Abstract
Topiramate (TMP) is a broad-spectrum anticonvulsant drug used to treat a wide variety of seizure disorders, for migraine prophylaxis, and for many other indications. An important side effect of TMP is metabolic acidosis, which is mediated by renal tubular defects. TMP inhibits carbonic anhydrase, an enzyme that is necessary for acid handling in the proximal renal tubule. Patients can present with asymptomatic serum electrolyte derangements, acute change in mental status, hyperventilation, cardiac arrhythmias, or other sequelae of metabolic acidosis and associated respiratory compensation. If taken chronically, TMP can cause renal stone formation, bone mineralization defects, and several other effects secondary to changes in serum and urine pH and electrolytes. There is no well-studied way to prevent metabolic acidosis in patients taking TMP, but physicians should be vigilant when prescribing this drug to patients with the history of renal diseases and other comorbidities, and aware of this potential etiology of metabolic acidosis. We present a literature review of the underlying mechanisms involved in the development of renal tubular acidosis secondary to TMP and its clinical consequences.
Keywords: renal tubular acidosis, topiramate
Introduction and background
Topiramate (TMP) is an anticonvulsant drug used to treat epilepsy in adults and children. According to American Academy of Neurology (AAN) guidelines, TMP can be used as initial therapy for newly diagnosed focal and mixed seizure disorders [1]; it is also used as monotherapy for refractory generalized tonic-clonic seizures and focal seizures in adults and children [2]. TMP has also been used for migraine prophylaxis, weight reduction, among other indications [3-5]. TMP is primarily excreted through the kidney and can cause renal tubular acid-base disturbances. With the wide and increasing use of this drug and its potential for renal side effects, it is imperative to consider a patient’s comorbidities before prescribing TMP and to be aware of possible drug toxicities when evaluating electrolyte derangements in patients taking this drug.
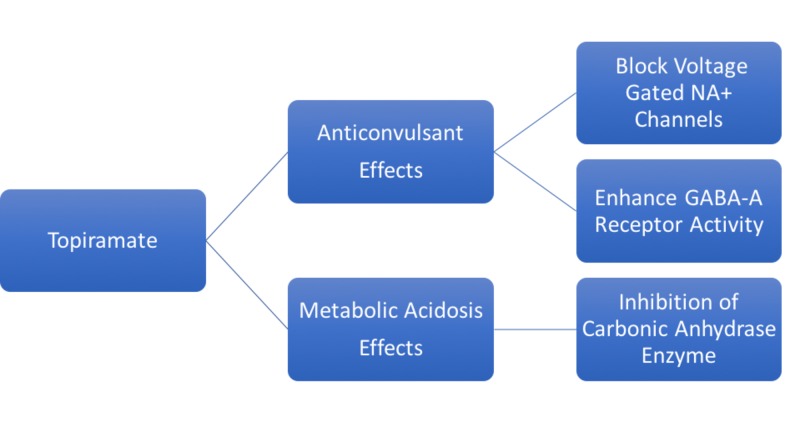
TMP is approved by the US Food and Drug Administration (FDA) for use in adults and children two years of age or older for epilepsy monotherapy or adjunctive therapy [3]. It is also approved for migraine prophylaxis [4], and for weight reduction in combination with phentermine [5]. Most patients achieve 90% of the maximum plasma concentration within two hours of oral administration [6]. TMP is unlikely to displace highly protein-bound drugs in the plasma, hence limiting its drug interactions. The predominant route of drug elimination is renal excretion, accounting for about 51% of TMP elimination [6]. The therapeutic activities and side effects of TMP are mediated by several different mechanisms of action (Figure 1).
Figure 1. Mechanisms of Action of Topiramate.
Review
Metabolic acidosis occurs when there is an imbalance between the body’s production of H+ ions, and the kidneys’ ability to excrete H+ ions and resorb HCO3-. Metabolic acidosis is classified as either high anion gap (caused by increased production or ingestion of acid and impaired renal acid excretion) or normal anion gap (caused by the loss of bicarbonate from the kidney or gastrointestinal tract).
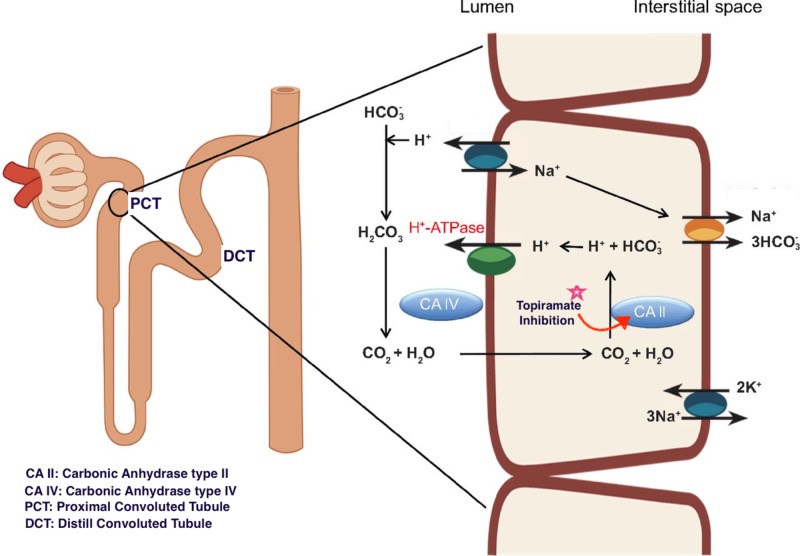
Renal tubular defects can result in metabolic acidosis due to impaired renal H+ excretion or HCO3- resorption. Carbonic anhydrase (CA) is an enzyme that catalyzes the formation of H+ and HCO3- from CO2 and H2O, which is necessary for renal excretion of H+ and resorption of HCO3- in the proximal convoluted tubule (PCT). Of the various types of CA, CA type II (CA-II) predominates in the human kidney, comprising about 95% of the total CA, while the remaining 5% consists of CA-IV and CA-XIII [7]. Supuran et al. reported that TMP is a potent inhibitor of CA-II and CA-XIII, and a medium potency inhibitor of CA-IV [8]. Because of its CA-inhibitory activity, especially against CA-II, TMP can impair H+ excretion and HCO3- absorption in the PCT, leading to increased delivery of HCO3- in the distal portion of the nephron and induce a normal anion gap metabolic acidosis (Figure 2).
Figure 2. Development of Proximal Renal Tubular Acidosis.
Distal (Type 1) renal tubular acidosis (RTA) is characterized by impaired acid secretion from the collecting tubules, leading to an inability to secrete acid, and resulting in progressive H+ ion accumulation which manifests clinically as decreased serum HCO3- [9]. Proximal (Type 2) RTA is caused by an inability to reabsorb HCO3- from the PCT. Since the PCT reabsorbs 85 to 90% of the filtered load of HCO3-, inhibition of CA-II by TMP can lead to Type 2 RTA. Type 2 RTA can also result from Fanconi syndrome, a generalized resorption defect affecting HCO3-, glucose, phosphate, amino acids, and tubular proteins. The differences between proximal and distal RTA are shown in Table 1.
Table 1. Type 1 and Type 2 Renal Tubular Acidosis (RTA).
| Distal (Type 1) RTA | Proximal (Type 2) RTA | Study Ref. | |
| Pathophysiology | Failure to secrete H+ and reabsorb K+ Ions | Failure to reabsorb HCO3 | |
| Location | Collecting & distal tubules | Proximal Tubules | |
| Urine pH | > 6.0 | <5.5 | 13 |
| Urine HCO3- | Normal | Increased | 13 |
| Fractional Excretion of HCO3- | <5% | >15% | 13 |
| Serum Potassium | Hypokalemia | Hypokalemia |
There are several reports in the literature of an association between TMP and hyperchloremic normal anion gap metabolic acidosis with an alkaline urine and positive urine anion gap (due to increased urine HCO3-) [10, 11]. In many cases, patients may be asymptomatic. Burmeister et al. [12] reported a case of severe metabolic acidosis in a 46-year-old woman who was prescribed TMP, 100 mg per day for three months; Ozer and Altunkaya described a similar presentation in a 58-year-old man [11]. Other studies have demonstrated the role of TMP in lowering plasma HCO3-; in a cross-sectional study, Welch et al. compared 32 TMP-treated subjects and 50 healthy volunteers and concluded that TMP-treated subjects had significantly lower serum total carbon dioxide content [13]. In another study, there was a 2.0 kg mean decline in weight after three months’ use of TMP [14].
Metabolic acidosis can present with complications due to respiratory compensation, notably hyperventilation, fatigue, altered mental status; more severe cases can lead to cardiac arrhythmia and coma. Stowe et al. [15] described a case of TMP-induced metabolic acidosis in a 20-year-old man presenting with disorientation, somnolence, agitation, and headache for two weeks.
Renal tubular acidosis caused by TMP can lead to decreased urine citrate concentration; hypocitraturia combined with decreased urine acidification in the distal convoluted tubules can contribute to the formation of calcium phosphate stones [16, 17]. The risk of the renal stone formation increases to 10 folds with the use of TMP. Warner et al. [18] in a study involving four subjects, reported that urinary citrate levels decreased significantly and rapidly after the start of TMP therapy and continued to decrease with escalating doses. Other common sides in adults are related to central nervous system (CNS) including paresthesia, fatigue, dizziness, somnolence, and mood symptoms [19]. TMP is also associated with oligohydrosis due to its inhibition of carbonic anhydrase within the sweat glands leading to impaired seat rate which is associated with heat intolerance and hyperthermia particularly in children [20].
There are no evidence-based or universally accepted management strategies to treat RTA secondary to TMP. TMP discontinuation should be considered in patients with persistent severe RTA. In some reports, normalization of mental status in patients with TMP-induced metabolic acidosis took 48 hours after the discontinuation of TMP [17]. Treatment with an alkali such as sodium bicarbonate or potassium citrate and citric acid can be used to restore normal serum HCO3- in patients with RTA. This can also decrease urinary calcium excretion and increase urinary citrate excretion which can prevent renal stone formation and improve bone disease in adults [21]. Adequate hydration whilst using TMP should be encouraged as it can reduce the risk of developing renal stones.
Conclusions
Topiramate has been demonstrated to be a potent inhibitor of some carbonic anhydrase isoenzymes, which can result in the development of type 2 renal tubular acidosis, normal anion gap metabolic acidosis, and nephrolithiasis as side effects from its use. Clinicians must remain vigilant to the clinical manifestations of metabolic acidosis, and aware of this possible etiology.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy. Report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. French JA, Kanner AM, Bautista J, et al. Neurology. 2004;62:1261–1273. doi: 10.1212/01.wnl.0000123695.22623.32. [DOI] [PubMed] [Google Scholar]
- 2.Clinical efficacy of topiramate as add‐on therapy in refractory partial epilepsy: the European experience. Ben‐Menachem E. Epilepsia. 1997;38:0. doi: 10.1111/j.1528-1157.1997.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration (FDA). FDA labelling information. [Nov;2018 ];http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm 2018
- 4.New and future migraine therapy. Ramadan NM, Buchanan TM. Pharmacol Ther. 2006;112:199–212. doi: 10.1016/j.pharmthera.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Supuran CT, Di Fiore A, De Simone G. Expert Opin Emerg Drugs. 2008;13:383–392. doi: 10.1517/14728214.13.2.383. [DOI] [PubMed] [Google Scholar]
- 6.Single-dose pharmacokinetics and effect of food on the bioavailability of topiramate, a novel antiepileptic drug. Doose DR, Walker SA, Gisclon LG, Nayak RK. J Clin Pharmacol. 1996;36:884–891. doi: 10.1002/j.1552-4604.1996.tb04754.x. [DOI] [PubMed] [Google Scholar]
- 7.The role of carbonic anhydrases in renal physiology. Purkerson JM, Schwartz GJ. Kidney Int. 2007;71:103–115. doi: 10.1038/sj.ki.5002020. [DOI] [PubMed] [Google Scholar]
- 8.Therapeutic applications of glycosidic carbonic anhydrase inhibitors. Winum JY, Poulsen SA, Supuran CT. Med Res Rev. 2009;29:419–435. doi: 10.1002/med.20141. [DOI] [PubMed] [Google Scholar]
- 9.Renal tubular acidosis. Laing CM, Unwin RJ. https://insights.ovid.com/nephrology/joneph/2006/03/009/renal-tubular-acidosis/8/00052477. J Nephrol. 2006;19:0. [PubMed] [Google Scholar]
- 10.Topiramate-induced renal tubular acidosis. Izzedine H, Launay-Vacher V, Deray G. Am J Med. 2004;116:281–282. doi: 10.1016/j.amjmed.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Topiramate induced metabolic acidosis. Ozer Y, Altunkaya H. Anaesthesia. 2004;59:830. doi: 10.1111/j.1365-2044.2004.03884.x. [DOI] [PubMed] [Google Scholar]
- 12.Topiramate and severe metabolic acidosis: case report. Burmeister JE, Pereira RR, Hartke EM, Kreuz M. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-282X2005000300032. Arq Neuropsiquiatr. 2005;63:532–534. doi: 10.1590/s0004-282x2005000300032. [DOI] [PubMed] [Google Scholar]
- 13.Biochemical and stone-risk profiles with topiramate treatment. Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. Am J Kidney Dis. 2006;48:555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Time course of adverse events in patients with localization-related epilepsy receiving topiramate added to carbamazepine. Majkowski J, Neto W, Wapenaar R, Van Oene J. Epilepsia. 2005;46:648–653. doi: 10.1111/j.1528-1167.2005.35904.x. [DOI] [PubMed] [Google Scholar]
- 15.Acute mental status changes and hyperchloremic metabolic acidosis with long-term topiramate therapy. Stowe CD, Bollinger T, James LP, Haley TM, Griebel ML, Farrar HC 3rd. Pharmacotherapy. 2000;20:105–109. doi: 10.1592/phco.20.1.105.34662. [DOI] [PubMed] [Google Scholar]
- 16.Topiramate-induced nephrolithiasis. Kuo RL, Moran ME, Kim DH, Abrahams HM, White MD, Lingeman JE. J Endourol. 2002;16:229–231. doi: 10.1089/089277902753752188. [DOI] [PubMed] [Google Scholar]
- 17.Acetazolamide-induced nephrolithiasis: implications for treatment of neuromuscular disorders. Tawil R, Moxley RT 3rd, Griggs RC. Neurology. 1993;43:1105–1106. doi: 10.1212/wnl.43.6.1105. [DOI] [PubMed] [Google Scholar]
- 18.Induction of progressive profound hypocitraturia with increasing doses of topiramate. Warner BW, LaGrange CA, Tucker T, Bensalem-Owen M, Pais VM Jr. Urology. 2008;72:29–32. doi: 10.1016/j.urology.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 19.A multicenter, outpatient, open-label study to evaluate the dosing, effectiveness, and safety of topiramate as monotherapy in the treatment of epilepsy in clinical practice. Sankar R, Ramsay E, McKay A, Hulihan J, Wiegand F. Epilepsy Behav. 2009;15:506–512. doi: 10.1016/j.yebeh.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Transient hypohidrosis induced by topiramate. de Carolis P, Magnifico F, Pierangeli G, Rinaldi R, Galeotti M, Cevoli S, Cortelli P. Epilepsia. 2003;44:974–976. doi: 10.1046/j.1528-1157.2003.40702.x. [DOI] [PubMed] [Google Scholar]
- 21.Incidence of radiographically evident bone disease, nephrocalcinosis, and nephrolithiasis in various types of renal tubular acidosis. Brenner RJ, Spring DB, Sebastian A, McSherry EM, Genant HK, Palubinskas AJ, Morris RC Jr. N Engl J Med. 1982;307:217–221. doi: 10.1056/NEJM198207223070403. [DOI] [PubMed] [Google Scholar]