Abstract
Since its recent reformulation, alfaxalone has gained popularity as an injectable veterinary anesthetic, including promising studies demonstrating the use of alfaxalone–xylazine for anesthesia in mice. Here we sought to expand these studies by testing additional dose ranges, elaborating on physiologic monitoring, testing sex- and strain-associated differences, and evaluating efficacy during actual surgical conditions. C57BL/6J mice showed significant sex-associated differences in anesthetic sensitivity, with males requiring higher doses of alfaxalone (80–120 mg/kg IP alfaxalone with 10 mg/kg IP xylazine) than females (40–80 mg/kg IP alfaxalone with 10 mg/kg IP xylazine) to achieve a surgical plane of anesthesia. In addition, female outbred CD1 mice were less sensitive to alfaxalone than female inbred C57BL/6J mice. When used during actual surgery, alfaxalone–xylazine administered intraperitoneally provided adequate anesthesia for a model of orthopedic surgery, whereas the same anesthetic regimen during laparotomy resulted in unacceptably high mortality; survival during laparotomy increased when drugs were administered subcutaneously. These results indicate that alfaxalone–xylazine may be a viable option for injectable surgical anesthesia in mice, although strain- and sex-associated differences and alternative routes of administration should be considered when optimizing the anesthetic regimen for specific experimental conditions.
Abbreviations: HR, heart rate; LORR, loss of righting reflex; LPWR, loss of pedal withdrawal reflex; PWR, pedal withdrawal reflex; RR, respiratory rate
Despite the routine and frequent use of anesthesia in mice for biomedical research, the techniques used need to be refined continually and options for new and improved anesthetic agents need to be explored. In rodents, inhalants such as isoflurane are generally the preferred anesthetic due to their reliability and ability to achieve rapid induction and recovery.15 However, inhalant anesthesia may not be a viable option for some procedures due to equipment limitations (for example, MRI), undesirable or confounding effects on physiologic parameters (heart rate [HR], blood pressure, respiration), or anatomic incompatibility (for example, procedures involving oral cavity or respiratory tract). In these cases, injectable anesthesia may be necessary. In larger species, injectable anesthetic dosing can be titrated easily through intravenous administration. Although intravenous administration is possible in mice, most injectable anesthetic drugs are administered as an intraperitoneal bolus. In addition, injectable anesthetics are appealing due to their ease of use, independence of specialized equipment, lack of personnel exposure to hazardous gases, and historical data for some research models.
A variety of injectable anesthetics are available, and combinations of ketamine and xylazine are the most commonly used regimens in mice.36 A key disadvantage of many bolus injectable drugs, including ketamine–xylazine, is a shallow dose–response curve, which produces unpredictable effects. Notably, comparable dosing regimens of ketamine–xylazine in mice can produce widely variable outcomes, ranging from sedation to surgical plane of anesthesia to death.1,6,24 This variability and high risk for adverse events necessitate the search for alternative injectable anesthetic protocols for intraperitoneal administration in mice.
Alfaxalone is a neuroactive steroid that functions as a GABA agonist.39 Previous formulations used as veterinary anesthetics were discontinued because the solubilizing agent induced histamine release and anaphylactic reactions. The drug was subsequently reformulated using 2-hydroxypropyl-β-cyclodextrin as the solubilizing agent, which eliminated these adverse reactions.40 Since its reformulation, alfaxalone has gained increasing popularity in veterinary medicine as an induction agent, a sedative, and a component of intravenous general anesthesia in a variety of species.3,4,8,9,11,17,20,30,33,37,40 Recently, 2 publications have evaluated alfaxalone as an anesthetic in mice. One of these studies34 demonstrated that alfaxalone, in combination with the α2-adrenergic agonist xylazine, could be administered intraperitoneally to safely induce a surgical plane of anesthesia in mice. Promisingly, the authors found that the alfaxalone– xylazine produced a longer duration of surgical anesthesia than ketamine–xylazine. The other recent study21 tested alfaxalone in combination with medetomidine and butorphanol and, interestingly, found several dosing combinations that were more effective when given subcutaneously rather than intraperitoneally.
The physiologic effects of alfaxalone anesthesia in mice and its suitability for use in surgical procedures need to be evaluated further. In the current study, we sought to build on previous studies of intraperitoneal alfaxalone–xylazine anesthesia in mice by testing wider dose ranges, expanding the monitoring of mice anesthetized with this drug combination, testing the ability of atipamezole to reverse its anesthetic effects, evaluating the response of the drug in 2 strains of mice, and assessing the efficacy of the anesthetic protocol in actual surgical conditions. Consistent with previous studies,21,34 intraperitoneal alfaxalone–xylazine immobilized C57BL/6J mice and achieved a surgical plane of anesthesia adequate for a model of orthopedic surgery. However, intraperitoneal alfaxalone–xylazine combined with laparotomy resulted in unacceptably high intraoperative mortality. In contrast, subcutaneous administration of alfaxalone–xylazine safely anesthetized mice during laparotomy.
Materials and Methods
Animals and facility.
The study population was comprised of 47 female and 24 male C57BL/6J mice (age, 8 to 32 wk; weight: females, 17 to 31 g; males, 21 to 38 g) and 12 female CD1 mice (age, 7 to 12 wk; weight, 26 to 35 g); all mice were obtained from Jackson Laboratories (Bar Harbor, ME). To minimize animal numbers, the sample size for each experiment was determined on the basis of previous published work and analysis of the data at the completion of each experiment in the current study. Animals were housed in an AAALAC-accredited facility at 72 °F (22.2 °C), 30% to 70% humidity, 10 to 15 air changes hourly, and a 12:12-h light:dark cycle with 3 to 5 mice per static polycarbonate microisolation cage (Max 75, Alternative Design, Siloam Springs, AR) on disposable bedding (0.12-in., Bed-O-Cobs, The Andersons, Maumee, OH). Mice were fed standard pelleted laboratory rodent chow (5001, LabDiet, St Louis, MO) and were provided municipal water in bottles without restriction. Dirty-bedding sentinel mice, one cage on each side of a rack, were tested quarterly and found to be free from fur mites and pinworms by cecal exam. In addition, sentinels were negative for antibodies to tested pathogens, including mouse hepatitis virus, mouse parvoviruses, rotavirus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus. All procedures were approved by the University of Pennsylvania's IACUC.
Mice were allowed at least 1 wk to acclimate to the housing facility and cage environment prior to the start of the study. Each mouse in this study underwent no more than 3 anesthetic events and no more than 2 anesthetic events using injectable anesthetics, with at least a 10-d washout period between procedures.
Experiment 1. Dose response and physiologic monitoring.
Female C57BL/6J (n = 14), male C57BL/6J (n = 16) mice, and female CD1 (n = 8) mice were used in this study. Mice were weighed individually on a digital scale (US-ACE, US Balance, Vincennes, IN) prior to dosing. Anesthetic drugs used were alfaxalone (10 mg/mL, Alfaxan, Jurox, Kansas City, MO) and xylazine (20 mg/mL, AnaSed, Lloyd Laboratories, Shenandoah, IA). Drugs were combined into a single syringe and diluted with sterile 0.9% NaCl to a concentration that delivered the appropriate doses at 0.01 mL per gram of body weight. Mice received 1 of 3 induction doses administered intraperitonally: 1) 40 mg/kg alfaxalone and 10 mg/kg xylazine (40A/10X); 2) 80 mg/kg alfaxalone and 10 mg/kg xylazine (80A/10X); or 3) 120 mg/kg alfaxalone and 10 mg/kg xylazine (120A/10X). The order of the doses was randomized among the experimental mice to prevent any systematic variation of the data, and the researcher was not blinded to the dose administered. Each mouse was manually restrained for intraperitoneal injection into the right lower quadrant of the abdomen by using a 25-gauge, 5/8-in. needle. Artificial tears ointment (Akwa Tears, Akorn, Lake Forest, IL) was applied to each mouse's eyes at the beginning of each anesthetic procedure.
After injection, mice were monitored for loss of righting reflex (LORR), which was defined as loss of the ability to return to standing or sternal recumbency after being placed in dorsal recumbency. Righting reflex was tested at 30-s intervals after mice stopped voluntarily ambulating and until LORR was confirmed. After LORR, mice were placed in dorsal recumbency on a circulating-water heating pad (Gaymar Industries, Orchard Park, NY) to maintain a body temperature of 35 to 37 °C. Body temperature was measured by using a rectal temperature probe (19 mm, model RET3, Thermoworks, Lindon, UT) connected to a thermometer (model TW2-193, MicroTherma, Thermoworks). HR was monitored during anesthesia through ECG (ECGenie and eMouse 11 Analysis Software, Mouse Specifics, Quincy, MA); leads were applied to both forelimbs and secured by using conductive putty (Mouse Specifics). Respiratory rate (RR) was measured visually by counting thoracic excursions.
Immobility was defined by absence of any spontaneous movement, including movement of limbs or vibrissae. A surgical plane of anesthesia was defined as immobility and the absence of motor response to a noxious stimulus. The pedal withdrawal reflex (PWR) was assessed by using a Touch Test Sensory Evaluator (300 g, 6.65 gauge, North Coast Medical, Gilroy, CA) as previously described.13,22,31 The point of compression was located on the dorsal aspect of the metatarsal region and was alternated between hindlimbs. A positive response was defined as withdrawal of the stimulated limb or any spontaneous motion of the mouse that was unassociated with the stimulated limb; because PWR testing occurred at 5-min intervals, a single negative PWR was interpreted as 5 min of surgical anesthesia. Time to loss of the PWR (LPWR) was recorded as the beginning of the longest continuous surgical plane and not necessarily the first negative PWR, although the first negative PWR and the beginning of the longest duration of surgical plane coincided for the majority of animals.
To evaluate autonomic responses to a noxious stimulus, HR and RR were measured within 5 s before and after PWR assessment every 5 min while mice were immobilized. An increase in HR of greater than 10 bpm or an increase in RR of more than 5 breaths per minute were established as clinically detectable positive responses with monitoring techniques routinely used in mice. The differences between HR before and after PWR and between RR before and after PWR were calculated, and the percentages of detectable positive responses were compared according to induction dose and whether mice were at a surgical plane of anesthesia as determined by PWR.
HR, RR, PWR, and rectal temperature were recorded every 5 min during anesthesia until return of spontaneous movement, at which point the monitoring equipment was removed. Times for LORR, return of righting reflex, loss and return of spontaneous movement, LPWR, and return of PWR were recorded. To achieve return of the righting reflex, the mouse had to right itself 3 successive times.
Four mice died during this experiment. Time of death was recorded when mice had exhibited complete respiratory arrest for 1 min; cardiac arrest was confirmed through ECG. Postmortem examinations were performed on all deceased mice in all of the experiments in this project to rule out gross anatomic abnormalities or trauma such as lacerations or hemorrhage caused by IP injection.
Experiment 2. Atipamezole reversal of alfaxalone–xylazine anesthesia.
Male (n = 9) and female (n = 3) C57BL/6J mice were tested in this experiment. The mice were anesthetized with 80A/10X as in experiment 1. At 35 min after anesthetic injection, mice received an intraperitoneal injection of either saline (control) or atipamezole (0.1 mg/kg; 5.0 mg/mL, Antisedan, Zoetis, Parsippany, NJ). The injection volume was the same for both groups, the solution given to each mouse was selected randomly, and the anesthetist was blinded to the syringe contents. Physiologic parameters and anesthetic depth (HR, RR, righting reflex, spontaneous movement, and PWR) were monitored as described for experiment 1. One mouse died immediately after the induction injection was given, and one mouse recovered from the surgical plane of anesthesia before the 35-min reversal time point; these 2 mice were excluded from further statistical analysis.
Experiment 3. Effects of alfaxalone on HR.
Female C57BL/6J (n = 6) and female CD1 (n = 4) mice were used to evaluate the effects of alfaxalone on HR, independent of other drugs. ECG data were recorded by using the ECGenie (Mouse Specifics) recording platform. Mice were placed on the platform, and a baseline HR recording was obtained. After the baseline ECG was recorded, the mice received alfaxalone (80 mg/kg IP), and HR was subsequently recorded at 5-min intervals. ECG was monitored continuously until the mouse was alert and no longer exhibited behavioral effects of anesthesia.
Experiment 4. Use of alfaxalone in a surgical procedure.
To test the ability of alfaxalone–xylazine to safely maintain a surgical plane of anesthesia in mice under actual surgical conditions, an experimental laparotomy was performed under alfaxalone (80 mg/kg IP) and xylazine (10 mg/kg IP) anesthesia. Given the results from this initial experiment, we performed 4 additional surgical experiments: 1) laparotomy with isoflurane anesthesia (n = 3); 2) laparotomy with a decreased dose of alfaxalone–xylazine (40A/10X IP; n = 8); 3) orthopedic surgery with alfaxalone–xylazine at 2 doses (80A/10X IP [n = 2] and 80A/8X IP [n = 3]); and 4) laparotomy with alfaxalone–xylazine (80A/10X) administered subcutaneously (n = 4). All surgeries were performed on female C57BL/6J mice. Procedures were conducted by 2 researchers, one who performed the surgeries and one who monitored anesthesia. The same researcher (JOM) was either the anesthetist or surgeon for all procedures and was responsible for the performance of the surgery and anesthetic monitoring.
For surgeries using isoflurane anesthesia, anesthesia was induced in a small induction chamber by using 4% isoflurane (Piramal Healthcare Limited, Andhra Pradesh, India). During the surgical procedure, anesthesia was maintained by using 1.7% to 2.5% isoflurane delivered in 600 mL/min oxygen and a closely fitting facemask. The isoflurane concentration was adjusted throughout each procedure, as is common practice during rodent surgeries—for example, the gas concentration might be increased to ensure negative PWR prior to incision or decreased during surgical closure. The isoflurane concentration delivered was verified by using an inhalant gas anesthetic monitor (Poet IQ2 Anesthetic Gas Monitor, Criticare Systems, Waukesha, WI), and waste anesthetic gas was scavenged through an activated charcoal canister (Omnicon F/Air Anesthesia Gas Filter Unit, AM Bickford, Wales Center, NY). For surgeries using subcutaneous alfaxalone–xylazine, mice were injected in the subcutaneous space between the scapulae. Anesthesia was monitored as for intraperitoneal drug administration.
The laparotomy procedure included opening of the abdomen and extensive manipulation of the abdominal contents and required approximately 30 min to complete with low risk of penetration of the gastrointestinal tract. Induction and anesthetic monitoring were performed as described for experiment 1, with recording of physiologic parameters every 5 min. LPWR was confirmed prior to the initial incision. After anesthesia induction, the mouse's abdomen was shaved and aseptically prepared. After surgical prep, mice received 0.05 to 0.1 mL local anesthetic, either lidocaine (Xylocaine 20 mg/mL, Fresenius Kabi, Lake Zurich, IL) or bupivacaine (5 mg/mL, Hospira, Lake Forest, IL) delivered subcutaneously along the ventral midline. The injection was delivered along the length of the incision. The choice of local anesthetic reflected the surgeon's preference and had no bearing on assessment of depth of anesthesia, which was based on PWR and thus anatomically distant from the site of the local anesthetic injection. After the abdomen and peritoneum were opened along the ventral midline by using Metzenbaum scissors, the abdominal contents were reflected to the left side of the abdomen, and the superior mesenteric artery was exposed for 5 min. The abdominal contents were then shifted to the right side of the abdomen, and the left kidney was isolated for 5 min. The abdominal contents were then replaced, and a routine 2-layer surgical closure was performed. The total surgery time was approximately 35 min, independent of type of anesthesia. For all of the surgical experiments, mice were euthanized by overdose of inhalant anesthetic once they had regained spontaneous movement.
In addition, an orthopedic surgical procedure was performed in C57BL/6J female mice (n = 5) by using either 80A/10X or 80A/8X intraperitoneally to test the efficacy of the anesthetic combination in a surgical procedure that did not involve the peritoneal cavity. After induction, the left hindlimb was shaved and aseptically prepared, and a subcutaneous injection of local anesthetic (as described earlier) was administered along the incision line. During the surgical approach, skin and muscle were incised over the lateral aspect of the femur, extending from the coxofemoral joint to the stifle. The femur was isolated, and a partial-thickness transverse incision was made through the periosteum and the cortex of the distal femur; after the incision, the bone remained exposed for 5 min. A second similar transverse incision was made proximal to the first femoral incision, followed by a second 5-min period of isolation. Muscle and skin were sutured closed in anatomic layers. When the mice regained spontaneous movement, they were euthanized through overdose of inhalant anesthetic.
Statistical analysis.
For experiment 1, the time to and duration of LORR and duration of LPWR (surgical plane anesthesia) were analyzed by 2-way ANOVA, with dose and sex as main effects through 45 min of anesthesia. Tukey posthoc analysis was performed when significant differences were found. HR and RR were analyzed by 3-way, repeated-measures ANOVA with dose, sex, and time after injection as the main effects; Tukey posthoc analysis was performed when significant differences were detected. Duration of LORR, duration of lack of spontaneous movement, and duration of LPWR were compared between female C57BL/6J and female CD1 mice by using t tests. To determine the responsiveness of the autonomic nervous system to a noxious stimulus, HR and RR were measured before and immediately after the touch test for PWR. Increases in HR of 10 bpm and in RR of 5 breaths per minute were considered to be the minimal detectable changes for routine monitoring in a research laboratory setting. Pearson correlation analysis was performed to determine the relationship between time to LORR, duration of LORR, and total time at a surgical plane of anesthesia (that is, duration of LPWR).
In experiment 2, the times from reversal injection to return of PWR and return of righting reflex were compared by using t tests, with reversal drug (atipamezole or saline control) as the main effect tested. For experiment 3, 2-way, repeated-measures ANOVA was used to compare HR at each time point, with main effects of time and strain. When significant differences were detected, Tukey posthoc analysis was performed. A second analysis compared HR between female C57BL/6J mice that received alfaxalone only (80 mg/kg) and female C57BL/ 6J mice from experiment 1 that received both alfaxalone (80 mg/kg) and xylazine (10 mg/kg), to determine the effects of xylazine in combination with alfaxalone. For experiment 4, the effects of subcutaneous compared with intraperitoneal alfaxalone–xylazine on HR and RR were compared between mice that underwent orthopedic surgery with intraperitoneal alfaxalone–xylazine (with data for both doses pooled) and those mice that received subcutaneous alfaxalone–xylazine for laparotomy. Significant differences were then tested by using Tukey posthoc analysis. All data were tested for a normal distribution and homogeneity of variance. In addition, values more than 3 SD from the mean were considered outliers and were excluded from statistical analysis. For all analyses, a P value of less than 0.05 was considered statistically significant. All statistical analyses were performed by using SigmaPlot 12.3 (Systat Software, San Jose, CA).
Results
Experiment 1. Dose response and physiologic monitoring.
This experiment comprised a total of 35 trials using C57BL/ 6J mice. Four mice (3 male, one female)—2 given 40 mg/kg alfaxalone–10 mg/kg xylazine, 1 given 80 mg/kg alfaxalone–10 mg/kg xylazine, and 1 given 120 mg/kg alfaxalone–10 mg/kg xylazine—failed to lose the righting reflex. Four mice (all female) died—3 given 120 mg/kg alfaxalone–10 mg/kg xylazine and one given 80 mg/kg alfaxalone–10 mg/kg xylazine; gross necropsy did not reveal any abnormalities. The time to LORR, duration of LORR, and duration of LPWR are reported in Table 1; the effect of drug dose on all 3 variables was significant (F2,24 = 13.14, P < 0.001; F2,24 = 12.40, P < 0.001; F2,24 = 10.93, P < 0.001, respectively). Significant sex-associated effects emerged for time to LORR (F1,24 = 19.01, P < 0.001) and duration LORR (F1,24 = 5.32, P = 0.03); however, duration of LPWR did not differ between sexes (P = 0.10). Data regarding strain-associated differences are reported in the section for experiment 3. Induction of anesthesia with alfaxalone–xylazine resulted in muscle twitching, but this side effect was generally mild and not considered to be a clinical or welfare concern for the mice in these experiments.
Table 1.
Response of the mice to the 3 doses of alfaxalone–xylazine anesthesia
| Dose (mg/kg) alfaxalone / xylazine | Strain | Sex | No. of mortalities | No. that achieved LORR | Time (min) to LORR | Duration (min) of LORR | No. that achieved LPWR | Duration (min) of LPWR |
| 40/10 | C57BL/6J | Female (n = 5) | 0 | 5 | 2.4 ± 0.4 (2.0–3.0)a | 41.2 ± 6.3 (35.5–50.0)e | 5 | 27.4 ± 15.4 (5–45)e |
| 40/10 | C57BL/6J | Male (n = 7) | 0 | 5 | 3.4 ± 0.3 (3.0–3.8)c | 34.1 ± 9.9 (19.0–46.3)c | 3 | 9.0 ± 10.8 (0–25)c |
| 80/10 | C57BL/6J | Female (n = 7) | 1 | 5 | 1.9 ± 0.1 (1.8–2.0) | 94.8 ± 48.9 (68.0–168.0)e,f | 5 | 70.0 ± 49.5 (15.0–135.0)e,f |
| 80/10 | C57BL/6J | Male (n = 5) | 0 | 5 | 2.2 ± 0.4 (1.8–2.5)d | 68.4 ± 7.2 (57.5–77.5)d | 5 | 55.8 ± 15.0 (35.0–75.0)d |
| 120/10 | C57BL/6J | Female (n = 5) | 3 | 2 | 1.8 ± 0.3 (1.2–2.0)a | 153.0 ± 14.1 (143.0–153.0)a,f | 2 | 107.5 ± 24.7 (90–125)f |
| 120/10 | C57BL/6J | Male (n = 6) | 0 | 5 | 2.6 ± 0.7 (2–3.8)d | 99.5 ± 28.6 (70.5–130.0)d | 5 | 79.0 ± 28.8 (50.0–110.0)d |
| 80/10 | CD1 | Female (n = 8) | 0 | 8 | 2.5 ± 0.5 (2.0–3.0) | 67.7 ± 18.1 (37.0–87.0) | 7 | 17.5 ± 10.3 (0–30.0)b |
LORR, loss of righting reflex; LPWR, loss of pedal withdrawal reflex.
Data are given as mean ± 1 SD (range)
Significant differences in duration of LORR were noted across doses (P > 0.001) and between sexes (P = 0.03). Significant differences in duration of LPWR were found between doses (P > 0.001).
Significant (P < 0.05) difference between male and female mice receiving the same dose of anesthetic.
Significant (P < 0.05) difference between strains for female mice receiving the same dose of alfaxalone–xylazine.
Different lowercase letters indicate significant (P <0.05) difference between doses within parameter and male sex.
Different lowercase letters indicate significant (P <0.05) difference between doses within parameter and female sex.
We tested the responsiveness of the autonomic nervous system by monitoring for a detectable change in HR (at least 10 bpm) or RR (at least 5 breaths per minute) in response to the Touch Test noxious stimulus. We measured this response in the presence of a positive PWR, when we were confident that mice were at an anesthetic plane with intact autonomic reflexes.7 Despite mice being at a lighter plane of anesthesia (that is positive PWR), detectable increases in HR occurred at only 6 of 70 time points, and detectable increases in RR were present at only 9 of 63 time points had after the noxious stimulus. The 95% CI were: for HR: before Touch Test, 315 to 325 bpm; after Touch Test, 315 to 325 bpm; for RR: before Touch Test, 148 to 150 breaths per minute; after Touch Test, 146 to 149 breaths per minute. These results demonstrate that subtle differences in depth of anesthesia after loss of the PWR are not reflected as changes in HR or RR in response to a noxious stimulus when mice have been anesthetized by using an alfaxalone–xylazine combination.
We were unable to use the current results to assess the HR and RR of survivors compared with mice that died, to analyze whether these parameters might function as predictors of impending anesthetic arrest. Because 7 of the 9 deaths occurred within 15 min of induction, no stable baselines or trends in HR and RR were established before cardiac arrest occurred.
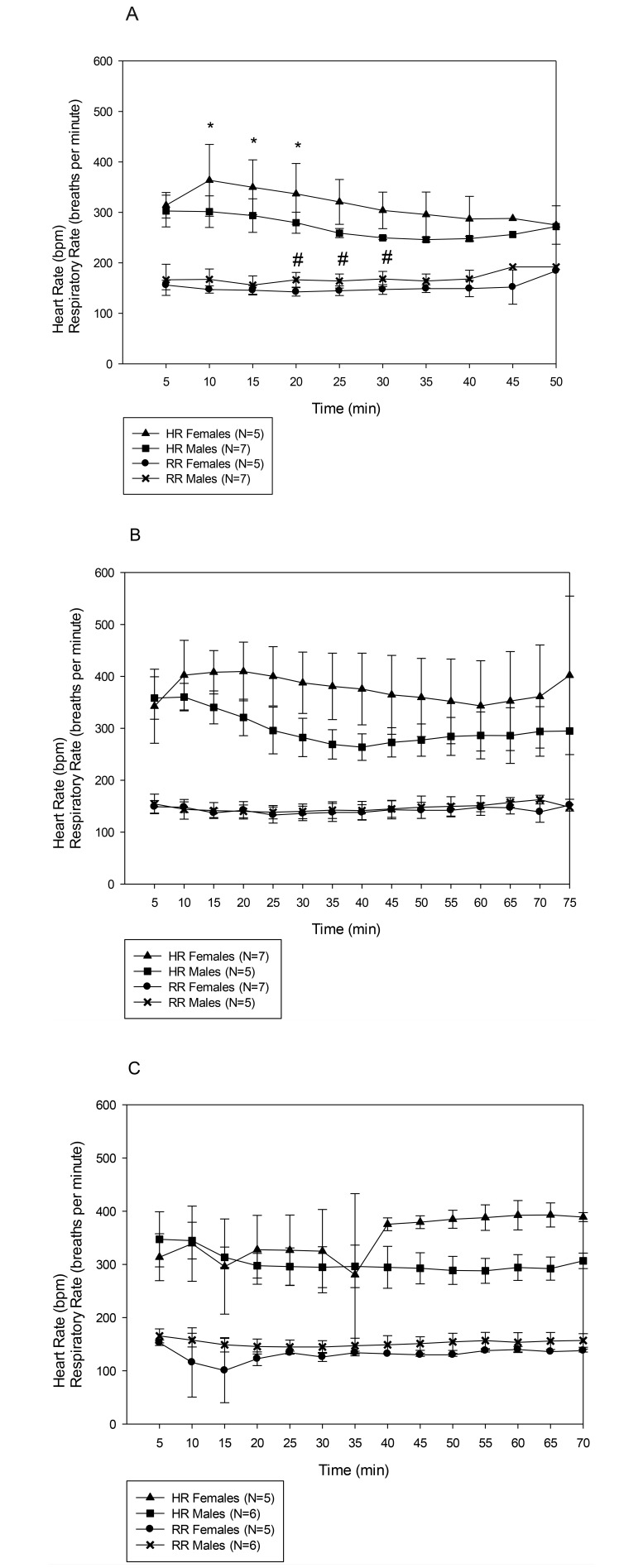
Figure 1 represents the HR and RR of male and female mice at 3 doses of alfaxalone–xylazine. Three-way ANOVA demonstrated that dose, sex, and time each exerted significant effects on both HR (F2,181 = 21.09, P < 0.001; F1,181= 53.61, P < 0.001; F8,181 = 3.43, P = 0.001, respectively) and RR (F2,175 = 19.95, P < 0.001; F1,175= 33.84, P < 0.001; F8,175 = 2.39, P < 0.018, respectively). In addition, the interaction between dose and sex was significant for both HR (F2,181 = 6.75, P = 0.001) and RR (F2,175 = 6.43, P = 0.002) and for the HR interaction between sex and time (F8,181 = 2.15, P = 0.033). On posthoc analysis, HR and RR differed significantly between male and female mice at the 40A/10X dose, HR differed between sexes at the 80A/10X dose, and RR differed between sexes at the 120A/10X dose (P < 0.05 for all comparisons).
Figure 1.
Heart rate (HR) and respiratory rate (RR) of male and female C57BL/6J mice under 3 doses of alfaxalone–xylazine: (A) 40A/10X. (B) 80A/10X. (C) 120A/10X . Results are given as mean ± 1 SD. Three-way ANOVA revealed significant differences in both HR and RR in dose (P < 0.001), sex (P < 0.001), and time (HR, P = 0.001; RR, P = 0.018). In addition, the interaction between dose and sex was significant for HR (P = 0.001) and RR (P = 0.002) and between sex and time (P = 0.033). Statistical analysis extended from 5 min to 45 min. *, HR significantly different between male and female mice; #, RR significantly different between male and female mice.
Strong correlation between time to LORR and the duration at a surgical plane of anesthesia would help anesthetists to predict the need for and timing of anesthetic intervention. However, neither the correlation between time to LORR and duration of LORR nor between time to LORR and duration at a surgical plane of anesthesia was significant (P = 0.31 and P = 0.36, respectively).
Experiment 2: Atipamezole reversal of alfaxalone–xylazine anesthesia.
Time to regain PWR differed (P < 0.001) between atipamezole (3.3 ± 1.3 min; range, 1.5 to 5 min) and saline control (26.7 ± 10.5 min; range, 15 to 35 min) , but the time to return of righting reflex did not differ (P = 0.17 between atipamezole (20.2 ± 16.3 min; range, 3 to 47 min) and saline control (37.6 ± 17.2 min; range, 21.8 to 56 min). One additional mouse was anesthetized for this experiment but died prior to atipamezole administration. On gross necropsy, a focal pinpoint hemorrhage was noted along the dorsal body wall just lateral to midline, indicating that the needle may have penetrated too deeply and caused additional trauma during injection. There were no significant differences in HR and RR between the mice used in this experiment and those that received the 80A/10X dose in experiment 1.
Experiment 3: Effects of alfaxalone on HR.
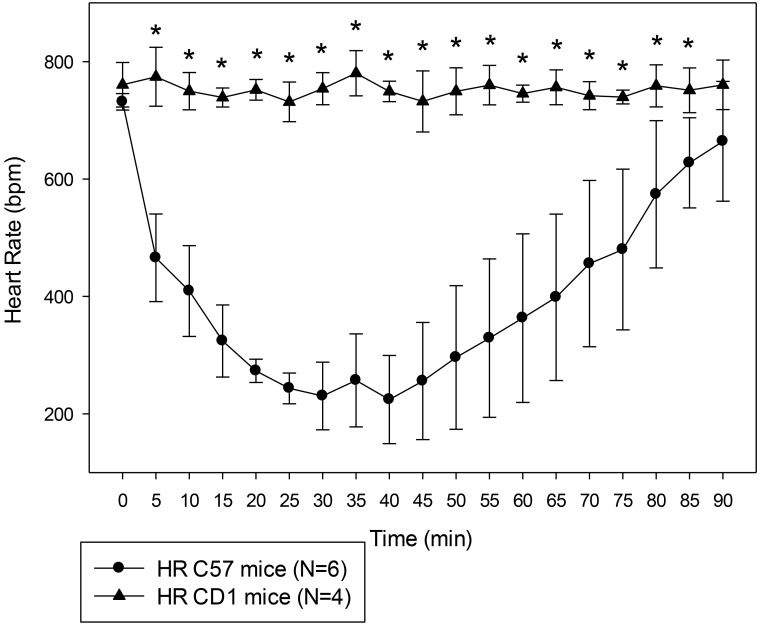
Mice that received alfaxalone only did not become fully anesthetized at any time point. The HR of C57BL/6J and CD1 female mice after alfaxalone administration are shown in Figure 2. The effects of time (F18,140 = 22.36, P < 0.001), strain (F1,140 = 72.27, P < 0.001), and their interaction (F18,140 = 20.73, P < 0.001) were all significant. All of the time points differed between strains except for the baseline reading before the injection and the last time point, with C57BL/6J mice having lower HR than CD1 mice. Given these results, we subsequently tested the differences between the anesthetic effects of 80 mg/kg alfaxalone–10 mg/kg xylazine on the 2 strains.
Figure 2.
HR of C57BL/6J and CD1 female mice that received 80 mg/kg alfaxalone only. Results are given as mean ± 1 SD. Statistical analysis extended from 5 to 45 min. *, Significant (P < 0.05) difference in HR between the 2 strains of mice.
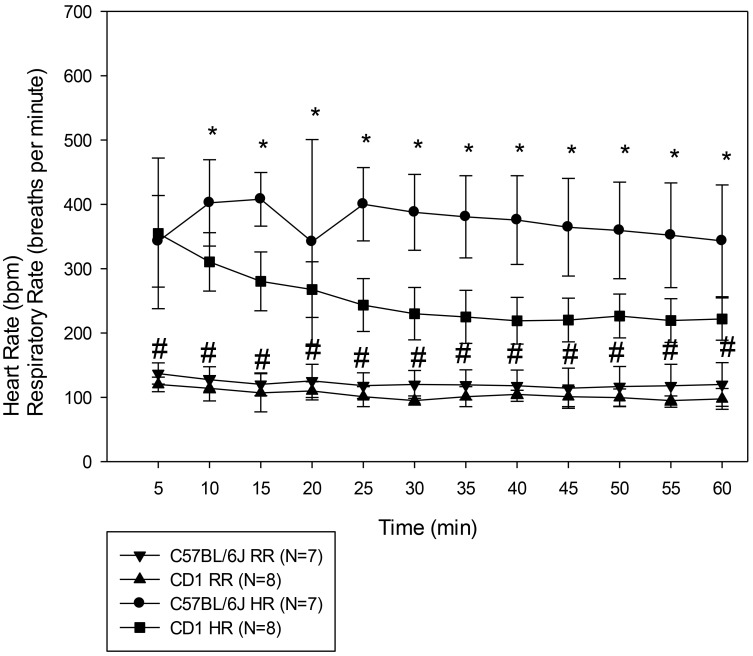
We found that CD1 mice were less sensitive to the drug combination than C57BL/6J mice (Table 1), with significantly shorter duration of LPWR and loss of spontaneous movement compared with C57BL/6J mice (P = 0.013 and P = 0.04, respectively). The total duration of LORR did not differ between strains (P = 0.24). Not only was the anesthetic response to the alfaxalone–xylazine protocol significantly different, but the HR and RR responses also differed between the strains (F1,105 = 13.31, P = 0.004 and F1,105 = 21.34, P < 0.001, respectively; Figure 3). Posthoc analysis revealed that both HR and RR differed between the 2 strains at all time points, except for HR at the 5-min time point.
Figure 3.
HR and RR of female C57BL/6J and CD1 mice that received 80 mg/kg alfaxalone and 10 mg/kg xylazine. Results are given as mean ± 1 SD. *, HR significantly (P < 0.05) different between the 2 strains of mice; #, RR significantly (P < 0.05) different between the 2 strains of mice.
Experiment 4: Use of alfaxalone in a surgical procedure.
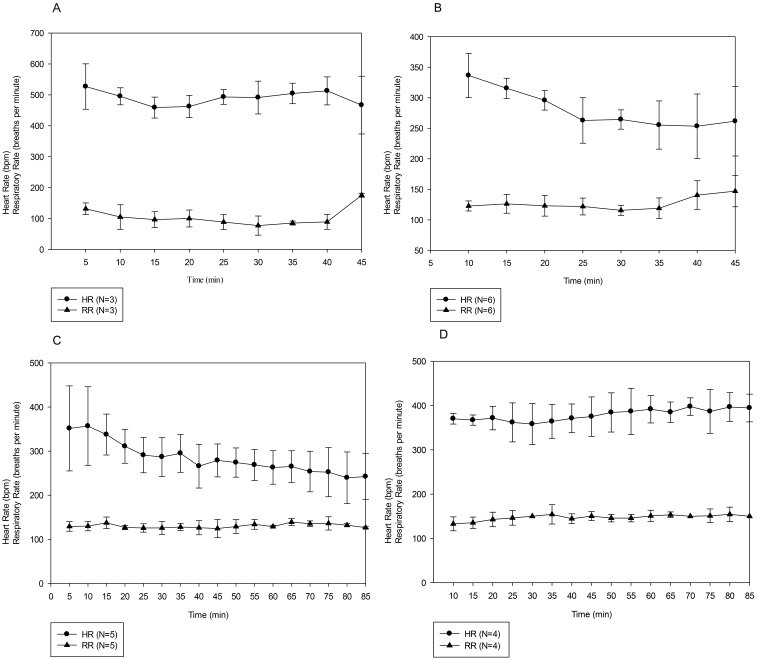
Our noxious stimulus, the 300-g Touch Test, provides a relatively mild stimulus to assess the surgical plane of anesthesia. To confirm that the alfaxalone–xylazine combination achieved a sufficient plane of anesthesia for more painful and invasive surgeries, we performed laparotomies on 4 mice anesthetized by using 80 mg/kg alfaxalone–10 mg/kg xylazine anesthesia. However, all 4 of the mice that received this dose died after the incision into the peritoneum; 3 of the 4 mice died within 10 min of the start of surgery, and the remaining mouse died 25 min after incision. In light of these results and those from the mouse that died in experiment 2 with evidence of focal, mild hemorrhage in the peritoneal lining, we hypothesized that peritoneal trauma and subsequent alteration in drug absorption may have contributed to the anesthetic deaths. To test this hypothesis, we performed 4 additional surgical experiments: 1) laparotomy with isoflurane anesthesia; 2) laparotomy with a decreased dose of intraperitoneal alfaxalone–xylazine (40A/10X); 3) orthopedic surgery with intraperitoneal 80A/8X or 80A/10X; and 4) laparotomy with 80A/10X administered subcutaneously. All 3 mice survived the laparotomy procedure using isoflurane anesthesia. A total of 8 mice underwent laparotomy at the decreased alfaxalone–xylazine dose: 4 mice survived, 2 mice died shortly after the abdominal incision, and 2 mice died after surgery completion but before the return of spontaneous movement. Of the 5 mice that underwent the orthopedic procedure, 4 survived, and the remaining animal died after the completion of surgery. All 4 mice that underwent laparotomy with subcutaneous administration of alfaxalone–xylazine survived the procedure. The subcutaneous anesthetic protocol provided 57.5 ± 22.5 min (range, 40 to 90 min) of surgical-plane anesthesia.
Figure 4 shows the HR and RR of the mice in each of these 4 additional surgical groups; the mice in the initial surgical group that received the higher dose of intraperitoneal alfaxalone–xylazine IP died too rapidly to collect reportable data and thus are not included. The HR and RR at the 40-min time in the isoflurane-anesthetized mice (Figure 4 A) are likely attributable to adjustments in isoflurane concentration, which was decreased during closure of the surgical incisions; the decrease in HR was probably a compensatory response to increased blood pressure, and the increase in RR likely reflects a direct response to the lower isoflurane concentration. The HR and RR of the 6 mice that received 40A/10X and survived through surgery are reported in Figure 4 B; those of the 5 mice undergoing orthopedic surgery are shown in Figure 4 C (responses did not differ significantly between doses, so data were pooled for analysis). The results from the 4 mice administered alfaxalone–xylazine subcutaneously for laparotomy (Figure 4 D) show significant differences due to route of administration for both HR (F1,87 = 16.35, P = 0.007) and RR (F1,90 = 11.47, P = 0.011). Posthoc analysis revealed that HR differed between routes at 25 min and thereafter. The RR differed significantly between the 2 routes of administration from 25 min after injection through 45 min and then again at the 60-min time point.
Figure 4.
HR and RR of mice undergoing (A) laparotomy with isoflurane anesthesia, (B) laparotomy with 40A/10X intraperitoneally, (C) orthopedic surgery with 80A/10X or 80A/8X intraperitoneally, and D) laparotomy with 80A/10X subcutaneously. Results are given as mean ± 1 SD. Panel B includes data from the 4 mice that survived the procedure and the 2 mice that died after the completion of surgery.
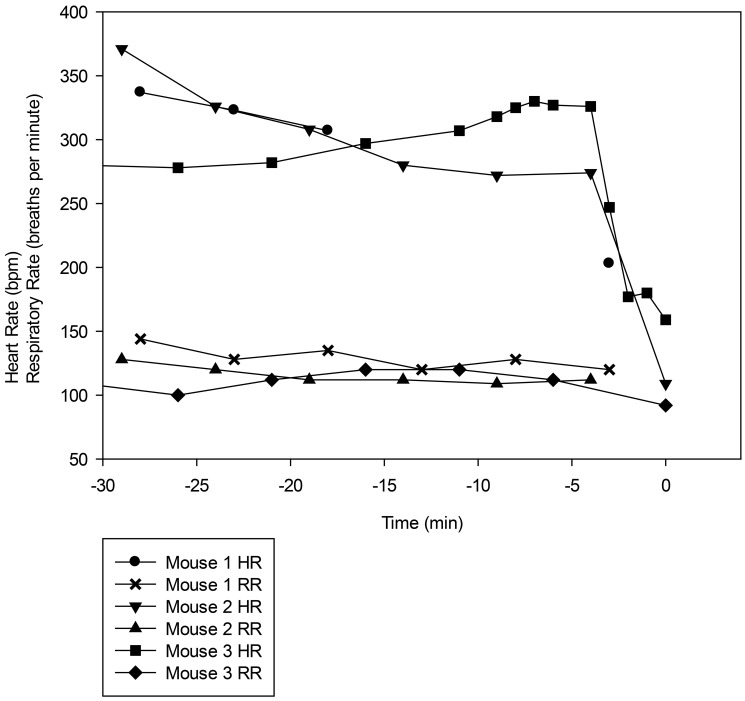
Three mice died after the completion of surgery and prior to regaining PWR (2 after laparotomy under alfaxalone–xylazine, and one after the orthopedic surgery). The HR and RR of these 3 mice (Figure 5) show rapid declines in HR and RR prior to the arrest, demonstrating the importance of careful monitoring of mice in the postsurgical period. Two additional mice died during this project that are not reported in the data elsewhere. One died during the pilot portion of the project, and the other inadvertently received an intraperitoneal injection of lidocaine prior to the laparotomy incision.
Figure 5.
HR and RR of the 3 mice that died after the completion of surgery. Mouse 1 underwent orthopedic surgery, and mice 2 and 3 underwent laparotomy at 40A/10X. T = 0 is defined as the onset of agonal breathing, from which no mice recovered in any of the experiments. Note that HR and RR were stable until immediately prior to the onset of agonal breathing.
Discussion
Identification and refinement of safe and reliable anesthetic regimens for rodents remains a challenge in biomedical research. Isoflurane is generally recommended for rodent anesthesia,15 but particular scenarios or scientific aims may preclude the use of gas anesthetics. Recent work has provided evidence that alfaxalone anesthetic combinations may offer a viable injectable alternative for anesthesia of mice.21,34 In the current study, alfaxalone–xylazine successfully produced sedation, immobilization, and surgical anesthesia in mice. However the use of alfaxalone–xylazine under actual surgical conditions yielded mixed results. Although intraperitoneal alfaxalone–xylazine was used successfully in a model of orthopedic surgery, the same regimen used during laparotomy resulted in unacceptably high mortality. For laparotomy procedures, subcutaneous administration of alfaxalone–xylazine is a viable alternative to intraperitoneal dosing.
Similar to a previous study,34 we found that alfaxalone–xylazine produced dose-dependent sedation and surgical anesthesia, with some mice remaining at a surgical plane for more than 2 h after a single injection at high doses. In addition, average time to loss of the righting reflex and time to achieve a surgical plane of anesthesia were similar to data from previous studies of alfaxalone–xylazine, ketamine–xylazine, and ketamine– xylazine–acepromazine.1,6,34 Unlike previous studies, which did not report any deaths after alfaxalone anesthesia, we observed a low rate of anesthetic mortalities at increased doses of alfaxalone–xylazine in surgically unmanipulated female mice and in animals undergoing experimental laparotomy.
We performed experimental laparotomy to evaluate the efficacy of alfaxalone–xylazine under actual surgical conditions using 80 mg/kg alfaxalone–10 mg/kg xylazine or 40 mg/kg alfaxalone–10 mg/kg xylazine, doses that we determined were successful in maintaining surgical anesthesia, as assessed through PWR. Unfortunately, the higher dose resulted in 100% intraoperative mortality, and the lower dose resulted in 25% acute mortality, with most animals arresting shortly after the initial surgical incision. To confirm that the deaths were associated with the anesthetic protocol, we performed the same surgery in isoflurane-anesthetized mice, all of which survived the procedure without adverse effects. To confirm that the alfaxalone–xylazine-induced deaths were associated with both intraperitoneal administration of the anesthetics and laparotomy, we tested the higher alfaxalone–xylazine regimen by using an orthopedic surgery model combined with IP administration and a laparotomy model with subcutaneous dosing. In these experiments, 2 of 3 mice having orthopedic surgery survived, and the third mouse died after the completion of the surgery—much later than the deaths that occurred during laparotomy. In addition, all 4 of the mice that received the anesthetic drugs subcutaneously survived laparotomy. Based on these results, we hypothesize that the deaths after intraperitoneal alfaxalone–xylazine and laparotomy were secondary to altered peritoneal blood flow—resulting from either visceral manipulation or peritoneal trauma due to injection or incision— and subsequent variation in peritoneal drug absorption. However, a definitive explanation, including the relative effects of alfaxalone and xylazine, requires additional analysis, including pharmacokinetics and measurement of anesthetic blood levels, and is beyond the scope of the present study. Ultimately, these results lead to our recommendation that alfaxalone–xylazine anesthesia may be administered intraperitoneally for procedures that do not require entry into the peritoneal cavity. Subcutaneous alfaxalone–xylazine may be a viable alternative for laparotomy and other procedures, although further study is recommended to determine optimal drug doses. For many experiments and surgeries, the route selected for alfaxalone–xylazine administration (intraperitoneal compared with subcutaneous) may depend on the preference of the researcher. Both a previous study21 and the current investigation compared subcutaneous with intraperitoneal administration; the previous study21 reported that the subcutaneous route of administration of alfaxalone, in combination with butorphanol and medetomidine, was significantly more effective in achieving a surgical plane of anesthesia than intraperitoneal administration, highlighting the potential for varying efficacy between different anesthetic combinations. In the current study, we found that either route was effective in producing a surgical plane of anesthesia but that the intraperitoneal route was contraindicated during laparotomy.
Alfaxalone can be combined with various other anesthetics to produce effective multimodal anesthesia, but pilot studies may be necessary to optimize dosage depending on the strain of mouse, sex, route of administration, duration of the procedure, and depth of anesthesia required. Another important consideration is the role of local anesthetics—both short-acting (lidocaine) and long-acting (bupivacaine)—in the anesthesia protocol. The degree of surgical stimulation is a critical variable in determining the anesthetic dose to administer, and local anesthetics modulate the level of stimulation during incision into the abdomen. Further testing of alfaxalone-based anesthesia in mice, focusing on the effects of experiment-dependent variables, will provide researchers with more options for safe anesthesia that minimize effects on experimental variables.
Our results demonstrated the efficacy of atipamezole at reversing alfaxalone–xylazine anesthesia in mice. Xylazine was incorporated into our anesthetic regimen because of previous work that found that combining xylazine with alfaxalone greatly reduced myoclonic activity and other abnormal behaviors observed with alfaxalone administered alone.34 Other injectable rodent anesthetic regimens—incorporating xylazine and ketamine–xylazine combinations most commonly—can be reversed through the administration of α2 antagonists such as yohimbine and atipamezole.26 Reversal agents can reduce anesthetic recovery time when administered at the end of a procedure and may even prevent mortality when given to animals in early stages of cardiopulmonary arrest.2,15,23,24,26,27 Three mice in the current investigation died after the completion of the surgical procedure (2 after low-dose alfaxalone and laparotomy and one after orthopedic surgery). Previous studies in dogs, cats, and rabbits have shown that more than 45% of anesthetic deaths occur after the completion of surgery and discontinuation of anesthesia.5,10,32 Anecdotal reports suggest that postanesthetic death is a common event in mice also. Atipamezole administration for anesthetic reversal when mice demonstrated agonal breathing after ketamine–xylazine–acepromazine anesthesia was successful in almost 50% of cases even at this advanced state of arrest.24 Although not used during surgical experiments in this study, we surmise that atipamezole reversal would have been beneficial, particularly for the mice that arrested and experienced bradycardia after surgery either due to the removal of the surgical stimulation or changes in blood pressure. Provided adequate perioperative analgesia has been given, there are few reasons to have an animal remain at a surgical plane of anesthesia postoperatively, and the use of reversal agents, like atipamezole, should be strongly encouraged in our field. In addition, the HR and RR data of the 3 arresting mice highlight the importance of the routine administration of reversal agents postoperatively. As Figure 5 shows, the HR and RR dropped very rapidly before the animals became agonal. This rapid drop occurred during a period when the intensity of monitoring may be reduced, such as during the postoperative period. As discussed earlier, the administration of atipamezole may have prevented these deaths from occurring. Our recommendation is that, whenever possible, reversal agents should be included as an integral component of injectable anesthetic regimens, and careful monitoring of the mice should continue even after the completion of the surgical procedure.
Our group's prior work has evaluated changes in HR and RR in mice anesthetized with ketamine–xylazine or isoflurane in response to a nonsurgical noxious stimulus.13 Consistent with the previous ketamine–xylazine results, experiment 1 showed that the HR and RR responses to a noxious stimulus during alfaxalone–xylazine anesthesia could not be used reliably to determine subtle changes in the depth of anesthesia. Inclusion of blood pressure monitoring might detect more subtle changes in autonomic nervous activity, but we did not include this feature in the current study because this monitoring is not routinely applied in the vast majority of experimental mouse surgeries. In addition, the current study provides the first data on HR and RR in mice anesthetized with isoflurane and alfaxalone–xylazine under actual surgical conditions. Direct comparison between the HR and RR in the current study with the 2 previous studies reporting these parameters in isoflurane-anesthetized mice is difficult, given that those studies maintained isoflurane at a constant 2%, whereas the current study varied the isoflurane concentration depending on the surgical events and depth of anesthesia. Despite this important difference, the mice receiving surgical stimulation in the current study tended to have higher respiratory rates than in the 2 previous studies, consistent with an autonomic response to a noxious surgical stimulation.13,37 It is important to remember that this stimulus is not considered a painful stimulus, because the animal is unconscious at a surgical plane of anesthesia and cannot, by definition, experience pain while unconscious. Although not performed in the present study, the addition of blood pressure monitoring during anesthesia would be of great value, because some evidence suggests that blood pressure is more sensitive to subtle changes in anesthetic depth than HR or RR.19,25 Although extensive anesthetic monitoring of physiologic parameters like blood pressure in mice would be ideal and potentially allow finer control of anesthetic depth, this level of monitoring is not always practical in a research setting due to the small size of murine patients and the reality that many rodent surgeries are performed by a single researcher who is responsible for all aspects of the procedure, including aseptic surgery and anesthetic monitoring.
Significant strain- and sex-associated differences occurred after alfaxalone–xylazine administration, including those affecting anesthetic response and physiologic parameters. Compared with outbred CD1 mice, C57BL/6J mice appeared more sensitive to the effects alfaxalone, demonstrating longer duration of sedation and anesthesia at identical drug doses. Similarly, when C57BL/6J mice received alfaxalone only, we observed profound changes in HR, whereas alfaxalone had little effect on HR in CD1 mice. Researchers and veterinarians can expect to see interstrain differences in anesthetic response.12,18,35 Although previous studies have evaluated the sensitivity of different mouse strains to anesthetics, little work has compared the sensitivities of the 2 strains used in the current investigation.
Sex-associated differences in response to anesthetics and other drugs have been recognized in many species, including mice.13,16,29,34,38 Consistent with previous findings in mice and rats,14,28,34 we found that female C57BL/6J mice experienced a longer duration of anesthesia in response to intraperitoneal alfaxalone–xylazine than did male mice. Notably, previous studies in rats found sex-associated differences in responses when alfaxalone was administered intraperitoneally but not intravenously,14,28 suggesting potential differences in peritoneal absorption between male and female mice. Further work is needed to elucidate sex-associated differences in response to alfaxalone–xylazine; in the meantime, these differences should be considered when administering this anesthetic protocol to mice and when developing new anesthetic protocols.
When anesthetizing mice for surgeries not involving the abdomen, we recommend the following intraperitoneal doses of alfaxalone–xylazine for future studies: 80 to 120 mg/kg alfaxalone with 10 mg/mg xylazine for male C57BL/6J mice, 40 to 80 mg/kg alfaxalone with 10 mg/kg xylazine for female C57BL/6J mice, and 80 mg/kg alfaxalone with 10 mg/kg xylazine in female CD1 mice. Given our findings, we advise against intraperitoneal administration of this combination for laparotomy. When performing a procedure involving the peritoneal cavity, subcutaneous administration of alfaxalone–xylazine is recommended, although further study is needed to optimize dose recommendations. These doses should be tested and modified as needed to suit the animals, scientific aims, and potential inclusion of systemic preoperative analgesics. As with any anesthetic regimen, researchers should consider a variety of anesthetics when developing new anesthetic regimens or applying them to novel procedures. This flexibility allows for optimization of the anesthetic protocol for the specific experimental needs and conditions, including the sex, strain, age, and health of the animals; the invasiveness and duration of the procedure; the experience of the surgeon; and the availability and quality of anesthetic monitoring.
In conclusion, the present study demonstrates that intraperitoneal alfaxalone–xylazine provided effective immobilization and anesthesia which may be suitable for orthopedic surgeries, imaging, or other minimally invasive procedures. Intraperitoneal administration of this anesthetic regimen is not recommended for mice undergoing laparotomy due to unacceptably high mortality rates - subcutaneous administration should be considered in these cases. Further evaluation is necessary to determine the efficacy of this anesthetic regimen for other types of surgeries, such as thoracotomies and surgeries involving the head and neck. Atipamezole provided effective anesthetic reversal, and we recommend the inclusion of anesthetic reversal as a component of any alfaxalone–xylazine regimen to shorten recovery times and to potentially prevent postsurgical anesthetic mortality. Finally, significant strain- and sex- associated differences were observed in both anesthetic responses and cardiovascular parameters, demonstrating the need for testing and titration of doses for specific experimental contexts.
Acknowledgments
Funding for this project was provided by the Office of the Vice Provost for Research, University of Pennsylvania. We thank Dr Lon Kendall for his support in the initiation of this project.
References
- 1.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 2.Baker NJ, Schofield JC, Caswell MD, McLellan AD. 2011. Effects of early atipamezole reversal of medetomidine–ketamine anesthesia in mice. J Am Assoc Lab Anim Sci 50:916–920. [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand HGMJ, Sandersen C, Murray J, Flecknell PA. 2017. A combination of alfaxalone, medetomidine and midazolam for the chemical immobilization of rhesus macaques (Macaca mulatta): preliminary results. J Med Primatol 46:332–336. 10.1111/jmp.12315. [DOI] [PubMed] [Google Scholar]
- 4.Bigby SE, Carter JE, Bauquier S, Beths T. 2017. The use of alfaxalone for premedication, induction, and maintenance of anaesthesia in pigs: a pilot study. Vet Anaesth Analg 44:905–909. 10.1016/j.vaa.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Brodbelt D. 2009. Perioperative mortality in small animal anaesthesia. Vet J 182:152–161. 10.1016/j.tvjl.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 7.Campagna JA, Miller KW, Forman SA. 2003. Mechanisms of actions of inhaled anesthetics. N Engl J Med 348:2110–2124. 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 8.Casoni D, Amen EM, Brecheisen M, Kuennecke B, Muggler T, Bergadano A. 2015. A combination of alfaxalone and medetomidine followed by an alfaxalone continuous-rate infusion in cynomolgus monkeys (Macaca fascicularis) undergoing pharmacoMRS. Vet Anaesth Analg 42:552–554. 10.1111/vaa.12267. [DOI] [PubMed] [Google Scholar]
- 9.Costa D, Leiva M, Moll X, Aguilar A, Pena T, Andaluz A. 2015. Alfaxalone versus propofol in dogs: a randomised trial to assess effects on peri-induction tear production, intraocular pressure and globe position. Vet Rec 176:73–73. 10.1136/vr.102621. [DOI] [PubMed] [Google Scholar]
- 10.DeLay J. 2016. Perianesthetic mortality in domestic animals: a retrospective study of postmortem lesions and review of autopsy procedures. Vet Pathol 53:1078–1086. 10.1177/0300985816655853. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch J, Ekiri A, de Vries A. 2017. Alfaxalone for maintenance of anaesthesia in ponies undergoing field castration: continuous infusion compared with intravenous boluses. Vet Anaesth Analg 44:832–840. 10.1016/j.vaa.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Dockstader CL, van der Kooy D. 2001. Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J Neurosci 21:9077–9081. 10.1523/JNEUROSCI.21-22-09077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson RL, Terzi MC, Jaber SM, Hankenson FC, McKinstry-Wu A, Kelz MB, Marx JO. 2016. Intraperitoneal continuous-rate infusion for the maintenance of anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 55:548–557. [PMC free article] [PubMed] [Google Scholar]
- 14.Fink G, Sarkar DK, Dow RC, Dick H, Borthwick N, Malnick S, Twine M. 1982. Sex difference in response to alphaxalone anaesthesia may be oestrogen-dependent. Nature 298:270–272. 10.1038/298270a0. [DOI] [PubMed] [Google Scholar]
- 15.Fish R, Danneman P, Brown M, Karas A. 2008. Anesthesia and analgesia in laboratory animals. Burlington (MA): Academic Press. [Google Scholar]
- 16.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and ‘depressed’ mice exposed to chronic mild stress. Neuroscience 290:49–60. 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Granfone MC, Walker JM, Smith LJ. 2018. Evaluation of an intramuscular butorphanol and alfaxalone protocol for feline blood donation: a pilot study. J Feline Med Surg 20:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griebel G, Belzung C, Perrault G, Sanger DJ. 2000. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148:164–170. 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 19.Haga HA, Tevik A, Moerch H. 2001. Electroencephalographic and cardiovascular indicators of nociception during isoflurane anaesthesia in pigs. Vet Anaesth Analg 28:126–131. 10.1046/j.1467-2987.2001.00051.x. [DOI] [PubMed] [Google Scholar]
- 20.Herbert GL, Bowlt KL, Ford-Fennah V, Covey-Crump GL, Murrell JC. 2013. Alfaxalone for total intravenous anaesthesia in dogs undergoing ovariohysterectomy: a comparison of premedication with acepromazine or dexmedetomidine. Vet Anaesth Analg 40:124–133. 10.1111/j.1467-2995.2012.00752.x. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi S, Yamada R, Hashimoto A, Miyoshi K, Yamashita K, Ohsugi T. 2016. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn J Vet Res 64:131–139. [PubMed] [Google Scholar]
- 22.Hill WA, Tubbs JT, Carter CL, Czarra JA, Newkirk KM, Sparer TE, Rohrbach B, Egger CM. 2013. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J Am Assoc Lab Anim Sci 52:176–179. [PMC free article] [PubMed] [Google Scholar]
- 23.Izer JM, Whitcomb TL, Wilson RP. 2014. Atipamezole reverses ketamine–dexmedetomidine anesthesia without altering the antinociceptive effects of butorphanol and buprenorphine in female C57BL/6J mice. J Am Assoc Lab Anim Sci 53:675–683. [PMC free article] [PubMed] [Google Scholar]
- 24.Jaber SM, Hankenson FC, Heng K, McKinstry-Wu A, Kelz MB, Marx JO. 2014. Dose regimens, variability, and complications associated with using repeat-bolus dosing of extend a surgical plane of anesthesia in laboratory mice. J Am Assoc Lab Anim Sci 53:684–691. [PMC free article] [PubMed] [Google Scholar]
- 25.Jaber SM, Sullivan S, Hankenson FC, Kilbaugh TJ, Margulies SS. 2015. Comparison of heart rate and blood pressure with toe pinch and bispectral index for monitoring the depth of anesthesia in piglets. J Am Assoc Lab Anim Sci 54:536–544. [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen CF, Maiello P, Wright MJ, Jr, Kracinovsky KB, Newsome JT. 2017. Comparison of atipamezole with yohimbine for antagonism of xylazine in mice anesthetized with ketamine and xylazine. J Am Assoc Lab Anim Sci 56:142–147. [PMC free article] [PubMed] [Google Scholar]
- 27.Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Saito Y, Takeuchi T. 2015. Anesthetic effects of a three-drugs mixture–comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp Anim 64:39–47. 10.1538/expanim.14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau C, Ranasinghe MG, Shiels I, Keates H, Pasloske K, Bellingham MC. 2013. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan in rats. J Vet Pharmacol Ther 36:516–520. 10.1111/jvp.12055. [DOI] [PubMed] [Google Scholar]
- 29.Levin-Arama M, Abraham L, Waner T, Harmelin A, Steinberg DM, Lahav T, Harlev M. 2016. Subcutaneous compared with intraperitoneal ketaminexylazine for anesthesia of mice. J Am Assoc Lab Anim Sci 55:794–800. [PMC free article] [PubMed] [Google Scholar]
- 30.Liao P, Sinclair M, Valverde A, Mosley C, Chalmers H, Mackenzie S, Hanna B. 2017. Induction dose and recovery quality of propofol and alfaxalone with or without midazolam coinduction followed by total intravenous anesthesia in dogs. Vet Anaesth Analg 44:1016–1026. 10.1016/j.vaa.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Lieggi CC, Artwohl JE, Leszczynski JK, Rodriguez NA, Fickbohm BL, Fortman JD. 2005. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci 44:17–22. [PubMed] [Google Scholar]
- 32.Matthews NS, Mohn TJ, Yang M, Spofford N, Marsh A, Faunt K, Lund EM, Lefebvre SL. 2017. Factors associated with anesthetic-related death in dogs and cats in primary-care veterinary hospitals. J Am Vet Med Assoc 250:655–665. 10.2460/javma.250.6.655. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz KA, Robertson SA, Wilson DV. 2017. Alfaxalone alone or combined with midazolam or ketamine in dogs: intubation dose and select physiologic effects. Vet Anaesth Analg 44:766–774. 10.1016/j.vaa.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Siriarchavatana P, Ayers JD, Kendall LV. 2016. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 55:426–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Sonner JM, Gong D, Eger EI., 2nd 2000. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg 91:720–726. 10.1213/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 36.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- 37.Tamura J, Ishizuka T, Fukui S, Oyama N, Kawase K, Miyoshi K, Sano T, Pasloske K, Yamashita K. 2015. The pharmacological effects of the anesthetic alfaxalone after intramuscular administration to dogs. J Vet Med Sci 77:289–296. 10.1292/jvms.14-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM. 2016. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res 312:305–312. 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 39.Visser SA, Smulders CJ, Gladdines WW, Irth H, van der Graaf PH, Danhof M. 2000. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnenolone in plasma using dansyl hydrazine as fluorescent label: application to a pharmacokinetic–pharmacodynamic study in rats. J Chromatogr B Biomed Sci Appl 745:357–363.. [DOI] [PubMed] [Google Scholar]
- 40.Warne LN, Beths T, Whittem T, Carter JE, Bauquier SH. 2015. A review of the pharmacology and clinical application of alfaxalone in cats. Vet J 203:141–148. 10.1016/j.tvjl.2014.12.011. [DOI] [PubMed] [Google Scholar]