Abstract
Mice routinely undergo surgical procedures for use in research; however, studies of skin preparation methods to achieve antisepsis are rare. The present study evaluated 4 skin preparation treatments: depilatory agent followed by povidone–iodine and alcohol scrub; depilatory agent followed by povidone–iodine and saline scrub; electric clippers followed by povidone–iodine and alcohol scrub; and electric clippers followed by povidone–iodine and saline scrub. Swabs for bacterial culture were obtained immediately after hair removal and after scrubbing to measure the reduction in bacterial load. Full-thickness incisions were assigned ASEPSIS wound scores and examined histologically on days 0, 1, and 7 after surgery. Neither bacterial load growth nor ASEPSIS wound scores differed among any of the treatments. Histopathology revealed statistically significant but biologically irrelevant differences. Overall all treatment methods achieved acceptable bacterial load reduction and surgical site healing.
Abbreviation: SSI, surgical site infection
Mice used in biomedical research frequently undergo surgical procedures as part of a research protocol. The appropriate conduct of surgery is important in maintaining animal welfare, and to that end, the American College of Laboratory Animal Medicine (among other groups with similar missions) recommends that all institutions using rodents for research, training, and testing establish written standards for performing surgical procedures.1 A vital component of aseptic surgery and one necessary to ensure a positive outcome is preparation of the skin prior to the procedure. Whether the patient is an animal or human, the end goal of preparing (prepping) the skin for surgery is to achieve adequate antisepsis. The principle of asepsis was established nearly 150 y ago, and this state is defined as creation of a skin surface sufficiently free of gross contamination and resident microbial flora so that the risk of surgical site infection (SSI) or systemic infection is acceptably reduced.26 The secondary goal is to prepare the skin so that irritation and trauma are minimized, which, when achieved in combination with the first goal, leads to a high likelihood of successful wound healing and increased animal welfare.
Skin preparation for rodent surgery is fundamentally the same as that for any heavily furred mammal and involves 2 steps: hair removal followed by cleaning of the bared skin with antimicrobial agents to achieve antisepsis. Hair removal can be accomplished either mechanically through the use of clippers or by using a chemical depilatory agent. Removing hair can be very traumatic to the skin and in fact is contraindicated in human surgery except when hair obscures the surgery site.39 Shaving requires a delicate touch, and mechanical damage to the stratum corneum layer of the skin can result.16 Depilatory agents are corrosive, thus raising concerns regarding chemical burns if the products are left in prolonged contact with the skin.29 For mice, the recommendation at our institution is to rinse away these creams within 15 s of application, which is far less time than the manufacturer's recommendation of 5 min for humans. Either method of hair removal carries potential for operator error leading to injury, general discomfort, and poor surgical outcomes including suboptimal wound healing and SSI.
Skin disinfection after hair removal can be achieved in a variety of ways, and a substantial body of literature provides guidance regarding how best to achieve skin antisepsis prior to surgery. Most of the recommendations are based on studies of SSI prevention in humans,5,12,23,37,46 in whom SSI are estimated to occur at a rate of 1% to 2% for ‘clean’ surgeries (that is, no signs of infection or inflammation). Depending on their severity, SSI increase hospitals costs by US$400 to US$30,000 per patient8,43 and result in patient pain and distress. These outcomes have prompted professional organizations such as the Association of Operating Room Nurses (AORN) to publish clear requirements regarding the presurgical preparation of human patients.39 Although no associated financial costs have been reported, the SSI rate for clean procedures in veterinary medicine is approximately 2.5%.27,45
Recommended best practices regarding the preparation of veterinary patients for surgery have been derived principally from companion animal practice. Povidone–iodine or chlorhexidine used in conjunction with alcohol have been studied most often and appear to enjoy the most widespread acceptance and use in both companion animal veterinary medicine as well as human medicine.3,5,10,12,25,36,38,40,50 Not surprisingly given the recommendations in human and companion animal medicine, the use of an iodophor–alcohol or chlorhexidine–alcohol combination is most commonly recommended for rodent surgery4,9,17,34 and, in the absence of data to the contrary, it's reasonable to presume that both methods are effective when used appropriately. The choice of which antiseptic to use is often dictated by the nature of the surgery and, in human medicine, the presence of allergies to a particular agent. For example, chlorhexidine is generally safe on intact skin and requires shorter contact time than iodophores, but chlorhexidine is cytotoxic to mucous membranes and other tissues and thus might secondarily promote bacterial colonization and infection.14,30,33,41,44,48 A complete discussion of available agents and considerations for their use is beyond the scope of the current study, and several excellent reviews are available in the human medical literature.37
Questions regarding effectiveness and concern over potential untoward outcomes associated with surgical skin preparation have driven many discussions within the Emory University IACUC. Recently, one research group noted difficulty in collecting satisfactory echocardiographic images from mice that had been prepared by using depilatory cream followed by an iodine and alcohol scrub combination and prompted the request to use sterile saline instead of alcohol. The researchers suggested that the combination of depilatory cream with alcohol led to increased skin inflammation, resulting in poor imaging quality. This request to deviate from current best practices and a paucity of data on the subject prompted the present study. Here we evaluated the antiseptic effectiveness of 4 skin preparation methods and their effects on surgical incision healing.
Materials and Methods
Mice.
The study population was composed of male C57BL/6 mice (n = 30; age, 10 to 15 wk) that were bred and housed at Emory University in accordance with AAALAC accreditation and the Guide for the Care and Use of Laboratory Animals.20 All experimental procedures were approved by the IACUC at Emory University. Mice in this study were included in a routine health surveillance program and were negative for all pathogens of interest.13 Mice were housed 2 to 5 animals per cage in ventilated caging, with corncob bedding (Bed-o'Cobs 1/8-in., The Andersons, Maumee, OH). Caging supplies were not sterilized for this experiment. Environmental conditions were maintained at 68 to 72 °F (20 to 22 °C) and 30% to 70% humidity with a 12:12-h light:dark cycle. Reverse-osmosis–filtered water and a standard rodent diet (Laboratory Rodent Diet 5001, LabDiet, St Louis, MO) were provided without restriction.
Experimental design.
Mice were randomly assigned to 1 of 2 hair-removal technique groups: using a depilatory agent (Nair, Church and Dwight, Trenton, NJ) or shaving by using electric clippers (Pocket Pro trimmer, Wahl Clipper, Sterling, IL). After hair removal, the left and right sides of each mouse were delineated, and each side was then randomly assigned 1 of 2 scrubbing regimens: povidone–iodine scrub (0.75% titratable iodine, Phoenix, St Joseph, MO) combined with either 70% isopropyl alcohol (Aspen Veterinary Resources, Liberty, MO) or sterile saline (10-mL vials, Hospira, Lake Forest, IL). We therefore evaluated 4 treatment conditions overall. Each of the 30 mice experienced 2 conditions, one each on the left and right sides, resulting in 60 treated sites (n = 15 sites per condition).
Skin preparation.
All animals were anesthetized with isoflurane. By using 1 of 2 methods (depilatory cream or shaving with clippers), hair was removed from the midthoracic region to the sacral vertebrae and extending to the lateral aspects of the abdomen. A marker was used to delineate the left side from the right; these sites were further prepared by using 1 of 2 combinations of scrubbing agents. For animals treated with depilatory cream, clippers were used to thin the hair prior to application of the cream, but the blades were not allowed to contact the skin. The depilatory cream was then applied by using cotton-tipped applicators in a circular motion; the cream remained on the skin for 10 s before being removed by using sterile gauze soaked in saline. For the remaining animals, clippers were used to remove hair using standard techniques, with particular care to avoid causing ‘razor burn.’ Before each new cage of mice, hair was physically removed from clippers by using a brush and then a cleaning spray (Oster Kool Lube) was applied. The scrubbing agents were applied in 3 alternating cycles, according to accepted practice in the medical field. More specifically, sterile cotton-tipped applicators soaked in povidone–iodine scrub were applied in a circular motion, starting at the proposed incision site and working outward to the edges of haired skin. The povidone–iodine scrub was allowed to remain on the skin for 1 min and then was removed by using sterile cotton-tipped applicators soaked in either alcohol or sterile saline; this process was repeated twice. The skin was allowed to air dry 30 s before starting the incision.
Bacterial load.
To determine the antimicrobial activity of each site preparation method, samples for bacterial culture and identification were taken from 20 prepared sites (n = 5 mice sampled per treatment group) immediately after hair removal and again after the skin was scrubbed on day 0. Samples were collected by using a sterile culturette (BBL, CultureSwab, EZ Single Swab Format, BD Diagnostics, Sparks, MD) that was moistened with sterile saline and rolled down the midline of the prepared site. The swabs were then placed into a sterile conical tube (Eppendorf Safe Lock Tubes, 1.5mL, Eppendorf Quality, Hamburg, Germany). An additional 100 µL of sterile saline was added to the tube, and this solution was vortexed with the swab for 15 s, plated (BBL Columbia Agar with 5% sheep's blood, BD Diagnostics, Sparks, MD), and then incubated at 37 °C for 18 h. The plates were transported to the University of Georgia–Athens (Athens, GA) for additional aerobic incubation and bacterial identification through MALDI-TOF analysis. Bacterial load was assessed and scored on a scale of 0 (no growth) to 4 (heavy growth). At the University of Georgia's Diagnostic Laboratory, very light growth was defined as having a few, isolated colonies on a plate, light growth involved 25% or less of the plate, moderate growth covered 26% to 75% of the plate, and heavy growth covered all 4 quadrants of the plate.
Surgical technique.
Animals were maintained on a surgical plane of isoflurane anesthesia after skin preparation. Buprenorphine (CIII Carpuject, Pfizer, New York, NY) was administered subcutaneously at 0.05 mg/kg to each mouse and repeated every 12 h for 1 d after surgery. The surgeon prepped aseptically, with hairnet, face mask, sterile gown, and gloves. After skin preparation, a sterile drape was placed over the patient, and an approximately 1.0-cm full-thickness skin incision was made in the center of the prepared area by using sterile surgical instruments. Hemostasis was achieved with sterile cotton-tipped applicators. The skin then was immediately closed with 5-0 polypropylene suture (Surgical Specialties, Angiotech Pharmaceuticals, Vancouver, Canada) in a simple interrupted pattern.
Gross evaluation.
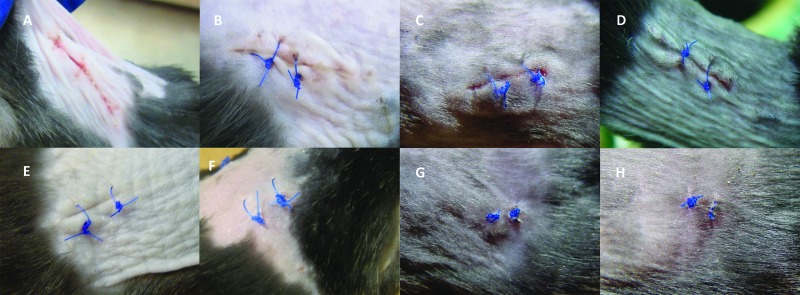
Surgical sites were photographed (Powershot SD1200 IS, Canon, Tokyo, Japan) at a distance of approximately 12 cm from the surgery site on day 1 (n = 10 samples per treatment) and day 7 (n = 5 samples per treatment). Day 0 was not photographed or evaluated because no healing would be evident at the gross level at that early time point. All photographs were visually assessed by 3 raters blinded to treatment condition. Assessors used a modified ASEPSIS wound score chart similar to that described elsewhere.47 Each assessor assigned scores for 4 criteria—erythema, serous exudate, swelling, and separation of deep tissues—and scores were summed to arrive at a total wound score. Composite scores of 0 to 10 indicated satisfactory healing; 11 to 20 demonstrated disturbance of healing; 20 to 30 were indicative of minor wound infection; 31 to 40 showed moderate wound infection; and scores greater than 40 revealed severe wound infection. The average ASEPSIS wound score for each treatment condition was then calculated and used for statistical analysis. Images representative of those evaluated are shown in Figure 1.
Figure 1.
Representative gross images of the average ASEPSIS wound score for each treatment group on days 1 and 7. Representative images of surgical sites on day 1 for mice treated with (A) depilatory cream, povidone–iodine, and alcohol; (B) depilatory cream, povidone–iodine, and saline; (C) shaving, povidone–iodine, and alcohol; and (D) shaving, povidone–iodine, and saline and on day 7 for mice treated with (E) depilatory cream, povidone–iodine, and alcohol; (F) depilatory cream, povidone–iodine, and saline; (G) shaving, povidone–iodine, and alcohol; and (H) shaving, povidone–iodine, and saline.
Microscopic evaluation.
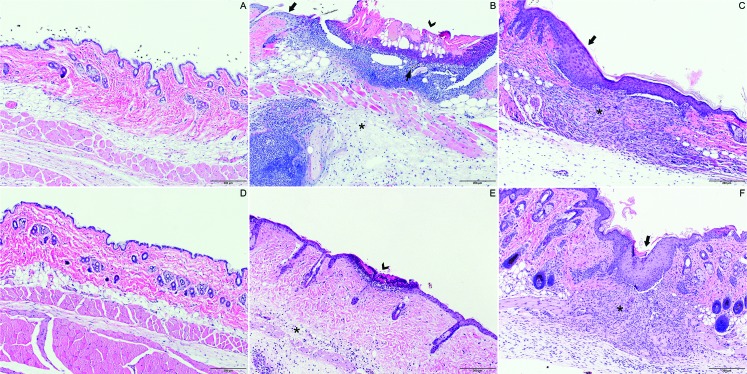
To microscopically examine surgical sites for bacterial contamination and wound healing, we used CO2 asphyxiation to euthanize 10 mice each on days 0, 1, and 7 after surgery. A 3×4-mm section of the wound was excised. Skin sections from areas not altered by a treatment group were also obtained to use as controls. Samples were fixed in 10% neutral buffered formalin, routinely processed, paraffin-embedded, sectioned at 5 µm, and stained with hematoxylin and eosin. The stained slides from each sample were examined by a board-certified veterinary pathologist blinded to treatment condition. Histopathology scoring was performed under both low-power (magnification, 100×) and high power field of view (magnification, 200×) by using methods similar to those published elsewhere.24 Criteria for assessment included dermal inflammation, follicular changes, fibroplasia, and epidermal hyperplasia. Each criterion was assigned a numeric score ranging from 0 (absent) to 3 (most robust change). Scores for each criterion were summed to obtain a cumulative histopathology lesion score for each section examined. A score of 12 was the maximum possible. Images of stained slides were captured at 200× magnification by using a microscope (model BX43, Olympus) equipped with a digital camera (model DP26, Olympus) by using digital imaging software (Cellsens 1.15, Olympus). Representative images are shown in Figure 2.
Figure 2.
Dermal pathology on days 1 and 7. (A) Normal skin with intact epidermis and absence of inflammation in dermis and subcutis. (B) At 24 h after shaving, skin tissue showed showed severe epidermal ulceration (arrowhead), furunculosis, and inflammation extending into dermis with a remnant of hair shaft (thin arrow). The thick arrow indicates intact skin adjacent to the ulceration site shows. Moderate subcutaneous edema, neutrophilic inflammation, and small numbers of macrophages (asterisk) were present. (C) At 7 d after shaving, skin tissue exhibited epidermal hyperplasia (arrow) and marked dermal fibroplasia with moderate infiltrates of neutrophils and macrophages (asterisk). (D) Normal skin with intact epidermis and absence of inflammation in dermis and subcutis. (E) At 24 h after application of the depilatory cream, focal epidermal erosion (arrowhead) and minimal inflammation and edema in dermis and subcutis (asterisk) were present. (F) At 7 d after application of the depilatory cream, skin sampes showed epidermal hyperplasia (arrow), marked dermal fibroplasia, and moderate infiltration of neutrophils and macrophages (asterisk). Hematoxylin and eosin staining; magnification, 100×. magnification.
Statistical analysis.
The Kendall coefficient of concordance score was calculated overall and for each combination of day and treatment to determine agreement among the 3 ASEPSIS visual assessors. Concordance values range from 0 (no agreement) to 1 (complete agreement). For bacterial load, ANOVA was used to test the difference between treatments. For ASEPSIS wound score, the median wound score for each mouse was calculated and used as the outcome in repeated measure model to test if differences existed by day, treatment, and interactions between those variables. A longitudinal generalized estimated equation model was fit to examine histopathology scores. The model included day, treatment, and interactions between those variables. For all 3 outcomes, the side of the mouse was evaluated and was included in the model only when statistically significant. All models were 2-sided with the significance level set at 0.05. All statistical analyses were performed by using SAS (version 9.3, SAS Institute, Cary, NC). All results are presented as mean ± SEM, unless otherwise noted.
Results
Interassessor agreement regarding ASEPSIS wound score.
The Kendall coefficient of concordance for the assessors’ ASEPSIS wound scores ranged from 0.495 to 0.692, indicating moderate to substantial agreement. In addition, assessor scores showed significant association.
Day 0.
For bacterial culture, only bacterial load after skin preparation was analyzed statistically. Prepreparation samples (that is, hair removal only) were not analyzed statistically and served only to describe normal skin flora and to ensure that animals were not ‘contaminated’ in some way by primary pathogens. After complete skin preparation, mice treated with shaving, povidone–iodine, and saline showed the highest bacterial load score (1.2 ± 0.5), followed by those prepped by using either depilatory cream, povidone–iodine, and saline or shaving, povidone–iodine, and alcohol (0.6 ± 0.5) and then those treated with depilatory cream, povidone–iodine, and alcohol (0.4 ± 0.5); none of the differences were statistically significant (P = 0.71). In total, 4 genera of bacteria were identified: Staphylococcus, Bacillus, Enterococcus, and Neisseria (Table 1). Staphylococcus xylosus was the most common isolate, identified in 57.5% (23 of 40) of the samples. Enterococcus gallinarum and Bacillus spp. each was present in 0.05% of samples (2 of 40). Neisseria spp. comprised 0.025% (1 of 40 cultures) of the samples. No growth was associated with 37.5% (15 of 40) of the samples, and of those, 86.6% (13 of 15) were samples obtained after scrubbing (Table 1). Histopathology scores for all treatment groups were 0 (data not shown).
Table 1.
Bacteria cultured from samples collected before and after skin disinfection
| Depilatory cream |
Shaving |
|||
| Before | After | Before | After | |
| Staphylococcus xylosus | 7 | 3 | 10 | 3 |
| Bacillus spp. | 0 | 0 | 0 | 2 |
| Enterococcus gallinarium | 1 | 0 | 1 | 0 |
| Neisseria spp. | 1 | 0 | 0 | 0 |
| No growth | 2 | 7 | 0 | 6 |
Data represent the number of plates on which each bacterial genus grew; some plates grew multiple bacterial species. Each mouse (n = 20) provided a ‘before’ (after hair removal but before surgical scrubbing) and ‘after’ (after scrubbing) sample. Each culture was incubated at 37 °C for 18 h; additional incubation and bacterial identification occurred at the University of Georgia–Athens.
Day 1.
The ASEPSIS wound score for mice treated with shaving, povidone–iodine, and alcohol was 1.15 ± 0.27, which was significantly (P = 0.046) greater than for those prepped with depilatory cream, povidone–iodine, and saline (0.35 ± 0.27) but not depilatory cream, povidone–iodine, and alcohol (0.60 ± 0.27) or shaving, povidone–iodine, and saline (0.50 ± 0.27; P > 0.10).
The mean histopathology score for mice prepped with shaving, povidone–iodine, and saline was 10.3 ± 0.23, the highest of any group. The next lower scores were for animals treated with shaving, povidone–iodine, and alcohol (9.8 ± 0.5); depilatory cream, povidone–iodine, and alcohol (6.8 ± 0.18); and depilatory cream, povidone–iodine, and saline (6.6 ± 0.36). Histopathology scores for the 2 groups that were shaved were significantly (P < 0.0001) greater than for those dehaired by using depilatory cream.
Day 7.
The mean ASEPSIS wound score for animals prepped by using shaving, povidone–iodine, and saline (0.20 ± 0.15) was the highest, whereas that of the group treated with shaving, povidone–iodine, and alcohol (0.10 ± 0.15) was the lowest; the mean score for mice treated with depilatory cream, povidone–iodine, and alcohol was 0.17 ± 0.15, and for those treated with depilatory cream, povidone–iodine, and saline it was 0.11 ± 0.15. None of the differences was statistically significant (P > 0.64. Treatment with shaving, povidone–iodine, and alcohol resulted in the highest mean histopathology score (7.3 ± 0.30), whereas depilatory cream, povidone–iodine, and saline was the lowest (5.7 ± 0.52); this difference was statistically significant (P = 0.008). The mean score for shaving, povidone–iodine, and saline was 6.6 ± 1.0 and for depilatory cream, povidone–iodine, and alcohol was 6.3 ± 0.78. None of the differences were significant, except for that between shaving, povidone–iodine, and alcohol compared with depilatory cream, povidone–iodine, and alcohol.
Discussion
Existing guidance for the preparation of mouse skin prior to surgery suggests the use of either shaving or depilatory agents for hair removal followed by chlorhexidine or povidone–iodine, both in combination with alcohol, for skin disinfection.4,7,34 Although these methods are generally accepted as best current practice, the effectiveness of presurgical skin preparation methods in rodents and their effects on surgical wound healing has recently prompted investigation.11 The goal of the present study was to evaluate 4 skin preparation methods for their ability to achieve antisepsis and to assess their effects on incisional wound healing. The data reported herein indicate that all methods were equally effective in achieving asepsis and resulted in satisfactory wound healing by day 7 after surgery.
In rodents, hair removal prior to cleaning of the skin with chosen disinfectants is a necessary step in preparation for surgery. In the present study, hair was removed either mechanically by using clippers or chemically by using a commercially available depilatory agent. We were uninterested in studying hair removal as an isolated endpoint because each method unquestionably is effective and, for skin preparation, it would always be done in conjunction with some form of disinfection. Although not done in this study, one option would have been to develop a scoring system and assess criteria such as erythema immediately after hair removal. In the absence of a formal method of assessment, we can report that the skin appeared grossly to be unharmed by using either method, and throughout the course of experimentation, no animals exhibited signs indicative of skin irritation such as pruritus. Similarly, all wounds healed satisfactorily, further supporting the notion that either method, when done appropriately, is acceptable. It is, however, important to recognize that hair removal was performed by an experienced veterinarian. In the hands of inattentive or inexperienced personnel, the risk for skin damage is increased, potentially resulting in discomfort for the animal and unsatisfactory wound healing, especially with depilatory agents that, if not applied for an appropriate duration, could cause considerable skin irritation.
Achieving satisfactory skin antisepsis requires the reduction of transient and resident microbial flora to the lowest achievable level. It is important to realize that the skin is never completely free of bacteria after preparation, and most studies show some proportion of samples with positive bacterial culture after preparation.15,25,31 Even when bacteria are superficially absent immediately after disinfection, they tend to recolonize over time.22 We therefore had no expectation that any treatment would completely eliminate bacteria from the skin surface and were interested to know which method resulted in the fewest bacteria.
All 4 treatments resulted in no or very low bacterial load scores. Because no benchmark for acceptable reduction in microbial flora is available, we cannot necessarily state that treatments were effective or ineffective—only that they resulted in similar outcomes. It is unsurprising that all disinfection regimens resulted in microbial reduction, because iodine was part of the regimen for all groups; however it is interesting that replacing alcohol with sterile saline had no appreciable effect. Alcohol has disinfectant properties that are well defined and accepted,9,19 and it exerts synergistic effects with other disinfectant agents,12 making it reasonable to expect that replacing it with saline would result in reduced antisepsis. Disinfection using iodine and saline after hair clipping did indeed result in the highest bacterial load score, but the difference was not significant statistically and likely of little consequence biologically, given that all wounds healed acceptably. The bacterial load for the other group treated with saline was essentially equal to those where alcohol was used, again supporting a conclusion that saline and alcohol are equally effective when used in combination with an iodine-based disinfecting agent. This finding suggests that the iodine contributed most of the antimicrobial activity in the preparation and that additional agents, whether saline or alcohol, serve little purpose except possibly some mechanical removal of bacteria. This outcome is not entirely unexpected, given that a substantial body of veterinary literature shows that povidone–iodine is an effective preoperative skin antiseptic.21,31,32,35,49 Whether this is true when alcohol or saline is combined with other disinfectants such as chlorhexidine is unknown and could serve as a focal point for a future study.
It is interesting to consider whether the method of hair removal itself contributes to antisepsis. In the present study, samples for bacterial cultures were collected after hair removal but prior to skin disinfection, primarily to describe the normal flora. Given that survival surgery would not take place in the absence of disinfection, the contribution of hair removal to antisepsis is ultimately inconsequential. With that said, it is reasonable to surmise that depilatory agents could have antimicrobial properties due to their corrosive nature. In addition, some bacteria are almost certainly removed mechanically through the act of wiping away the agent after application. We noted less bacterial growth in samples from skin treated with depilatory cream regardless of whether alcohol or saline was used after povidone–iodine, but whether the difference was clinically meaningful was not further explored.
Staphylococcus xylosus was most commonly isolated—unsurprising given that Staphylococcus spp. are ubiquitous in nature and considered to be one of the most prevalent components of the normal skin flora of several species, including mice. The other bacteria that were identified included Bacillus spp., Enterococcus gallinarium, and Neisseria spp. and all can likewise be considered normal commensals in the mouse.28,42 Although characterization of mouse skin flora was not a primary objective of this study, it was important to know from the onset that no primary pathogens were present in the event that untoward outcomes were observed at later time points. In addition, our findings further provided some assurance that SPF status was maintained in the mice during housing.
Although measurement of microbial burden is a good direct assessment of the effectiveness of a skin preparation regimen, the ultimate goal is to eliminate the risk for SSI and allow successful healing of the surgical incision. Several factors, including aseptic surgical technique, contribute to SSI prevention, but skin antisepsis is considered to be the foundation.39 In the present study, a single experienced surgeon performed all procedures and given the simplicity of and short duration necessary for creating a single skin incision, the risk of a break in aseptic technique was minimal. Excessive grooming of the incision site could contribute to SSI or delayed healing, but this factor likely was equally spread across all treatment groups and was never observed. We therefore presumed that skin preparation was the major factor determining whether an SSI would occur.
Assessing incisional wounds for evidence of an SSI in a clinical setting is done by simple examination of the surgical site, and human healthcare has formal guidelines for making a determination.18 No similar guidance exists in veterinary medicine, but a limited survey of the literature suggests that the appearance of purulent discharge is the central criterion.27,45 These reports, however, are centered on dogs and cats in a clinical setting, and there are no published studies of SSI in rodents. More than 80 methods for assessing surgical wounds in human medicine are described.8 Among those, the ASEPSIS grading scale is used most commonly and was chosen for the current study. To bolster gross observations, histologic measurement of wound healing was done with the idea that a contaminated wound would exhibit changes suggestive of infection or delayed healing. Although several methods are available for measuring incisional wound healing, histologic methods are considered the ‘gold standard.’2
ASEPSIS wound scores for all treatment groups at both days 1 and 7 were extremely low, barely eclipsing a score of 1 for the group that was shaved and scrubbed by using alcohol. Although this group was scored significantly higher at day 1 than one other group—the one treated with depilatory cream, povidone–iodine, and saline—this difference likely was inconsequential biologically, and by day 7, the differences diminished. This pattern strongly suggests that wounds were healing well in all cases. In general agreement with this conclusion were the histopathology scores that were lower on day 7 relative to day 1. On day 1, the scores ranged from 6.6 to 10.3 on a scale of 12, and although they could be interpreted as ‘high’, it's more appropriate to conclude that they reflect an acute phase in the healing process. Animals that were shaved overall had increased follicular inflammation and epidermal damage with ulcerations—these events all may increase the likelihood of SSI. Although this outcome was not necessarily expected, previous work has shown that shaving skin can be very traumatic, resulting in significant changes in structure and integrity.16 Most importantly, histologic changes diminished in all groups on day 7, suggesting that all wounds were healing satisfactorily with no indicators of infection such as increased infiltration by inflammatory cells. In humans, complete wound healing, as measured by cessation of remodeling, can require up to a year,6 and although comparable studies of complete wound healing in mice are not available, it is almost certain that remodeling is still occurring, as indicated primarily by fibroplasia, at this relatively early (7 d) time point. Clinically, a 10- to 14-d time point is often defined as the time when incisional wounds are considered healed adequately to remove materials used in wound closure, and the presumption is that our scores would continue to decline had we included a more distant time point.
In conclusion, all methods of skin preparation used in this study resulted in satisfactory antisepsis and wound healing, and we believe that all are safe and effective for use in rodent surgery. Some evidence suggested that the use of a depilatory agent was less traumatic to the skin than shaving, but with appropriate care and attention to detail, either hair removal method is acceptable. It is important to acknowledge that a simple incisional wound created by an experienced surgeon was used in this study. Procedures that are more complex, of longer duration, or performed by less experienced surgeons will carry a greater risk of SSI and other untoward outcomes. Regardless of the efficacy of the skin preparation regimen, the use of aseptic surgery principles remains vital to a successful outcome.
Acknowledgments
We thank Maya Encantada Meeks, Kristy Calderon, Michelle Hull, and Amy Dryman for their technical assistance. Thank you to Dr Susan Sanchez and the Athens Veterinary Diagnostic Laboratory at the University of Georgia– Athens.
References
- 1.American College of Laboratory Animal Medicine. 2016. ACLAM position statement on rodent surgery. J Am Assoc Lab Anim Sci 55:822–823. [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell DM, Campbell L, Thomason HA, Brass A, Hardman MJ. 2014. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen 22:281–287. 10.1111/wrr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association of Surgical Technologists. [Internet] 2008. AST standards of practice for skin prep of the surgical patient. [Cited 15 December 2017] Available at: http://www.ast.org/uploadedFiles/Main_Site/Content/About_Us/Standard_Skin_Prep.pdf
- 4.Bernal J, Baldwin M, Gleason T, Kuhlman S, Moore G, Talcott M. 2009. Guidelines for rodent survival surgery. J Invest Surg 22:445–451. 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- 5.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans J, Donlan R, Schecter WP, Healthcare Infection Control Practices Advisory Committee 2017. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152:784–791. 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 6.Broughton G, 2nd, Janis JE, Attinger CE. 2006. The basic science of wound healing. Plast Reconstr Surg 117 7 Suppl:12S–34S. 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 7.Brown MJ, Pearson PT, Tomson FN. 1993. Guidelines for animal surgery in research and teaching. AVMA Panel on Animal Surgery in Research and Teaching, and the ASLAP (American Society of Laboratory Animal Practitioners). Am J Vet Res 54:1544–1559. [PubMed] [Google Scholar]
- 8.Bruce J, Russell EM, Mollison J, Krukowski ZH. 2001. The measurement and monitoring of surgical adverse events. Health Technol Assess 5:1–194. 10.3310/hta5220. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DM, McIver R, Bianco R. 2000. The thin blue line: a review and discussion of aseptic technique and postprocedural infections in rodents. Contemp Top Lab Anim Sci 39:27–32. [PubMed] [Google Scholar]
- 10.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC, Alsharif A, Berger DH. 2010. Chlorhexidine–alcohol versus povidone–iodine for surgical-site antisepsis. N Engl J Med 362:18–26. 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 11.Del Valle JFE, Pak D, Zhang J, Crim MJ, Lawrence F, Steficek B, Hankenson F. 2017. Comparison of contemporary aqueous and alcohol-based surgical scrub agents for rodent surgical skin preparation. In: Abstracts of scientific papers presented at the 2017 AALAS National Meeting, Austin, Texas, 15–19 October 2017. J Am Assoc Lab Anim Sci 56:669. [Google Scholar]
- 12.Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. 2015. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev CD003949.doi: 10.1002/14651858.CD003949.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emory University. [Internet]. 2018. Emory mouse colony health surveillance and maintenance. [Cited 1 June 2018] Available at: http://www.dar.emory.edu/vetcare/sentinel_mouse.php.
- 14.Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A. 2008. Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigation. Toxicol In Vitro 22:308–317. 10.1016/j.tiv.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Gibson KL, Donald AW, Hariharan H, McCarville C. 1997. Comparison of 2 presurgical skin preparation techniques. Can J Vet Res 61:154–156. [PMC free article] [PubMed] [Google Scholar]
- 16.Hamza M, Tohid H, Maibach H. 2014. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol 34:335–343. 10.3109/15569527.2014.966109. [DOI] [PubMed] [Google Scholar]
- 17.Hoogstraten-Miller SL, Brown PA. 2008. Techniques in aseptic rodent surgery. Curr Protoc Immunol 82:1.12.1–1.12.14. 10.1002/0471142735.im0112s82 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. 1992. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13:606–608. 10.1017/S0195941700015241. [DOI] [PubMed] [Google Scholar]
- 19.Huerkamp MJ. 2002. Alcohol as a disinfectant for aseptic surgery of rodents: crossing the thin blue line? Contemp Top Lab Anim Sci 41:10–12. [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 21.Johnson J, Messier S, Meulyzer M, Vinardell T, Marcoux M, David F. 2015. Effect of presurgical iodine-based disinfection on bacterial colonization of the equine peripodal region. Vet Surg 44:756–762. 10.1111/vsu.12338. [DOI] [PubMed] [Google Scholar]
- 22.Johnston DH, Fairclough JA, Brown EM, Morris R. 1987. Rate of bacterial recolonization of the skin after preparation: 4 methods compared. Br J Surg 74:64–64. 10.1002/bjs.1800740121. [DOI] [PubMed] [Google Scholar]
- 23.Kamel C, McGahan L, Polisena J, Mierzwinski-Urban M, Embil JM. 2012. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review. Infect Control Hosp Epidemiol 33:608–617. 10.1086/665723. [DOI] [PubMed] [Google Scholar]
- 24.Klopfleisch R. 2013. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology—a systematic review. BMC Vet Res 9:1–15. 10.1186/1746-6148-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrechts NE, Hurter K, Picard JA, Goldin JP, Thompson PN. 2004. A prospective comparison between stabilized glutaraldehyde and chlorhexidine gluconate for preoperative skin antisepsis in dogs. Vet Surg 33:636–643. 10.1111/j.1532-950X.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- 26.Lister BJ. 2010. The classic: on the antiseptic principle in the practice of surgery. 1867. Clin Orthop Relat Res 468:2012–2016. 10.1007/s11999-010-1320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayhew PD, Freeman L, Kwan T, Brown DC. 2012. Comparison of surgical site infection rates in clean and clean-contaminated wounds in dogs and cats after minimally invasive versus open surgery: 179 cases (2007–2008). J Am Vet Med Assoc 240:193–198. 10.2460/javma.240.2.193. [DOI] [PubMed] [Google Scholar]
- 28.Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, Kawano J. 2002. Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci 64:245–250. 10.1292/jvms.64.245. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health, Health and Human Resources. [Internet]. 2018. Household products database. Church and Dwight Co., MSDS-1236. Nair hair remover lotion for body and legs, baby oil, fresh scent-11/11/2015. [Cited 26 December 2018]. Available at: https://hpd.nlm.nih.gov/cgi-bin/household/brands?tbl=brands&id=3005218&query=Nair&searchas=TblBrands.
- 30.Nonami K, Saitoh S, Nishimura-Danjobara Y, Ishida S, Oyama Y. 2016. Chlorhexidine possesses unique cytotoxic actions in rat thymic lymphocytes: its relation with electrochemical property of membranes. Environ Toxicol Pharmacol 48:17–21. 10.1016/j.etap.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Osuna DJ, DeYoung DJ, Walker RL. 1992. Comparison of an antimicrobial adhesive drape and povidone–iodine preoperative skin preparation in dogs. Vet Surg 21:458–462. 10.1111/j.1532-950X.1992.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 32.Park HM, Han SS, Lee EC, Lee SD, Yoon HM, Eom BW, Kim SH, Ryu KW, Park SJ, Kim YW, Park B. 2016. Randomized clinical trial of preoperative skin antisepsis with chlorhexidine gluconate or povidone–iodine. Br J Surg 104:e145–e150. 10.1002/bjs.10395. [DOI] [PubMed] [Google Scholar]
- 33.Penn-Barwell JG, Murray CK, Wenke JC. 2012. Comparison of the antimicrobial effect of chlorhexidine and saline for irrigating a contaminated open-fracture model. J Orthop Trauma 26:728–732. 10.1097/BOT.0b013e31826c19c4. [DOI] [PubMed] [Google Scholar]
- 34.Pritchett-Corning KR, Luo Y, Mulder GB, White WJ. 2011. Principles of rodent surgery for the new surgeon. J Vis Exp 47:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rochat MC, Mann FA, Berg JN. 1993. Evaluation of a one-step surgical preparation technique in dogs. J Am Vet Med Assoc 203:392–395. [PubMed] [Google Scholar]
- 36.Rodrigues AL, Simões Mde L. 2013. Incidence of surgical site infection with pre-operative skin preparation using 10% polyvidone–iodine and 0.5% chlorhexidine–alcohol. Rev Col Bras Cir 40:443–448. [Article in English, Portuguese]. 10.1590/S0100-69912013000600004. [DOI] [PubMed] [Google Scholar]
- 37.Sidhwa F, Itani KM. 2015. Skin preparation before surgery: options and evidence. Surg Infect (Larchmt) 16:14–23. 10.1089/sur.2015.010. [DOI] [PubMed] [Google Scholar]
- 38.Sistla SC, Prabhu G, Sistla S, Sadasivan J. 2010. Minimizing wound contamination in a ‘clean’ surgery: comparison of chlorhexidine–ethanol and povidone–iodine. Chemotherapy 56:261–267. 10.1159/000319901. [DOI] [PubMed] [Google Scholar]
- 39.Spruce L. 2016. Back to basics: surgical skin antisepsis. AORN J 103:96–100. [DOI] [PubMed] [Google Scholar]
- 40.Stubbs WP, Bellah JR, Vermaas-Hekman D, Purich B, Kubilis PS. 1996. Chlorhexidine gluconate versus chloroxylenol for preoperative skin preparation in dogs. Vet Surg 25:487–494. 10.1111/j.1532-950X.1996.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 41.Tabor E, Bostwick DC, Evans CC. 1989. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA 261:557–558. 10.1001/jama.1989.03420040091021. [DOI] [PubMed] [Google Scholar]
- 42.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, James GA, Olerud JE, Fleckman P. 2010. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci 49:588–591. [PMC free article] [PubMed] [Google Scholar]
- 43.Urban JA. 2006. Cost analysis of surgical site infections. Surg Infect (Larchmt) 7 Suppl 1:S19–S22. 10.1089/sur.2006.7.s1-19. [DOI] [PubMed] [Google Scholar]
- 44.van Huyssteen AL, Bracey DJ. 1999. Chlorhexidine and chondrolysis in the knee. J Bone Joint Surg Br 81:995–996. 10.1302/0301-620X.81B6.9719. [DOI] [PubMed] [Google Scholar]
- 45.Vasseur PB, Levy J, Dowd E, Eliot J. 1988. Surgical wound infection rates in dogs and cats. Data from a teaching hospital. Vet Surg 17:60–64. 10.1111/j.1532-950X.1988.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 46.Waltz PK, Zuckerbraun BS. 2017. Surgical site infections and associated operative characteristics. Surg Infect (Larchmt) 18:447–450. 10.1089/sur.2017.062. [DOI] [PubMed] [Google Scholar]
- 47.Wilson AP, Treasure T, Sturridge MF, Gruneberg RN. 1986. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 327:311–312. 10.1016/S0140-6736(86)90838-X. [DOI] [PubMed] [Google Scholar]
- 48.Wyganowska-Swiatkowska M, Kotwicka M, Urbaniak P, Nowak A, Skrzypczak-Jankun E, Jankun J. 2016. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int J Mol Med 37:1594–1600. 10.3892/ijmm.2016.2550. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda T, Hasegawa T, Yamato Y, Kobayashi S, Togawa D, Arima H, Matsuyama Y. 2015. Optimal Timing of preoperative skin preparation with povidone–iodine for spine surgery: a prospective, randomized controlled study. Asian Spine J 9:423–426. 10.4184/asj.2015.9.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zubrod CJ, Farnsworth KD, Oaks JL. 2004. Evaluation of arthrocentesis site bacterial flora before and after 4 methods of preparation in horses with and without evidence of skin contamination. Vet Surg 33:525–530. 10.1111/j.1532-950X.2004.04074.x. [DOI] [PubMed] [Google Scholar]