Abstract
Background:
The value of quantitative interferon-γ release assay results for predicting progression from Mycobacterium tuberculosis infection to active disease is unknown.
Methods:
We analysed data from a reported vaccine efficacy trial of MVA85A in South Africa. QuantiFERON-TB Gold In-Tube (QFT) negative, HIV uninfected infants aged 18–24 weeks were enrolled. We stratified participants by quantitative QFT result (IFN-γ <0.35, 0·35–4·00, >4·00 IU/ml) at the intermediate study visit (Day 336) and determined risk of progression to active tuberculosis disease over the subsequent 6–24 months. No QFT differences were observed between placebo and MVA85A groups; therefore, both groups were included in analyses. Study clinicians were not blinded to QFT values, but strict case definitions were used and excluded QFT results.
Findings:
Among 2,512 infants with QFT tests performed at Day 336, 172 (6·8%) were positive. Compared with QFT non-converters (disease incidence 0·7 per 100 person-years; 95% CI: 0·4–1·1), children with QFT conversion at IFN-γ values between 0·35–4·00 IU/ml did not have significantly increased risk of disease (2·5 per 100 person-years; 95% CI: 0·4–9·4; IRR 3·7; p=0·23). However, QFT conversion at IFN-γ values >4·00 IU/ml was associated with markedly increased disease incidence (28·0 per 100 person-years; 95% CI: 14·9–45·7) compared to nonconverters (IRR 42·5; p<0·0001); and compared to children with IFN-γ values between 0·35–4·00 IU/ml (IRR: 11·4; p=0·00047). Among 91 QFT converters with a repeat test, 53 (58·2%) reverted from positive to negative. QFT reversion risk was inversely associated with IFN-γ value at QFT conversion and was highest (47/61; 77·0%) with IFN-γ values <4·00 IU/ml.
Interpretation:
In young children, tuberculosis disease risk was not significantly elevated, and QFT reversion was common, following QFT conversion at IFN-γ values up to 10 times the recommended test threshold. By contrast, QFT conversion at very high IFN-γ values (>4·00 IU/ml) warrants intensified diagnostic and preventive intervention for extremely high risk of tuberculosis disease.
Background
Interferon-γ release assays (IGRAs) are diagnostics for Mycobacterium tuberculosis (MTB) infection, widely used in high-resource clinical settings to investigate contacts of tuberculosis cases and to guide isoniazid preventive therapy (IPT).1 Among children, a positive IGRA result may improve performance of clinical algorithms for diagnosis of pauci-bacillary pulmonary tuberculosis, by demonstration of prior MTB exposure and infection, despite the modest sensitivity and specificity of IGRAs for active disease, though evidence is conflicting.2–8
Upon MTB infection, young children are at very high risk of progression to tuberculosis disease and, compared to adults, at higher risk of severe tuberculosis morbidity and mortality.9 There has been considerable interest in the use of chemoprophylaxis among high-risk children; however, randomized trials of untargeted IPT among children in high-transmission settings have had mixed results.10,11 IPT reduces incidence of disease in MTB infected or exposed children by >50% and is recommended in national and international guidelines.12,13 However, implementation of IPT is poor in the resource-limited countries where effective tuberculosis prevention is most needed.
One of the key obstacles to targeted tuberculosis screening and preventive therapy is differentiating only those MTB-infected individuals at highest risk of disease from the majority who will remain healthy. Individuals who test IGRA positive have only two- to three-fold elevated risk for tuberculosis disease,14–18 and the positive predictive value for disease progression among IGRA-positive household contacts, or among individuals with IGRA conversion, is <2%.19,20
Further, because of the lack of natural quantitative breakpoints in the distribution, the conversion threshold value of the QuantiFERON-TB Gold In-Tube test (QFT) (IFN-γ ≥ 0·35 IU/ml), one of the most widely used IGRAs, is subject to debate [16]. It is not clear whether higher IFN-γ values represent more recent MTB exposure, greater aerosolized inoculum, or sustained infection; or simply reflect heterogeneity in human immune responses to MTB. Importantly, evidence on whether higher QFT conversion IFN-γ values are associated with increased risk of progression to active disease is conflicting,19,21–23 and current management algorithms do not distinguish between IFN-γ values above the QFT manufacturer’s recommended test threshold.
We analysed longitudinal data from a tuberculosis vaccine efficacy trial, in which no protective effect was seen on incidence of MTB infection or disease in a large cohort of Bacille Calmette Guerin (BCG) vaccinated South African infants, all of whom were QFT negative at enrolment.24 We hypothesized that QFT conversions and quantitative QFT values predict tuberculosis risk among infants. We therefore evaluated the relationship between QFT conversion IFN-γ values and risk of subsequent active tuberculosis disease and of QFT reversion.
Methods
Study Design and Participants
The MVA85A 020 trial was a double-blinded, randomised, placebo-controlled clinical trial conducted in a rural region near Cape Town, South Africa (2009 – 2012). Healthy infants aged 4–6 months who had received BCG vaccination within 7 days of birth were enrolled if they were HIV ELISA negative and had no known household or other close exposure to a tuberculosis patient; QFT testing was performed and those with a positive test were excluded.
Study Procedures
Study procedures and results have been described previously.24 In brief, infants were randomized to receive one dose of MVA85A or Candida skin test antigen (placebo control). QFT testing was repeated at Day 336 and at end of study, which ranged from 6 to 24 months following Day 336. Infants were actively followed every 3 months to identify signs or symptoms of disease, or history of exposure to tuberculosis. Children with persistent cough, failure to thrive, weight loss, tuberculin skin test (TST) or QFT conversion, or close contact with a TB patient, were admitted for standardised investigation. Investigation included QFT and TST if not already performed; chest radiography; HIV testing; and two paired, consecutive induced sputa and early morning gastric lavages tested by auramine staining and smear microscopy, MGIT liquid culture; and from January 2011, Xpert MTB/RIF. Children with positive QFT or TST results were referred to public clinics for IPT.
QuantiFERON
QFT was performed according to manufacturer’s instructions (Qiagen, Venlo, The Netherlands). Briefly, QFT is used to detect in vitro MTB-specific immune responses by measuring IFN-γ concentration in plasma harvested from whole blood incubated with MTB-specific antigens (TB Ag) minus IFN-γ detected in unstimulated control (Nil). The amount of IFN-γ is quantified by ELISA, using IFN-γ standards between 0 and 4 IU/ml (outside the USA). To evaluate the detectable range of IFN-γ concentration beyond the standard curve, blood samples from 42 healthy adult volunteers were stimulated in the TB Ag tube. Undiluted and diluted (1:5, 1:10 and 1:25) plasma samples were analysed by QFT IFN-γ ELISA. Linearly extrapolated results were compared with each of the diluted results to assess reliability, which was evaluated by Pearson’s product-moment correlation coefficient.
Outcome Definitions
We defined a positive QFT as TB antigen – Nil IFN-γ value greater than or equal to 0·35 IU/ml, as per manufacturer’s instructions. QFT conversion was defined as a positive test that followed a negative test and QFT reversion as a negative test that followed a positive test. For the original trial, the primary disease endpoint (Endpoint 1) required either microbiological confirmation, defined as at least one positive liquid culture for M. tuberculosis or Xpert MTB/RIF from any clinical specimen; or, in the absence of microbiological confirmation, specific chest radiographic and clinical findings indicative of tuberculosis in addition to positive TST or QFT conversion. Study clinicians were not blinded to QFT results. Since QFT was a component of the latter composite definition, we utilized a Revised Endpoint 1 for this analysis that removed QFT conversion from the diagnostic criteria to avoid bias towards association with QFT status. Additionally, after a tuberculosis diagnosis, most children did not have QFT repeated, as they had met the main study endpoint.
Analysis
Consistent with the primary trial publication that QFT conversion and tuberculosis disease rates did not differ by study arm,24 we analysed QFT dynamics and tuberculosis risk in control and MVA85A vaccine participants together. We analysed QFT conversions at Day 336 and at end of study. We compared QFT converters and non-converters at the Day 336 study visit and analysed subsequent tuberculosis disease incidence through the end of study. We included tuberculosis cases that were diagnosed within 60 days of the end of study visit, as evaluation for suspected disease typically began at end of study. We further stratified QFT positive results by IFN-γ value at the Day 336 study visit, first as continuous values, and then using ordinal categories based on inspection of model results of disease risk, reversion risk, and positive predictive value of various thresholds. For our main results, we selected thresholds of 0·35 IU/ml (manufacturer’s recommended value) and 4·00 IU/ml, because the latter is the maximum standard provided in international kits. The relationship between QFT conversion value, disease incidence and probability of QFT reversion at end of study were analysed using logistic regression models and generalised additive models with penalised splines. We evaluated effect mediation of sex, age, vaccine assignment and isoniazid preventive therapy on the relationship between QFT value and tuberculosis risk in generalized linear models. We calculated exact 95% Poisson confidence intervals and compared tuberculosis disease incidence rates in groups using a two-sample Poisson test. All statistical analyses were performed using R 3·2·4 (R Foundation for Statistical Computing, Vienna, Austria).25
Ethical Approval
The trial was approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee, Oxford University Tropical Research Ethics Committee, and the Medicines Control Council of South Africa. Parents or legal guardians provided written, informed consent.
Role of the Funding Source
Aeras was the sponsor of the MVA85A 020 Trial. BL is an employee of Aeras who participated in writing the manuscript. JRA is supported by the National Institutes of Health (K01 AI104411). No additional funding was obtained for this analysis of trial data. JRA, EN, TJS, and MH had complete access to the data. All authors reviewed and interpreted analyses and contributed to the writing of the manuscript. MH had final responsibility for the decision to submit for publication.
Results
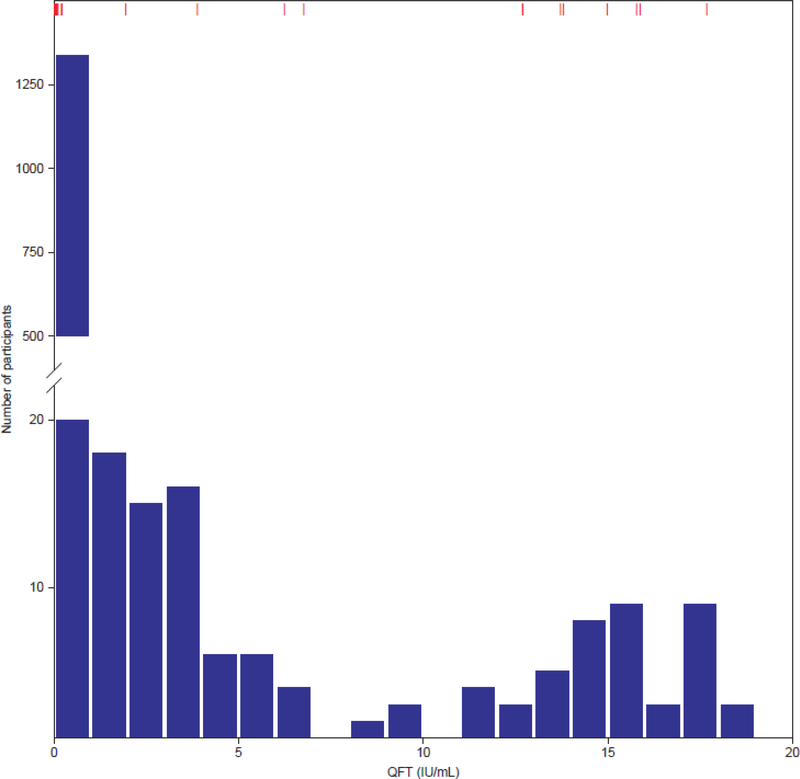
Among the 2,797 infants enrolled in the trial at median age 20·4 weeks (interquartile range [IQR]: 19·3, 22·0), 2,772 infants had a negative QFT at enrolment, 5 had no quantitative results available, and 20 (0·7%) had an indeterminate result. Among those with a negative QFT at baseline, 2,512 (90·6%) had a QFT performed at the Day 336 visit. In this group, 172 (6·8%) were positive and 13 (0·5%) were indeterminate. Among QFT converters with a positive test at Day 336, median QFT conversion IFN-γ value was 3·41 IU/ml (IQR: 0·82, 13·72). A bimodal distribution of IFN-γ values was observed, with a nadir of approximately 7·0 IU/ml (Figure 1). QFT conversion rate was no different among infants receiving the MVA85A vaccine (88/1254; 7·0%) and those receiving placebo (89/1281; 6·9%) (p=1·00). QFT conversion values similarly did not differ for these two groups (MVA85A vaccine group median: 3·46 [IQR: 0·86, 13·19]; placebo group median: 3·41 [IQR: 0·72, 13·80]; p=0·83). At the end of study visit, 2,045 of those children who were QFT negative at Day 336 had a repeat QFT performed and 116 (5·7%) had converted. A non-significant trend towards higher QFT conversion among those negative at day 336 was seen in the MVA85A vaccine group at end of study (p=0.090).
Figure 1. Distribution of QuantiFERON-TB Gold In-Tube IFN-γ values at the intermediate study visit (Day 336).
Bars represent numbers of individuals with QFT values between numbers (e.g. 0.01–1.00, 1.01–2.00)
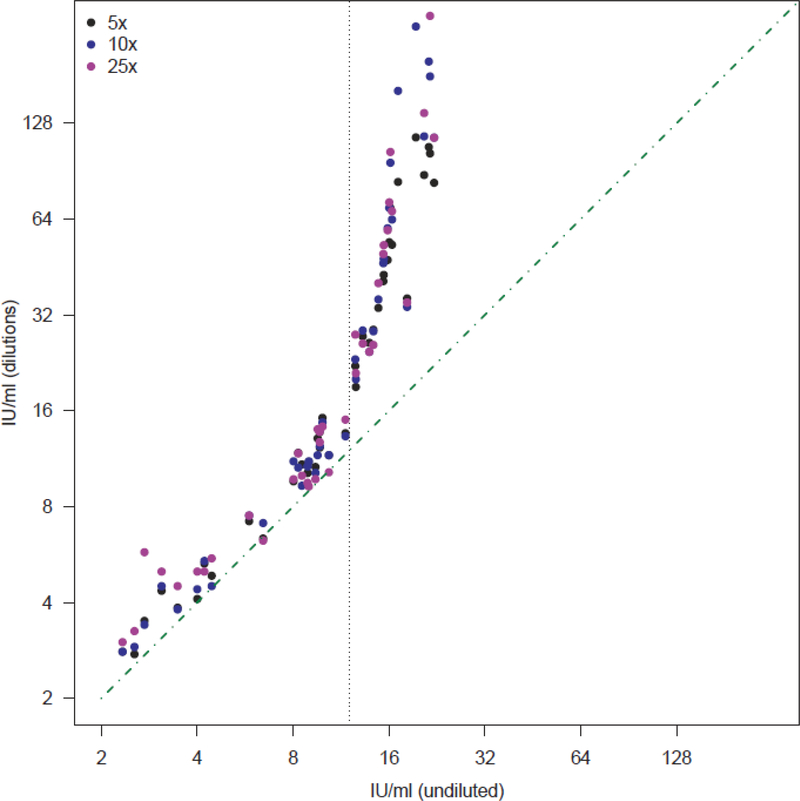
Because of the bimodality, we investigated whether very high QFT conversion IFN-γ values, which exceed the maximum value of the standard curve and were therefore estimated through linear extrapolation, were a reliable estimate of true IFN-γ concentrations. Among 42 samples from healthy volunteers with varying IFN-γ values that were diluted 5-, 10-, and 25-fold, we found high correlation between extrapolated measurements in undiluted samples and actual measurements in diluted samples up to approximately 12·00 IU/ml (Pearson’s r: 0.94–0.97). Measurements in undiluted samples underestimated IFN-γ concentrations above 12·00 IU/ml (Pearson’s r: 0.66–0.88; Figure 2).
Figure 2. Comparison of QFT IFN-γ values estimated by dilutions with QFT IFN-γ estimates from undiluted values, with linear extrapolation above 4 IU/ml.
To estimate whether IFN-γ values extrapolated above the assay higher standard (4 IU/ml) were reliable, measurements obtained from undiluted samples were correlated with those obtained by diluting the same samples 5- (black marker), 10- (blue marker) and 25-folds (purple marker).
Measurements obtained from diluted samples were multiplied by the dilution factor to estimate the pre-dilution IFN-γ concentration and correlated with values measured in the corresponding undiluted samples. The green diagonal dot-dashed line represents perfect correlation. The vertical dotted line depicts an undiluted IFN-γ value of 12, above which extrapolated results became less reliable.
Overall, 58 children (2·1%) met diagnostic criteria for active tuberculosis disease, including 42 (1·5%) who had microbiologically confirmed tuberculosis. Thirty-seven cases occurred between Day 336 and end of study, but 9 of them were missing Day 0 or Day 336 QFT results. An additional 107 children were treated for tuberculosis, without meeting the study definition, prior to day 336 and were excluded from further analysis. Among the 172 converters at day 336, 30 were diagnosed and treated for tuberculosis prior to day 336, leaving 142 converters available for analysis. Among the 2,232 children with negative QFT at Day 336, 16 were diagnosed with tuberculosis by end of study (incidence 0·7 per 100 person-years), and 11 of them had microbiologically confirmed tuberculosis (incidence 0·5 per 100 person-years, Table 1).
Table 1.
Incidence of tuberculosis (cases per 100 person-years) according to Day 336 QFT IFN-γ value·
| TB Case Definition | Day 336 QFT IFN-γ value | N | Cases | Incidencea | 95% CI | IRR | IRR 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Revised Case Definition 1 | < 0·35 | 2232 | 16 | 0·7 | (0·4–1·1) | ref | ref | ref |
| 0·35–4·00 | 79 | 2 | 2·5 | (0·4–9·4) | 3·7 | (0·4–15·8) | 0·23 | |
| > 4·00 | 63 | 10 | 28·0 | (14·9–45·7) | 42·5b | (17·2–99·7) | <0·0001 | |
| Culture or Xpert Positive | < 0·35 | 2232 | 11 | 0·5 | (0·2–0·8) | ref | ref | ref |
| 0·35–4·00 | 79 | 2 | 2·5 | (0·4–9·4) | 5·4 | (0·6–24·8) | 0·13 | |
| > 4·00 | 63 | 7 | 19·6 | (8·9–36·8) | 43·3c | (14·2–122·3) | <0·0001 |
Incidence reported in cases per 100 person-years
IRR of >4·00 vs 0·35–4·00 for Revised Case Definition 1: 11·4 (95% CI: 2·4–107·2), p<0·0.00047
IRR of >4·00 vs 0·35–4·00 for Culture or Xpert Positive: 8·0 (95% CI: 1·5–78·8); p=0·0094
QFT: QuantiFERON-TB Gold In-Tube; IRR: Incidence Rate Ratio; CI: Confidence Interval
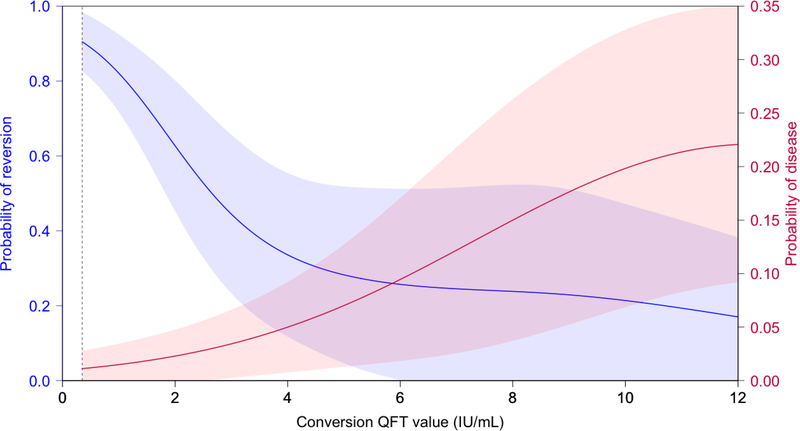
Tuberculosis risk increased substantially above conversion QFT value of 4·00 IU/ml (Figure 4). Among 79 children with a QFT conversion IFN-γ value between 0·35–4·00 IU/ml, two were subsequently diagnosed with tuberculosis (incidence 2·5 per 100 person-years) and both cases were microbiologically confirmed (Table 1). Among 63 children with a QFT conversion IFN-γ value >4·00 IU/ml, 10 were diagnosed with tuberculosis (28·0 cases per 100 person-years) and seven were microbiologically confirmed (19·6 cases per 100 person-years, Table 1).
Figure 4. Probability of QuantiFERON-TB Gold In-Tube reversion between the intermediate and final study visit and prospective risk of disease, as a function of the QFT conversion IFN-γ value.
The shaded areas reflect 95% confidence intervals from a generalized additive model. Vertical dashed line reflects the current recommended cutoff (0·35 IU/ml).
Compared to QFT non-converters, children with Day 336 QFT conversion IFN-γ values greater than 4·00 IU/ml had markedly increased incidence of active tuberculosis disease by both case definitions (IRR for Revised Endpoint 1: 42·5, 95% CI: 17·2–99·7; and IRR for microbiologically-confirmed cases: 43·3, 95% CI: 14·2–122·3; both p<0·0001). Using the threshold of 4·00 IU/ml, the sensitivity and specificity for subsequent disease were 36% and 99% and the positive and negative predictive values were 16% and 99%, respectively. The positive and negative predictive values using the manufacturer’s cut-off of 0·35 IU/ml were 8% and 99%, respectively. Median time to diagnosis among QFT non-converters and QFT converters from the time of QFT testing was 210 days (IQR: 134–373) and 44 days (IQR: 30–72) (p=0.0020), respectively (Figure 3). Among the 16 individuals with a negative QFT at day 336 who were subsequently diagnosed with tuberculosis, 14 had QFT repeated at diagnosis, and all (14/14; 100%) were positive. We found no effect mediation of vaccine assignment (p=0·93), age (p=0·96), sex (p=0·16) or receipt of IPT (p=0·79) on the relationship between QFT values and tuberculosis risk.
Figure 3. Tuberculosis-free survival from day 336 study visit, stratified by quantitative QFT result.
Black: IFN-γ <0·35 IU/ml; Blue: IFN-γ 0·35–4·00; Red: IFN-γ >4·00
Among the 172 QFT converters at Day 336, 91 children had a repeat QFT at end of study, and 53 (58·2%) reverted from positive to negative QFT. QFT reversion rates did not differ by vaccine/placebo assignment (MVA85A vaccine group: 29/52; control group 24/39; p=0·74) or among those who subsequently received IPT or four-drug curative therapy (received: 29/55; did not receive: 24/36; p=0·27). We analysed risk of QFT reversion at end of study as a function of the QFT conversion IFN-γ value at Day 336 (Table 2 and Figure 4). At QFT conversion IFN-γ values between 0·35–4·00 IU/ml, subsequent reversion rates were high (47/61; 77·0%). Very high QFT conversion IFN-γ values were associated with substantially lower risk of QFT reversion (Odds Ratio 0·07 [95% CI: 0·02–0·21] for QFT conversion IFN-γ value >4 IU/ml vs ≤4 IU/ml) (Figure 4), though probability of QFT reversion for QFT conversion IFN-γ values even above 4 IU/ml remained moderately high (6/30; 20·0%) (Table 2).
Table 2.
Risk of QFT reversion at end of study, among children who converted at the intermediate study visit, according to the quantitative QFT conversion IFN-γ value.
| Day 336 IFN-γ value (IU/ml) | N | Reverted | % | 95% CI |
|---|---|---|---|---|
| 0·35 – 4·00 | 61 | 47 | 77·0 | (64·2–86·5) |
| > 4·00 | 30 | 6 | 20·0 | (8·4–39·1) |
| Total (>0·35) | 91 | 53 | 58·2 | (47·4–68·3) |
Discussion
We found high rates of QFT conversion and high incidence of tuberculosis disease, using a rigorous endpoint definition, in a cohort of young children living in a rural community in South Africa. Consistent with our prior studies in older populations, we found an increased risk of incident tuberculosis disease following QFT conversion [18]. However, contrary to traditional interpretation of the QFT result, we additionally found that tuberculosis disease incidence rates were significantly higher among children with very high QFT conversion IFN-γ values (>4·00 IU/ml), with tuberculosis disease incidence >40-fold that of QFT non-converters and 11-fold that of QFT converters with IFN-γ value between 0·35–4·00 IU/ml. By contrast, children with QFT conversion IFN-γ values less than 4·00 IU/mL (more than 10 times higher than the assay threshold value) had a high risk of QFT reversion and did not have significantly increased risk of progression to active disease, compared to QFT non-converters. Taken together, these results indicate that very high QFT conversion IFN-γ values, much higher than previously considered clinically useful, are a powerful indicator of markedly increased risk for progression from MTB infection to active tuberculosis disease in this vulnerable population.
Despite the elevated incidence and mortality from tuberculosis among children in high-burden countries, there has been a dearth of large cohort studies characterizing the role of IGRAs for predicting progression from infection to disease in this population. The existing literature consists predominantly of cross-sectional studies demonstrating the accuracy of IGRAs in diagnosis of active or latent tuberculosis, frequently in comparison with tuberculin skin testing. Results have been at times conflicting, with some studies demonstrating comparable sensitivity and specificity to TST, while others finding higher or lower sensitivity.3,26–32 Rates of indeterminate QFT tests have been higher in young children than in adults;26,33–36 although in this study, we found a low rate of indeterminate tests (0·4% of all tests).
It is possible that very high QFT conversion IFN-γ values among children are a marker of very early, incipient disease, or subclinical active disease that was present at the time of QFT testing. This hypothesis is supported by the relatively short interval between QFT testing and TB disease diagnosis. The findings also echo a recent adolescent study that identified elevated IFN-regulated genes as a predictor of subsequent tuberculosis risk.37 The critical question is whether identification of those MTB infected children at highest risk offers an opportunity for 1) early case detection of subclinical tuberculosis; and 2) in those children in whom aggressive diagnostic measures do not reveal tuberculosis, the use of preventive therapy to interrupt progression to active disease. Untargeted IPT failed to prevent tuberculosis among HIV-exposed infants in a recent randomized trial;11 and whether QFT-targeted preventive therapy—with one or more drugs—could reduce tuberculosis morbidity and disease in this very high-risk population merits further study. Such strategies would need to be balanced against the risk of undertreating undiagnosed active disease, causing selective pressure for antibiotic resistance.
Several studies have addressed the question of whether QFT IFN-γ values have additional value for prediction of incident tuberculosis, and results have been conflicting. Diel et al found that 16 of 19 contacts (84%) who subsequently progressed to tuberculosis had IFN-γ values >3·5 IU/ml at initial investigation.21 In a German study, Geis et al reported that 5 of 6 contacts (83%) with disease had IFN-γ values >10 IU/ml, and suggested that raising the QFT test threshold could improve the positive predictive value and reduce the number needed to treat. By contrast, Haldar et al did not find an association between QFT conversion IFN-γ value and tuberculosis progression among adult contacts in the United Kingdom,23 and Zellweger et al found minimal variability in risk by IFN-γ value in a cohort of contacts in a large European network.19 It should be noted that these studies measured IFN-γ values in primarily adult tuberculosis contacts in low endemicity areas, whereas our study enrolled infants in a high transmission area who were known to be QFT negative and were then followed prospectively for QFT conversion and subsequent disease.
The lack of an inflection point in the distribution of IFN-γ values in previous studies has called into question the validity of the 0·35 IU/ml test threshold [19]. For the first time, we have shown in infants that the distribution of IFN-γ values is clearly bimodal, as has been shown previously for the distribution of TST in older age groups [19]. In the absence of a gold standard test, it is impossible to determine how this distribution relates to MTB infection, but we suggest that based on the nadir of measured QFT conversion IFN-γ values at 7·0–8·0 IU/ml, it is unlikely that the bimodal distribution differentiates MTB uninfected from infected children. We note that this bimodal distribution differs from the unimodal distribution of QFT values observed in adolescent and adult studies, including one from the same laboratory and community, suggesting that the naïve infant immune response to MTB may differ from that of adults.20
For our main analyses, we selected a threshold of 4·00 IU/ml based on disease and reversion risks above this inflection point (Figure 4) and availability of IFN-γ standards up to 4·00 IU/ml in the international kits. We note that high rates of disease (2·5 per 100) were found even among those with QFT values 0·35–4·00, and the optimal threshold from a perspective of targeting interventions may be lower. Because IFN-γ values greater than 4·00 IU/ml lie beyond the maximum value within the standard curve of the assay, we performed multiple dilution experiments using plasma from healthy volunteers and found that observed IFN-γ values estimated through linear extrapolation were a reliable estimate of true IFN-γ concentrations up to approximately 12·00 IU/ml, suggesting that the observed distribution nadir is not an analytical artefact. We infer that the bimodal distribution of QFT conversion IFN-γ values may be related to other factors such as recent or transient MTB infection, size of inoculum or bacillary load.
Several recent studies have reported high rates of IGRA reversion (>60%) among healthcare workers in low-burden countries.38–41 In a study of adolescents in the same high-burden community, we previously documented a reversion rate of 23·7% following QFT conversion.20 There have been few data on QFT reversion risk among young children. Shah et al found low rates of reversion (15%) among QFT-positive household contacts of tuberculosis cases. In the present study, we found high overall rates of QFT reversion among infants (58·2%) and, as seen in our adolescent study, we found an inverse relationship between QFT conversion IFN-γ value and reversion risk; however, reversion rates remained fairly high (20%) even at very high IFN-γ values (>4·00 IU/ml).
The traditional approach to early detection of childhood tuberculosis has been the investigation of household contacts of adult tuberculosis cases. However, recent studies from South Africa have demonstrated that half or more of tuberculosis cases in children are not linked to an adult case in the household or are diagnosed before the adult.42 The use of serial IGRA testing represents a potential tool for early identification of children with subclinical or incipient tuberculosis; whether this would be cost-effective as a testing strategy, either standalone or in conjunction with contact tracing, remains to be determined.
The results of this analysis should be interpreted within the context of the limitations of the study design and available data. First, while we excluded QFT from the rigorous case definition for tuberculosis used in this study, clinicians were not blinded to QFT results and it is possible that decision to investigate on the basis of a positive QFT led to increased diagnosis of tuberculosis disease; however, it is unlikely that this would have explained higher disease rates associated with greater quantitative IFN-γ values. Additionally, our findings were consistent when using a microbiologically confirmed endpoint. To avoid potential for boosting of QFT responses, TSTs were not routinely performed in this study, precluding direct comparison. Children who lived in a household with a smear-positive tuberculosis case were screened for active tuberculosis; a number of such children were identified as having active tuberculosis prior to day 336 and therefore not included in the prospective analysis. We are unable to assess effect mediation between exposures and QFT IFN-γ values on tuberculosis risk. Participants with a positive QFT who did not have tuberculosis were referred for IPT, but IPT did not appear to confound the relationship between IFN-γ values and risk of disease. We used undiluted samples with QFT values extrapolated from the standard curve above 4.00 IU/ml in our main analyses, as we did not have samples available from this cohort to perform measurements on diluted samples; our analysis from healthy controls suggests that this would be reliable up to 12.00 IU/ml. A number of technical factors can influence quantitative results of QFT testing, as recently outlined by Banaei and colleagues.43 To minimize risk of variation, all assays were performed according to a trial protocol in a single laboratory accredited with the South African National Accreditation System (SANAS), by well-trained technicians with tested competency, under a rigorous quality assurance programme and external monitoring by SANAS and the trial sponsor, Aeras. Finally, we conducted this study in a community with extremely high rates of tuberculosis; whether these findings will hold true in lower burden settings should be investigated.
Currently, WHO guidelines recommend that IGRAs not be used to replace TSTs in high-burden, low- and middle-income countries, and a number of national and regional guidelines recommend TSTs over IGRAs in young children.12,44 However, many of the same guidelines recommend IGRAs preferentially over TSTs in BCG-vaccinated individuals, which leaves an evidence gap for BCG-vaccinated children. We found that QFT testing in a high-burden, BCG-vaccinated population can identify children at highest risk of subclinical disease or imminent progression to tuberculosis disease, who may benefit from more intensive diagnostic and chemotherapeutic interventions. Very high QFT conversion IFN-γ values (>4·00 IU/ml) in particular were associated with lower probability of QFT reversion and markedly elevated risk of tuberculosis disease. By contrast, conversion values in the range of 0·35–4·00 IU/ml had low predictive value for tuberculosis and were associated with high rates (77%) of reversion. The current recommended QFT threshold IFN-γ value of 0·35 IU/ml may therefore be too low in this population, and a higher test threshold may be indicated for risk-targeted intervention. Similarly, infant QFT conversion values in the range of 0·35–4·00 IU/ml might warrant repeat testing if preventive therapy is considered. If validated in other study populations, these findings justify revision of current international guidelines for use of IGRAs in young children.12,44
Research In Context.
Evidence before this study
We searched PubMed for relevant articles published in English before July 26, 2016, using the search terms “(Quantiferon OR T-Spot.TB OR ‘interferon gamma release assay’) AND (child OR children OR pediatric OR infant) AND tuberculosis”. While several studies reported on sensitivity and specificity of interferon gamma release assays (IGRAs) for active tuberculosis in children, the search identified no studies reporting prognostic value of IGRA conversions or predictive value of quantitative interferon-γ values for tuberculosis among young children. Among household contacts of tuberculosis patients, positive IGRAs were associated with increased risk of tuberculosis, but the positive predictive value was <2%. Additionally, several studies have noted high rates of indeterminate IGRA results among children.
Added value of this study
In this study, we present data from a large prospective cohort of infants from a tuberculosis-endemic community who were QuantiFERON-negative at baseline and followed prospectively for QuantiFERON conversion and/or tuberculosis disease. We found that very high QuantiFERON IFN-γ conversion values (>4·00 IU/ml) were associated with markedly elevated risk of tuberculosis disease compared with non-converters or converters with lower QuantiFERON IFN-γ values (0·35–4·00 IU/ml). Among children who did not convert QuantiFERON at one year but were subsequently diagnosed with disease, all whose QuantiFERON was repeated were positive at time of diagnosis. Additionally, QuantiFERON IFN-γ values above the manufacturer’s threshold but below 4·00 IU/ml were associated with very high rates of reversion.
Implications of all the available evidence
Guidelines from the World Health Organization, United States Centers for Disease Control and Prevention and other bodies currently recommend tuberculin skin testing over IGRAs in young children from high-burden settings, in part due to lack of evidence from this age group. These data suggest that high QuantiFERON IFN-γ conversion values may have strong prognostic or diagnostic value for tuberculosis in this population. If validated in other study populations, these findings justify revision of current international guidelines for use of IGRAs in young children.
Acknowledgements
We thank study participants and their families; the community of Cape Winelands East district; and South African Tuberculosis Vaccine Initiative (SATVI) personnel; Deborah Abrahams; and Gregory Hussey.
Funding: Aeras was the sponsor of the MVA85A 020 Trial. BL is an employee of Aeras who participated in writing the manuscript. JRA is supported by the National Institutes of Health (K01 AI104411). No additional funding was obtained for this analysis of trial data.
Footnotes
Conflicts of Interest
MH reports grants to University of Cape Town from Aeras and Wellcome Trust, during the conduct of the study. TJS reports grants from Aeras, Wellcome Trust and Oxford-Emergent Tuberculosis Consortium during the study. BL is an employee of Aeras, the funder of the Phase 2 study from which the data for the manuscript were collected. All other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hawn TR, Day TA, Scriba TJ, et al. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev MMBR 2014; 78: 650–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clin Infect Dis Off Publ Infect Dis Soc Am 2015; 61Suppl 3: S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenum S, Selvam S, Mahelai D, et al. Influence of age and nutritional status on the performance of the tuberculin skin test and QuantiFERON-TB gold in-tube in young children evaluated for tuberculosis in Southern India. Pediatr Infect Dis J 2014; 33: e260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodha R, Mukherjee A, Saini D, et al. Role of the QuantiFERON®-TB Gold In-Tube test in the diagnosis of intrathoracic childhood tuberculosis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis 2013; 17: 1383–8. [DOI] [PubMed] [Google Scholar]
- 5.Moyo S, Isaacs F, Gelderbloem S, et al. Tuberculin skin test and QuantiFERON® assay in young children investigated for tuberculosis in South Africa. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis 2011; 15: 1176–81, i. [DOI] [PubMed] [Google Scholar]
- 6.Schopfer K, Rieder HL, Bodmer T, et al. The sensitivity of an interferon-γ release assay in microbiologically confirmed pediatric tuberculosis. Eur J Pediatr 2014; 173: 331–6. [DOI] [PubMed] [Google Scholar]
- 7.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 2011; 30: 694–700. [DOI] [PubMed] [Google Scholar]
- 8.Togun TO, Egere U, Gomez MP, et al. No added value of interferon-γ release to a prediction model for childhood tuberculosis. Eur Respir J 2016; 47: 223–32. [DOI] [PubMed] [Google Scholar]
- 9.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis 2004; 8: 392–402. [PubMed] [Google Scholar]
- 10.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ 2007; 334: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med 2011; 365: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva, Switzerland, 2015. [PubMed] [Google Scholar]
- 13.Luabeya KKA, Tameris MD, Geldenhuys HD, et al. Risk of Disease After Isoniazid Preventive Therapy for Mycobacterium tuberculosis Exposure in Young HIV-uninfected Children. Pediatr Infect Dis J 2015; 34: 1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahomed H, Hawkridge T, Verver S, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PloS One 2011; 6: e17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nenadić N, Kirin BK, Letoja IZ, Plavec D, Topić RZ, Dodig S. Serial interferon-γ release assay in children with latent tuberculosis infection and children with tuberculosis. Pediatr Pulmonol 2012; 47: 401–8. [DOI] [PubMed] [Google Scholar]
- 17.Lienhardt C, Fielding K, Hane AA, et al. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PloS One 2010; 5: e10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamford ARJ, Crook AM, Clark JE, et al. Comparison of interferon-gamma release assays and tuberculin skin test in predicting active tuberculosis (TB) in children in the UK: a paediatric TB network study. Arch Dis Child 2010; 95: 180–6. [DOI] [PubMed] [Google Scholar]
- 19.Zellweger J-P, Sotgiu G, Block M, et al. Risk Assessment of Tuberculosis in Contacts by IFN-γ Release Assays. A Tuberculosis Network European Trials Group Study. Am J Respir Crit Care Med 2015; 191: 1176–84. [DOI] [PubMed] [Google Scholar]
- 20.Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med 2015; 191: 584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med 2011; 183: 88–95. [DOI] [PubMed] [Google Scholar]
- 22.Geis S, Bettge-Weller G, Goetsch U, Bellinger O, Ballmann G, Hauri AM. How can we achieve better prevention of progression to tuberculosis among contacts? Eur Respir J 2013; 42: 1743–6. [DOI] [PubMed] [Google Scholar]
- 23.Haldar P, Thuraisingam H, Patel H, et al. Single-step QuantiFERON screening of adult contacts: a prospective cohort study of tuberculosis risk. Thorax 2013; 68: 240–6. [DOI] [PubMed] [Google Scholar]
- 24.Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet Lond Engl 2013; 381: 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Australia: R Foundation for Statistical Computing, 2016. http://www.R-project.org. [Google Scholar]
- 26.Ferrara G, Losi M, D’Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet Lond Engl 2006; 367: 1328–34. [DOI] [PubMed] [Google Scholar]
- 27.Kampmann B, Whittaker E, Williams A, et al. Interferon-gamma release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J 2009; 33: 1374–82. [DOI] [PubMed] [Google Scholar]
- 28.Tsolia MN, Mavrikou M, Critselis E, et al. Whole blood interferon-γ release assay is a useful tool for the diagnosis of tuberculosis infection particularly among Bacille Calmette Guèrin-vaccinated children. Pediatr Infect Dis J 2010; 29: 1137–40. [DOI] [PubMed] [Google Scholar]
- 29.Rose MV, Kimaro G, Nissen TN, et al. QuantiFERON®-TB gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PloS One 2012; 7: e37851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhagen LM, Maes M, Villalba JA, et al. Agreement between QuantiFERON®-TB Gold In-Tube and the tuberculin skin test and predictors of positive test results in Warao Amerindian pediatric tuberculosis contacts. BMC Infect Dis 2014; 14: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiappini E, Bonsignori F, Mazzantini R, et al. Interferon-gamma release assay sensitivity in children younger than 5 years is insufficient to replace the use of tuberculin skin test in western countries. Pediatr Infect Dis J 2014; 33: 1291–3. [DOI] [PubMed] [Google Scholar]
- 32.Garazzino S, Galli L, Chiappini E, et al. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J 2014; 33: e226–31. [DOI] [PubMed] [Google Scholar]
- 33.Bergamini BM, Losi M, Vaienti F, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 2009; 123: e419–24. [DOI] [PubMed] [Google Scholar]
- 34.Tebruegge M, de Graaf H, Sukhtankar P, et al. Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J Clin Microbiol 2014; 52: 2694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu Roy R, Sotgiu G, Altet-Gómez N, et al. Identifying predictors of interferon-γ release assay results in pediatric latent tuberculosis: a protective role of bacillus Calmette-Guerin?: a pTB-NET collaborative study. Am J Respir Crit Care Med 2012; 186: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Critselis E, Amanatidou V, Syridou G, et al. The effect of age on whole blood interferongamma release assay response among children investigated for latent tuberculosis infection. J Pediatr 2012; 161: 632–8. [DOI] [PubMed] [Google Scholar]
- 37.Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet Lond Engl 2016; 387: 2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai M, Joshi R, Dogra S, et al. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med 2006; 174: 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zwerling A, Benedetti A, Cojocariu M, et al. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PloS One 2013; 8: e54748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 2013; 188: 1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorman SE, Belknap R, Graviss EA, et al. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 2014; 189: 77–87. [DOI] [PubMed] [Google Scholar]
- 42.Middelkoop K, Mathema B, Myer L, et al. Transmission of Tuberculosis in a South African Community With a High Prevalence of HIV Infection. J Infect Dis 2015; 211: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banaei N, Gaur RL, Pai M. Interferon Gamma Release Assays for Latent Tuberculosis: What Are the Sources of Variability? J Clin Microbiol 2016; 54: 845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkinger CM, Dheda K, Pai M. Guidelines on interferon-γ release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2011; 17: 806–14. [DOI] [PubMed] [Google Scholar]