Abstract
Background
Antiretroviral therapy has surely increased the life expectancy of people living with HIV. However, long term complications like HIV associated sensory neuropathy has a negative impact on quality of life among people living with HIV (PLHIV). In Ethiopia, lack of data on magnitude of the burden and predictors of HIV associated sensory neuropathy in many resource limited setting has led to under diagnosis and eventually under management of HIV-SN. Hence, this study was set out to establish the burden of HIV-associated sensory neuropathy and, its association with demographic, health and clinical characteristics among people living with HIV in Ethiopia.
Methods
Cross-sectional study was conducted to assess the prevalence of HIV-associated sensory neuropathy and the associated factors among adult HIV patients at University of Gondar Teaching Hospital, Gondar, Ethiopia. Brief Peripheral Neuropathy Screening tool validated by AIDs Clinical trial group was used for screening HIV-associated sensory neuropathy. Data were analyzed descriptively and through uni- and multivariate logistic regression.
Results
In total 359 adult PLHIV with a mean age of 36.5± 9.07 years participated, their median duration of HIV infection was 60 months (IQR 36–84) and their median CD4 count 143cells/μL (IQR 69.5–201.5). Age above 40 years, anti-tuberculosis regimen, tallness, and exposure to didanosine contained antiretroviral therapy were found to be associated with HIV-associated sensory neuropathy (AOR 1.82, 1.84, 1.98 and 4.33 respectively).
Conclusions
More than half of the HIV patients who attended HIV care clinic at University of Gondar hospital during the study period were found to present with peripheral sensory neuropathy. Higher age, tallness, TB medication, and didanosine in ART were significantly associated with HIV-SN as screened by effective diagnostic (BPNS) tool.
Background
Life expectancy of people living with HIV has considerably increased after antiretroviral therapy (ART) era making HIV infection a chronic illness [1]. An estimated 5.5 HIV infected people among 6.6 million those who were saved from death between 1995 and 2012 live in low-middle income countries [2], [3]. Many debilitating complications related to HIV infection and ART regime are the recent spotlight [4]. HIV-associated sensory neuropathy (HIV-SN) is one of those complications among people living with HIV and the most common cause of chronic neuropathic pain, loss sensation, paraesthesia, foot ulcers, unemployment, poor, patient adherence to treatment, follow-ups, and poor quality of life [5–8]. The absence of neuro-regenerative therapies and proven ineffectiveness of analgesics in treatment of neuropathic pain among patients with HIV-SN clearly demonstrates the lack of effective treatments and the need for early diagnosis of patients at risk of HIV-SN [9–12].
Approximately 50% of the HIV- infected individuals receiving ART in developing countries reside in sub-Saharan [2], [3], [13]. Although there is an on-going decline in HIV-associated CNS disease and opportunistic infections in recent years, peripheral sensory neuropathies associated with HIV and ART remains to be high and frequent [14], [15].
The global estimate of HIV-SN varies widely from 1.73 to 69.4% in different HIV infected population. Studies done in resource-limited settings in sub-Saharan Africa, reported the risk of HIV-SN in ART patients with a range of estimates, from 30% to as high as 64% and the constraints in treatment availability in these settings [1], [5], [16]–[18]. These differences probably reflect the use of varying definitions of peripheral neuropathy and lack of use of a validated screening tool to diagnose HIV-SN [19], [20]. Hence, this study used Brief Peripheral Neuropathy Screening (BPNS) tool validated by AIDs Clinical trial group[21].
Moreover, the development of HIV-SN is likely to be influenced by various demographic, bio-clinical characteristics, among patients with HIV and some first-line drugs of ART. In Ethiopia, where the prevalence of adult HIV has been estimated to be 1.1% or nearly 1.2 million PLHIV [22], the burden of HIV-SN has not yet been estimated. Furthermore, the ART regime contains drugs some of which have been demonstrated in other studies to increase the risk of HIV-SN [23]–[26]. Therefore, this study was set to determine the burden of HIV-SN of the lower extremity, and the associated demographic, health and clinical characteristics, among PLHIV attending HIV care clinic at University of Gondar hospital (UOGH), North West Ethiopia.
Methods
Study design, setting, and participants
An institutional based cross-sectional descriptive study was carried out from February 2017 to June 2017. UOGH HIV care clinic is located in Gondar town, Ethiopia. UOGH is a 700 bedded governmental referral hospital that serves poor both urban and rural community. The HIV care and treatment started in 2003. As of 2016, the clinic cares for 11,127 HIV infected patients that is about 90 to 130 patients every day. This institution provides HIV testing including CD4 cell count monitoring every 6 months, health care consultation, counseling, and ART medication for those with CD4 count ≤ 350 cells/mm3 free of cost. Ethical approval was obtained from the Gondar University School of Medicine research and ethical review committee (SOM-2310706). Consent form was explained and written consent was obtained from all the participants.
Patients of both sexes attending UOGH HIV care clinic and aged ≥18 years were eligible for inclusion. All the participants signed written informed consent agreeing on participation and permitting to use their medical records/files. From the information, a total of 29 participants with additional medical conditions like; active tuberculosis (TB) patients and receiving treatment during study period (37.9%), cognitive disorders (3.4%), history of peripheral nerve injury (10.3%), chronic renal conditions (13.8%), active opportunistic infection (17.2%), leprosy (3.4), vitamin B12 deficiency (10.3), severe communication impairments (3.4), and were excluded from this study. Among the 29 excluded participants, 9 diabetic HIV patients with additional medical conditions like current TB, and renal disease were excluded.
Sample determination and sampling procedure
The sample size required for this study was determined using single population proportion formula by assuming the prevalence to be 35% from previous research [27], with a 95% confidence interval, and 5% marginal error. The derived sample size was n = 326. Accounting for an estimated non-response or refusal rate of 10%, the required sample size was n = 359.
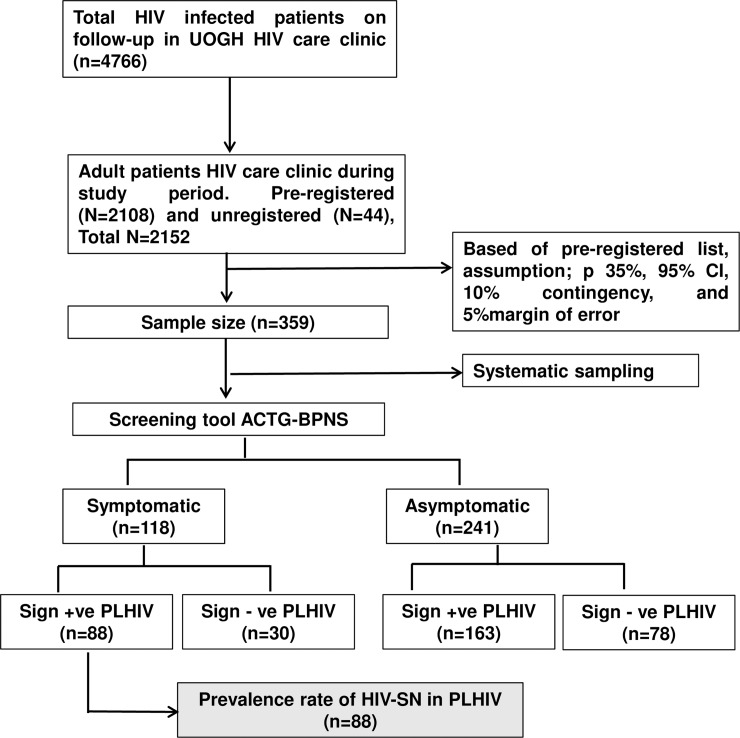
The study population was considered homogeneous and as indicated by the unpublished prior feasibility pilot study, a systematic random sampling was implemented. From the HIV-infected patients attending HIV care clinic each day during the study period, Kth patient was selected and the first person between 1 and K was randomly chosen, then taking every Kth number thereafter, where Kth was a sampling interval on the register list made for the day. The Kth (K = 6) was determined by dividing the total number of participants on the list (N), by the sample size. The procedure was repeated until estimated eligible sample size was reached; a sample of 359 was included from the total pre-registered number of 2108 for the month (Fig 1).
Fig 1. Study flow diagram.
UOGH-University of Gondar Hospital, BPNS-Brief Peripheral Neuropathy Screening, PLHIV-People living with HIV, HIV-SN-HIV associated sensory neuropathy.
Study procedure
Demographic and clinical data
Participants demographic, clinical and laboratory data were extracted from the medical chart and by using a purposely prepared structured questionnaire by trained nurses. Behavioural characteristics like alcohol consumption, smoking, khat-chewing, and physical activity were self-reported. Clinical staging based on WHO guidelines [28] were done as a part routine by clinicians. Physical measurements such as height and weight were measured and results of the screening were recorded onto a standardized form.
HIV-associated sensory neuropathy data
Prior to data collection, a two-day intensive training was given to data collectors (physiotherapist) by the principal investigator (KS) and clinical neurologist of UOGH following which a pre-test of data collection was carried out with 15 PLHIV. Brief peripheral neuropathy screening tool validated by the AIDS Clinical trial Group (ACTG) [20] was administered by a trained physiotherapist. In this screening, first patients were questioned to rate presence and severity of symptoms in feet and/or legs, using a scale of 1 (mild) to 10 (severe) for both legs separately. Symptoms included pain, aching, or burning; a sensation of ‘‘pins and needles” and numbness. The highest of the 3 scores were converted into a subjective PN grade, as follows: symptoms absent, grade 0; the score of 1–3, grade 1; the score of 4–6, grade 2; and score of 7–10, grade 3. Objective findings included in the BPNS were a loss of vibration perception and abnormal ankle deep tendon reflexes. Vibration perception was evaluated using a 128-Hz tuning fork, maximally struck and applied at the great toe distal interphalangeal joint of each foot.
Vibration sense was defined and graded as normal or grade 0 for vibration felt for >10seconds, as mild loss or grade 1 for vibration felt for 6–10 seconds, as moderate loss or grade 2 for vibration felt for < 5 seconds, and as severe loss or grade 3 for no feeling of vibration. Ankle reflexes were tested using a reflex hammer and graded as follow (0: absent, 1: hypoactive, 2: normal, 3: hyperactive, 4: clonus). Based on PNS validation study, the presence of HIV-SN was defined when someone had both subjective neuropathy (grade, >0) and ≥1 abnormal finding bilaterally on physical examination. A neurologist randomly examined (3 patients/day/30 days on random visits) the previously evaluated patients by the trained physiotherapist using BPNS to ensure accuracy and all were concordant.
Data management and analysis
The collected data was re-checked for its completeness and entered into EPI info version 7.0 (Centres for Disease Control and prevention, USA) and then transferred to IBM Statistical Package for Social Sciences (SPSS) version 20.0 for Windows for statistical analysis. Descriptive statistics were examined to determine the representativeness of study sample to the research setting population during the study period in percentages, frequency, and margin of error. Formulas proposed by Cochrane [29] were used to calculate the normal approximation frequency [S1 File]. The magnitude and severity in relation to socio-demographic and health variables were analysed using descriptive analysis. HIV-SN was defined by the presence of symptoms and at least an abnormal perception of vibration or abnormal ankle reflexes or both and expressed as percentage of the study population.
With HIV-SN as the dependent variable (categorized; present or absent), bivariate and multivariate binary logistic regression analyses were executed to establish the association with different independent variables. Chi-square or appropriate Fisher’s exact test was used to analyse prevalence distribution of HIV-SN and estimate its association with categorized independent variables. Independent variables included in the model were age, gender, height, weight, level of education, marital status, occupation, place of residence, duration of HIV, current and baseline CD4 count, ART regimen combination, duration on ART, and previous/history of TB regimen. Model fit was also assessed by described method [30]. Results were considered statistically significant when 95% confidence intervals not containing unity (equal to p-value <0.05). Initially, bivariate analyses were conducted and independent variables that were found statistically significant were included in multivariate analysis. Crude and adjusted Odds Ratios (OR) together with its 95% Confidence Intervals (95%CI) was reported. Potential confounding variables were entered into the model as covariates and variability in association checked. When clear subgroups seemed present in the data, significance testing (Pearson χ2) and, if appropriately sized subgroups per category remained, logistic regression were performed.
Results
Patient characteristics
A total of 359 adult PLHIV consented and participated in the study and this is more than 100% of the power calculated sample size (n = 326). Of the 359 participants majority of them 234 (65.2%) were females. The mean age, weight and height were 36.5 + 9.07 years, 55 + 10.4kg, 1.61 + 0.82m respectively. In addition to those who had registered (N = 2108), forty four unregistered HIV patients attended HIV care clinic for consultations during study period. The average age of the population (n = 2152) was 48 years. Nearly one fourth (26.5%) of them were underweight, almost two in five (40.4%) were married, almost a quarter (25.6%) of them were uneducated, 68(18.9%) were governmental employees, 314 (94.7%) were Orthodox Christian and the majority (94.7%) of them live in urban area. None of the patient had diabetes. Most of the subject (93.6%) self-reported no previous or current alcohol consumption and almost all (98.6%) subjects reported no history of smoking Table 1. Except Pre-HAART participants 3.6%, the frequency of the remaining demographic and clinical variables of the research sample fell within the total population range (95% CI) and the research sample was determined to be representative of the research setting population (S1 File).
Table 1. Distribution of HIV-SN and socio-demographic characteristics of 359 patients living with HIV [PLHIV] who attended HIV care clinic, North West Ethiopia, 2017.
| Variable | Sample total | HIV-SN | ||
|---|---|---|---|---|
| Present | Absent | |||
| n (%) | n (%) | n (%) | ||
| All participants | 359 (100%) | 88 (24.5%) | 271 (75.5%) | |
| Sex | Male | 125 (34.8) | 29 (8.1) | 96 (26.7) |
| Female | 234 (65.2) | 59 (16.4) | 175 (48.8) | |
| Age group (years) | < 30 | 74 (20.6) | 13 (3.6) | 61 (17) |
| 30–39 | 177 (49.3) | 38 (10.6) | 139 (38.7) | |
| 40–49 | 68 (18.9) | 18 (5.01) | 50 (13.9) | |
| 50–59 | 32 (8.9) | 14 (3.9) | 18 (5.01) | |
| ≥60 | 8 (2.2) | 5 (1.4) | 3 (0.8) | |
| Residence | Urban | 340 (94.5) | 82 (22.8) | 258 (71.7) |
| Rural | 19 (5.29) | 6 (1.7) | 13 (3.59) | |
| Marital status | Married | 145 (40.4) | 37 (10.3) | 108 (30.1) |
| Single | 45 (12.5) | 7 (1.9) | 38 (10.6) | |
| Divorced | 85 (23.7) | 15 (4.2) | 70 (19.5) | |
| Windowed | 71 (19.8) | 25 (7.0) | 46 (12.8) | |
| Separated | 13 (3.6) | 4 (1.1) | 9 (2.5) | |
| Level of Education | Uneducated | 92 (25.6) | 30 (8.4) | 62 (17.2) |
| Grade 1–6 | 70 (19.5) | 14 (3.9) | 56 (15.6) | |
| Grade 7–8 | 43 (12) | 6 (1.7) | 37 (10.3) | |
| Grade 9–10 | 59 (16.4) | 16 (4.5) | 43 (11.9) | |
| Grade 11–12 | 43 (12) | 12 (3.3) | 31 (8.7) | |
| College & above | 52 (14.5) | 10 (2.9) | 42 (11.6) | |
| Occupational status | Governmental | 68 (18.9) | 18 (5.0) | 50 (13.9) |
| Private employee | 53 (14.8) | 9 (2.5) | 44 (12.3) | |
| Daily labourer | 48 (13.5) | 15 (4.2) | 33 (9.3) | |
| House wife | 66 (18.4) | 14 (3.9) | 52 (14.6) | |
| Unemployed | 49 (13.6) | 12 (3.3) | 37 (10.3) | |
| Farmer | 17 (4.7) | 4 (1.1) | 13 (3.6) | |
| Other | 59 (16.4) | 16 (4.5) | 43 (11.9) | |
| Religion | Orthodox | 314 (87.5) | 79 (22.0) | 235 (65.5) |
| Muslim | 37 (10.4) | 6 (1.7) | 31 (8.7) | |
| Protestant | 8 (2.2) | 2 (0.5) | 6 (1.7) | |
| Others | 1 (0.3) | 0 (0.0) | 1 (0.3) | |
| Alcoholic history | Yes | 23 (6.4) | 4 (1.1) | 19 (5.3) |
| No | 336 (93.6) | 84 (23.4) | 252 (70.2) | |
The median duration of HIV infection since diagnosed as on 31st March 2015 was 60 months (IQR; 36–84).The use of highly active antiretroviral therapy (HAART) was also 60 months (IQR; 28–84). The median baseline and recent CD4 cell count were 143 cell/mm3 (IQR; 69.5–201.5) and 368 cell/mm3 (IQR; 246–546.8) respectively. 346 (96.4%) were on HAART, 158 (44%) were on a regimen containing AZT+3TC+NVP and majority of the subject 326 (90.8%) were in the clinical stage between I and II. Of the 359 patients, 160 (44.6%) participants reported a history of anti-TB treatment and 157 (43.7%) reported being on both anti TB and ART regimen in the past Table 2.
Table 2. Distribution of HIV-SN and clinical characteristics of 359 patients living with HIV [PLHIV] for adults aged 18 and above years, North West Ethiopia, 2017.
| Variable | Sample total | HIV-SN | |
|---|---|---|---|
| Present | Absent | ||
| N (%) | n (%) | n (%) | |
| All participants | 359 (100%) | 88 (24.5%) | 271 (75.5%) |
| Baseline CD4 cell count | |||
| > 200 cell/mm3 | 94 (26.2) | 30 (8.36) | 58 (16.16) |
| ≤ 200 cell/mm3 | 263 (73.3) | 58 (16.16) | 205 (57.10) |
| Recent CD4 cell count | |||
| ≥ 350 cell/mm3 | 195 (54.3) | 49 (13.65) | 146 (40.67) |
| 200–350 cell/mm3 | 100 (27.9) | 21 (5.85) | 79 (22.01) |
| ≤ 200 cell/mm3 | 61 (17) | 17 (4.74) | 44 (12.26) |
| WHO disease stages | |||
| Stage I and II | 326 (90.8) | 80 (22.28) | 246 (68.52) |
| Stage III and IV | 33 (9.19) | 8 (2.23) | 25 (6.96) |
| History of anti TB treatment | |||
| Yes | 160 (44.6) | 51 (14.21) | 109 (30.36) |
| No | 199 (55.4) | 37 (10.31) | 162 (45.13) |
| Type of HAART regimen | |||
| AZT+3TC+NVP | 158 (44) | 35 (9.75) | 123 (34.26) |
| AZT+3TC+EFV | 12 (3.3) | 5 (1.39) | 7 (1.95) |
| TDF+ 3TC+EFV | 107 (29.8) | 23 (6.41) | 84 (23.4) |
| TDF+ 3TC+NVP | 43 (12) | 13 (3.62) | 30 (8.36) |
| ABC+DDI+LPV/R | 15 (4.2) | 10 (2.79) | 5 (1.39) |
| Others regimen | 11 (3.1) | 1 (0.28) | 10 (2.795) |
| Pre-HAART | 13 (3.6) | 1 (0.28) | 12 (3.34) |
HIV-associated sensory neuropathy
Using BPNS tool, the prevalence of neuropathic symptoms was found to be 32.9% (118/359) and the most common were the sensation of aching and burning type of pain on leg/ feet (Table 3). Among patients who self-reported symptoms, 32.2% had both diminished ankle reflexes and impaired perceptions of tuning fork vibration, 28.8% had only diminished ankle reflex, 13.5% (16/118) had only abnormal perception of vibration, 24.6% (30/118) had neither abnormal perception of vibration nor diminished reflex.
Table 3. Prevalence of sign and symptoms consistent with HIV-SN among adult HIV patient 2017, Ethiopia.
| Symptoms (at legs or feet) | n (%) | |
|---|---|---|
| Pain, aching or burning | 95(26.5) | |
| Pins and needles | 24(6.7) | |
| Numbness (lack of sensation) | 49(13.6) | |
| Asymptomatic | 241(67.1) | |
| Ankle reflex | Right leg | Left leg |
| Grade 0: absent reflex | 28(7.8) | 28 (7.8) |
| Grade 1: hypoactive | 169 (47.1) | 167(46.5) |
| Grade 2: normal | 152 (42.3) | 153(42.6) |
| Grade 3: hyperactive | 10 (2.8) | 11(3.1) |
| Grade4: clonus | 0(0) | 0(0) |
| Perception of tuning fork vibration | Right leg Left leg | |
| Grade 0: max. perception > 10 sec | 216 (60.2) | 118 (52.4) |
| Grade 1: perception for 6–10 sec | 113 (31.5) | 145 (40.4) |
| Grade 2: perception for ≤ 5 sec | 22 (6.1) | 19 (5.3) |
| Grade 3: no perception | 8 (2.2) | 6 (1.7) |
Among those without symptoms, 163 (68%) at the least reported either the impaired perception of vibration or diminished ankle reflex, 49 (20.3%) had both diminished ankle reflex and abnormal vibration perception, 76 (31.5%) had only diminished reflex, 38 (15.7%) had only abnormal vibration perception. The prevalence of HIV-SN according to ACTG-BPNS definition among the PLHIV was 24.5% (88/359) of the total study samples Table 1. The median duration of symptoms reported was 12 months (IQR; 2–42). Among asymptomatic, 21% of participants were found to be normal using BPNS examination.
Patient characteristics predicting prevalence of HIV-SN
HIV-SN in people living with HIV was significantly associated with patient characteristics such as advanced age, height, history of anti-tuberculosis treatment and didanosine (DDI) contain in HAART regimen. Thus, in this study HIV-infected patients with advanced age (> 40 years old) were nearly two times more likely to develop HIV-SN than younger (< 40 years old) patients [AOR = 1.82, 95% CI: 1.05,3.13], patients with history of anti- tuberculosis regimen were nearly two twice more likely to develop HIV-SN than no history of antituberculosis treatment [AOR = 1.84, 95% CI: 1.09,3.10], patients with increased height(> 170 cm) were very nearly two folds more likely to develop HIV-SN than reduced height (< 170 cm) [AOR = 1.98, 95% CI: 1.01,3.91], and DDI contain in HAART regimen were more than four times more likely to develop HIV-SN in HIV-infected patients [AOR = 4.33, 95% CI: 1.34,14.00] Table 4. Hence, age, height, history of TB medication, and DDI in HAART were found to be major predictors of HIV-SN in this study.
Table 4. Univariate and multivariate analysis for the associated and predicting demographic and health status characteristics to HIV-SN, 2017, Ethiopia.
| Variables | HIV-SN | Univariate | p | Multivariate | p | |
|---|---|---|---|---|---|---|
| Yes | No | COR (95% CI) |
AOR (95%,CI) |
|||
| Age | ||||||
| ≤40 years | 51 | 200 | 1(ref) | 1(ref) | ||
| >40 years | 37 | 71 | 2.04(1.23,3.37) | 0.005 | 1.82(1.05,3.13) | 0.031* |
| Height | ||||||
| ≤170 cm | 68 | 240 | 1(ref) | 1(ref) | ||
| >170 cm | 20 | 31 | 2.27(1.22,4.24) | 0.01 | 1.98(1.01,3.91) | 0.047* |
| History of anti TB treatment | ||||||
| No | 37 | 162 | 1(ref) | 1(ref) | ||
| Yes | 51 | 109 | 2.04(1.25,3.33) | 0.004 | 1.84(1.09,3.10) | 0.021* |
| Duration with HIV since diagnosis. | ||||||
| ≤5 years | 38 | 146 | 1(ref) | 1(ref) | ||
| >5years | 50 | 125 | 1.53(0.94,2.49) | 0.082 | 1.24(0.43,3.5) | 0.68 |
| Duration of ART | ||||||
| ≤5 years | 43 | 152 | 1(ref) | 1(ref) | ||
| >5years | 44 | 107 | 1.45(0.89,2.36) | 0.133 | 1.01(0.35,2.9) | 0.974 |
| DDI contain in HAART regimen. | ||||||
| No | 77 | 254 | 1(ref) | 1(ref) | ||
| Yes | 10 | 5 | 6.59(2.18,19.88) | 0.001 | 4.33(1.34,14.00) | 0.014* |
*Denotes significant association of characteristics with HIV-SN in multivariate model, AOR- Adjusted odds ratio, CI -Confidence Interval, COR-Crude odds ratio
Discussion
The BPNS as an screening tool in examining HIV-SN in HIV-infected patients has been validated using physiologic (quantitative sensory threshold testing) and pathologic (epidermal nerve fiber density) testing as the gold standards [20]. Definition of HIV-SN consistent with BPNS tool is presence of both subjective and objective findings. However, the criteria of BPNS that a patient must have both self-reported neuropathic symptoms and clinical signs to be classified as HIV-SN may eventually result in omission of some patients with mild HIV-SN [31]. With this diagnostic criterion of BPNS tool, there are always chances for substantial underestimation of peripheral neuropathy (PN) [32].
Using BPNS screening and definition of HIV-SN used in the validation study, the prevalence of HIV-associated sensory neuropathy in our study participants was 24.5%. A study which used clinical criteria (signs and symptoms of neuropathy) along with more standardized electrophysiology studies reported that two-thirds of HIV-SN is subclinical [33]. This means even if an asymptomatic patient present with abnormal clinical signs relevant to HIV-SN may not fulfill the diagnostic criteria for HIV-SN as per the BPNS tool. Similarly, in our study 45.7% (163/359) of asymptomatic patients had an abnormal perception of clinical signs relevant to HIV-SN. Some authors [4], [33], [34] categorize this group as asymptomatic PN or early HIV-SN and also suggest that they are more likely to become symptomatic when exposed to risk factors.
However, the most striking finding in this study is, if we are to combine both the group of patients identified with HIV-SN as per ACTG-BPSN definition and asymptomatic PN with relevant clinical sign, the prevalence of PN in our study will alarmingly rise to 70.2%. In addition, a further 10% PLHIV reported neuropathic pain with absences of objective findings indicating early HIV-SN and they are more likely to become symptomatic when challenged with other risks for HIV-SN. Which suggest that the prevalence will expand to near 80% and only a mere 20% patients seems to be unaffected with normal examination using BPNS tool. This method of identifying PN will surely reduce the chances of underestimation by BPNS tool and will improve chances of early detection of PN among HIV in the low resource setups like UOGH.
A study done among rural dwellers in Rwanda [15] reported a prevalence of 40% peripheral neuropathy from one rural district hospital. But, the Rwandan study used the Subjective Peripheral Neuropathy Screen (SPNS) that evaluates HIV-SN subjectively, unlike BPSN that evaluates signs objectively in addition to the subjective assessment. Furthermore, the prevalence found in this study is lower than studies done in Africa, such as Jimma, Ethiopia 34.6%, Nigeria 39% and Mombasa, Kenya 36% [18], [25], [27], [35]. A similar prevalence was found in studies done in Douala, Cameron 21% [34] and in Tanzania 20.9% [36]. The possible reasons might be due to differences in methodologies, characteristics of study population, exposure to risk factors, sample size, intervening variables and unidentifiable environmental factors. Moreover, the prevalence of HIV-SN reported in this study falls lower than the range of 30 to 60% reported elsewhere. Even so, it is important to note that as much as 85% of HIV-infected patients in Ethiopia are on ART and aging (2) and some ARTs regimen along with other coexisting-risk factors are likely to influence the development of HIV-SN. Eventually, the prevalence of HIV-SN in Ethiopia is more likely to continue increasing. In contrast, factors like less ART-naïve patients, better managed HIV, and frequent screening for HIV-SN may result in decline of HIV-SN rate. Thus efforts are needed to frequently identify the prevalence of HIV-SN so as to develop cost-effective strategies to curb the burden.
Similar to other studies [15], [32], [37] our study identified higher age (> 40 years) to be a predictor variable for developing HIV-SN. With the rapidly aging HIV population and improved life expectancy due to successful therapy may further increase the burden of HIV-SN and amplify the challenges in HIV therapeutics.
Our study confirms the clinical notion that HIV-SN in HIV infected patients is height dependent, with a small, but the consistently significant HIV-SN risk in taller HIV-infected patients. The height of > 170 cm was two times more likely to develop HIV-SN when compared with patients <170 cm [AOR = 2.144, 95% CI: 1.076, 4.269], which is consistent with other sub-Saharan studies [16], [34], [36], [38]. Unlike other studies [17], this study demonstrated no association between gender (men), weight and HIV-SN. Gender association deserves concern in future studies done in Ethiopia.
Low enrolment of PLHIV with a known history of diabetes, history of alcohol consumption and smoking had limited the power to evaluate their association and possible interaction effect of diabetes mellitus with HIV infection in our study where several other studies have suggested a strong association. Moreover, in resource-limited settings, diabetes is often not diagnosed early and the possibility of undiagnosed diabetes among PLHIV prevails. History of anti TB medication and concurrent use of ART was found to be strongly associated to HIV-SN in our study with isoniazid being the most common anti TB drug administered. Though isoniazid exposure is reported as risk factor elsewhere [34], its sole association with HIV-SN is beyond the scope of the data set of this study.
In contrast to many studies [23], [24], [27], this study found out that, HIV-SN with or without symptom did not associate with low CD4 counts or greater WHO stage of the disease. Limited enrolment of patients with low CD4 counts and the criteria to commence ART had been revised to CD4 count < 350 cells/mm3might be reasons behind it. Previous studies [25], [26] also reported that after successful therapy neither CD4 count nor viral load suppression decreased the odds of developing peripheral neuropathy. Several studies [10], [17], [39] postulate D-drugs related mitochondrial toxicity as a cause of HIV-SN. The majority of our study population were on HAART for more than 6 years and previous exposure to stavudine may also have contributed.
A few limitations can be mentioned to benefit future research and the findings must be considered in the light of these limitations; this study includes its definition of SN which was chosen based on previous work validating the ACTG-BPNS but resulted in patients who had isolated neuropathic symptoms or asymptomatic signs being classified as neuropathy free. We were not able to distinguish DSP and ATN due to the limited enrolment of the pre-ART patient and a longitudinal design, unlike our cross-sectional design would enable us to determine temporal relationships. Furthermore, the non-availability of golden standard electrophysiological devices limited diagnostic accuracy. Despite these limitations, this study included a relatively larger representative sample of the clinical population, Ethiopia, which enhances external validity of results and provides a well-powered insight and estimate of prevalence of HIV-SN in resource limited settings.
Conclusions
HIV-SN was found to be highly prevalent among HIV infected patient at the University of Gondar Hospital, Ethiopia As the life expectancy of people with HIV increases the prevalence of HIV-SN will also increase, making it a major clinical issue and burden particularly in resource limited settings. It is known that managing neuropathic pain is a challenging problem in HIV infected. Hence, early detection of HIV-SN permits patient education, control of modifiable risk and confounding factors, and better chances of improvement with added choices of managements. Simple and validated neurological assessment tools to detect early HIV-SN or those at risk among all HIV patients should be used routinely. We found that the occurrence of HIV-SN increases with advanced age and height thereby necessitating early diagnosis in these risk groups. Physician’s drug choice in HAART and the anti-tuberculosis regime shall consider the role of these drugs and possible association with HIV-SN especially in Sub-Saharan terrains.
Ethical approval
Ethical approval was secured from the Ethical Review Committee of the College of Medicine and Health Sciences, University of Gondar, Ethiopia.
Supporting information
S1 File Legend Sample representativeness.
(PDF)
Acknowledgments
Firstly, we would like to express our deepest gratitude to the University of Gondar for ethical approval and funding. Our gratitude and appreciation go to data collectors, adults living with HIV attending HIV care clinic who participated in this study. We are also grateful to all hospital authorities and HIV care clinic staffs for their cooperation.
Abbreviations
- ACTG
AIDs Clinical trial group
- AOR
Adjusted odds ratio
- ART
antiretroviral therapy
- ATN
antiretroviral therapy toxic neuropathy
- BPNS
Brief Peripheral Neuropathy Screening
- cART
combination antiretroviral therapy
- CI
Confidence Interval
- DDI
didanosine
- HIV-SN
HIV associated sensory neuropathy
- HIV-DSP
HIV associated distal sensory polyneuropathy
- HAART
highly active antiretroviral therapy
- IQR
Inter-Quartile Range
- PLHIV
People living with HIV
- PN
Peripheral neuropathy
- SN
Sensory neuropathy
- TB
Tuberculosis
Data Availability
The datasets supporting the conclusions of this article are available in the GitHub repository in https://github.com/Asmare/HIV-AIDS-sensory-neuropathy, The data is sole property of UOG, Ethiopia; contains patient confidential information and is also part of on-going project. Additional data could be obtained upon formal request from corresponding author or Academic coordinator, Dept of Physiotherapy, CMHS, UOG, physiomelaku2008@gmail.com.
Funding Statement
The total budget and other necessary equipment were totally funded by the University of Gondar. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen H. et al. , “Peripheral neuropathy in ART-experienced patients: prevalence and risk factors,” J. Neurovirol., vol. 19, no. 6, pp. 557–564, 2013. 10.1007/s13365-013-0216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.J. U. N. P. on HIV/AIDS, UNAIDS Report on the global AIDS epidemic. December 2013. 2013.
- 3.Colvin M, Gorgens-Albino M, Kasedde S. Analysis of HIV prevention response and modes of HIV transmission: the UNAIDS-GAMET-supported synthesis process. ND. http://www.unaidsrstesa.org/files/MoT_0.pdf (last accessed 20 October 2010). 2008.
- 4.Chai N. C. and McArthur J. C., “HIV and peripheral neuropathy,” Chronic Pain HIV Pract. Approach A, pp. 51–62, 2016. [Google Scholar]
- 5.Pettersen J. A. et al. , “Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: Protease inhibitor–mediated neurotoxicity,” Ann. Neurol., vol. 59, no. 5, pp. 816–824, 2006. 10.1002/ana.20816 [DOI] [PubMed] [Google Scholar]
- 6.Evans S. R. et al. , “HIV peripheral neuropathy progression: protection with glucose-lowering drugs?,” J. Neurovirol., vol. 18, no. 5, pp. 428–433, 2012. 10.1007/s13365-012-0119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jember G., Melsew Y. A., Fisseha B., Sany K., Gelaw A. Y., and Janakiraman B., “Peripheral Sensory Neuropathy and associated factors among adult diabetes mellitus patients in Bahr Dar, Ethiopia,” J. Diabetes Metab. Disord., vol. 16, no. 1, p. 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balamurugan J. and Raja P. A., “Bilateral object exploration training to improve the hand function in stroke subjects,” Int. J. Med. Res. Health Sci., vol. 2, no. 3, pp. 479–486, 2013. [Google Scholar]
- 9.Chou R. et al. , “Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain,” J. Pain, vol. 10, no. 2, p. 113–130. e22, 2009. 10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson D. M., Brown S., Tobias J., and N.-4010 C. S. Group, “Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy,” Neurology, vol. 70, no. 24, pp. 2305–2313, 2008. 10.1212/01.wnl.0000314647.35825.9c [DOI] [PubMed] [Google Scholar]
- 11.Dunn K. M. et al. , “Opioid Prescriptions for Chronic Pain and OverdoseA Cohort Study,” Ann. Intern. Med., vol. 152, no. 2, pp. 85–92, 2010. 10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Zhang T., and Ho W.-Z., “Opioids and HIV/HCV infection,” J. Neuroimmune Pharmacol., vol. 6, no. 4, p. 477, 2011. 10.1007/s11481-011-9296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granich R. et al. , “Trends in AIDS deaths, new infections and ART coverage in the top 30 countries with the highest AIDS mortality burden; 1990–2013,” PLoS One, vol. 10, no. 7, p. e0131353, 2015. 10.1371/journal.pone.0131353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theroux N., Phipps M., Zimmerman L., and Relf M. V., “Neurological complications associated with HIV and AIDS: clinical implications for nursing,” J. Neurosci. Nurs., vol. 45, no. 1, pp. 5–13, 2013. 10.1097/JNN.0b013e318275b1b2 [DOI] [PubMed] [Google Scholar]
- 15.Biraguma J. and Rhoda A., “Peripheral neuropathy and quality of life of adults living with HIV/AIDS in the Rulindo district of Rwanda,” SAHARA-J J. Soc. Asp. HIVAIDS, vol. 9, no. 2, pp. 88–94, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Saylor D. et al. , “Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda,” Neurology, vol. 89, no. 5, pp. 485–491, 2017. 10.1212/WNL.0000000000004136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenas-Pinto A. et al. , “Peripheral neuropathy in HIV patients in sub-Saharan Africa failing first-line therapy and the response to second-line ART in the EARNEST trial,” J. Neurovirol., vol. 22, no. 1, pp. 104–113, 2016. 10.1007/s13365-015-0374-7 [DOI] [PubMed] [Google Scholar]
- 18.Lifson A. R. et al. , “Prevalence and factors associated with use of khat: a survey of patients entering HIV treatment programs in Ethiopia,” Addict. Sci. Clin. Pract., vol. 12, no. 1, p. 3, 2017. 10.1186/s13722-016-0069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkataramana A. B., “Diagnostic utility of the subjective peripheral neuropathy screen in HIV-infected persons with peripheral sensory polyneuropathy,” AIDS Read., pp. 341–341, 2005. [PubMed] [Google Scholar]
- 20.Cherry C. L., Wesselingh S. L., Lal L., and McArthur J. C., “Evaluation of a clinical screening tool for HIV-associated sensory neuropathies,” Neurology, vol. 65, no. 11, pp. 1778–1781, 2005. 10.1212/01.wnl.0000187119.33075.41 [DOI] [PubMed] [Google Scholar]
- 21.Mehta S. A. et al. , “Implementation of a validated peripheral neuropathy screening tool in patients receiving antiretroviral therapy in Mombasa, Kenya,” Am. J. Trop. Med. Hyg., vol. 83, no. 3, pp. 565–570, 2010. 10.4269/ajtmh.2010.09-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.“Ethiopia:Analytical summary—HIV/AIDS—AHO.” [Online]. Available: http://www.aho.afro.who.int/profiles_information/index.php/Ethiopia:Analytical_summary_-_HIV/AIDS. [Accessed: 20-Nov-2017].
- 23.Menezes C. N., Maskew M., Sanne I., Crowther N. J., and Raal F. J., “A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects,” BMC Infect. Dis., vol. 11, no. 1, p. 244, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modayil R. R. et al. , “Adverse drug reactions to antiretroviral therapy (ART): an experience of spontaneous reporting and intensive monitoring from ART centre in India,” Pharmacoepidemiol. Drug Saf., vol. 19, no. 3, pp. 247–255, 2010. 10.1002/pds.1907 [DOI] [PubMed] [Google Scholar]
- 25.Hawkins C., Achenbach C., Fryda W., Ngare D., and Murphy R., “Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya,” JAIDS J. Acquir. Immune Defic. Syndr., vol. 45, no. 3, pp. 304–310, 2007. 10.1097/QAI.0b013e318050d66c [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann C. J. et al. , “Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events,” AIDS Lond. Engl., vol. 22, no. 1, p. 67, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shurie J. S. and Deribew A., “Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals.,” Ethiop. Med. J., vol. 48, no. 2, pp. 85–93, 2010. [PubMed] [Google Scholar]
- 28.W. H. Organization, “WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children,” 2007.
- 29.Cochran W. G., Sampling Techniques: 3d Ed Wiley New York, 1977. [Google Scholar]
- 30.Evans S. and Li L., “A comparison of goodness of fit tests for the logistic GEE model,” Stat. Med., vol. 24, no. 8, pp. 1245–1261, 2005. 10.1002/sim.2023 [DOI] [PubMed] [Google Scholar]
- 31.Cherry C. L. et al. , “Age and height predict neuropathy risk in patients with HIV prescribed stavudine,” Neurology, vol. 73, no. 4, pp. 315–320, 2009. 10.1212/WNL.0b013e3181af7a22 [DOI] [PubMed] [Google Scholar]
- 32.Evans S. R. et al. , “Peripheral neuropathy in HIV: prevalence and risk factors,” AIDS Lond. Engl., vol. 25, no. 7, p. 919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skopelitis E. E. et al. , “Distal sensory polyneuropathy in HIV-positive patients in the HAART era: an entity underestimated by clinical examination,” Int. J. STD AIDS, vol. 17, no. 7, pp. 467–472, 2006. 10.1258/095646206777689062 [DOI] [PubMed] [Google Scholar]
- 34.Luma H. N., Tchaleu B. C. N., Doualla M. S., Temfack E., Sopouassi V. N. K., and Mapoure Y. N., “HIV-associated sensory neuropathy in HIV-1 infected patients at the Douala General Hospital in Cameroon: a cross-sectional study,” AIDS Res. Ther., vol. 9, no. 1, p. 35, 2012. 10.1186/1742-6405-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshinaike O. et al. , “Influence of age and neurotoxic HAART use on frequency of HIV sensory neuropathy,” AIDS Res. Treat., vol. 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullin S., Temu A., Kalluvya S., Grant A., and Manji H., “High prevalence of distal sensory polyneuropathy in antiretroviral‐treated and untreated people with HIV in Tanzania,” Trop. Med. Int. Health, vol. 16, no. 10, pp. 1291–1296, 2011. 10.1111/j.1365-3156.2011.02825.x [DOI] [PubMed] [Google Scholar]
- 37.Kamerman P. R., Wadley A. L., and Cherry C. L., “HIV-associated sensory neuropathy: risk factors and genetics,” Curr. Pain Headache Rep., vol. 16, no. 3, pp. 226–236, 2012. 10.1007/s11916-012-0257-z [DOI] [PubMed] [Google Scholar]
- 38.Wadley A. L., Cherry C. L., Price P., and Kamerman P. R., “HIV neuropathy risk factors and symptom characterization in stavudine-exposed South Africans,” J. Pain Symptom Manage., vol. 41, no. 4, pp. 700–706, 2011. 10.1016/j.jpainsymman.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein K. A. et al. , “Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort,” Clin. Infect. Dis., vol. 40, no. 1, pp. 148–157, 2005. 10.1086/426076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 File Legend Sample representativeness.
(PDF)
Data Availability Statement
The datasets supporting the conclusions of this article are available in the GitHub repository in https://github.com/Asmare/HIV-AIDS-sensory-neuropathy, The data is sole property of UOG, Ethiopia; contains patient confidential information and is also part of on-going project. Additional data could be obtained upon formal request from corresponding author or Academic coordinator, Dept of Physiotherapy, CMHS, UOG, physiomelaku2008@gmail.com.