Abstract
Scope:
The tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) has been shown to ameliorate metabolic abnormalities and fatty liver. The present study investigated the mechanisms of actions of EGCG on bile acid homeostasis and lipid metabolism.
Methods:
Male C57BL/6J mice were fed a low-fat diet, a high-fat western-style diet or a high-fat western-style diet containing 0.32% EGCG. The effects of the treatments on biochemical parameters, gene expression and lipidomics were analyzed.
Results:
EGCG treatment significantly reduced body weight gain, mesenteric fat mass, fasting blood glucose, insulin resistance, serum cholesterol and severity of fatty liver after treatment for 17 weeks, but most of these effects were less apparent at week 33. At week 17, EGCG treatment significantly elevated the mRNA levels of cholesterol 7α-hydroxylase, HMG-CoA reductase, low-density lipoprotein receptor and scavenger receptor B1, and partially normalized the high-fat diet induced lipidomic profile. The intestinal bile acid content was significantly decreased by EGCG, while fecal excretion of bile acids, cholesterol and total lipids were increased.
Conclusion:
EGCG decreases bile acid reabsorption, results in lower intestinal bile acid levels, which further decreased the absorption of lipids. These actions contribute to the alleviation of metabolic abnormalities and fatty liver disease caused by the high-fat diet.
Keywords: EGCG, fatty liver, lipidomics, bile acid homeostasis, cholesterol 7α-hydroxylase
1. Introduction
Green tea, a popular beverage worldwide, is derived from the plant Camellia sinensis and contains high levels of polyphenols. Among all the tea polyphenols, (−)-epigallocatechin-3-gallate (EGCG) is the most abundant and most bioactive component [1, 2]. In the past decades, green tea has been shown to reduce body weight, alleviate metabolic syndrome, and prevent diabetes, cardiovascular diseases and other diseases in animal models and humans [2]. However, the time-dependent changes and the fundamental mechanisms of these health benefits of green tea still remain unclear. Recently, several studies have shown that green tea and EGCG affect hepatic bile acid homeostasis. Hirsova et al. found that EGCG (50 mg/kg body weight, i.p.) significantly increased the gene expression of hepatic cholesterol 7α-hydroxylase (Cyp7a1), a rate-limiting enzyme of hepatic bile acids synthesis, as well as the plasma 7α-hydroxy-4-cholesten-3-one levels in female Wistar rats [3]. The upregulation of hepatic Cyp7a1 by EGCG was also shown in a cholesterol gallstone mouse model and hepG2 cell line [4, 5]. On the other hand, EGCG treatment was shown to markedly decrease the mRNA levels of major bile acid transporters (Asbt and Ostα) in the rat ileum [3].
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities generally characterized by abdominal obesity, elevated blood pressure, elevated levels of fasting plasma glucose, high levels of serum triglycerides, and low levels of high-density lipoprotein cholesterol (HDL-C) [6, 7]. MetS is becoming a major public health and clinical challenge worldwide [7]. It significantly increases the risk of diabetes and cardiovascular diseases. Fatty liver is a disorder with excess fat in the liver, and is thought to be a cause and a consequence of MetS [6, 7]. In recent years, bile acids have been shown to regulate lipid and carbohydrate metabolism as well as energy homeostasis in both hepatic and extrahepatic tissues [8]. Li et al. showed that a Cyp7a1/SREBP2/miR-33a axis played a critical role in regulation of hepatic cholesterol, bile acid and fatty acid synthesis, and an antagonist of miR-33a increased bile acid synthesis to maintain lipid homeostasis and prevent fatty liver diseases [9]. However, there have been no studies examining the role of bile acid homeostasis in the regulation of lipid metabolism by tea catechins in mice.
In this study, we investigated the effects of dietary EGCG on parameters related to MetS and fatty liver disease in mice fed a high-fat western-style diet (HFW). The HFW was used in our previous study to mimic the Western dietary risk factors for the induction of MetS in mice [10]. It contained fat contributing 60% of the total calories and lower levels of calcium, vitamin D3, choline, folate and fiber than the regular AIN76 or AIN93 diets [10]. A dose of 3.2g EGCG/kg diet was selected for study based on our previous work [10] and our review of the literature [2]. This is an effective dose without adverse side effects. In the present work, the beneficial effects of EGCG were observed at 17 weeks, but less pronounced at 33 weeks. The effects of EGCG on bile acid and lipid metabolism were characterized in samples collected at 17 weeks. Lipidomic analysis was conducted to characterize alterations in lipid species, and quantitative PCR was used to study key gene expression changes pertaining to lipid metabolism. The results showed that EGCG lowered intestinal bile acid levels and decreased absorption of lipids, and these actions contributed to the alleviation of metabolic abnormality and fatty liver.
2. Materials and Methods
2.1. Diet and treatment of animals
Male C57BL/6J mice (8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). All animal studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Rutgers, the State University of New Jersey. All efforts were made to minimize the suffering of experimental animals. The animals were housed in plastic cages with corncob bedding, five mice per cage, in an environmentally controlled mouse room (temperature 24 ± 0.5°C, relative humidity 70-75% and artificial illumination from 6:00 am to 6:00 pm). All the mice received diets and tap water ad libitum throughout the experiment.
The rodent diets were low-fat diet (LF, AIN93M diet with 10% calories from fat), high-fat western-style diet (HFW, 60% calories from fat), and HFW + EGCG diet (HFWE, containing 3.2g EGCG/kg diet). These diets were prepared by Research Diets Inc. (New Brunswick, NJ), and the compositions were published previously [10]. The EGCG (purity of 94.5%) used in this study was a gift from Dr. Yukihiko Hara of Tea Solutions, Hara Office, Inc. (Tokyo, Japan).
After one week of acclimation on laboratory chow, the mice were randomly divided into three groups, and subjected to the following treatments: LF (n=10), HFW (n=20) and HFWE (n=20) for 33 weeks. Body weight, diet consumption and water consumption of experimental animals were monitored weekly. Fecal samples (each for a 48-h period) were collected biweekly and stored at −80°C for further analysis.
2.2. Monitoring of blood parameters
The fasting blood glucose was monitored using test strips on the Ascensia Contour Blood glucose meter (Bayer Healthcare LLC, Mishawaka, IN). One day before the measurement, cage bedding was changed in order to avoid coprophagy, and the mice were fasted overnight (9:00 p.m. to 9:00 a.m.). After blood glucose measurement, a blood sample (about 100 μl) was collected from the tail vein. Serum was prepared, divided into small aliquots and stored frozen at −80°C. Serum alanine aminotransferase (ALT) was determined using ALT Discrete Pak kit (Catachem Inc., Bridgeport, CT). The concentrations of serum triacylglycerols (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and HDL-C were measured using commercial kits (Pointe Scientific, Inc. Canton, MI). Serum insulin levels were measured using a Rat/Mouse Insulin ELISA kit (Millipore Corporation, Billerica, MA), and insulin resistance was calculated according to the homeostasis assessment model (HOMA-IR). HOMA-IR = [Glucose] x [Insulin] / 22.5
2.3. Sample harvesting from mice
After 17 and 33 weeks of treatment, five mice from the LF group and ten mice from the other two groups at each time point, without fasting, were euthanized by carbon dioxide asphyxiation. The blood was obtained via cardiac puncture; serum was prepared and stored at −80°C. Immediately after blood collection, the abdominal cavity was opened, the gallbladder was collected, transferred into a tube, frozen on dry ice and stored at −80°C for further bile acid analysis. The liver was quickly dissected, weighed and one third of the left lateral lobe was cut and fixed in 10% buffered formalin for histopathological analyses. Another piece of liver (~100mg) from the same site of the liver was stored in RNAlater solution (Ambion, NJ) for RNA extraction and gene expression analysis. The remaining liver tissue was rapidly clamped with a Wollenberg clamp in liquid nitrogen and stored at −80°C for lipidomics and other biochemical analyses.
The distal end of ileum (3cm) was cut, washed with ice cold saline and stored in RNAlater solution (Qiagen Inc., Valencia, CA) for gene expression analysis. The remaining small intestine (including the content) was collected, frozen on dry ice and stored at −80°C for bile acid analysis. The interscapular brown adipose tissue (BAT) and visceral white adipose tissue (VAT), including mesenteric, epididymal and perirenal fat pad, were collected, weighed and stored at −80°C.
2.4. Hepatic and fecal lipids
The method of hepatic and fecal lipid determination was based on Manley et al. [11]. In brief, 100mg liver tissues or fecal samples were homogenized in 1 mL of buffer (18mM Tris pH 7.5, 300 mM mannitol, 50 mM EGTA, and 0.1 mM phenylmethysulfonyl fluoride). The homogenate (400μl) was mixed with 2 mL of chloroform-methanol (2:1), and shaken on a rotating shaker overnight at room temperature. Then, 1 mL of H2O was added and the mixture was centrifuged for 5 min at 3000g at 4°C. The lower lipid phase was then collected and dried by vacuum centrifugation. The dried lipid pellet was weighed and then dissolved in a mixed solvent (tert-butanol: Triton X-114: methanol, 9:4:2). The TG and cholesterol levels were determined using commercial kits according to manufacturer’s instruction (Pointe scientific, Inc. Canton, MI).
2.5. Liver histology
For histological examination of the liver, the formalin-fixed liver tissue samples were embedded in paraffin, sectioned serially at 4-μm thickness and stained with hematoxylin and eosin. The severity of fatty liver was evaluated by a steatosis scoring system described by Liang et al. [12]. Macrovesicular steatosis, microvesicular steatosis and hepatocellular hypertrophy were scored and the severity in each category was graded, based on the percentage of the total area affected, into the following categories: 0 (<5%), 1 (5–33%), 2 (34–66%) and 3 (>66%). The unweighted sum of the scores for steatosis (macrovesicular steatosis, microvesicular steatosis and hypertrophy) thus ranged from 0~9.
2.6. Lipidomic analysis of liver and serum
Frozen liver samples (~30mg) were weighed and pulverized in a CryoMill machine (Retsch, Germany) with a stainless ball at liquid nitrogen temperature. Pulverized tissue powder was mixed by vortexing with 1 mL of 0.1 M HCl in 50% methanol and kept at −20°C for 30 min. Then 0.5 mL of chloroform was added to the mixture and vortexed to mix. After sitting on ice for 10 min, the samples were centrifuged at >15,000 RCF for 10 min. The chloroform phase was then transferred to a glass vial using a Hamilton syringe. For the second extraction, chloroform (0.5 mL) was added to the remaining mixture and the extraction was repeated. The combined extract was dried under a stream of nitrogen and re-dissolved in a mixture of methanol: chloroform: 2-propanol (1:1:1). For serum samples, 50 μl of serum was extracted with 500 μL of ethyl acetate three times and the combined extract was processed as above. Fatty acids and lipids were analyzed on an Agilent 6550 iFunnel Q-TOF mass spectrometer coupled to a 1290 Infinity UPLC system. Each sample was analyzed twice using the same LC gradient but different ionization modes on the mass spectrometer to cover both the positive charged and negative charged species. The LC separation was achieved on an Agilent Poroshell 120 EC-C18 column (150 × 2.1 mm, 2.7 μm particle size) at a flow rate of 150 μl/min. The gradient was generated with solvents A and B as follows: 0 min, 25% B; 2 min, 25% B; 4 min, 65% B; 16 min, 100 % B; 20 min, 100% B; 21 min, 25% B; 27 min, 25% B. Solvent A contained 1mM ammonium acetate and 0.2% acetic acid in 10% methanol. Solvent B contained 1mM ammonium acetate and 0.2% acetic acid in methanol:2-propanol (2:98).
2.7. Total bile acid contents in the liver, gallbladder, small intestine and fecal samples
The bile acid levels in different tissues were determined using the method of Kim et al.[13]. In brief, liver, intestine, gallbladder and fecal samples were homogenized in PBS, mechanically shaken in 100% ethanol to extract the bile acids, and centrifuged to obtain the supernatant. Then ethanol was added to the pellet, vortexed to dispense the tissues, and shaken again to repeat the extraction. After centrifugation, the supernatants were combined for total bile acid analysis. The concentrations of bile acids were determined using a total bile acid assay kit (Diazyme Laboratories, Poway, CA). The total bile acid pool size was the sum of the bile acids in the liver, small intestine and gallbladder, including their contents.
2.8. Gene expression analysis
Real-time quantitative PCR (RT-qPCR) was employed to study the effects of EGCG on genes involved in lipid and bile acid metabolism. Total RNA was extracted from liver and ileum samples using a RNeasy mini kit (Qiagen Inc., Valencia, CA) and reverse transcription reaction was conducted using a Superscript kit (Invitrogen, Carlsbad, CA), following the manufacture’s instruction. The quality of RNA was checked by spectrophotometry and electrophoresis. Real time quantitative PCR was performed using a Power SYBR® Green PCR Master Mix kit (Life Technologies, Warrington, UK) on an ABI ViiATM 7 system. The expression level of a target gene mRNA was normalized to the mRNA level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The amount of the target gene expression was calculated by the 2−ΔΔCT method. The sequences of the genes involved in the present study were obtained from Gene bank (www.ncbi.nlm.nih.gov/Genbank), and the sequences of primers used are listed in supplementary table 1.
2.9. Statistical analysis
All data were expressed as the mean ± standard error of mean (SEM), and analyzed using the IBM SPSS Statistics 22.0 (IBM, Armonk, NY). Student’s t-test was used to assess the difference between two groups. One-way ANOVA followed by Tukey multiple range analysis methods were employed to assess the differences among different groups. A significant level p < 0.05 was set for all tests. Statistical analyses of lipidomic data are shown in the respective sections.
3. Results
3.1. Body weight, food intake and levels of blood glucose and insulin
The body weight and food consumption were monitored weekly throughout the experiment. There was no significant difference in body weight among the three groups in the first week. Afterwards, the body weights of mice in HFW and HFWE groups were significantly higher than that of the LF group. EGCG treatment significantly suppressed the body weight gain of mice during weeks 3-16 (Figure 1 A). However, the body weights of mice in the HFW and HFWE group were not significantly different after week 17 (Figure 1 A).
Figure 1. Effects of dietary EGCG on body weight, food consumption and levels of fasting blood glucose and insulin.
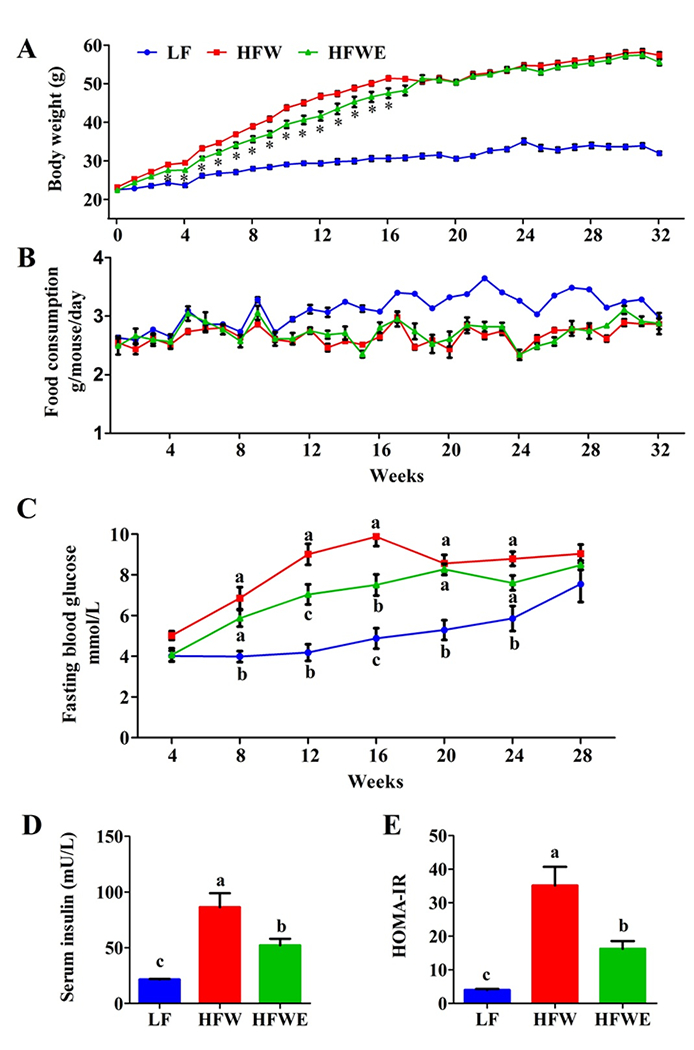
The mice received low-fat diets (LF), high-fat western-style diet (HFW), and HFW + EGCG diet (HFWE, containing 0.32% EGCG). The number of mice used in the three groups were 10, 20 and 20, respectively. On week 17, 50% of the mice from each group were sacrificed and the remaining mice were sacrificed on week 33. A, body weights; B, food consumptions (monitored weekly for 5 mice in each cage); C, blood glucose levels in mice after overnight fasting; D and E, serum insulin and HOMA-IR on week 16. All data are expressed as mean ±SEM for A (n=10 and 5 before and after week 17 for LF group; n=20 and 10 before and after week 17 for HFW and HFWE groups), B (n=2 and 1 before and after week 17 for LF group, 4 and 2 before and after week 17 for HFW and HFWE groups); and C, D, E (n=5). Error bars smaller than the dimension of the symbol cannot be seen in the figure. a,b,cSignificant difference among groups (ANOVA, p < 0.05); *Significant difference from the HFW group (t-test, p < 0.05).
There was also no difference in food consumption of animals among three groups in the first 10 weeks. During weeks 11 to 16, the average diet intake of mice in the LF group appeared to be higher than the two other groups (Figure 1 B). However, the food consumption of mice among the three groups was not significantly different at the end of the experiment.
To observe the development of metabolic abnormalities, fasting blood glucose levels were determined in samples collected at weeks 4, 8, 12, 16, 20, 24 and 28. The blood glucose levels of mice in HFW and HFWE groups were significantly higher than those of mice in LF group after week 8 (Figure 1 C). At weeks 12 and 16, EGCG treatment significantly decreased the blood glucose (by 22-24%). However, there were no significant differences in blood glucose levels between HFW and HFWE groups at weeks 20, 24 and 28 (Figure 1 C). At week 12, the fasting serum insulin levels were increased by 8-fold by HFW and the increase was partially prevented by EGCG (Figure 1 D). A similar pattern of changes was also observed in the calculated insulin resistance index (HMOA-IR) (Figure 1 E).
3.2. Adipose tissue mass, hepatic lipids and serum lipids
Compared to the LF group, the liver weight was significantly augmented by the HFW and this effect was prevented by EGCG at week 17 (Table 1). However, this preventive effect of EGCG was not observed at week 33. Consistent with the results on body weight, the adipose tissue mass of mice in the HFW group were notably higher than those of the LF group, especially for the mesenteric adipose, which was 5-fold higher (Table 1). EGCG treatment significantly decreased the mesenteric adipose tissue (at week 17 only), but not other types of fat.
Table 1.
Liver and adipose tissue weights of mice from different experimental groups
| LF | HFW % of body weight |
HFWE | |
|---|---|---|---|
| Week 17 | |||
| Liver | 4.35±0.03b | 5.20±0.39a | 4.00±0.34b |
| Mesenteric adipose | 0.62±0.12c | 3.19±0.25a | 2.10±0.28b |
| Perirenal adipose | 0.47±0.08b | 1.11±0.06a | 1.39±0.14a |
| Epididymal adipose | 1.74±0.24b | 3.60±0.44a | 4.65±0.38a |
| Brown adipose | 0.59±0.04b | 1.21±0.07a | 1.00±0.07a |
| Week 33 | |||
| Liver | 4.70±0.70b | 5.75±0.18a | 5.74±0.17a |
| Mesenteric adipose | 0.65±0.03b | 1.95±0.09a | 2.23±0.09a |
| Perirenal adipose | 0.47±0.03c | 1.53±0.19b | 2.20±0.11a |
| Epididymal adipose | 2.25±0.12a | 2.31±0.11a | 2.73±0.15a |
| Brown adipose | 0.36±0.05b | 0.89±0.05a | 1.02±0.05a |
Data are expressed as the mean ± SEM (n = 10).
Significant difference among groups (ANOVA, p < 0.05)
The levels of serum lipid at weeks 8, 12 and 16 were examined (Table 2). HFW significantly increased the serum concentrations of TC and LDL-C at all-time points, and EGCG only partially prevented this effect at week 16. However, no significant difference in the levels of serum total TG was observed among the LF, HFW and HFWE groups.
Table 2.
Serum lipids and ALT indexes of mice from different experimental groups
| Week 8 | Week 12 | Week 16 | |
|---|---|---|---|
| TG mmol/L | |||
| LF | 1.33±0.14 | 1.01±0.06 | 1.29±0.09 |
| HFW | 1.13±0.05 | 1.16±0.03 | 1.07±0.05 |
| HFWE | 1.29±0.11 | 1.06±0.03 | 1.04±0.04 |
| TC mmol/L | |||
| LF | 3.54±0.12b | 2.68±0.33b | 2.96±0.06b |
| HFW | 4.45±0.17a | 4.40±0.11a | 4.53±0.16a |
| HFWE | 4.41±0.17a | 3.78±0.32a | 3.59±0.22b |
| LDL-C mmol/L | |||
| LF | 1.28±0.10b | 1.02±0.09 | 0.24±0.04c |
| HFW | 2.03±0.14a | 1.47±0.20 | 1.22±0.10a |
| HFWE | 1.83±0.09a | 1.19±0.13 | 0.88±0.08b |
| HDL-C mmol/L | |||
| LF | 1.65±0.07 | 1.10±0.20b | 2.10±0.07b |
| HFW | 1.97±0.09 | 1.31±0.16b | 3.05±0.10a |
| HFWE | 1.71±0.12 | 1.70±0.09a | 2.23±0.19b |
| ALT U/L | |||
| LF | 32.18±3.70b | 37.75±6.86b | 17.94±4.98c |
| HFW | 66.57±7.07a | 66.47±5.37a | 131.57±13.54a |
| HFWE | 39.86±4.29b | 48.95±6.26ab | 74.28±13.44b |
Samples were collected on weeks 8, 12 or 16. TC, total cholesterol; TG, triacylglycerol(s); LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; ALT, alanine aminotransferase. Values are shown as mean ± SEM (n = 10).
Significant difference among groups, by (ANOVA, p < 0.05)
The levels of hepatic total lipid content, TC and TG were affected by dietary treatment (Figure 2). HFW significantly increased (almost doubled) the total lipid and cholesterol contents, and EGCG treatment prevented these increases. EGCG, however, had no effect on the elevated hepatic TG levels caused by HFW, which was several-fold higher than the LF group.
Figure 2. Effects of dietary EGCG on hepatic lipid contents and severity of fatty liver.
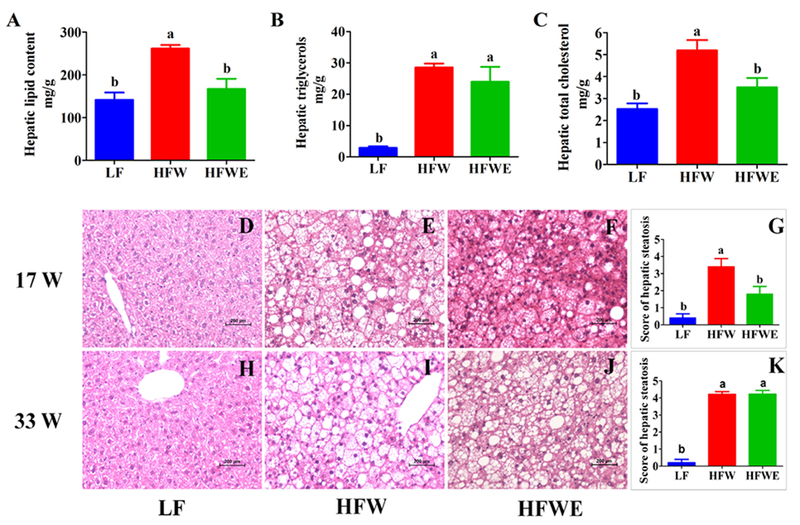
A, hepatic total lipid contents; B, hepatic total cholesterol contents; C, hepatic total triacylglycerol contents; and D, E, F and G are liver slices and hepatic steatosis score of mice at week 17; H, I, J and K are hepatic histological data from mice at week 33. D, H mice in LF groups; E, I, mice in HFW groups; F, J, mice in HFWE groups. All data are expressed as mean ±SEM (for LF group, n=5; for HFW and HFWE groups, n=10) a,b,cSignificant difference (ANOVA, p < 0.05).
On weeks 8, 12 and 16, mice in the HFW group had significantly elevated ALT levels as compared to the LF control group, suggesting the development of hepatotoxicity. EGCG treatment significantly prevented this event (Table 2).
3.3. Hepatic pathology
Histopathological analysis revealed no sign of fatty liver in the LF group (Figure D, H). In contrast, liver sections of mice from the HFW group were occupied by large areas of microvesicular steatosis, regions of evolving macrovesicular fatty change and hepatocellular hypertrophy (Figure 2 E, I). EGCG treatment markedly attenuated the severity of fatty liver on week 17, with decreased microvesicular steatosis, macrovesicular steatosis and hepatocellular hypertrophy (Figure 2 F). Compared to the LF group, the average histological hepatic steatosis score of mice in the HFW group was several-fold higher, and that for the HFWE group was significantly lower on week 17 (Figure 2 G). On week 33, there was much more macrovesicular change, with marked increase in acute and chronic inflammatory cells in sinusoids and around portal triad than on week 17. There was also occasional hepatocyte necrosis, but not fibrosis, of the liver (Figure 2 I). At this time point, however, EGCG treatment did not have a statistically significant effect on liver histopathology and on steatosis score (Figure 2 J, K).
3.4. Lipidomic analysis of liver and serum samples
The liver and serum samples on week 17 were analyzed by UPLC-ESI-QTOF/MS operating in both positive and negative ionization modes, and compared with a standard chemical compound database. A total of 33 free fatty acid species (FFA, negative ion mode) and 179 lipid species (positive ion mode) were identified in the liver (Supplementary Table 2), including cholesteryl esters (CE), ceramides (CM), phosphatidylcholines (PC), phosphatidylethanolamines (PE), sphingomyelins (SM), diacylglycerols (DG) and TG. Principal component analysis (PCA) distinguished mice among three groups using hepatic lipidomic data, and the first principal component factor placed the HFWE group in between the HFW and LF groups, suggesting that EGCG abrogate some of the effects of the HFW diet (Figure 3 A). However, ion signals of FFA (acquired in negative mode) were indistinguishable among experimental animals in the three groups (Figure 3 B). OPLS-DA (orthogonal partial least squares – discriminant analysis) [14] of the lipidomic data also clearly separated mice from HFW and HFWE group as shown by the score plot (Supplementary Figure 1). This clustering is mainly due to changes in the levels of DG (34:1), DG (34:2), DG (36:2), DG (36:3), PC(38:3), TG(52:2), TG(52:3), TG(52:4) and SM(18:1/22:0), as suggested by the S-plot. These lipid species are generally the more abundant species also found in the later analysis as shown in Figure 4. The S-plot can decrease the risk of false positives in the selection of potential biomarkers and thus complements the analysis in Figure 4.
Figure 3. Principal component analysis (PCA) score plots of liver and serum samples at week 17 using the lipidomics analyzing database.
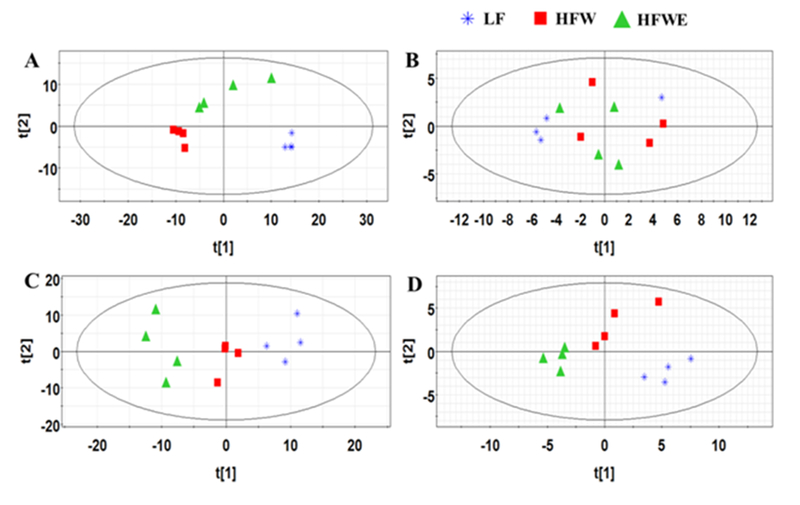
A, Hepatic positive ionization analysis; B, Hepatic negative ionization analysis; C, Serum positive ionization analysis; D, Serum negative ionization analysis; each point represents an individual liver or serum sample from LF, HFW and HFWE groups. The t[1] and t[2] correspond to principal components 1 and 2, respectively.
Figure 4. Hepatic lipid profiles analyzed by lipidomics.
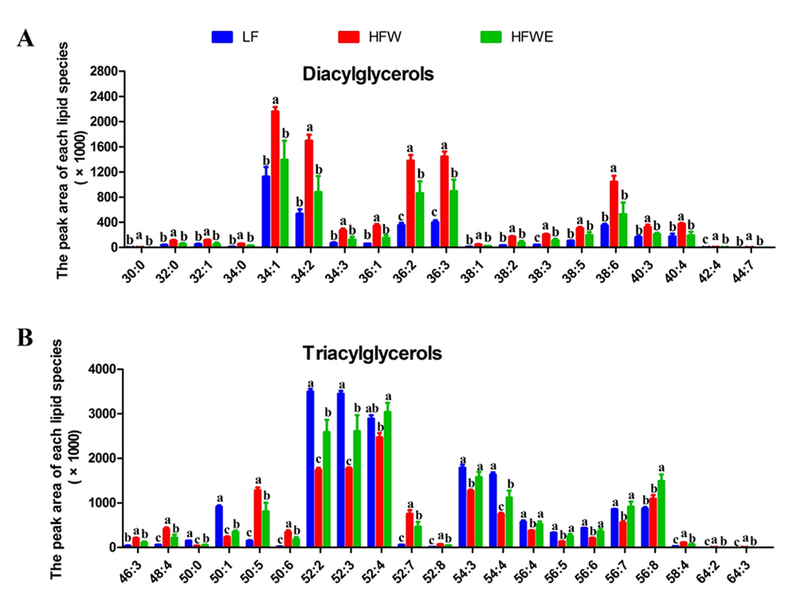
The designations of each panel are indicated in the figure. Data are presented as mean ± SEM, (for LF group, n=4; for HFW and HFWE groups, n=5). a,b,cSignificant difference among groups (ANOVA, p < 0.05).
With the liver samples collected on week 33, both PCA and partial least squares-discriminant analysis (PLS-DA) analysis of the lipidomic data distinguished mice in the LF group from the other two groups. However, both models could not differentiate mice between HFW and HFWE groups (Supplementary Figure 2). The contents (peak heights) of all the lipid species analyzed in the liver of mice at week 33 are listed in Supplementary Table 4.
The lipidomic data of liver samples from week 17 were examined by ANOVA. There were 138 individual lipids that showed significant difference between the HFW and LF groups. EGCG treatment significantly changed the contents of 72 lipid species, mostly in neutralizing the effects of HFW. DG and TG were the two most abundant classes altered by dietary treatments. Specifically, HFW significantly elevated the levels of 24 DG species (compared to the LF group). EGCG treatment prevented the HFW-induced elevation of almost all of DG species (Figure 4 A). In TG, the species with more unsaturated fatty acids (from dietary sources) are expected to be increased by HFW, while those with less unsaturated fatty acids (from biosynthesis) are expected to be decreased. This pattern was observed in many but not all TG species (Supplementary Table 2). Among the 21 TG species that were affected by EGCG, the more abundant ones (TG 52:2, 52:3, 52:4, 54:3, 54:4) were decreased by HFW, and EGCG partially or fully prevented this decrease (Figure 4 B). The levels of many PC species were decreased and some were increased by HFW; EGCG prevented the HFW-induced lowering of PC species with 4-6 double bonds (PC 36:5, 36:6, 40:4, 42:5) and HFW-induced increasing of some PC species with less double bonds (PC 36:3, 38:3) (Supplementary Table 2). HFW lowered the levels of most of the SM and PE species, and EGCG partially prevented some of these effects (Supplementary Table 2). Of the 24 CM species, many species were increased and many others were decreased by HFW, and some of these actions were prevented by EGCG. In all the 33 FFA species measured, no significant difference among the three groups was observed (Supplementary Table 2), consistent with the results of the PCA (Figure 3B).
In lipidomic analysis of serum samples collected at week 17, a total of 37 FFA (negative ionization mode) and 175 other lipid species (positive ionization mode) were identified. PCA distinguished mice among three groups using serum lipid species identified both in positive and negative ionization modes (Figure 3 C, D). The first principal component factors of both two modes placed the HFWE group further away from the LF group than the HFW group, suggesting EGCG treatment had activities beyond neutralizing the HFW effects. The score plot in the OPLS-DA also clearly separated mice from HFW and HFWE groups, and the S-plot suggested that the clustering were mainly due to changes in the levels of PC(34:1), PC (36:4), PC (38:4), PC (38:5), PC(38:6), FFA(16:0), FFA(18:0), FFA(18:1), FFA(18:2), FFA(20:3), FFA(20:4) and FFA(22:6) (Supplementary Figure 2). These species are generally the more abundant species found in the analysis shown below (Figure 5).
Figure 5. Serum lipid profiles analyzed by lipidomics.
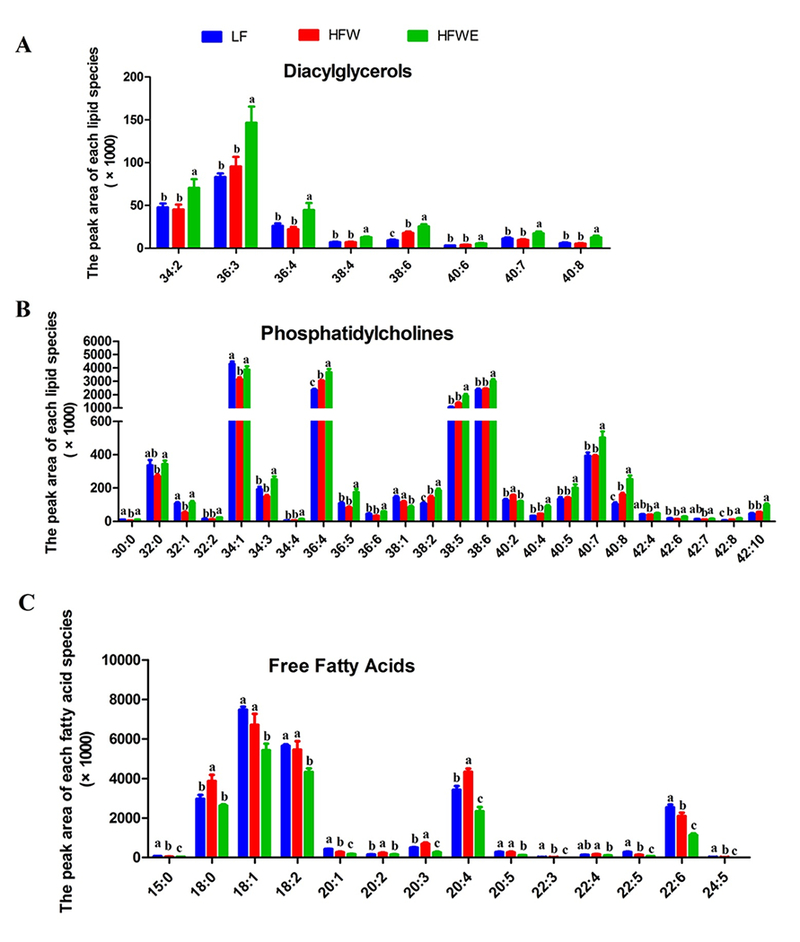
The designations of each panel are indicated in the figure. Data are presented as mean ± SEM, (for LF group, n=4; for HFW and HFWE groups, n=5). a,b,cSignificant difference among groups (ANOVA, p < 0.05).
ANOVA analysis showed that HFW altered 66 lipid species and 24 free fatty acid species in the serum (compared to the LF group). In comparison to the HFW group, EGCG treatments significantly changed the levels of 64 lipid species, including 2 CM, 24 PC, 11 PE, 8 SM, 8 DG and 11 TG species, and 14 FFA species (Supplementary Table 3). The levels of DG in the serum were rather low and were not significantly altered by HFW (Supplementary Table 3); however, 50% of the DG species were increased by EGCG treatment (Figure 5A). Out of the 72 TG species measured, five species were decreased and four species were increased by HFW; nine species were increased by EGCG treatment and all these TG species contained ≥ 8 double bonds (Supplementary Table 3). Some PC species were decreased and some other species increased by HFW, and EGCG increased the levels of more abundant PC species (Figure 5 B). The total levels of serum FFA species were increased by HFW and decreased by EGCG (Supplementary Table 3). Specifically, EGCG decreased the levels of five abundant and eight less abundant FFA species, mostly to levels even lower than the LF groups (Figure 5C), with only one minor species increased in level by EGCG. While HFW decreased many PE species and increased some SM species, EGCG treatment increased the levels of most of the lipid species in PE and SM classes. Of the 13 CM species measured, six were increased by HFW, of which two species were decreased by EGCG (Supplementary Table 3).
3.5. Bile acid pool size, fecal bile acids and fecal lipids
To examine the role of bile acid homeostasis in the lipid-lowering effect of EGCG, the bile acid pool size was investigated. The intestinal bile acid contents were markedly increased (doubled) by HFW resulting in an enlarged bile acid pool, and EGCG prevented these increases (Figure 6 A, D). The dietary treatments, however, did not significantly affect the hepatic and gallbladder contents of bile acids (Figure 6 B, C). HFW appeared to decrease fecal excretion of bile acids (non-significantly), but EGCG significantly increased the fecal total bile acid content (by 1.5-fold) (Figure 6 E). Mice in the HFW group also had significantly higher fecal total lipid and cholesterol levels than those of the LF group, and EGCG further increased the fecal lipid and cholesterol excretion (Figure 6 F, G).
Figure 6. Effects of dietary treatments on total bile acids, fecal bile acids and fecal lipids.
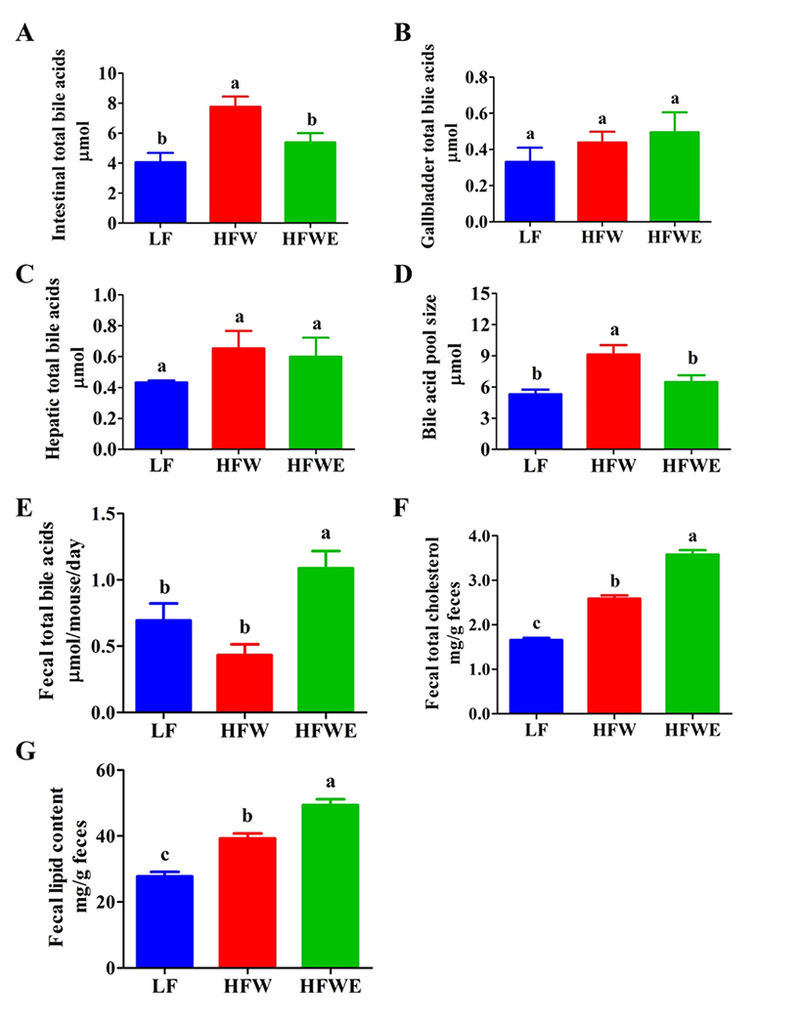
On week 17, the total bile acid contents were measured in the small intestine (A), gallbladder (B), and liver (C). The bile acid pool size (D) is the sum of total bile acid contents in these samples. Also shown are the fecal bile acid contents (E) on week 17 and the fecal levels of total fat (F) and cholesterol (G) on week 20. All data are expressed as mean ± SEM (for LF group, n=5; for HFW and HFWE groups, n=10). a,b,cSignificant difference, (ANOVA, p < 0.05).
3.6. Hepatic mRNA expressions of lipid and bile acid metabolizing genes
The expressions of hepatic and ileal genes related to bile acid homeostasis were investigated. The hepatic mRNA level of Cyp7a1 was induced by EGCG to 5.6-fold or 3-fold the value of the HFW or LF group, respectively (Figure 7 A). EGCG also prevented the HFW-induced downregulation of hepatic mitochondrial sterol 27-hydroxylase (Cyp27a1, a key enzyme for the bile acid alternative synthesis pathway). The gene expression of farnesoid X receptor (FXR) was also significantly increased by EGCG (Figure 7 A). The mRNA level of the nuclear receptor small heterodimer partner (SHP), however, was not changed. Similarly, EGCG did not alter the mRNA levels of ATP-binding cassette transporter 5 (ABCG5), apical sodium-dependent bile acid transporter (ASBT) or intestinal fibroblast growth factor 15 (FGF15) in the ileum (Figure 7 B).
Figure 7. Effects of treatment on mRNA expression levels of genes in the liver and ileum.
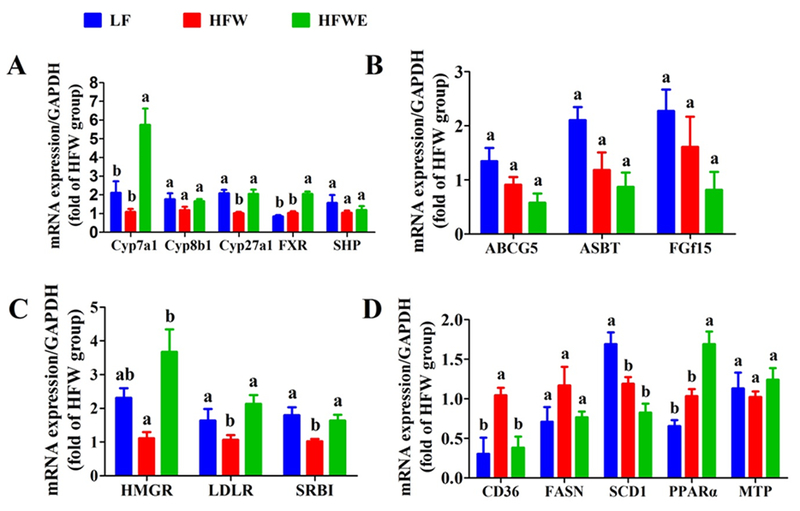
Mice were sacrificed on week 17. mRNA expression of key genes related to bile acid metabolism were measured in the liver (A) and ileum (B). mRNA expression of key genes related to cholesterol and fatty acid metabolism in the liver were also shown (C, D). All data are expressed as mean ± SEM (for LF group, n=5; for HFW and HFWE groups, n=10). a,b,cSignficant difference among groups (ANOVA, p < 0.05).
The expression of hepatic HMG-CoA reductase (HMGR), low-density lipoprotein receptor (LDLR) and scavenger receptor B1 (SR-B1) was significantly elevated by EGCG, neutralizing the downregulation effect of the HFW (Figure 7 C). The hepatic mRNA expression of cluster of differentiation 36 (CD36), also known as fatty acid translocase (FAT), was decreased by EGCG (neutralizing the effects of HFW), and that of peroxisome proliferator-activated receptor-alpha (PPARα) was further increased (Figure 7 D). However, the gene expression of fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1) and mitochondrial trifunctional protein (MTP) were not altered by EGCG treatments (Figure 7 D).
4. Discussion
The objective of the present study was to characterize the effects of EGCG in alleviating metabolic abnormalities and fatty liver disease, as well as to explore the underlying mechanisms. Our data clearly demonstrate that supplementation of EGCG to the HFW significantly reduces the body weight gain, mesenteric fat mass, fasting blood glucose levels, insulin resistance, serum cholesterol and ALT levels, hepatic lipid contents and severity of hepatic steatosis around week 17. These observations are consistent with our previous results [10]. However, upon prolonging the experiment to 33 weeks, some of the beneficial health effects of EGCG were no longer apparent (Figure 1 and Tables 1 and 2). Such a trend was also observed in previous studies [10]. The mechanisms of this time-dependent change are not clearly known. It may reflect the growth pattern, homeostasis and biological adaptation of the mice in this model system. The body weight gain of mice in the HFW group slowed down or plateaued after week 16, whereas the body weight of mice in the HFWE group still increased; thus, the growth-inhibition effect of EGCG was no longer observed around week 32. Similarly, the increase of fasting blood glucose levels stopped in the HFW group after week 16, but continued in the HFWE group. Even the LF group showed an increase in fasting blood glucose levels after week 12 and accelerated after week 24. By week 28, no significant differences in fasting blood glucose levels were observed among the three groups. In human studies, it has also been speculated that the beneficial effects of tea polyphenols against obesity were more robust in short term studies than long term studies [15].
The alleviation of obesity, MetS and fatty liver disease by tea polyphenols, in particular EGCG, has been demonstrated, and two types of mechanisms of action have been proposed [2]. 1) In the gastrointestinal tract, EGCG lowering the absorption of lipids and protein and/or affects microbes and other factors. 2) After absorption, EGCG promotes catabolism and inhibits biosynthesis in the liver and other tissues, possibly by the activation of AMP-activated protein kinase (AMPK) [2]. Our results highlight the importance of modulating bile acid homeostasis by EGCG in lowering lipid absorption.
The observed lower intestinal bile acid levels and the higher levels of fecal bile acids in EGCG-treated mice suggest that EGCG decreases bile acid reabsorption. It is estimated that about 90-95% of bile acids released from gallbladder into the intestinal tract are reabsorbed [16]. The intestinal reabsorption mainly occurs at the terminal ileum by the apical sodium-dependent bile salt transporter (ASBT; SLC10A2) [17]. The inhibition of ABST function by EGCG has been observed in vitro in cell lines [18], and may occur in our experimental system. In addition, EGCG may bind bile acids and alter the structure of micelles consisting of neutral lipids, phospholipids and cholesterol [19], leading to decreased lipid absorption or reabsorption. The biliary secreted bile acids are reabsorbed in distal ileum, predominantly as conjugated forms. Microbial deconjugation carried out by gut bacterial bile salt hydrolase, which removes the glycine or taurine moiety, could decrease intestinal active reuptake of bile acids and increase their fecal elimination [20–23]. EGCG has been reported to have significant effects on intestinal microbiota [24–26] and could alter the deconjugation of bile acids. The lower bile acid pool would decrease fat absorption, leading to the observed lower hepatic levels of fats and cholesterol accompanied by increased fecal excretion.
PCA analysis of liver lipidomic data suggest that EGCG partially prevented the changes caused by HFW. However, the results from serum lipidomic PCA analysis suggest the activity of EGCG is beyond neutralizing the effects of HFW (Figure 3). These results are consistent with the conclusion drawn from the analysis in Figures 4 and 5. The effects of EGCG in affecting different lipid species in the liver and blood are not fully understood. Suppression of CD36 expression by EGCG (Figure 7D) is expected to decrease hepatic uptake of fatty acids from the blood. This mechanism may contribute to the decreased levels of hepatic DG species and increased levels of serum DG species caused by EGCG. The basis for many of the changes on specific lipid species due to the treatments with HFW and EGCG are unclear and need to be investigated further. Some of the described changes in lipid species may be false positives, and the species suggested by the S-plots in Supplementary Figures 1 and 3 may carry more weight.
It is interesting that EGCG decreased the serum levels of many of the free fatty acids species that were elevated by HFW. DG and FFA are lipid species associated with lipotoxicity [27–30]. It was reported that the increased hepatic DG content led to the activation of novel protein kinase Cϵ resulting in decreased insulin signaling in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) associated hepatic insulin resistance and type 2 diabetes [31], and that serum FFA levels correlated with NAFLD and could be used as an indicator for predicting advanced fibrosis in NAFLD patients [32]. The lower levels of hepatic DG and serum FFA in the EGCG-treated group may be related to the protective effect of EGCG.
PC is the major phospholipid component of all plasma lipoprotein classes [33]. Hepatic PC is necessary for the packaging and export of triglycerides in very low density lipoprotein (VLDL) [34] and for the solubilization of bile salts for secretion [35]. The HFW was used in this study to mimic dietary risk factors for NAFLD [10]. The lower dietary levels of choline and folate (than the LF diet), however, only decreased the levels of some PC in the liver. The effect of the low choline/folate status on the development of fatty liver in our study is unclear. It was reported that the concentration of serum PC in individuals with steatohepatitis was significantly lower than that of healthy, age- and sex-matched controls [25]. The elevated levels of serum PC levels by EGCG is consistent with the alleviation of fatty liver by EGCG as shown in Figure 2.
In the present study, the hepatic mRNA levels of Cyp7a1 and Cyp27a1 were markedly increased by EGCG. This result is consistent with recent reports on the up-regulation of Cyp7a1 by tea catechins in vitro and in vivo [3, 4, 36, 37]. Cyp7a1 and Cyp27a1 are the rate-limiting enzymes of classical and alternative pathways for bile acid synthesis, respectively [16]. The elevated expressions of these two genes indicate a potential enhanced biosynthesis of bile acids, possibly a compensatory response of the decreased levels of hepatic bile acids. The enhanced bile acid biosynthesis would use more cholesterol as the substrate and reduce hepatic cholesterol levels. This would elevate the expression of HMGR (the rate-limiting enzyme of cholesterol synthesis) as we observed. LDLR and SR-BI are key receptors for cells to absorb cholesterol from circulation [16], and the enhanced expression of these genes by EGCG in the liver (Figure 7C) would enhance hepatic uptake of cholesterol. This is consistent with the decreased levels of serum LDL-C and HDL-C (Table 2). Collectively, our data demonstrated that EGCG treatment significantly altered hepatic bile acid and cholesterol metabolism in mice maintained on HFW diet.
In conclusion, our results demonstrate that dietary EGCG modulates bile acid homeostasis and alleviates the metabolic abnormality and fatty liver disease caused by a high-fat diet. These beneficial effects are proposed to be due to the decreased intestinal bile acid reabsorption, decreased lipid absorption, reduced hepatic lipid load and altered hepatic and lipid metabolism. The results on gene expression and lipidomic analysis provide more detailed information on these events. The time-dependent action of EGCG on its beneficial health effects needs further investigation.
Supplementary Material
Acknowledgements
This work was supported by grants from the U.S. National Institutes of Health CA120915 (to CSY), CA211437 (to WL) and shared facilities funded by CA72720 and ES005022, as well as the John L. Colaizzi Chair Endowment fund (to CSY). The authors thank the personnel of Laboratory Animal Service in the Department of Chemical Biology for taking care of our research mice, and thank Ms. Vi P. Dan for her assistance in the preparation of this manuscript. All authors and contributors of this manuscript declare no conflict of interests.
Abbreviations:
- ABCG5
ATP binding cassette transporter 5
- ALT
alanine aminotransferase
- BAT
brown adipose tissue
- CD36
cluster of differentiation 36
- CE
cholesteryl esters
- CM
ceramides
- Cyp7a1
cholesterol 7α-hydroxylase gene
- Cyp8b1
sterol 12α-hydroxylase gene
- Cyp27a1
sterol 27-hydroxylase gene
- DG
diacylglycerols
- EGCG
(−)-epigallocatechin-3-gallate
- FAS
fatty acid synthase
- FFA
free fatty acids
- FGF15
fibroblast growth factor 15
- FXR
farnesoid X receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HDL-C
high-density lipoprotein cholesterol
- HFW
high-fat western-style diet
- HFWE
HFW + EGCG diet
- HMGR
HMG-CoA reductase
- LDL-C
low-density lipoprotein cholesterol
- LDLR
low density lipoprotein receptor
- LF
low-fat diet
- LXRα
liver X receptors-α
- MetS
metabolic syndrome
- NAFLD
non-alcoholic fatty liver disease
- OPLS-DA
orthogonal PLS-DA
- PLS-DA
partial least squares - discriminant analysis
- PC
phosphatidylcholines
- PCA
principal component analysis
- PE
phosphatidylethanolamines
- RT-qPCR
real-time quantitative PCR
- SCD1
stearoyl-CoA desaturas-1
- SHP
small heterodimer partner
- SM
sphingomyelins
- SR-B1
scavenger receptor B1
- TC
total cholesterol
- TG
triacylglycerols
- VAT
visceral white adipose tissue
- VLDL
very low density lipoprotein
5 References
- [1].Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X, The anti-obesity effects of green tea in human intervention and basic molecular studies. European journal of clinical nutrition 2014, 68, 1075–1087. [DOI] [PubMed] [Google Scholar]
- [2].Yang CS, Zhang J, Zhang L, Huang J, Wang Y, Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Molecular nutrition & food research 2016, 60, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hirsova P, Karlasova G, Dolezelova E, Cermanova J, Zagorova M, Kadova Z, Hroch M, Sispera L, Tomsik P, Lenicek M, Vitek L, Pavek P, Kucera O, Cervinkova Z, Micuda S, Cholestatic effect of epigallocatechin gallate in rats is mediated via decreased expression of Mrp2. Toxicology 2013, 303, 9–15. [DOI] [PubMed] [Google Scholar]
- [4].Lee M-S, Park J-Y, Freake H, Kwun I-S, Kim Y, Green tea catechin enhances cholesterol 7α-hydroxylase gene expression in HepG2 cells. British Journal of Nutrition 2007, 99. [DOI] [PubMed] [Google Scholar]
- [5].Shan D, Fang Y, Ye Y, Liu J, EGCG reducing the susceptibility to cholesterol gallstone formation through the regulation of inflammation. Biomed Pharmacother 2008, 62, 677–683. [DOI] [PubMed] [Google Scholar]
- [6].Yki-Järvinen H, Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. The Lancet Diabetes & Endocrinology 2014, 2, 901–910. [DOI] [PubMed] [Google Scholar]
- [7].Kaur J, A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014, 2014, 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [8].Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M, Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li T, Francl JM, Boehme S, Chiang JY, Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 2013, 58, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS, Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. Journal of agricultural and food chemistry 2011, 59, 11862–11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manley S, Ni HM, Williams JA, Kong B, DiTacchio L, Guo G, Ding WX, Farnesoid X receptor regulates forkhead Box O3a activation in ethanol-induced autophagy and hepatotoxicity. Redox Biol 2014, 2, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, Havekes LM, Kleemann R, van den Hoek AM, Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One 2014, 9, e115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ, Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. Journal of lipid research 2007, 48, 2664–2672. [DOI] [PubMed] [Google Scholar]
- [14].Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J, Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 2008, 80, 115–122. [DOI] [PubMed] [Google Scholar]
- [15].Janssens PL, Hursel R, Westerterp-Plantenga MS, Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. The Journal of nutrition 2015, 145, 864–870. [DOI] [PubMed] [Google Scholar]
- [16].Li T, Chiang JY, Bile acid signaling in metabolic disease and drug therapy. Pharmacological reviews 2014, 66, 948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ, Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. Journal of Clinical Investigation 1995, 95, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Annaba F, Kumar P, Dudeja AK, Saksena S, Gill RK, Alrefai WA, Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. American Journal of Physiology-Gastrointestinal and Liver Physiology 2010, 298, G467–G473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ogawa K, Hirose S, Nagaoka S, Yanase E, Interaction between Tea Polyphenols and Bile Acid Inhibits Micellar Cholesterol Solubility. J Agric Food Chem 2016, 64, 204–209. [DOI] [PubMed] [Google Scholar]
- [20].Wahlstrom A, Sayin SI, Marschall HU, Backhed F, Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 2016, 24, 41–50. [DOI] [PubMed] [Google Scholar]
- [21].Dawson PA, Karpen SJ, Intestinal transport and metabolism of bile acids. Journal of lipid research 2015, 56, 1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG, Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proceedings of the National Academy of Sciences 2014, 111, 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS, Bile acids and the gut microbiome. Current opinion in gastroenterology 2014, 30, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI, Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 2013, 24, 1415–1422. [DOI] [PubMed] [Google Scholar]
- [25].Most J, Penders J, Lucchesi M, Goossens G, Blaak E, Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. European Journal of Clinical Nutrition 2017. [DOI] [PubMed] [Google Scholar]
- [26].Martel J, Ojcius DM, Chang C-J, Lin C-S, Lu C-C, Ko Y-F, Tseng S-F, Lai H-C, Young JD, Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nature Reviews Endocrinology 2017, 13, 149–160. [DOI] [PubMed] [Google Scholar]
- [27].Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, Duan J, Sullards MC, Leiker TJ, Barkley RM, Quehenberger O, Armando AM, Milne SB, Mathews TP, Armstrong MD, Li C, Melvin WV, Clements RH, Washington MK, Mendonsa AM, Witztum JL, Guan Z, Glass CK, Murphy RC, Dennis EA, Merrill AH Jr., Russell DW, Subramaniam S, Brown HA, Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res 2015, 56, 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hirsova P, Ibrabim SH, Gores GJ, Malhi H, Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. Journal of lipid research 2016, 57, 1758–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE, The lysosomal‐mitochondrial axis in free fatty acid–induced hepatic lipotoxicity. Hepatology 2008, 47, 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cantley JL, Yoshimura T, Camporez JPG, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ, Kahn M, Guigni BA, CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proceedings of the National Academy of Sciences 2013, 110, 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Birkenfeld AL, Shulman GI, Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y, Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep 2014, 4, 5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bursill CA, Abbey M, Roach PD, A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 2007, 193, 86–93. [DOI] [PubMed] [Google Scholar]
- [34].Remely M, Ferk F, Sterneder S, Setayesh T, Roth S, Kepcija T, Noorizadeh R, Rebhan I, Greunz M, Beckmann J, EGCG Prevents High Fat Diet-Induced Changes in Gut Microbiota, Decreases of DNA Strand Breaks, and Changes in Expression and DNA Methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Oxidative medicine and cellular longevity 2017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Z, Agellon LB, Vance DE, Phosphatidylcholine homeostasis and liver failure. The Journal of biological chemistry 2005, 280, 37798–37802. [DOI] [PubMed] [Google Scholar]
- [36].Suzuki T, Kumazoe M, Kim Y, Yamashita S, Nakahara K, Tsukamoto S, Sasaki M, Hagihara T, Tsurudome Y, Huang Y, Maeda-Yamamoto M, Shinoda Y, Yamaguchi W, Yamada K, Tachibana H, Green tea extract containing a highly absorbent catechin prevents diet-induced lipid metabolism disorder. Sci Rep 2013, 3, 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim YJ, Houng SJ, Kim JH, Kim YR, Ji HG, Lee SJ, Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J Nutr Biochem 2012, 23, 186–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


