ABSTRACT
Purpose: To determine the positive yield (utility rate) of temporal artery biopsy (TAB) in patients with suspected giant cell arteritis (GCA).
Study Design: Systematic review (CRD42017078508) and meta-regression.
Materials and Methods: All articles concerning TAB for suspected GCA with English language abstracts from 1998 to 2017 were retrieved. Articles were excluded if they exclusively reported positive TAB, or only cases of known GCA. Where available, the pre-specified predictors of age, sex, vision symptoms, jaw claudication, duration of steroid treatment prior to TAB, specimen length, bilateral TAB, and use of ultrasound/MRI (imaging) were recorded for meta-regression.
Results: One hundred and thirteen articles met eligibility criteria. The I2 was 92%, and with such high heterogeneity, meta-analysis is unsuitable. The median yield of TAB was 0.25 (95% confidence interval 0.21 to 0.27), with interquartile range 0.17 to 0.34. On univariate meta-regression age (coefficient 0.012, p = 0.025) was the only statistically significant patient factor associated with TAB yield.
Conclusions: Systematic review revealed high heterogeneity in the yield of TAB. The median utility rate of 25% and its interquartile range provides a benchmark for decisions regarding the under/overutilization of TAB and aids in the evaluation of non-invasive alternatives for the investigation of GCA.
KEYWORDS: Giant cell arteritis, temporal artery biopsy, yield, systematic review, meta-regression
Introduction
Giant cell arteritis (GCA) is the most common primary systemic vasculitis in the elderly and can cause irreversible blindness and occasionally aortitis, myocardial infarction, and stroke. The diagnosis of GCA is clinical, but the 1990 American College of Rheumatology (ACR) classification criteria were not intended for diagnosis,1 and may not be accurate for ophthalmic GCA patients.2 Temporal artery biopsy (TAB) for histologic analysis is the reference standard for the diagnosis of cranial GCA.3,4 However TAB is an invasive procedure that requires at least 20–40 minutes to perform,5 and is subject to false-negatives from skip lesions or inadequate length specimens. Clinicians may request TAB fearing the consequences of a missed diagnosis of GCA, and for pathologic confirmation of disease due to the many potential side effects of glucocorticoid treatment. We undertook a systematic review of the literature from the last two decades to determine the positive yield of TAB for biopsy-proven GCA. The positive yield of TAB provides a utility benchmark for practitioners, may aid in decision-making for GCA, and also help in the evaluation of alternative tests for GCA.
Materials and methods
The Prospero registration ID for this project was CRD42017078508. All articles with patients undergoing TAB for suspected GCA and with English language abstracts were retrieved using the search terms (giant cell arteritis OR temporal arteritis) AND biopsy. Relevant articles were sourced from PubMed, Embase, Cochrane Central Register of Controlled Trials, Google Scholar, Open Grey, and hand search from 1 January 1998 to 31 December 2017. Studies were excluded if they reported patients with positive TAB only, or patients with negative TAB only, or selected only patients with an established clinical diagnosis of GCA. To determine the yield, the number of patients undergoing biopsy rather than the number of TAB performed was recorded. A positive TAB biopsy was considered to be mononuclear cell infiltrate of the arterial wall with or without giant cells, or intimal thickening/degeneration of the media with destruction of the internal elastic laminae. Healed arteritis was considered a positive TAB (see Supplemental Files), but periarterial lymphocytic infiltrates in isolation was not considered a positive TAB. Equivocal reports were considered negative TAB. TAB series from the same authors, institution(s), or regions with overlapping time periods were only counted once. (See Supplemental Table II for specific article details.) When series partially overlapped, the new information was separated out when possible.
Five authors independently reviewed and selected eligible studies and performed the quality analysis (DW, EB, JM, AK, and GS). Disagreements were adjudicated by the principle author (EI).
The quality analysis (see Figure 1) was performed from the perspective of the potential for bias in the TAB results. The major criteria for selection bias were non-consecutive TAB in the study group and verification bias. Although articles investigating ultrasound/magnetic resonance imaging (MRI) for GCA may have had little bias with respect to the imaging investigation, TAB may not have been obtained in all patients, or the decision to perform TAB may have been influenced by the result of the imaging study, leading to verification bias. In a large TAB series of unilateral and bilateral biopsies, if only results of the bilateral biopsies were reported this was considered selection bias. If only subjects with high ACR scores underwent TAB, this was considered a possible performance bias. If the pathologist was not blinded to the patient’s symptoms, bloodwork results, or ACR score, this was a possible detection bias. Withdrawals from TAB (e.g. patient refusal to undergo TAB, or a vein or nerve specimen rather than the artery) were considered an attrition bias. If the pathology results from all patients that underwent TAB were not listed, this was considered a reporting bias. The main reason for “other bias” was because TAB series from the same city, author, or institution had partial overlap of patients that we could not completely eliminate.
Figure 1.
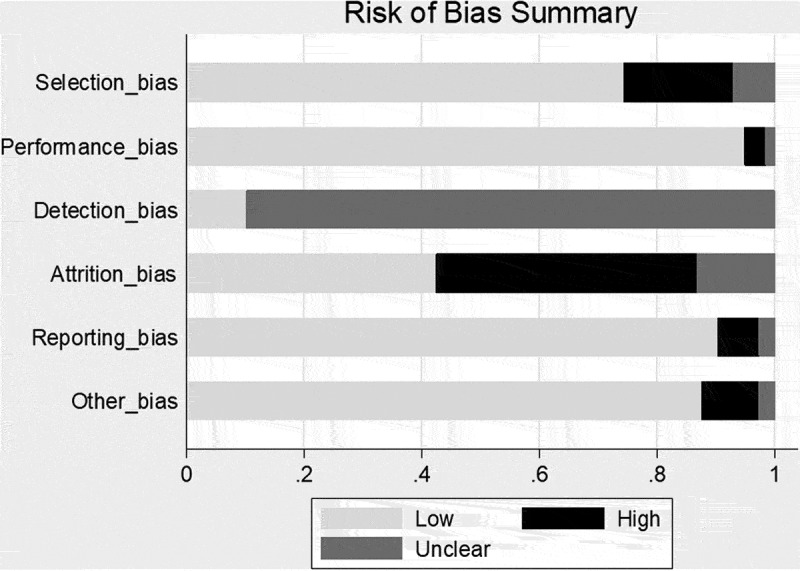
Risk of bias summary.
The majority of the articles were retrospective studies, and although it is acknowledged that retrospective reports may be more prone to bias than their prospective counterparts, this factor was not included in the “other bias” category. The decision to perform TAB was rarely randomized with few exceptions.6
Initial analysis was performed with Stata 14.2 (StataCorp LLC, College Station, TX, USA) using a random-effects meta-analysis of proportions (metaprop) with Freeman–Tukey double arcsine transformation to stabilize the variances and exact confidence intervals.7 Stata was then used to perform a random-effects meta-regression of the aggregate-level data (metareg). If heterogeneity exceeded 75%,8 we would also compare the results with MetaXL 5.3 (EpiGear International Pty Ltd) fixed-effect inverse variance heterogeneity model (IVHet) with double arcsine prevalence transformation, 0.5 continuity correction, and normalized prevalence.9–11 If heterogeneity exceeded 90%, we would report the median yield and interquartile range.
When available, the pre-specified predictors of patient age, proportion of females, specimen length, bilateral TAB, ACR scores greater than or equal to three,12 vision symptoms, duration of steroid use prior to TAB, study size, decade of study, and use of arterial imaging (ultrasound or MRI) were recorded from each study for meta-regression. A p value < 0.05 was considered to be statistically significant. The utility rate of TAB by country and yield from first decade versus second decade of reports were also compared.
Results
Four thousand three hundred and forty-four GCA studies were identified. (See Figure 2: PRISMA flow diagram.)
Figure 2.
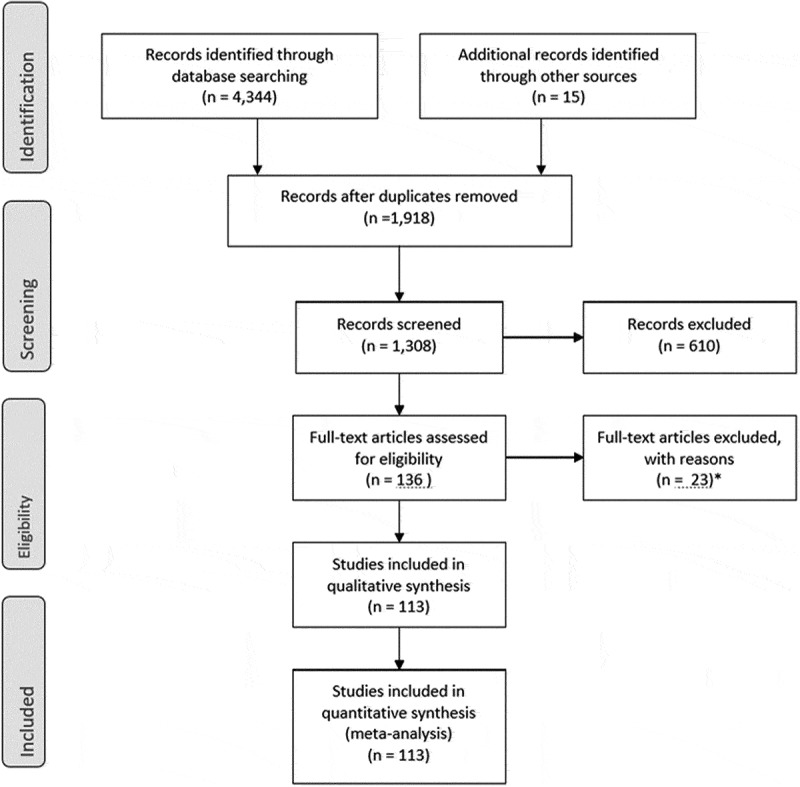
PRISMA flow diagram.
After duplicate records were removed, 1918 records remained. After screening, 610 studies were removed. One hundred and thirty-six articles were assessed for eligibility with 23 subsequent exclusions. Qualitative and quantitative synthesis of the 113 remaining articles was performed.
The 113 articles are listed in Table I of the Supplemental Files and encompass 30,898 TAB, of which 7379 (23.9%) were positive. The yield of TAB from the articles had a right skew distribution (Figure 3) with a non-weighted mean 27.7%, mode 33.3%, and median 25.0% with interquartile range 16.6–34.3%. The 95% confidence interval of the median was 21.4% to 27.0%.
Figure 3.
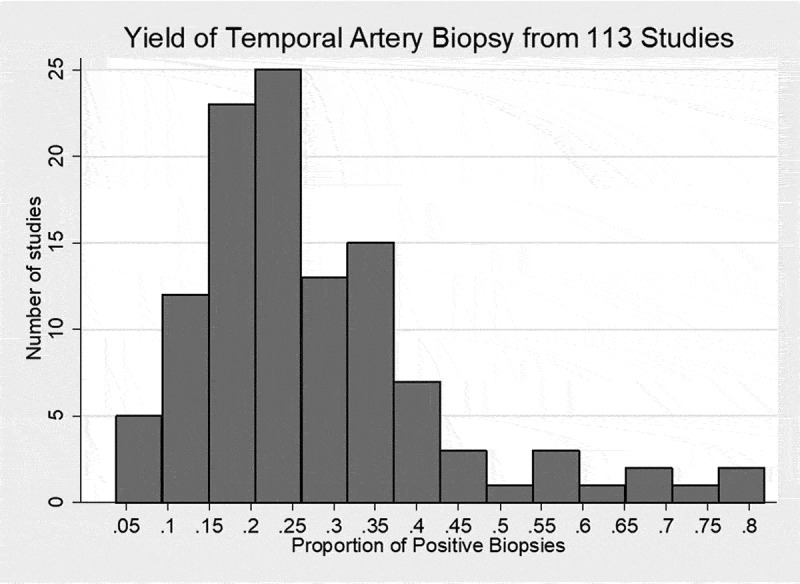
Histogram of the yield of temporal artery biopsy from 113 studies.
On meta-analysis of proportions using Stata random-effects analysis and Freeman–Tukey double arcsine transformation, the pooled estimate was 0.25 (95% confidence interval 0.23, 0.27) but I2 was 92.1%. With such high heterogeneity, the weights for meta-analysis techniques are compromised, including the fixed-effect IVHet model which showed pooled estimate of 0.23 (95% confidence interval 0.20, 0.27) and I2 = 92%. It is inaccurate to report the results for meta-analysis in the setting of such high heterogeneity; the forest plots and funnel plots are also not meaningful but are included (Supplemental Figure I and Figure II) for interested readers.
Although the yield could be calculated for all articles, information of the pre-specified predictors was not uniformly available. Sixty-five percent of the articles indicated the patient age was on average 72.7 ± 2.8 years. Sixty-six percent of the articles indicated subject gender, and on average 68% of the subjects were female.
To determine the possible causes of the high heterogeneity, meta-regression was performed. (Table 1) On univariate random-effects meta-regression, age (p = 0.025) and the use of arterial imaging (p = 0.001) were the only variables with a statistically significant association with TAB yield (see Table 1 and Figure 4). Subgroup analysis is more appropriate for categorical variables11 but examined to see the comparative IVHet yields of TAB for each predictor variable. (see Supplemental Table III). IVHet found a lower proportion of females and higher proportion of biopsies within the 2-week steroid window and studies with imaging were statistically significant at the p = 0.05 level. As such, multivariable meta-regression incorporating age, gender, duration of steroid use prior to TAB, and imaging was performed, and 21 articles provided information on all these variables. None of the predictors remained statistically significant on multivariable meta-regression of 21 articles (see Supplemental Files).
Table 1.
Aggregate means and random-effects meta-regression of temporal artery biopsy yield.
| Predictor | n | Mean (SD) | I2_res | Adj. R2 | Coefficient | p value |
|---|---|---|---|---|---|---|
| Age (years) | 73 | 72.7 (2.8) | 59% | 14% | 0.0119 | 0.025 |
| % Female | 75 | 68 (8) | 60% | 5% | −0.2236 | 0.131 |
| Length (mm) | 42 | 14.1 (6.3) | 39% | 5% | 0.0017 | 0.443 |
| % Bilateral biopsy | 55 | 20 (33) | 57% | −3% | 0.0143 | 0.766 |
| % Vision symptoms | 37 | 27 (18) | 45% | 24% | −0.1932 | 0.091 |
| % Jaw claudication | 34 | 19 (12) | 38% | −8.81% | −0.0014 | 0.993 |
| % Steroids <2 weeks | 28 | 85 (26) | 60% | −3.21% | 0.0573 | 0.688 |
| % ACR criteria ≥3 | 31 | 62 (23) | 59% | −8.1% | 0.0780 | 0.500 |
| Study size | 113 | 273 (562) | 55% | 0.61% | −0.00002 | 0.244 |
| Decade of study (1st vs 2nd) | 113 | N/A | 59% | −1.53% | −0.0070 | 0.790 |
| Imaging vs. No-image study | 113 | N/A | 54% | 14% | .1349287 | 0.001 |
n = number of articles that provided details on the predictor variable.
Mean = aggregate mean.
SD = standard deviation of the aggregate mean.
ACR ≥3 = American College of Rheumatology classification criteria score for GCA with value of three or greater.
Study size = number of patients in each study.
Adj. R2 = adjusted R2; the percentage of between-study variance (heterogeneity) explained by the variable(s) in the model. Proportion of total between-study variance explained by the model.
I2 res = residual heterogeneity.
Coefficient = the coefficient of the meta-regression.
N/A = not applicable.
Imaging = colour duplex ultrasound or MRI.
No-image = temporal artery biopsy only.
Figure 4.
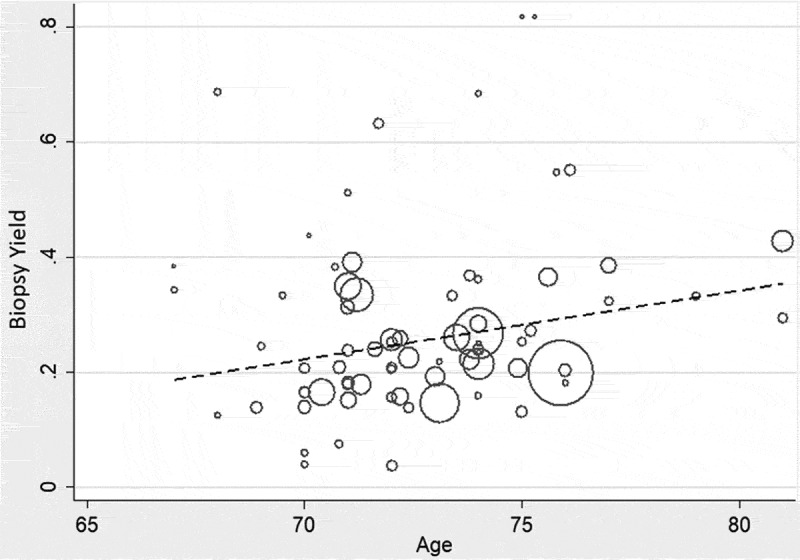
Random-effects meta-regression of age versus the yield of temporal artery biopsy.
The TAB yield from countries with at least three TAB series is shown in Table 2. The IVHet technique was employed as there was high heterogeneity for every country with the exception of Italy. France had the lowest yield of TAB and Germany the highest utility rate. There was selection bias with non-consecutive TAB in most of the German studies, as five out of six of the studies were primarily concerned with colour duplex sonography (CDS) or MRI (imaging) in the investigation of GCA, and not all the patients underwent TAB. Except for one study from Sweden, with a utility rate of 19.9%, we did not find any TAB reports from Scandinavian countries that met our eligibility criteria.
Table 2.
Meta-analysis of the yield of temporal artery biopsy by country presented in ascending order, using the inverse variance heterogeneity model.
| Country/Region | n | Pooled estimate | 95% confidence interval | I2 |
|---|---|---|---|---|
| France | 4 | 0.16 | 0.03, 0.31 | 91% |
| Australia/New Zealand | 9 | 0.22 | 0.18, 0.25 | 59% |
| United States | 26 | 0.23 | 0.16, 0.30 | 95% |
| United Kingdom | 37 | 0.24 | 0.20, 0.29 | 87% |
| Canada | 7 | 0.25 | 0.17, 0.32 | 86% |
| Israel | 3 | 0.30 | 0.20, 0.40 | 69% |
| Spain | 9 | 0.31 | 0.22, 0.40 | 90% |
| Italy | 3 | 0.40 | 0.36, 0.44 | 9% |
| Germany | 6 | 0.47 | 0.24, 0.70 | 94% |
n = number of studies.
pooled estimate = yield of temporal artery biopsy.
I2 = the percentage of variation across studies attributed to heterogeneity rather than chance.
Discussion
The I2 statistic indicates the degree of inconsistency across studies or the percentage of variation in point estimates across studies attributed to heterogeneity instead of chance. Given the I2 statistic of 92%, it was not advisable to report the pooled results with a meta-analysis even using high heterogeneity models such as IVHet.10 With skewed data, the median is the preferred indicator of central tendency.
There are at least two applications for the median estimate derived from this systemic review: (i) If billing data on the number of TAB is known for a particular region, but the aggregate pathology results are not available, the median can be used to estimate the incidence of biopsy-proven GCA and facilitate medical resource planning.13 (ii) The median yield provides a benchmark figure that allows decision-making regarding the under/overutilization of TAB and aids in the evaluation of non-invasive alternative investigations for GCA.
Advocating a 25% utility rate for TAB as appropriate is debatable,14 but the risk and expense of TAB are low, the side effects of prolonged glucocorticoid treatment are substantial, and the potential risks of irreversible blindness and occasionally death15 from a missed diagnosis of GCA are devastating. The sensitivity of a single TAB is 87.1% (95% confidence interval, 81.8–91.7%).16 The sensitivity and yield of TAB may be influenced by variables such as the local prevalence of the disease, risk factor scores, available surgical resources, institutional preferences for clinical work-up, patient age, gender, ethnicity, timing of biopsy and glucocorticoid treatment, biopsy length, unilateral versus bilateral biopsy, pathology routines, and the availability of ancillary imaging techniques. On univariate meta-regression, the only study predictors that affected the yield of TAB were increasing age and imaging. GCA is regarded as a disease of immunosenescence, and age is a strong risk factor for GCA.17–19 It makes intuitive sense to perform TAB at the site of an ultrasound abnormality, but Germano did not find that the yield of TAB increased when guided by colour duplex ultrasound halo.6 The issue of possible selection bias in the series of TAB that accompanied imaging studies was previously mentioned. We acknowledge the potential pitfalls of meta-regression including ecologic fallacy, confounding, and false-positive results from multiple testing. On multivariable meta-regression, none of our predictors were statistically significant.
Using the interquartile range as a guide, it is possible that institutions with a utility rate markedly lower than 17% may be performing too many TAB or perhaps inadequate length TAB, while those with a yield much higher than 34% might be underutilizing TAB.
Performing TAB based on the ACR risk score is conjectured as a method to improve the yield of TAB.14,20 However, meta-regression did not show that the ACR score had a significant impact on TAB yield. The ACR classification criteria were not intended for diagnosis,1 and may not be accurate for ophthalmic GCA patients.2 A multivariable prediction model with geographic external validation has been shown to outperform the pre-TAB ACR criteria,18 and may be more accurate in selecting candidates for TAB. Although some feel that a high ACR risk score may obviate TAB,14 we advocate confirmatory TAB given the risks of systemic glucocorticoid treatment, and because other diseases can mimic or overlap the clinical presentation of GCA, including amyloidosis,21 granulomatosis with polyangiitis and other antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides,22 calciphylaxis,23 Mönckeberg’s medial calcific sclerosis,24 and zoster sine herpete.25 Without TAB, these alternative diagnoses may not be determined in an expedient fashion.
Although GCA is a clinical diagnosis, a positive TAB is the reference standard for the diagnosis of GCA.26 Non-invasive technologies such as CDS, MRI, and positron emission tomography are being continually evaluated as non-invasive alternatives to TAB. The most recent European League Against Rheumatism (EULAR) consensus proposes CDS first in patients with suspected large-vessel vasculitis, provided it is promptly available and the test is performed and interpreted by trained, experienced specialists using the appropriate machine settings and protocols.27 As of 2018 though, “CDS use is still not widespread in routine clinical practice.”28 The pooled sensitivity of the CDS “halo” sign (homogeneous and hypoechoic vessel wall thickening due to arterial oedema) is 77%, and false-positive CDS halos may occur with ANCA-associated vasculitis, infectious diseases, and severe arteriosclerosis.27 Germano did not find that the yield of TAB increased when guided by CDS halo,6 and recommended confirmatory TAB even when CDS suggests temporal artery abnormality because of the possible side effects of prolonged glucocorticoid therapy.29 The 2018 EULAR consensus states that their imaging guidelines “should not be understood as a recommendation against performing TAB” and that “many physicians still consider TAB as the gold standard test for the diagnosis of GCA.” To best compare the performance of imaging modalities against TAB, the utility rate (positive yield) of TAB is a helpful metric.
In summary, our systematic review of 113 articles in the GCA literature from the last two decades showed marked heterogeneity in the diagnostic yield of TAB. In such circumstances, results are usually not pooled for meta-analysis. The median yield of TAB was 25% (interquartile range 17–33%) and is useful for clinical decision-making, medical resource planning, and comparative studies of alternative investigations for GCA. Although the yield of TAB may vary with the regional prevalence of GCA, gender, biopsy length, bilateral biopsy, timing of biopsy in relation to glucocorticoid treatment, and proportion of study patients with vision symptoms or jaw claudication, our univariate meta-regression found that increasing age was the only statistically significant patient factor associated with biopsy yield.
Funding Statement
None.
Supplemental data
Supplemental data for this article can be access on the publisher’s website.
Acknowledgments
The authors thank Professor Suhail A. Doi for his advice on the iv Het model.
Declaration of conflicts of interest
None of the authors have any conflicts of interest to declare.
References
- 1.Hunder G. The use and misuse of classification and diagnostic criteria for complex disease. Ann Intern Med. 1998;129(5):417–418. doi: 10.7326/0003-4819-129-5-199809010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Murchison AP, Gilbert ME, Bilyk JR, et al. Validity of the American college of rheumatology criteria for the diagnosis of giant cell arteritis. Am J Ophthalmol. 2012. October;154(4):722–729. doi: 10.1016/j.ajo.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371:50–57. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford). 2010. August;49(8):1594–1597. doi: 10.1093/rheumatology/keq039a. [DOI] [PubMed] [Google Scholar]
- 5.Albertini JG, Ramsey ML, Marks VJ. Temporal artery biopsy in a dermatologic surgery practice. Dermatol Surg. 1999. June;25(6):501–508. doi: 10.1046/j.1524-4725.1999.08296.x. [DOI] [PubMed] [Google Scholar]
- 6.Germanò G, Muratore F, Cimino L. Is colour duplex sonography-guided temporal artery biopsy useful in the diagnosis of giant cell arteritis? A randomized study. Rheumatology. 2015;54:400–404. doi: 10.1093/rheumatology/keu241. [DOI] [PubMed] [Google Scholar]
- 7.Nyaga VN, Arbyn M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014. November;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003. September;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013. November;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 10.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015. November;45(Pt A):130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Barendregt JJ, Doi SA. MetaXL User Guide Version 5.3. pdf. Sunrise Beach: EpiGear International PTY Ltd; 2016. [Google Scholar]
- 12.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990. August;33(8):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 13.Ing EB, Lahaie Luna G, Pagnoux C, et al. The incidence of giant cell arteritis in Ontario, Canada. Can J Ophthalmol. Published online. May 15 2018. [DOI] [PubMed] [Google Scholar]
- 14.Davies CG, May DJ. The role of temporal artery biopsies in giant cell arteritis. Ann R Coll Surg Engl. 2011. January;93(1):4–5. doi: 10.1308/003588411X12851639107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammad AJ, Nilsson JÅ, Jacobsson LT, Merkel PA, Turesson C. Incidence and mortality rates of biopsy-proven giant cell arteritis in southern Sweden. Ann Rheum Dis. 2015. June;74(6):993–997. doi: 10.1136/annrheumdis-2013-204652. [DOI] [PubMed] [Google Scholar]
- 16.Niederkohr RD, Levin LA. A Bayesian analysis of the true sensitivity of a temporal artery biopsy. Invest Ophthalmol Vis Sci. 2007. February;48(2):675–680. doi: 10.1167/iovs.06-1106. [DOI] [PubMed] [Google Scholar]
- 17.Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13(4):231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ing EB, Lahaie Luna G, Toren A, et al. Multivariable prediction model for suspected giant cell arteritis: development and validation. Clin Ophthalmol. 2017. November 22;11:2031–2042. doi: 10.2147/OPTH.S151385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ing EB, Ing R. The use of a nomogram to visually interpret a logistic regression prediction model for giant cell arteritis. Neuro-Ophthalmology. 2018. February 5. doi: 10.1080/01658107.2018.1425728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieri A, Milligan R, Hegde V, Hennessy C. Temporal artery biopsy: are we doing it right? Int J Health Care Qual Assur. 2013;26(6):559–563. doi: 10.1108/IJHCQA-06-2012-0055. [DOI] [PubMed] [Google Scholar]
- 21.Ing EB, Woolf IZ, Younge BR, Bjornsson J, Leavitt JA. Systemic amyloidosis with temporal artery involvement mimicking temporal arteritis. Ophthalmic Surg Lasers. 1997. April;28(4):328–331. [PubMed] [Google Scholar]
- 22.Ong Tone S, Godra A, Ing E. Polyangiitis overlap syndrome with granulomatosis with polyangiitis (Wegener’s) and giant cell arteritis. Can J Ophthalmol. 2013. February;48(1):e6–8. doi: 10.1016/j.jcjo.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Roverano S, Ortiz A, Henares E, Eletti M, Paira S. Calciphylaxis of the temporal artery masquerading as temporal arteritis: a case presentation and review of the literature. Clin Rheumatol. 2015. November;34(11):1985–1988. doi: 10.1007/s10067-015-2942-x. [DOI] [PubMed] [Google Scholar]
- 24.Belliveau MJ, Almeida DRP, Eneh A, Farmer J. Mönckeberg medial calcific sclerosis mimicking giant cell arteritis clinically. Can J Ophthalmol. 2013. February;48(1):71–72. doi: 10.1016/j.jcjo.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Ing EB, Ing R, Liu X, et al. Does herpes zoster predispose to giant cell arteritis: a geo-epidemiologic study. Clin Ophthalmol. 2018;12:113–118. doi: 10.2147/OPTH.S151893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danesh-Meyer HV. Temporal artery biopsy: skip it at your patient’s peril. Am J Ophthalmol. 2012. October;154(4):617–619. doi: 10.1016/j.ajo.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018. January 22 p. Epub ahead of print. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 28.Monti S, Floris A, Ponte C, et al. The use of ultrasound to assess giant cell arteritis: review of the current evidence and practical guide for the rheumatologist. Rheumatology (Oxford). 2018. February;57(2):227–235. doi: 10.1093/rheumatology/kex173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González-Gay MA, Pina T, Blanco R. The search for improvement in the sensitivity of temporal artery biopsy in giant cell arteritis. Rheumatology. 2015. March;54(3):379–380. doi: 10.1093/rheumatology/keu312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


