ABSTRACT
A 73-year-old female with a past medical history of breast cancer, who 10 years earlier experienced complete remission, complained of bilateral visual field disturbances and photopsia, 2 months prior. Tumour recurrence and metastatic lesions were not found during the medical examination, but antibodies against recoverin were detected in her serum. Despite immunosuppressive treatment with prednisolone and plasmapheresis, rapid and diffuse degeneration of the patient’s photoreceptors and deterioration of her visual field were observed. This is a rare case of cancer-associated retinopathy with a long interval (10 years) between the diagnosis of the malignancy and visual loss.
KEYWORDS: Cancer-associated retinopathy, recoverin, breast cancer
Introduction
Cancer-associated retinopathy (CAR) is a well-known autoimmune paraneoplastic retinopathy. It is thought to be a form of autoimmune retinopathy, which is characterized by pan-retinal degeneration, fast deterioration of visual functions, severe electroretinographic changes, anti-retinal antibodies, and a history of carcinoma.1,2 Typical visual symptoms of CAR include bilateral and progressive, painless, severe, and permanent visual loss.1,3 Immunity against the cancer and cross-reactivity with retinal antigens are thought to be the cause of the disease.4–7 Frequent primary malignancies are small-cell lung carcinoma, gynaecological malignancies, and breast cancer.4 Based on a cohort study of 209 patients, the interval between the diagnosis of cancer and the onset of CAR varied from weeks to years, depending on the cancer.8 According to a study of CAR in breast cancer, the interval was an average of 4.6 years, but its peak was 2–3 years after the clinical diagnosis in autoantibody-positive patients.5 Cases with long intervals between the clinical diagnosis of malignancy and visual loss, especially >10 years, have not been well-documented. We describe an anti-recoverin, antibody-positive CAR patient who showed rapid deterioration of the visual field and progressive loss of ellipsoid zones as revealed by spectral domain-optical coherence tomography (SD-OCT) after 10 years of complete remission from breast cancer.
Case report
A 73-year-old female was admitted to our hospital because of visual field disturbances and photopsia in both eyes, 2 months prior. She had a past history of breast cancer; she underwent resection and chemotherapy 10 years prior, resulting in complete remission.
Initial neuroophthalmic testing at our hospital revealed a visual acuity of 20/50 in the right eye and 20/25 in the left eye. A perfect arrangement was obtained for both eyes using the Farnsworth dichotomous test (panel D-15). The patient’s extraocular movements were normal. A relative afferent pupillary defect in the right eye was noted. No inflammatory cells were observed in the anterior chamber or anterior vitreous. Ophthalmoscopy revealed a mottled fundus in both eyes (Figure 1A). Fluorescein angiography showed marked segmental leakage from perivascular lesions in both eyes (Figure 1B). On autofluorescence (FAF) imaging, hyperfluorescence was observed along the arcade vessels in both eyes (Figure 1C). Electroretinography showed a severe decrease in both the a- and b-waves in the combined rod-cone response, as well as severely diminished cone and flicker responses (Figure 2). A Humphrey visual field test revealed bilateral ring-like peripheral scotoma, especially in the right eye (Figure 3A). SD-OCT revealed a diffuse irregularity and segmental disappearance of the ellipsoid zone outside of the macula, thinning of the outer nuclear layer, macular traction by the epiretinal membrane (ERM) in the right eye, and segmental irregularity of the ellipsoid zone in the left eye. These findings coincided with severe visual field defects (Figure 3A). Blood tests revealed antibodies against recoverin, but no anti-neuronal antibody to collapsing response mediator protein-5, amphiphysin, paraneoplastic antigen MA2, Ri, Yo, Hu, Sox-2, titin, zic4, glutamic acid decarboxylase 65, or Tr was detected. The results of Treponema pallidum hemagglutination assays and rapid plasma regain tests were negative. T-spot testing and tests for antibodies against HIV were negative. Serum tests for IgG antibodies against cytomegalovirus, herpes simplex virus, and varicella zoster virus were positive; however, tests for IgM antibodies against these viruses was negative, indicating past infections (not an active state). Because CAR was strongly suspected, we performed whole body fluorodeoxyglucose positron emission tomography to investigate any underlying malignancy. No recurrence of breast cancer, metastatic lesion, or other solid tumour was found (Figure 4). Haematological malignancies were not suspected based on blood tests. The patient was treated with oral prednisolone (initial dose: 30 mg/kg/day) for 6 months. However, it did not stabilize the condition, and the patient’s visual acuity deteriorated to light perception in the right eye and 20/100 in the left eye. A Humphrey field analyser revealed no preserved visual field in the right eye and a remaining visual field only in the upper nasal region in the left eye (Figure 3B). SD-OCT revealed an undetectable ellipsoid zone and atrophy of the outer nuclear layer in the right eye, and progressive loss of the ellipsoid zone and thinning of the outer retina in the left eye (Figure 3B). Plasmapheresis was performed five times in 2 weeks, followed by oral azathioprine treatment. Transient improvement in the patient’s visual acuity to 20/50 in the left eye and slight enlargement of the visual field in the lower nasal region in the left eye were observed. However, 2 weeks after the completion of plasmapheresis, the patient complained of darkness in her left eye, resulting in a loss of light perception in both eyes. On visual field testing, the patient’s sensitivity degraded to 0 in whole visual fields. The ellipsoid zone was no longer evident on SD-OCT, and atrophy of the outer nuclear layer was observed in the left eye (Figure 3C). FAF showed diffuse hypofluorescence in the whole retina (Figure 1D). During a follow-up visit at >1.5 years, no solid malignancy was detected.
FIGURE 1.
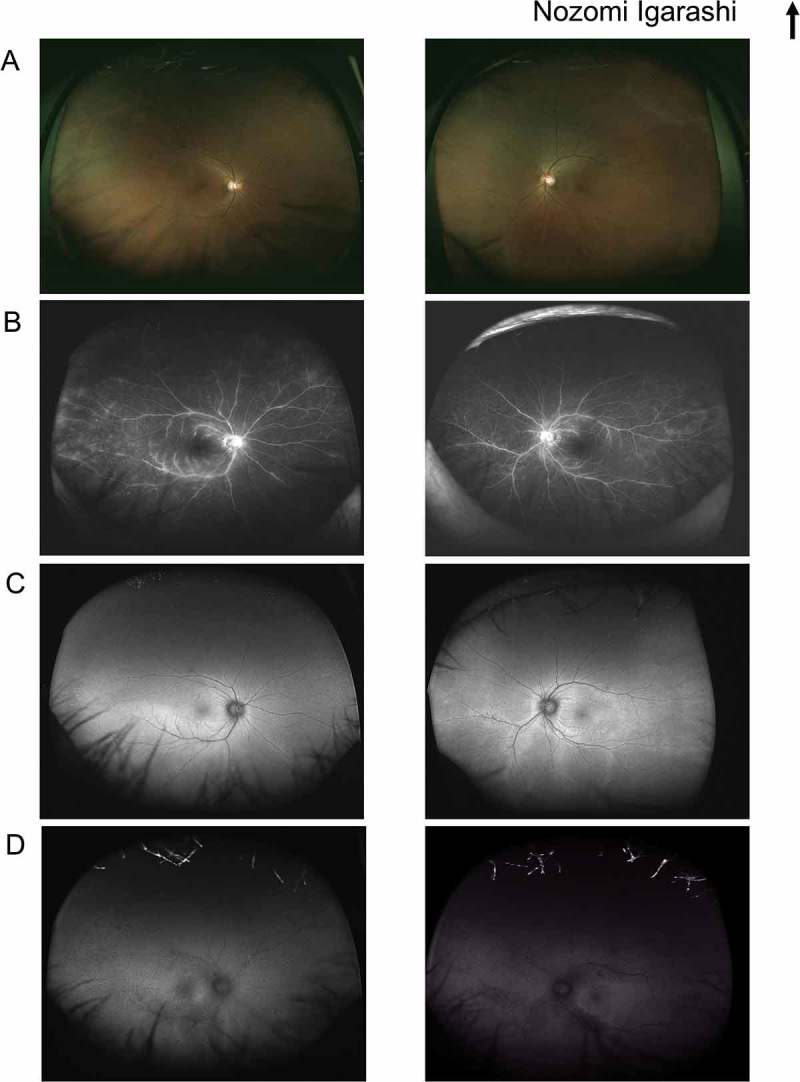
A–C. Color fundus photography (A), fluorescein angiography (B), and autofluorescence imaging (C) at the initial presentation. (D) Autofluorescence imaging after the loss of bilateral visual function.
Figure 2.
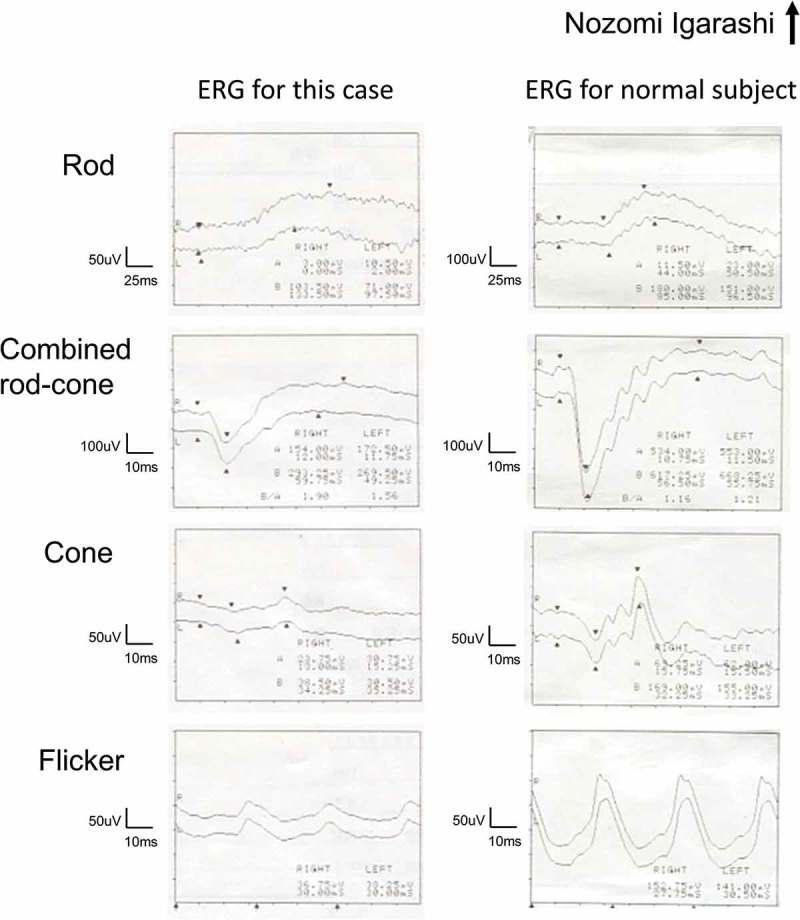
Full-field flash electroretinography from the patient (left column) and from a normal subject (right column) for comparison. From top to bottom, rod response, combined rod-cone response, cone response, and flicker response.
Figure 3.
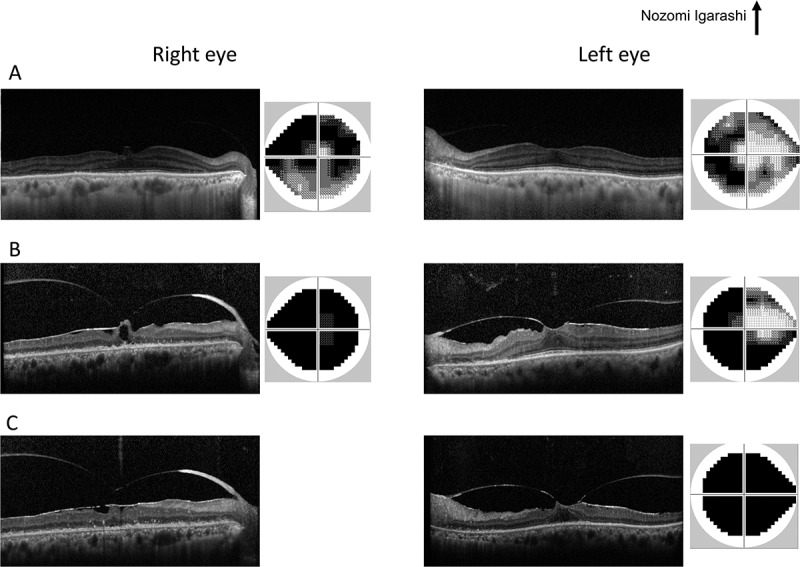
The time course of SD-OCT images and Humphrey visual fields at initial presentation (A), during oral prednisolone treatment (B), and after plasmapheresis treatment (C). The results from the right and left eyes are displayed in the left and right halves, respectively.
Figure 4.
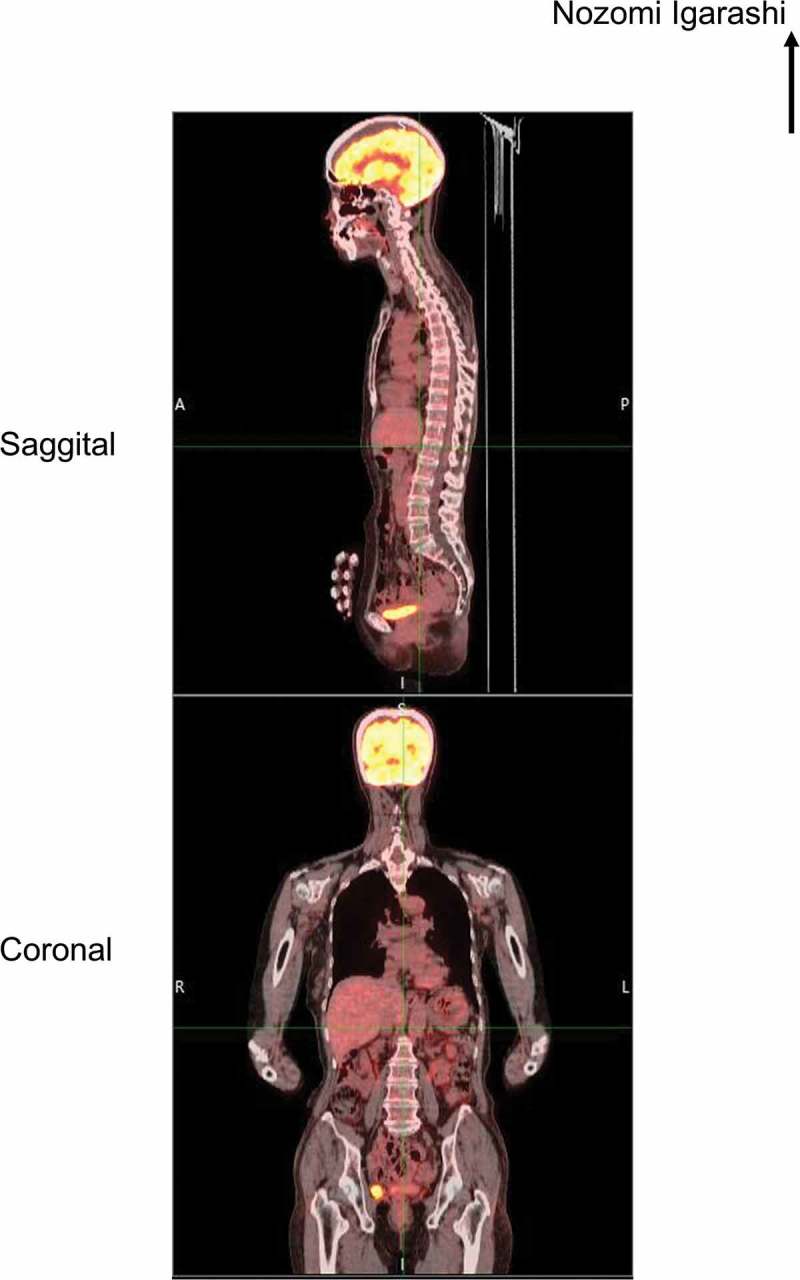
Whole body fluorodeoxyglucose positron emission tomographic images at 1 month after the initial presentation. A and P in the upper panel indicate the anterior and posterior, respectively, while R and L in the lower panel indicate right and left, respectively.
Discussion
In this patient, CAR developed 10 years after complete breast cancer remission. According to a frequency histogram of the intervals between the clinical diagnosis of the malignancy and visual symptom onset, long intervals (more than 10 years) are uncommon.5 One possibility is that there was a new, undetectable small malignancy whose growth was suppressed by the anti-recoverin antibodies because it was reported that anti-recoverin antibodies may act as an inhibitor against tumour growth.6 Furthermore, 5% of breast cancer-related CAR patients showed both visual symptoms and the existence of anti-retinal autoantibodies prior to the clinical diagnosis of the malignancy.5
The pathogenic mechanism of CAR is thought to be autoimmune cross-reactivity of the antibody against common antigens of the retina and the cancer.4–7 Recoverin antigen is expressed on some types of tumour cells9,10 as well as on the outer segments of photoreceptors.4,6 Cross-reactivity between cancerous and photoreceptor recoverin antigens induced the apoptosis of photoreceptor cells, causing a severe visual loss.6,7,11,12 The time course of our findings, including progressive loss of the ellipsoid zone, visual field deterioration, and decreased visual acuity, is consistent with the reported underlying mechanism of CAR.
At the initial examination, except for the macular region, SD-OCT showed a loss or irregularity in the ellipsoid zone. This observation is consistent with a previous study showing that retinal degenerative changes were prominent in the paramacular and equatorial regions.13 In our case, periphlebitis was observed, though it is not common in CAR.4,13,14 The underlying mechanism has been suggested as impairment of the blood-retinal barrier during remodelling of the retinal vasculature.15,16 Hyperfluorescence on FAF imaging has also been observed, possibly caused by a decrease in retinal pigment epithelium function related to ongoing metabolic demands.17 Hypoautofluorescence has been suggested to reflect photoreceptor death and retinal pigment epithelial atrophy.17 This may explain our case, which exhibited hyperfluorescence at an early stage and an active autoimmune reaction against photoreceptors followed by hypofluorescence at a later stage, involving an inactive autoimmune reaction state due to photoreceptor loss. This mechanism is consistent with our SD-OCT findings (Figure 3).
There is no established treatment for CAR.3,4 Although treatment with immunosuppressive agents such as systemic corticosteroids, plasmapheresis, immunoglobulins, or rituximab has been performed, no treatment has been shown to result in high efficacy.13,18–21 In our case, plasmapheresis was transiently and partially effective, but it could not maintain the patient’s vision. The detection of anti-recoverin serum antibodies without malignancy suggests that the patient’s immune system recognized the retina as an original antigen, resulting in the continuous production of autoantibodies against recoverin and causing photoreceptor degeneration. Therefore, plasmapheresis functioned only as a supportive treatment to reduce the amount of autoantibodies during treatment. This explains why the patient’s vision deteriorated despite treatment with plasmapheresis and immunosuppressive agents. It is assumed that earlier intervention might have preserved the patient’s vision for a longer period because the rate of photoreceptor loss would have decrease with reduced autoantibodies.
Infectious uveitis due to tuberculosis, syphilis, herpes viruses, and HIV could be a differential diagnosis based on the findings of periphlebitis and ERM. However, it is unlikely because of the blood test results and the finding of no inflammatory cells in the anterior or vitreous chambers. Acute zonal occult outer retinopathy (AZOOR) could be an alternative diagnosis. However, it is unlikely because of the detection of anti-recoverin antibodies,2,22 a mottled fundus appearance at the initial visit in contrast to a previous report,23 and the absence of hyperautofluorescent demarcating lines between the normal retina and the AZOOR lesion, which was recently reported as a typical feature of AZOOR.24
In conclusion, with the increase in cancer and former cancer patients, the frequency of CAR is expected to rise. Clinicians should therefore be aware that CAR can occur long after a diagnosis of malignancy, even if no malignancy is present.
Funding Statement
H.S. was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number [JP16K11319];
Declaration of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the writing and content of the article.
References
- 1.Gordon LK. Paraneoplastic syndromes in neuro-ophthalmology. J Neuro-Ophthalmol. 2015;35(3):306–314. doi: 10.1097/WNO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 2.Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;30(2):127–134. doi: 10.1007/s00281-008-0114-7. [DOI] [PubMed] [Google Scholar]
- 3.Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina. 2014;34(5):827–845. doi: 10.1097/IAE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 4.Rahimy E, Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Surv Ophthalmol. 2013;58(5):430–458. doi: 10.1016/j.survophthal.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Adamus G. Latest updates on antiretinal autoantibodies associated with vision loss and breast cancer. Invest Ophthalmol Vis Sci. 2015;56(3):1680–1688. doi: 10.1167/iovs.14-15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thirkill CE. Cancer-induced, immune-mediated ocular degenerations. Ocul Immunol Inflamm. 2005;13(2–3):119–131. doi: 10.1080/09273940590928733. [DOI] [PubMed] [Google Scholar]
- 7.Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48(1):12–38. doi: 10.1016/S0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 8.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009;8(5):410–414. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda A, Ohguro H, Maeda T, et al. Aberrant expression of photoreceptor-specific calcium-binding protein (recoverin) in cancer cell lines. Cancer Res. 2000;60(7):1914–1920. [PubMed] [Google Scholar]
- 10.Matsuo S, Ohguro H, Ohguro I, Nakazawa M. Clinicopathological roles of aberrantly expressed recoverin in malignant tumor cells. Ophthalmic Res. 2010;43(3):139–144. doi: 10.1159/000253486. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Maeda A, Maruyama I, et al. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001;42(3):705–712. [PubMed] [Google Scholar]
- 12.Makiyama Y, Kikuchi T, Otani A, et al. Clinical and immunological characterization of paraneoplastic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(8):5424–5431. doi: 10.1167/iovs.13-11868. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi Y, Ohara S, Sakamoto T, Kohno T, Nakao F. Cancer-associated retinopathy with retinal phlebitis. Br J Ophthalmol. 1993;77(12):795–798. doi: 10.1136/bjo.77.12.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Toma HS, Thirkill CE, Dunn JP Jr.. Cancer-associated retinopathy with retinal periphlebitis in a patient with ovarian cancer. Ocul Immunol Inflamm. 2010;18(2):107–109. doi: 10.3109/09273940903457441. [DOI] [PubMed] [Google Scholar]
- 15.Anastasakis A, Dick AD, Damato EM, Spry PG, Majid MA. Cancer-associated retinopathy presenting as retinal vasculitis with a negative ERG suggestive of on-bipolar cell pathway dysfunction. Documenta Ophthalmologica Advanc Ophthalmol. 2011;123(1):59–63. doi: 10.1007/s10633-011-9277-y. [DOI] [PubMed] [Google Scholar]
- 16.Cao R, Cao Y. Cancer-associated retinopathy: a new mechanistic insight on vascular remodeling. Cell Cycle. 2010;9(10):1882–1885. doi: 10.4161/cc.9.10.11521. [DOI] [PubMed] [Google Scholar]
- 17.Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012;32(7):1385–1394. doi: 10.1097/IAE.0b013e3182398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MA, Thirkill CE, Hart WM Jr.. Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell lung carcinoma. J Neuro-Ophthalmol. 1997;17(2):77–83. doi: 10.1097/00041327-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Keltner JL, Thirkill CE, Tyler NK, Roth AM. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol. 1992;110(1):48–53. doi: 10.1001/archopht.1992.01080130050025. [DOI] [PubMed] [Google Scholar]
- 20.Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117(4):471–477. doi: 10.1001/archopht.117.4.471. [DOI] [PubMed] [Google Scholar]
- 21.Dy I, Chintapatla R, Preeshagul I, Becker D. Treatment of cancer-associated retinopathy with rituximab. J Natl Compr Cancer Netw. 2013;11(11):1320–1324. doi: 10.6004/jnccn.2013.0156. [DOI] [PubMed] [Google Scholar]
- 22.Ohguro H, Yokoi Y, Ohguro I, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137(6):1117–1119. doi: 10.1016/j.ajo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002;134(3):329–339. doi: 10.1016/S0002-9394(02)01640-9. [DOI] [PubMed] [Google Scholar]
- 24.Mrejen S, Khan S, Gallego-Pinazo R, Jampol LM, Yannuzzi LA. Acute zonal occult outer retinopathy: a classification based on multimodal imaging. JAMA Ophthalmol. 2014;132(9):1089–1098. doi: 10.1001/jamaophthalmol.2014.1683. [DOI] [PubMed] [Google Scholar]


