Abstract
Rice rats (Oryzomys palustris) are an unconventional laboratory species that has been used to study photoperiodicity, periodontitis, and osteonecrosis of the jaw. Interventional procedures that require anesthesia, including oral procedures, are sometimes necessary in preclinical settings. The use of anesthetics including isoflurane and ketamine combined with α2-adrenoreceptor agonists, such as dexmedetomidine and xylazine, is well-established for laboratory rodents. However, their effects have been studied only modestly in rice rats. The aims of this study were to 1) determine the safety and consistency of 3 common anesthetic modalities in rice rats; 2) compare the physiologic and clinical responses to these anesthetics, and 3) verify the effectiveness of the most successful modality by testing it during an oral procedure (tooth extraction). Isoflurane, intraperitoneal ketamine–dexmedetomidine, and intraperitoneal ketamine–xylazine were evaluated by using a crossover design, in which each rat received all of the anesthetics. Compared with ketamine–dexmedetomidine and ketamine–xylazine, isoflurane inhalation through a nose cone produced more rapid induction, entry to a surgical plane of anesthesia, and initial recovery. In addition, isoflurane produced optimal anesthesia throughout the procedure for most rats. Unlike ketamine–dexmedetomidine and ketamine–xylazine, isoflurane did not alter rectal temperature, SpO2, or respiratory rate during the surgical tolerance period, whereas ketamine–dexmedetomidine and ketamine–xylazine decreased rectal temperature during the last stage of anesthesia and induced cardiorespiratory depression. Furthermore, 2 rats experienced negative outcomes warranting euthanasia: one after receiving ketamine–dexmedetomidine, and the other after ketamine–xylazine anesthesia. In conclusion, isoflurane was the most reliable and effective anesthetic in rice rats and maintained a surgical depth of anesthesia for as long as 30 min, thus supporting successful tooth extractions.
Abbreviations: SBP, systolic blood pressure; TP, toe pinch
Marsh rice rats (Oryzomys palustris) are a well-established animal model for periodontitis. Rice rats have a marked susceptibility to a spontaneous form of generalized periodontitis that closely resembles the human disease,1,3,20,22,23,36,47,48 and more recently, we described a distinctive, localized form of periodontitis in this species, which we defined as food impaction-induced localized periodontitis.38 We used rice rats with both forms of periodontitis to develop 2 novel models of medication-related osteonecrosis of the jaw,2,39 a rare but serious side effect of potent antiresorptive medications, such as bisphosphonates and denosumab, which are commonly prescribed for management of bone malignancy or osteoporosis.33,45,46 Medication-related osteonecrosis of the jaw occurs primarily in patients that receive antiresorptive agents in combination with recent oral trauma (for example, tooth extraction) or preexisting or coexisting inflammatory oral disease (for example, established periodontitis, dental disease).33,45,46
The potential benefits of rice rats as models in dental and periodontal research suggests that interventions in the oral cavity that require general anesthesia are imminent. Isoflurane, ketamine–dexmedetomidine, ketamine–xylazine, and ketamine–midazolam are well-established anesthetic modalities that are routinely used in conventional laboratory rodents.13,14,16,18,24,25,26,37,40,43,50,51,54 However, because rice rats are an emerging research model, little is known about optimal conditions for general anesthesia of this species.35 Anesthesia with isoflurane has a wide safety margin. Although isoflurane ideally is administered through endotracheal intubation, physical interference by the tracheal tube would prohibit access to the oral cavity and compromise the ability to perform oral procedures and surgeries. Administration of isoflurane through a nasal cone could be a viable alternative, but whether an adequate anesthesia level could be reached by using isoflurane is unknown, and placement of the nasal cone might still interfere with access to the oral cavity.
The use of injectable anesthetic modalities circumvents the potential issues of accessibility to the oral cavity encountered with inhalation anesthesia. Typical modalities in rodents include a combination of ketamine, a dissociative agent, and an α2-adrenoreceptor agonist, such as medetomidine, dexmedetomidine, or xylazine.14,16,18,25,37,43,50 However, injectable modalities sometimes fail to provide an adequate surgical plane of anesthesia in mice and rats7,9,10,15,28,54 and require a prolonged induction time compared with isoflurane.19,49 Furthermore, these drug combinations can produce variable effects even within rodents of the same species,9,19,27,49 and there are limited published data for efficacy of injectable anesthetics in the rice rat.35
The overall purpose of the current study was to develop a reliable anesthesia protocol for rice rats that consistently provides a surgical level of anesthesia for approximately 30 min and that allows full access to the oral cavity. The aims of this study were to 1) determine the safety and consistency of 3 common anesthetic modalities in rice rats; 2) compare the physiologic and clinical responses to these anesthetics, and 3) verify the effectiveness of the most successful modality by testing it during an oral procedure (tooth extraction).
We hypothesized that 1) isoflurane would be a safer and more reliable anesthetic to use in rice rats compared with the injectable anesthetics ketamine–dexmedetomidine and ketamine–xylazine and 2) oral interventions, such as tooth extractions, can be achieved successfully under anesthesia with isoflurane administered through a nasal cone.
Materials and Methods
Animals.
A monogamous continuous-breeding system was used to generate pups for this study as previously described.2,39 At weaning (age, 4 wk), 22 clinically normal rice rats (9 male and 13 female; weight, 28 g or greater; body condition score, 3.0 or greater)53 were selected for this study. After weaning, rats ate pelleted rodent chow (Teklad LM485 [irradiated 7912] Rodent Diet, Envigo, Tampa, FL) throughout the study.
All rats were housed (3 to 5 rats of the same sex per cage) in static filter-top cages (area, 143 in.2) with pine shavings as bedding and continuous access to food and water. Cages were enriched with manipulanda (wooden tongue depressors) and additional shelter for nesting (paper huts). In addition, rats in the breeding colony were provided weekly with sunflower seeds for foraging. The housing room was maintained at 20 to 26 °C with an average humidity of 30% to 70% and a 12:12-h light:dark cycle. Breeder pairs were housed in the same conditions as experimental rats but with a 14:10-h light:dark cycle. The Animal Care Services resource at the University of Florida is an AAALAC-accredited animal care and use program. The research protocol was approved by the University of Florida IACUC (protocol nos. 2001609488, 201408452, and 201408453).
Origin of the breeding colony and health status of the colony.
We received 17 pairs of marsh rice rats from D K Edmonds (Department of Biology, Indiana University Southeast, New Albany, IN) in 2010 and an additional 30 pairs in 2014. On arrival, the colony-derived rodents were maintained in a biocontainment facility (BSL2) for 6 mo before transfer to a conventional rodent housing room. During quarantine, sentinels (CD1 mice and CD rats) and randomly chosen rice rats were euthanized and necropsied every 4 wk to monitor for the presence of clinical disease, pathogens, and antibodies to infectious agents as previously described.5 After quarantine, rats were moved to a conventional facility and continued to be monitored by using a quarterly cycle sentinel program. Briefly, sentinels were exposed to soiled bedding from rice rat cages for a minimum of 6 wk before necropsy, serology, and parasitology analysis. Sentinels were monitored for the following rat and mouse viruses: coronavirus (sialodacryoadenitis virus, rat coronavirus), Hanta (Hantaan) virus, lymphocytic choriomeningitis virus, pneumonia virus of mice, rat parvoviruses (rat parvor viruses 1 and 2, rat minute virus, Kilham rat virus, H1 virus), reovirus type 3, Sendai virus), Theiler murine encephalomyelitis virus, ectromelia virus, epizootic diarrhea of infant mice virus, rotavirus, K virus, polyoma virus, minute virus of mice, mouse adenovirus types 1 and 2, mouse cytomegalovirus, mouse hepatitis virus, and mouse parvoviruses. At least once every quarter, we surveyed for Mycoplasma pulmonis by serology and for fur mites and pinworms through tape tests and fecal flotation, respectively. The following bacteria and fungi were monitored at least annually: cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutcheri, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Encephalitozoon cuniculi, and Helicobacter spp. In addition, we monitored for fur mites and pinworms annually. Superficial skin scrapes, perianal tape tests, and zinc sulfate fecal flotation preparations were routinely examined microscopically for the presence of ecto- and endoparasites in breeders and experimental animals. In addition, direct pelt and cecal content examinations were performed in euthanized rice rats and sentinels.
Drugs.
Atipamezole HCl (Antisedan, Zoetis, Kalamazoo, MI), atropine sulfate (0.04 mg/kg; Vedco, St Joseph, MO), isoflurane (Butler Animal Health Supply, Dublin, OH), ketamine HCl (Ketaset, Zoetis), dexmedetomidine HCl (Putney, Portland, ME), xylazine (AnaSed, Akorn, Lake Forest, IL), and meloxicam (Alloxate, Patterson Veterinary, St Paul, MN) were purchased from Patterson Veterinary.
Justification for the selected anesthetics and doses.
Anesthetics and doses were selected according to data published for other rodent species (including mice, rats, and ham sters),13,14,16,18,24,25,26,37,40,43,50,51,54 and a single previous report that used ketamine–xylazine and ketamine–dexmedetomidine combinations in rice rats.35 Initial dose combinations (approved animal use protocol) of ketamine–dexmedetomidine (greater than 50 mg/kg IP, greater than 0.125 mg/kg IP), ketamine–xylazine (greater than 50 mg/kg IP, greater than 4 mg/kg IP) and ketamine–midazolam (greater than 50 mg/kg IP, greater than 5 mg/kg IP) produced unpredictable acute mortality and respiratory distress in surviving animals (data not shown). After these observations, procedures were suspended, and veterinary staff was consulted regarding recommendations to improve survival and reduce respiratory distress. The addition of subcutaneous injection of atropine sulfate (0.04 mg/kg), approximately 10 min prior to administration of ketamine–dexmedetomidine and ketamine–xylazine and at the end of the anesthesia procedure was recommended to reduce excessive bradycardia and to decrease the accumulation of secretions in the oropharynx and upper respiratory tract that could negatively affect airflow to the lungs. In addition, administration of oxygen through a nose cone (flow rate, 1.5% to 2% v/v) was recommended for rats that received injectable anesthetics to maintain SpO2 at greater than or equal to 95%. Oxygen administration was withdrawn when rice rats recovered the toe pinch (TP) and righting reflexes.
For the remainder of the study, the reduced ketamine dose of 50 mg/kg IP was used in combination with either 0.125 mg/kg IP dexmedetomidine or 4 mg/kg IP xylazine. The ketamine–midazolam combination was discontinued according to veterinary recommendation. Atropine was not administered to rats during isoflurane administration.
Experimental design.
Male (n = 9) and female (n = 3) rice rats (age, 22 to 24 wk) received each of the anesthetic treatments with 7-d washout period between evaluations. The washout period between treatments was chosen in light of the estimated half-life of the drugs measured in various other species;44 atropine had the longest half-life (3 h) among the drugs used in this study. Considering that approximately 97% of a drug is eliminated after 5 half-lives, complete elimination of any of the drugs we used would take no longer than 15 h, and the 7-d washout period likely resulted in the complete elimination of all administered doses. For analysis, male and female rats were consolidated into a single group because no sex-associated differences were observed (P > 0.05, see Data and Statistical Analyses section). The anesthetics were administered in the following order: 1) isoflurane, 2) ketamine–dexmedetomidine (50 mg/kg IP, 0.125 mg/kg IP), and 3) ketamine–xylazine (50 mg/kg IP, 4 mg/kg IP). To allow thorough monitoring of each rat during anesthesia, only 3 rats were tested each day.
Initial procedures and reflex monitoring.
For all procedures, rats were placed on a warmed surgical platform (Kent Scientific, Torrington, CT) in dorsal recumbency and wrapped with multipurpose sealing wrap (Press'n Seal, Glad, Clorox, Oakland, CA) to support body temperature after the induction stage and throughout the surgical tolerance stage. Ophthalmic lubricant (Puralube Vet Ointment, Dechram, Leawood, KS) was placed in each eye. Loss of the righting reflex marked the beginning of the induction period and was defined as the inability of the rat to resume a normal position, with all 4 feet on the ground, after being placed in dorsal recumbence. The TP reflex was monitored after anesthesia induction by using forceps to pinch the skin between the toes of the hindlimbs and observing whether the limb was withdrawn. Rice rats were considered in a surgical plane of anesthesia when the TP reflex was negative (that is, limb was not withdrawn). TP was monitored every approximately 2 min throughout the procedure to assess depth of anesthesia.
According to the presence or absence of the righting and TP reflexes, the anesthesia procedure was divided into the stages previously defined in genus Rattus6 but with minor modifications: 1) induction, the time from the administration of the anesthetic(s) to the loss of the righting reflex; 2) nonsurgical tolerance, the time from loss of the righting reflex to loss of the TP in the hindlimbs; 3) surgical tolerance, the time from the absence of the TP reflex to the point when isoflurane-treated rats were withdrawn from the inhalant or when ketamine–dexmedetomidine- and ketamine–xylazine-treated rats were injected with atipamezole to partially reverse the effects of dexmedetomidine or xylazine; and 4) initial recovery, the time from the end of the surgical tolerance stage to the time when rats regained the righting reflex and first became ambulatory.
Throughout the surgical tolerance stage, rectal temperature (in °C), SpO2 (SpO2, as percentage), and heart rate (bpm) were monitored (MouseOx Plus, Braintree Scientific, Braintree, MA). Respiratory rate (breaths per minute) was assessed by counting the number of inspiratory movements reflected on the thoracic cavity. Systolic blood pressure (SBP; in mm Hg) was assessed automatically (BP2000 Series II Blood Pressure Analysis System, RA Platform, Visitech Systems, Apex, NC). All parameters were recorded every approximately 2 min during the surgical tolerance stage for a minimum of 30 min. Measurements were terminated when rats were withdrawn from isoflurane or reversal agent was injected. The rationale to monitor for as long as 30 to 35 min was to mimic the time needed to perform a standard surgical procedure in laboratory rodents, such as tooth extraction, ovariectomy, or placement of a vascular catheter. To exclude potential effects of circadian rhythm, the anesthetic evaluations were always performed during the daytime, from 0900 to 1200.
Inhalation anesthesia by using isoflurane through a nose cone.
Rice rats were anesthetized with isoflurane by using a laboratory animal anesthesia machine (VetEquip, Vivermore, CA). Rats were placed in an induction chamber which was then filled with isoflurane (4% v/v) and oxygen until loss of the righting reflex (1.5 to 2 min). Next, rats were placed on the surgical platform and a nasal cone was placed on their noses to continue isoflurane administration at 4% v/v for 1 to 2 min; isoflurane concentration was reduced to 2% to 3% (v/v) for the remainder of the procedure. After rats spent 30 to 35 min within the surgical tolerance stage, nasal cones were removed, and the anesthetic machine was turned off.
Injectable anesthesia with ketamine–dexmedetomidine and ketamine–xylazine.
Rats were weighed and subcutaneously injected with atropine sulfate (0.04 mg/kg) approximately 10 min before anesthetic induction and again at the end of the procedure. Rice rats were injected intraperitoneally with the indicated drug mixtures (ketamine–dexmedetomidine or ketamine–xylazine) in the right caudal quadrant, lateral to the midline next to the umbilicus. Ketamine–dexmedetomidine was administered to the rats 7 d after they received isoflurane, followed by ketamine–xylazine, which was administered to the rice rats 7 d after they received ketamine–dexmedetomidine. Any rat showing a positive TP reflex received supplemental anesthesia comprising ketamine alone at 50% the concentration of the initial dose. When rats continued to display positive TP reflexes, they again were injected with a 50% dose of ketamine (3rd dose overall), but only after at least 15 min had elapsed after the previous injection. After completing 30 to 35 min within the surgical tolerance stage, rats were injected with atipamezole (1 mg/kg IP) to partially reverse the effects of dexmedetomidine and xylazine.
Tooth extraction procedure.
Tooth extraction was performed in isoflurane-anesthetized female rice rats (n = 10) to verify that this anesthetic modality was optimal for procedures in the oral cavity. Rats were anesthetized with isoflurane at 4% (v/v) for induction and 2% to 3% (v/v) for maintenance, respectively. All rats received a subcutaneous injection of meloxicam (2 mg/kg daily) to reduce postoperative pain prior to the procedure. After anesthesia induction, rats were placed on the heated surgical platform in dorsal recumbency, and the body was stabilized by using a custom-made support apparatus. The head was secured by placing a surgical wire caudal to the maxillary incisors, which was affixed to 2 magnetic stabilizers situated laterocranially to the head. After verification of the surgical tolerance stage (that is, absence of TP reflex), the second maxillary molar (M2) was extracted by using dental explorers (no. 23) and toothed tissue forceps as previously described for genus Rattus4 but with minor modifications. The tip of the probe was first placed at the distobuccal gingival margin at M1M2 and M2M3. The dental explorer or tissue forceps were repeatedly rotated in a dorsal and mesial direction to loosen M2. The probe was then removed from its original position, placed at the roof of the root furcation, and repeatedly rotated to loosen and elevate the tooth. The tooth was then removed by using a pair of toothed forceps. Each tooth extraction procedure lasted 20 to 30 min.
Postprocedural care.
After the nose cone was removed, rats were placed in sternal recumbency in a clean recovery cage with fresh bedding. A warming disc was placed underneath one corner of the recovery cage to maintain body temperature and to ensure that the rat could move from the warm area when needed. Rice rats that underwent tooth extractions were examined daily by lab personnel and veterinary staff for signs of pain or distress for 72 h after the extractions. Subcutaneous administration of meloxicam once daily was continued for the first 72 h after extraction. Moist chow and purified soft diet (DietGel 76A, ClearH2O, Westbrook, ME) were offered to the rats during the first 5 d after extraction. There were no clinical signs of pain or distress after extraction: rats continued to gain weight and were observed eating moistened chow and soft diet within hours of extraction.
Data and statistical analyses.
Data are expressed as mean ± 1 SD for each group. The SigmaStat 3.5 statistical package (Systat Software, Point Richmond, CA) was used to analyze the data. To determine potential differences between sexes, 2-way ANOVA with Holm–Sidak posthoc testing was performed, with sex and anesthetic modality as the main effects. Data were evaluated to assess differences among the 3 anesthetic modalities by using one-way ANOVA for repeated measures and Holm–Sidak posthoc testing. When data were not normally distributed, repeated-measures ANOVA followed by the Friedman test was applied. A P value less than 0.05 was considered statistically significant.
Results
Comparison of isoflurane, ketamine–dexmedetomidine, and ketamine–xylazine anesthesia in rice rats.
Anesthesia stages.
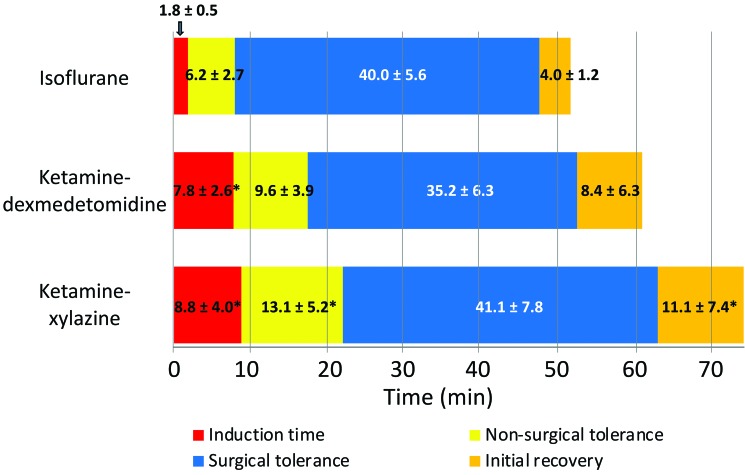
Isoflurane rapidly induced anesthesia in rice rats, with faster (P < 0.05) loss of the righting reflex (1.8 ± 0.5 min) compared with ketamine–dexmedetomidine (7.8 ± 2.6 min) and ketamine–xylazine (8.8 ± 4.0 min; Figure 1). The nonsurgical tolerance stage (duration between a negative righting reflex and a negative TP reflex) was longer (P < 0.05) after ketamine–xylazine treatment than isoflurane (Figure 1). In addition, ketamine–dexmedetomidine treatment showed a trend (P = 0.07) toward a longer nonsurgical tolerance stage than that for isoflurane. Furthermore, rats recovered faster (P < 0.05) after isoflurane administration (4 ± 1.2 min) compared with ketamine–xylazine (11.1 ± 7.4 min) but not ketamine–dexmedetomidine (8.4 ± 6.3 min; Figure 1).
Figure 1.
Duration of the anesthesia stages due to isoflurane, ketamine–dexmedetomidine, and ketamine–xylazine. Compared with ketamine–dexmedetomidine or ketamine–xylazine, isoflurane produced a shorter induction time and more rapid entry into the surgical tolerance phase of anesthesia. At 35 to 40 min after induction, isoflurane was withdrawn by removing the nose cone, and ketamine–dexmedetomidine and ketamine–xylazine anesthesia were partially reversed by injecting atipamezole to block the effects of dexmedetomidine and xylazine. Isoflurane produced approximately 2-fold and 2.5-fold shorter time to initial recovery compared with ketamine–dexmedetomidine and ketamine–xylazine, respectively. Values are given as mean ± 1 SD; *, value significantly different (P < 0.05) from that for isoflurane-treated rats. Data were analyzed by using ANOVA; a P value less than 0.05 was considered statistically significant.
Anesthesia depth during the surgical tolerance stage.
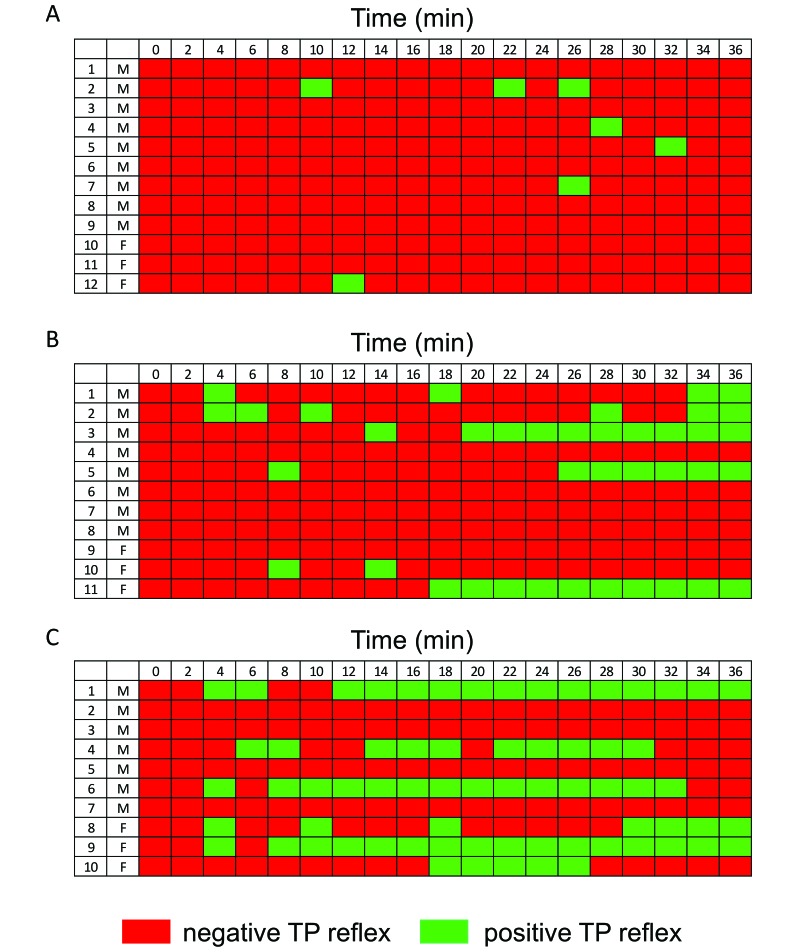
Rice rats were considered to be in the surgical tolerance stage as soon as the TP reflex was negative (Figure 2 A through C, red rectangles). Under isoflurane anesthesia, the majority of rats had negative TP reflexes throughout most of the surgical tolerance stage (Figure 2 A, red rectangles). Only 5 rats elicited sporadic, positive TP reflex events (green rectangles), which were abolished by temporarily (less than 2 min) increasing the concentration of isoflurane from 2% to 3.5%. All isoflurane-treated rats survived the study.
Figure 2.
Quality of anesthesia during the surgical tolerance stage due to (A) isoflurane, (B) ketamine–dexmedetomidine, and (C) ketamine– xylazine (C). Rice rats were considered to be at a surgical level of anesthesia when the TP reflex was negative (red rectangles). Rats under isoflurane anesthesia had negative TP reflexes throughout the surgical tolerance stage (A, red rectangles), except for 5 rats that yielded sporadic positive TP reflexes (A, green rectangles), which were eliminated by transiently increasing isoflurane concentration. In contrast, (B) 55% of rats treated with ketamine–dexmedetomidine and (C) 60% given ketamine–xylazine displayed isolated or consecutive positive TP reflex events throughout anesthesia. Numbers indicate individual rats (F, female; M, male).
In contrast to isoflurane, ketamine–dexmedetomidine and ketamine–xylazine resulted in marked variability in the depth of anesthesia (Figure 2 B and C). Indeed, 55% of ketamine–dexmedetomidine-injected and 60% of ketamine–xylazine-injected rats displayed isolated or consecutive positive TP reflex events throughout the surgical tolerance stage. Half of the rats were supplemented with additional doses of anesthetic (ketamine), but doing so did not increase the depth of anesthesia in these rats (Figure 2 B and C). One rat died that was treated with ketamine–dexmedetomidine and that received supplemental ketamine after a positive TP reflex; another rat, treated with ketamine–xylazine, also died.
Assessment of physiologic and clinical parameters.
We found no significant differences in the rectal temperature of rice rats among the 3 anesthesia groups, and rectal temperature values were stable throughout the initial 28 to 30 min of monitoring (Figure 3 A). However, compared with isoflurane, ketamine–dexmedetomidine and ketamine–xylazine induced significant, sustained decreases in rectal temperature after 35 min (Figure 3 A). Isoflurane produced continuous, stable rectal temperature until the end of the surgical tolerance stage. For all anesthetic regimens, SpO2 values were consistently 95% to 99% in all rats throughout the entire surgical tolerance stage (Figure 3 B); however, rats anesthetized with either ketamine–dexmedetomidine or ketamine–xylazine received O2 through a nasal cone throughout the procedure. Both respiratory and heart rates were consistently stable throughout the surgical tolerance phase regardless of anesthetic modality (Figure 3 C and D). However, the injectable anesthetics produced significantly (P < 0.05) lower values compared with isoflurane-treated rats throughout most of the surgical tolerance stage (Figure 3 C and 3 D). SBP was consistently stable throughout the surgical tolerance stage regardless of anesthetic modality, and there were no significant differences among anesthesia groups at any time point (Figure 3 E).
Figure 3.
Physiologic parameters monitored in rice rats during the surgical tolerance period. (A) Isoflurane (blue) resulted in sustained rectal temperature until the end of the procedure, but rectal temperature was significantly decreased under ketamine–dexmedetomidine (orange) and ketamine–xylazine (gray) at the last 2 time points of the surgical tolerance period. (B) SpO2 was within 96% to 99% in all rats with the 3 anesthetics during the complete surgical tolerance stage. (C) Respiratory rate, (D) heart rate, and (E) SBP were consistently stable in rice rats for all 3 anesthesia modalities. However, respiratory and heart rates in ketamine–dexmedetomidine- and ketamine–xylazine-treated rats were lower than those in isoflurane-treated rats. *, Values for isoflurane group (light blue) are significantly (P < 0.05) different from that for the ketamine–dexmedetomidine (orange) or ketamine–xylazine (gray) group. Data were analyzed by using ANOVA.
Tooth extraction procedure.
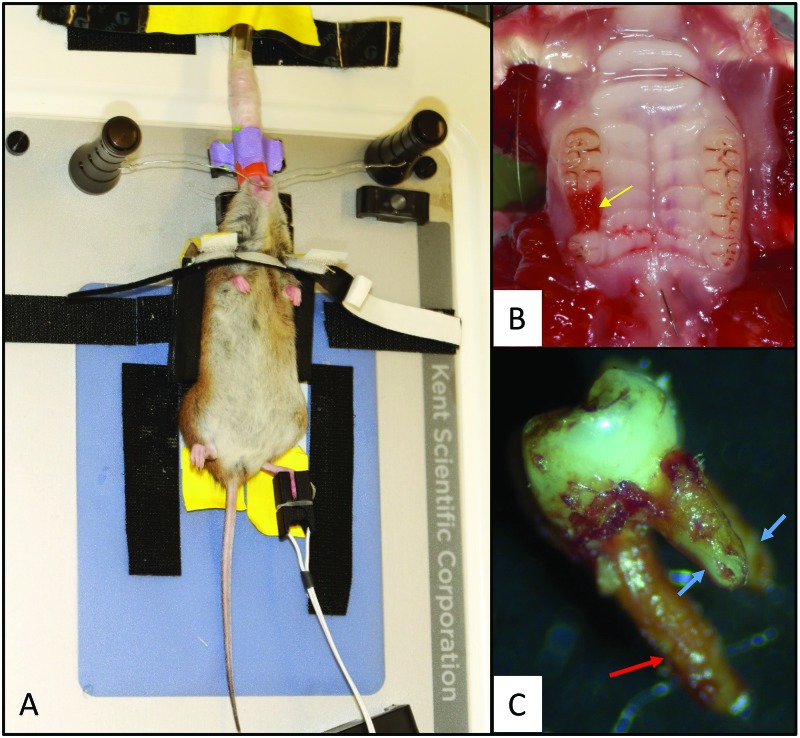
The study data suggested that isoflurane is the most effective and reliable among the 3 modalities to use for surgical procedures lasting 30 to 35 min in rice rats. We performed maxillary tooth extraction in a group of female rice rats to determine the feasibility of isoflurane administered through a nasal cone for oral procedures. The customized stabilization device for the head (Figure 4 A) and body (Figure 4 A) allowed placement of tubing and nose cone (Figure 4 A) in a manner that did not obstruct the oral cavity. In all subjects, the second maxillary molar was successfully extracted within 20 to 30 min (Figure 4 B). In most (80%) of the extractions, the complete second molar was removed with its 3 intact roots, whereas in the remaining 20% of rats, a small apical piece of one of the small buccal roots was broken (Figure 4 C).
Figure 4.
Tooth extraction procedure in rice rats. (A) Rice rats were placed on a heated surgical platform in dorsal recumbency and stabilized by using a custom-made support device. The head was stabilized by placing surgical wire affixed to 2 magnetic stabilizers caudal to the maxillary incisors. Isoflurane was administered to the rats through a nasal cone. A pulsimeter was placed on the foot to measure SpO2 and heart rate. (B) Dorsal aspect of the palate; the yellow arrow indicates the extraction socket after removal of the right second maxillary molar. (C) A complete extracted second molar, showing the larger, palatal root (red arrow) and 2 smaller, mesial and distal buccal roots (blue arrows). All 3 roots were intact after extraction in 80% of the rats, as depicted in the photo, whereas in 20% of rats, a small apical piece of one of the small buccal roots was broken.
Discussion
Inhalation anesthesia with isoflurane is the most widely accepted method of general anesthesia in biomedical research, particularly for mice and rats.18,25,27,34 However, the effect of isoflurane on physiologic and clinical anesthesia parameters have not been reported for rice rats (Oryzomys palustris), which is an emerging laboratory model that is especially important for oral disease research. This species belongs to family Cricetidae and therefore is evolutionarily divergent from more conventional family Muridae models (Mus and Rattus), suggesting the necessity for a systematic evaluation of general anesthetic modalities in rice rats. Therefore, we tested 3 commonly used modalities—isoflurane, ketamine–dexmedetomidine, and ketamine–xylazine. Our results showed that isoflurane was the most reliable and effective anesthetic modality for maintaining a surgical plane of anesthesia for as long as 30 min in rice rats. Compared with ketamine–dexmedetomidine and ketamine–xylazine, isoflurane had a shorter period of induction and rats recovered from anesthesia faster. Furthermore, the majority of the rats had an optimal surgical tolerance depth of anesthesia throughout most of the anesthesia procedure; the remaining rats required only a transient increase in isoflurane concentration to return them to a surgical depth level of anesthesia.
Isoflurane is frequently used in other rodent species but with variable results depending on the species. In mice, isoflurane exerts only a moderate cardiodepressive effect compared with the more pronounced depression induced by injectable agents, such as pentobarbital, urethane, and ketamine-containing combinations.29,30 Isoflurane levels of 1.5% (v/v) result in mean arterial pressure and heart rate values comparable to those of alert, unanesthetized C57BL/6 mice.11 In Wistar rats, however, isoflurane caused mild hypotension and increased heart rate, whereas ketamine–xylazine was less suppressive on blood pressure, heart rate, and body temperature but prolonged return to consciousness and recovery.6 In addition, isoflurane dose-dependently decreased heart rate, blood pressure, and left ventricular systolic and diastolic function in Sprague–Dawley rats,55 and 3% (v/v) isoflurane significantly decreased myocardial contractility, blood pressure, heart rate, and impaired left ventricular diastolic function.55 In the current study, 2% to 3% (v/v) isoflurane did not induce significant changes in heart rate or SBP for approximately 35 min, suggesting that rice rats are more resilient to the isoflurane-induced cardiovascular depression observed in Rattus rats. Furthermore, isoflurane did not induce significant changes in rectal temperature, SpO2, or respiratory rate during the surgical tolerance period.
Ketamine–α2 agonist combinations have the ability to produce short-term surgical anesthesia with satisfactory analgesia and give researchers the option of hastening recovery through reversal of the α2 agonist effect by using atipamezole.14,16,18,25,37,43,44,50 In addition, injectable anesthetics preclude the need for intubation or nasal cones that are necessary for isoflurane administration but that might obstruct access to the face and oral cavity. Despite these advantages, combinations of injectable anesthetics do not always provide a reliable surgical plane of anesthesia and can be insufficient for major surgical procedures in many strains of mice and some rat strains.9,27,49 Likewise, our study showed that ketamine–dexmedetomidine and ketamine–xylazine did not provide a consistent surgical depth level of anesthesia. Specifically, ketamine–dexmedetomidine (50 mg/kg IP, 0.125 mg/kg IP) and ketamine–xylazine (50 mg/kg IP, 4 mg/kg IP) effectively maintained the surgical depth stage of anesthesia in only 45% and 40% of the rats, respectively. In addition, the majority of rats displayed isolated or consecutive positive TP reflexes throughout the anesthesia procedure, and these positive responses persisted even when rats were redosed with ketamine more than once. It is unclear why these effects occurred. Performing the TP reflex every 2 min in a hindlimb might have induced sensitization, with noxious peripheral stimuli in the tissue area inducing hyperalgesia and increased positive responses to the stimuli. However, this explanation seems unlikely, because isoflurane-treated rats did not demonstrate this behavior. Furthermore, previous studies evaluating anesthetics in rodents have used TP reflex tests as frequently as once per minute in alternating hindlimbs and forelimbs49 or every 5 min in alternate hindlimbs.9 Despite the variability in anesthetic depth among rats treated with ketamine–dexmedetomidine or ketamine–xylazine, there were no significant differences in the analyzed physiologic parameters among these rats, suggesting that these parameters are not directly dependent on the quality of anesthesia. Higher doses of ketamine–dexmedetomidine and ketamine–xylazine likely could have induced a more consistent surgical depth level of anesthesia that eliminated positive TP reflexes. However, given our initial findings in a preliminary test (see the section Justification for the selected anesthetics and doses), it is also possible that higher doses would have generated significantly greater mortality, respiratory distress, or delayed recovery time.
Postmortem examination of the rats that died during the preliminary test revealed the presence of foamy fluid in the lumen of the tracheas and lung, and histopathologic examination revealed congestion of kidneys, liver, and lungs (data not shown). Therefore, the veterinary staff recommended the addition of atropine, which reduces bronchial secretions and protects the heart from the vagal stimulation that can occur during surgical procedures.12,16,17 In the present study, we found that administering atropine stabilized the ketamine–dexmedetomidine- and ketamine–xylazine-treated rice rats during anesthesia and improved recovery compared with rice rats in the preliminary experiment, which did not receive atropine. Specifically, atropine produced a marked improvement of airway function during ketamine–xylazine and ketamine–dexmedetomidine anesthesia. In addition to its protective effects on airway function, atropine sulfate reduces bradycardia in rats (Rattus) anesthetized with ketamine–xylazine and ketamine-detomidine.42 Therefore atropine might similarly prevent or reduce bradycardia during ketamine–dexmedetomidine and ketamine–xylazine treatments in rice rats, but further studies are needed to firmly establish these effects, because none of the rice rats in our current study received ketamine–dexmedetomidine or ketamine–xylazine in the absence of atropine.
Although atropine alleviated respiratory distress in rice rats and decreased mortality, atropine might also have produced effects in the cardiovascular system52 and CNS32 through its anticholinergic effects, which might have negatively influenced the depth of anesthesia produced by ketamine–dexmedetomidine and ketamine–xylazine. Although other anticholinergics, such as glycopyrrolate and scopolamine, are available, they are associated with severe adverse events, such as respiratory distress21 and arrhythmia.8 Therefore, these more potent, longer-lasting effects could be disadvantageous for short-term surgeries.
Some of the effects observed with the injectable drugs might have been due to carryover from the previous treatments. In the current study, ketamine and atropine could have induced receptor sensitization in the rats that influenced subsequent sedation.31 Furthermore, frequent use of α2-adrenoceptor agonists can mask pain responses in some species.41 Therefore, although the washout period between each treatment was extensive and the frequency was limited, carryover analgesic effects at peripheral, spinal, and brainstem sites might have been present during the second exposure. However, if pain responses were being masked, then we would have expected greater efficacy of ketamine–xylazine and ketamine–dexmedetomidine than what we observed. The effect of carryover cannot be fully addressed without further studies, in which the order of anesthetic modalities is varied.
A limitation of our study is that we did not measure physiologic parameters in conscious animals; therefore there are no baseline values for comparison with those obtained during anesthesia. Consequently, we cannot establish the very early effects of the anesthetics. Future studies to describe these effects would necessitate internal placement of electronic probes to telemetrically monitor these parameters before, during and after anesthesia. Despite this limitation, we were able to show the effects of each modality on physiologic parameters during the surgical tolerance stage.
In conclusion, we found that, in contrast to ketamine– dexmedetomidine and ketamine–xylazine, isoflurane anesthesia administered through a nasal cone is a reliable and effective anesthetic modality for performing 30- to 35-min surgical procedures, including oral interventions, in rice rats. Our study further demonstrates variability in these anesthetic modalities between species and underlines the importance of empirically determined anesthesia conditions for emerging laboratory species.
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research (NIDCR), grant no. R01DE023783-01A.
References
- 1.Aguirre JI, Akhter MP, Kimmel DB, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee JY, Reinhard MK, Battles AH, Kesavalu L, Wronski TJ. 2012. Enhanced alveolar bone loss in a model of noninvasive periodontitis in rice rats. Oral Dis 18:459–468. 10.1111/j.1601-0825.2011.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ. 2012. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res 27:2130–2143. 10.1002/jbmr.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre JI, Akhter MP, Neuville KG, Trcalek CR, Leeper AM, Williams AA, Rivera M, Kesavalu L, Ke HZ, Liu M, Kimmel DB. 2017. Age-related periodontitis and alveolar bone loss in rice rats. Arch Oral Biol 73:193–205. 10.1016/j.archoralbio.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. 2010. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis 16:674–685. 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre JI, Edmonds K, Zamora B, Pingel J, Thomas L, Cancel D, Schneider L, Reinhard MK, Battles AH, Akhter MP, Kimmel DB, Wronski TJ. 2015. Breeding, husbandry, veterinary care, and hematology of marsh rice rats (Oryzomys palustris), a small animal model for periodontitis. J Am Assoc Lab Anim Sci 54:51–58. [PMC free article] [PubMed] [Google Scholar]
- 6.Albrecht M, Henke J, Tacke S, Markert M, Guth B. 2014. Effects of isoflurane, ketamine–xylazine and a combination of medetomidine, midazolam, and fentanyl on physiological variables continuously measured by telemetry in Wistar rats. BMC Vet Res 10:198–212. 10.1186/s12917-014-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 8.Bachman L, Freeman A. 1961. The cardiac rate and rhythm in infants during induction of anesthesia with cyclopropane. Atropine versus scopolamine as preanesthetic medication. J Pediatr 59:922–927. 10.1016/S0022-3476(61)80324-7. [DOI] [PubMed] [Google Scholar]
- 9.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 10.Chaves AA, Dech SJ, Nakayama T, Hamlin RL, Bauer JA, Carnes CA. 2003. Age and anesthetic effects on murine electrocardiography. Life Sci 72:2401–2412. 10.1016/S0024-3205(03)00137-1. [DOI] [PubMed] [Google Scholar]
- 11.Constantinides C, Mean R, Janssen BJ. 2011. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 12.Correa FC, Ciminelli PB, Falcao H, Alcantara BJ, Contador RS, Medeiros AS, Zin WA, Rocco PR. 2001. Respiratory mechanics and lung histology in normal rats anesthetized with sevoflurane. J Appl Physiol (1985) 91:803–810. 10.1152/jappl.2001.91.2.803. [DOI] [PubMed] [Google Scholar]
- 13.Cruz JI, Loste JM, Burzaco OH. 1998. Observations on the use of medetomidine–ketamine and its reversal with atipamezole for chemical restraint in the mouse. Lab Anim 32:18–22. 10.1258/002367798780559383. [DOI] [PubMed] [Google Scholar]
- 14.Curl JL. 1988. Ketamine-xylazine anaesthesia in the Djungarian hamster (Phodopus sungorus). Lab Anim 22:309–312. 10.1258/002367788780746269. [DOI] [PubMed] [Google Scholar]
- 15.Dittmar MS, Fehm NP, Vatankhah B, Horn M. 2004. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp Med 54:652–655. [PubMed] [Google Scholar]
- 16.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2010. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press. [Google Scholar]
- 17.Flecknell PA. 1996. Anesthesia of common laboratory species, p 159–224. In: Laboratory animal anesthesia, London (United Kingdom): Academic Press. [Google Scholar]
- 18.Flecknell PA. 1996. Laboratory animal anaesthesia: a practical introduction for research workers and technicians. San Diego (CA): Academic Press. [Google Scholar]
- 19.Gaertner DJ, Boschert KR, Schoeb TR. 1987. Muscle necrosis in Syrian hamsters resulting from intramuscular injections of ketamine and xylazine. Lab Anim Sci 37:80–83. [PubMed] [Google Scholar]
- 20.Gotcher JE, Jee WS. 1981. The progress of the periodontal syndrome in the rice rat. J Periodontal Res 16:275–291. 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 21.Green SM, Roback MG, Krauss B, Emergency Department Ketamine Metaanalysis Study Group 2010. Anticholinergics and ketamine sedation in children: a secondary analysis of atropine versus glycopyrrolate. Acad Emerg Med 17:157–162. 10.1111/j.1553-2712.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 22.Gupta OP, Shaw JH. 1956. [Periodontal disease in the rice rat. I. Anatomic and histopathologic findings.] Oral Surg Oral Med Oral Pathol 9:592–603. 10.1016/0030-4220(56)90319-xylazine.[Article in French]. [DOI] [PubMed] [Google Scholar]
- 23.Gupta OP, Shaw JH. 1956. [Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease.] Oral Surg Oral Med Oral Pathol 9:727–735. 10.1016/0030-4220(56)90249-3.[Article in French]. [DOI] [PubMed] [Google Scholar]
- 24.Hahn N, Eisen RJ, Eisen L, Lane RS. 2005. Ketamine–medetomidine anesthesia with atipamezole reversal: practical anesthesia for rodents under field conditions. Lab Anim (NY) 34:48–51. 10.1038/laban0205-48. [DOI] [PubMed] [Google Scholar]
- 25.Hawk CT, Leary SL, Morris TH. 2005. Formulary for laboratory animals, Ames (IA): Blackwell Publishing. [Google Scholar]
- 26.Hedenqvist P, Roughan JV, Flecknell PA. 2000. Effects of repeated anaesthesia with ketamine/medetomidine and of preanaesthetic administration of buprenorphine in rats. Lab Anim 34:207–211. 10.1258/002367700780457536. [DOI] [PubMed] [Google Scholar]
- 27.Hrapkieweicz KL, Medina L, Holmes D. 1998. Clinical laboratory animal medicine: an introduction, 2nd ed Ames (IA) :Iowa State University Press. [Google Scholar]
- 28.Hrapkieweicz K, Medina L. 2007. Clinical laboratory animal medicine. Ames (IA): Blackwell Publishing. [Google Scholar]
- 29.Janssen BJ, De Celle CT, Debets JJ, Brouns AE, Callahan MF, Smith TL. 2004. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287:H1618–H1624. 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 30.Janssen BJ, Smits JF. 2002. Autonomic control of blood pressure in mice: basic physiology and effects of genetic modification. Am J Physiol Regul Integr Comp Physiol 282:R1545–R1564. 10.1152/ajpregu.00714.2001. [DOI] [PubMed] [Google Scholar]
- 31.Kanda T, Iguchi A, Yoshioka C, Nomura H, Higashi K, Kaya M, Yamamoto R, Kuramoto T, Furukawa T. 2015. Effects of medetomidine and xylazine on intraocular pressure and pupil size in healthy Beagle dogs. Vet Anaesth Analg 42:623–628. 10.1111/vaa.12249. [DOI] [PubMed] [Google Scholar]
- 32.Ketchum JS, Sidell FR, Crowell EB, Jr, Aghajanian GK, Hayes AH., Jr 1973. Atropine, scopolamine, and ditran: comparative pharmacology and antagonists in man. Psychopharmacology (Berl) 28:121–145. 10.1007/BF00421398. [DOI] [PubMed] [Google Scholar]
- 33.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer M, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. 2007. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491. 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 34.Kohn DF, Wixson SK, White WJ, Benson GJ. 1997. Anesthesia and analgesia in laboratory animals. New York (NY): Academic Press. [Google Scholar]
- 35.Leber M, Kijtawornrat A, Freed CL, Hueni S, Huja SS. 2009. Meeting Comparison of ketamine–xylazine and ketamine/dexmedetomidine anesthesia and their effects on electrocardiograms in rice rat (Oryzomys palustris). Abstracts of Scientific Presentations, Denver, Colorado. AALAS National. J Am Assoc Lab Anim Sci 48:628–628. [Google Scholar]
- 36.Leonard EP. 1979. Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris). Am J Pathol 96:643–646. [PMC free article] [PubMed] [Google Scholar]
- 37.Maddison JE, Page SW, Church DB. 2008. Small animal clinical pharmacology. Edinburgh: Elsevier. [Google Scholar]
- 38.Messer JG, Jiron JM, Chen HY, Castillo EJ, Mendieta Calle JL, Reinhard MK, Kimmel DB, Aguirre JI. 2017. Prevalence of food impaction-induced periodontitis in conventionally housed marsh rice rats (Oryzomys palustris). Comp Med 67:43–50. [PMC free article] [PubMed] [Google Scholar]
- 39.Messer JG, Mendieta Calle JL, Jiron JM, Castillo EJ, Van Poznak C, Bhattacharyya N, Kimmel DB, Aguirre JI. 2018. Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose-dependent manner in rice rats (Oryzomys palustris) with localized periodontitis. Bone 108:79–88. 10.1016/j.bone.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina AM, Moyano MR, Serrano-Rodriguez JM, Ayala N, Lora AJ, Serrano-Caballero JM. 2015. Analyses of anaesthesia with ketamine combined with different sedatives in rats. Vet Med (Praha) 60:368–375. 10.17221/8384-VETMED. [DOI] [Google Scholar]
- 41.Muir WW, Hubbell JA. 2009. Equine anesthesia. Monitoring and emergency therapy. St Louis (MO): Saunders Elsevier; 10.1016/B978-1-4160-2326-5.00026-2 [DOI] [Google Scholar]
- 42.Olson ME, Vizzutti D, Morck DW, Cox AK. 1994. The parasympatholytic effects of atropine sulfate and glycopyrrolate in rats and rabbits. Can J Vet Res 58:254–258. Erratum: 1995. Can J Vet Res 59: 25. [PMC free article] [PubMed] [Google Scholar]
- 43.Payton AJ, Forsythe DB, Dixon D, Myers PH, Clark JA, Snipe JR. 1993. Evaluation of ketamine–xylazine in Syrian hamsters. Cornell Vet 83:153–161. [PubMed] [Google Scholar]
- 44.Plumb DC. 2011. Plumb's veterinary drug handbook. Hoboken (NJ): Wiley and Sons. [Google Scholar]
- 45.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, American Association of Oral and Maxillofacial Surgeons 2009. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67 5 Suppl:2–12. [DOI] [PubMed] [Google Scholar]
- 46.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O'Ryan F, American Association of Oral and Maxillofacial Surgeons 2014. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg 72:1938–1956. 10.1016/j.joms.2014.04.031. Erratum: 2015 J Oral Maxillofac Surg 73:1440, 1879. [DOI] [PubMed] [Google Scholar]
- 47.Ryder MI. 1980. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J Periodontal Res 15:502–515. 10.1111/j.1600-0765.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 48.Shaw JH, Gupta OP. 1956. The relation of a chelating agent to smooth-surface lesions in the white rat. J Nutr 60:311–322. 10.1093/jn/60.3.311. [DOI] [PubMed] [Google Scholar]
- 49.Struck MB, Andrutis KA, Ramirez HE, Battles AH. 2011. Effect of a short-term fast on ketamine–xylazine anesthesia in rats. J Am Assoc Lab Anim Sci 50:344–348. [PMC free article] [PubMed] [Google Scholar]
- 50.Swindle MM, Vogler GA, Fulton LK, Marini RP, Popilskis S. 2002. Preanesthesia, anesthesia, analgesia, and euthanasia, p 955–1003. Chapter 22. In: Fox WC, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press; 10.1016/B978-012263951-7/50025-9 [DOI] [Google Scholar]
- 51.Szczęsny G, Veihelmann A, Massberg S, Nolte D, Messmer K. 2004. Long-term anaesthesia using inhalatory isoflurane in different strains of mice—the haemodynamic effects. Lab Anim 38:64–69. 10.1258/00236770460734416. [DOI] [PubMed] [Google Scholar]
- 52.Toft P, Romer UD. 1987. Glycopyrrolate compared with atropine in association with ketamine anaesthesia. Acta Anaesthesiol Scand 31:438–440. 10.1111/j.1399-6576.1987.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 53.Ullman-Culleré MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
- 54.Wellington D, Mikaelian I, Singer L. 2013. Comparison of ketamine–xylazine and ketamine–dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci 52:481–487. [PMC free article] [PubMed] [Google Scholar]
- 55.Yang CF, Chen MYC, Chen TI, Cheng CF. 2014. Dose-dependent effects of isoflurane on cardiovascular function in rats. Tzu Chi Med J 26:119–122. 10.1016/j.tcmj.2014.07.005. [DOI] [Google Scholar]