side effects were considerable and study removal due to toxicity or patient refusing further treatment was high with the combination regimen (41%) compared to bevacizumab alone (17%).
The advantage of this trial was its multicenter and randomized design. The inclusion of a placebo in the design was intended to reduce bias. However, there was an accidental unblinding at several sites due to a distributor error (exposed label of the un-blinding to multiple site pharmacies) and therefore, the power of the placebo inclusion was lost in the final analysis. It is unlikely, however, this had a significant factor to the final conclusions of the study – as most patients on everolimus could easily be determined by the high reports of toxicities compared to the placebo group.
In conclusion, we cannot recommend further exploration of the combination of everolimus and bevacizumab in women with recurrent ovarian cancer.
RESEARCH HIGHLIGHTS.
Bevacizumab monotherapy is active therapy for recurrent ovarian cancer, but resistance is common
BV+EV combination had a higher measurable response (22% vs 12%) compared to BV alone
BV+EV combination had higher toxicity and study removal (29% vs 12%) compared to BV alone
BV+EV combination did not improve progression free or overall survival
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1 U10 CA180822) and NRG Operations (U10CA180868). Drs. Aghajanian and Tew are supported in part by the MSK Cancer Center Support Grant P30 CA008748.
The following Gynecologic Oncology institutions participated in this study: University of Oklahoma Health Sciences Center, Memorial Sloan Kettering Cancer Center, Duke University Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Cancer Research for the Ozarks NCORP, Women and Infants Hospital, Michigan Cancer Research Consortium Community Clinical Oncology Program, University of Wisconsin Hospital and Clinics, University of California Medical Center at Irvine-Orange Campus, The Hospital of Central Connecticut, Carolinas Medical Center/Levine Cancer Institute, Baystate Medical Center, University of California at Los Angeles Health System, University of Iowa Hospitals and Clinics, Case Western Reserve University, Washington University School of Medicine, Carle Cancer Center, Abington Memorial Hospital, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Abramson Cancer Center of The University of Pennsylvania, Cleveland Clinic Foundation, Ohio State University Comprehensive Cancer Center, Rush University Medical Center, Cooper Hospital University Medical Center, Iowa-Wide Oncology Research Coalition NCORP and Upstate Carolina CCOP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abstract presented as poster presentation at the 50th Annual ASCO meeting 5/30/14 – 6/3/14 in Chicago, IL
CONFLICT OF INTEREST STATEMENT
Dr. Angeles Secord reports grants from NCTN Grant and Gynecologic Oncology Group Grant during the conduct of the study; grants from Astra Zeneca, Eisai, Bristol Myers Squibb, Incyte, Amgen, Genentech, Endocyte, Exelixis, Boerhinger Ingelheim, Astex Pharmaceuticals Inc., Prima Biomed, Abbie-Vie, Astellas Pharma Inc., Tesaro, PharmaMar, Merck, other from Janssen, Clovis, Genentech, Astra Zeneca, Astex, Tesaro, Alexion, Boerhinger Ingelheim, Myriad, Arivave, outside the submitted work.
Dr. Jeanne Schilder received a grant from the NRG/GOG.
Dr. Krishnansu Tewari received grant monies from Genentech for Clinical Trial at UC Irvine. He also received payment for lectures including service on speakers bureaus for CME activities only from Genentech/Roche.
Dr. Carol Aghajanian received money paid to her for consultancy from Tesaro, Mateon Therapeutics, Clovis, Cerulean, Bayer and VentriRx.
All other co-authors had no conflicts of interest to declare.
REFERENCES
- 1.Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol 1999; 6(4):373–378. [DOI] [PubMed] [Google Scholar]
- 2.Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, et al. Prognostic significance of VEGF expression in human ovarian carcinoma. Br J Cancer 2000; 83(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CA, Cheng WF, Lee CN, Chen TM, Kung CC, Hsieh FJ, et al. Serum VEGF in epithelial ovarian neoplasms: correlation with patient survival. Gynecol Oncol 1999; 74(2):235–240. [DOI] [PubMed] [Google Scholar]
- 4.Burger RA, Sill M, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007. November 20; 25(33):5165–5171. [DOI] [PubMed] [Google Scholar]
- 5.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 2007. November 20; 25(33):5180–5186. [DOI] [PubMed] [Google Scholar]
- 6.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago and Princess Margaret Hospital phase II consortia. J Clin Oncol 2008; 26(1):76–82. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lawrence E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014; 32(13):1302–1308. [DOI] [PubMed] [Google Scholar]
- 8.Stockleer MR, Hilpert F, Friedlander M, King MT, Wenzel L, Lee CK, et al. Patientreported outcome results from the open label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol 2014; 32(13):1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A Randomized, double blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012; 30:2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Gynecology Oncology Group incorporation of bevacizumab in the primary treatment of ovarian cancer. N Eng J Med 2011; 365:2473–2483. [DOI] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Eng J Med 2011; 365:2484–2496. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017; 18(6):779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 2004; 2: 5853–5857. [DOI] [PubMed] [Google Scholar]
- 14.Mabunchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) Delays Tumor Onset and Progression in a Transgenic Mouse Model of Ovarian Cancer. Cancer Res 2007; 67(6):2408–2413. [DOI] [PubMed] [Google Scholar]
- 15.Mabunchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. RAD001 Inhibits Ovarian Cancer Cell Proliferation, Enhances Cisplatin-induced Apoptosis and Prolongs Survival in an Ovarian Cancer Model. Clin Cancer Res 2007; 13(14):4261–4270. [DOI] [PubMed] [Google Scholar]
- 16.Treeck O, Wackwitz B, Haus U, Ortmann O. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol 2006; 102(2):292–299. [DOI] [PubMed] [Google Scholar]
- 17.Afinitor (Everolimus) Tablets for Oral Administration [Prescribing Information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012. [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474(7353):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behbakht K, Sill MW, Rubin SC, Mannel RS, Waggoner S, Schilder RJ, et al. Phase II trial of mTOR inhibitor, temsirolimus, and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Study. Gynecol Oncol 2011; 123(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emons G, Kurzeder C, Schmaideldt B, Neuser P, de Gregorio N, Pfisterer J, et al. Temsirolimus in women with platinum-refractory/resistant ovarian cancer or advanced/recurrent endometrial carcinoma. A phase II study of the AGO-study group (AGO-GYN8). Gynecol Oncol 2016; 140(3):450–456. [DOI] [PubMed] [Google Scholar]
- 21.Zafar Y, Bendell J, Lager J, et al. Preliminary results of a phase 1 study of bevacizumab in combination with everolimus (RAD001) in patients with advanced solid tumors. J Clin Oncol 24:145s, 2006. (suppl, abstr 3097). [Google Scholar]
- 22.Hainsworth JD, Spigel DR, Burris HA 3rd, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell cancer. J Clin Oncol 2010; 28(13):2131–2136. [DOI] [PubMed] [Google Scholar]
- 23.Piha-Pail SA, Wheler JJ, Levenback C, Lu K, Falchook GS, Naing A, et al. Advanced gynecologic malignancies treated with a combination of the VEGF inhibtor bevacizumab and the mTOR inhibior temsirolimus. Oncotarget 2014; 5(7):1846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieand S, Schroeder G, O’Fallon JR: Stopping when the experimental regimen does not appear to help Stat Med 1994; 13:1453–1458. [DOI] [PubMed] [Google Scholar]
- 25.Jennison C, Turnbull BW: Group Sequential Methods With Applications to Clinical Trials Boca Raton, FL, Chapman & Hall/CRC, 2000, pp 49. [Google Scholar]
- 26.Monk BJ, Sill MW, Walker J, Darus CJ, Sutton G, Tewari KS, et al. Randomized phase II evaluation of bevacizumab versus bevacizumab plus fosbretabulin in recurrent ovarian, tubal, or peritoneal carcinoma: a NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 2016; 34(19):2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rustin GJ, Quinn M, Thigpen T, Du Bois A, Pujade-Lauraine E, Jakobsen A, et al. New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst 2004; 96: 487–488 [DOI] [PubMed] [Google Scholar]