Abstract
Among patients undergoing cardioversion for atrial fibrillation, the presence of left ventricular thrombus is a relatively uncommon and challenging clinical dilemma. While left atrial appendage thrombus is a contraindication to cardioversion, there is paucity of data regarding the safety of cardioversion in with the presence of left ventricular apical thrombus. Also, thrombus characteristics such as protrusion and mobility on echocardiography are known risk factors for systemic embolism. In this article, we present a case highlighting the management of atrial fibrillation in the setting of left ventricular dysfunction, acute heart failure, and echocardiographic evidence of acute left ventricular apical thrombus.
Keywords: atrial fibrillation, cardioversion, embolism, thrombus
1 |. INTRODUCTION
A 65-year old man presented to the emergency department with a one-week history of exertional dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. He denied chest pain or palpitations. Prior medical history was unremarkable except for mild renal insufficiency attributed to nonsteroidal anti-inflammatory drug use. Vital signs at presentation showed heart rate 148 beats per minute (BPM) and regular and blood pressure of 117/83 mm Hg. Oxygen saturation was 97% on 2 L of O2 by nasal cannula. Physical examination revealed jugular venous distention to the angle of the jaw. On palpation, the cardiac apical impulse was displaced laterally. No cardiac murmurs were appreciated on auscultation, and gallops could not be ascertained due to the patient’s tachycardia. Bibasilar rales and bilateral lower extremity pitting edema were present.
Electrocardiogram (ECG) demonstrated atrial flutter with 2:1 atrioventricular block (Figure 1). Chest x-ray revealed cardiomegaly with diffuse haziness and interstitial opacities consistent with pulmonary edema (Figure 2). Laboratory investigations showed initial troponin T level 0.02 ng/dL, increasing to 0.03 ng/dL at 6 hours and 0.03 ng/dL at 12 hours (normal reference <0.01 ng/dL). Creatinine was elevated at 1.6 mg/dL (reference range 0.8–1.3 mg/dL). Thyroid-stimulating hormone, electrolytes, and complete blood count were within normal limits.
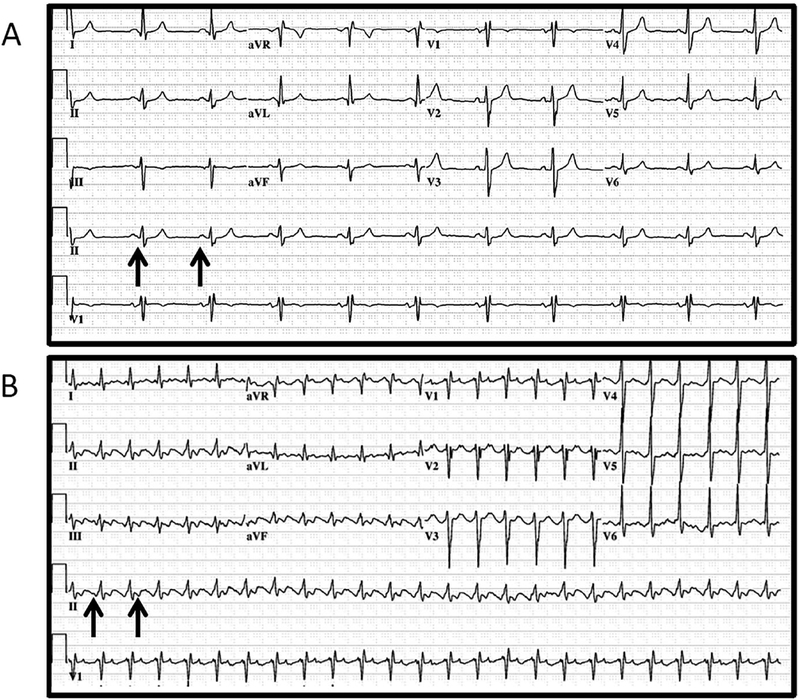
FIGURE 1.
Last available electrocardiogram (ECG) obtained 6 years prior to admission demonstrates sinus rhythm (arrows) with incomplete right bundle branch block (A). ECG obtained upon admission (B) reveals atrial flutter (arrows) with 2:1 atrioventricular (AV) block, rapid ventricular response, and a nonspecific T-wave abnormality in the anterolateral leads (V3–V6)
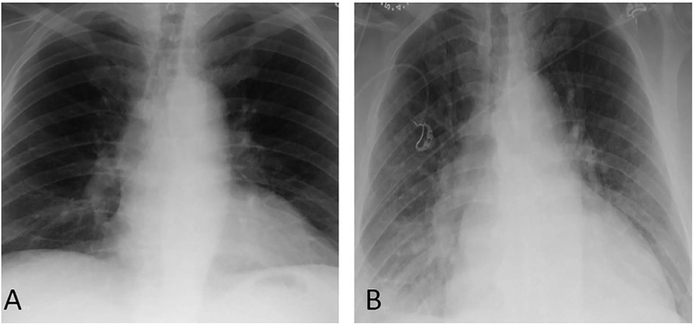
FIGURE 2.
Last available posteroanterior (PA) chest film obtained 6 years prior to admission notes normal cardiac size and no evidence of pulmonary infiltrates (A). PA chest x-ray upon admission (B) notes interval development of diffuse hazy interstitial opacities predominately at the base, cardiomegaly, and pulmonary venous hypertension
The patient was treated with intravenous (IV) diltiazem and oral metoprolol, which were both subsequently discontinued due to hypo-tension without significant effect on reducing the heart rate. Diuresis with IV furosemide was commenced along with IV heparin. The following morning a transesophageal echocardiogram (TEE) was performed prior to planned electrical cardioversion (ECV). TEE revealed moderate biventricular enlargement with severely reduced global left ventricular (LV) function and a LV ejection fraction (LVEF) of 15%. Although the left atrial appendage was free of thrombus, a discrete 2.3 × 2.5 cm echo dense mobile mass with defined margins distinct from the endocardium was present at the LV apex (Figure 3, Movies S1 and S2), consistent with a thrombus.
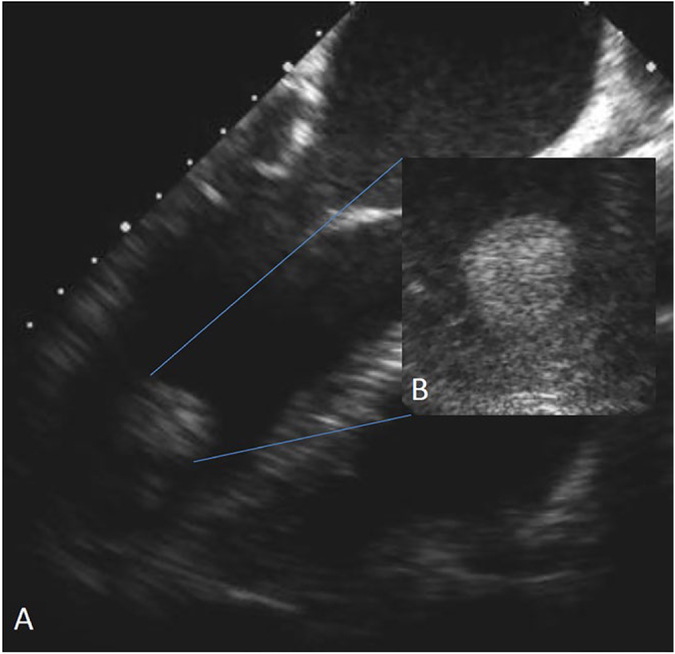
FIGURE 3.
Transesophageal echocardiogram (TEE) (A) with zoomed in view of left ventricular apex (B), demonstrating a protruding, mobile echo density suggestive of an acute thrombus
ECV was canceled due to the presence of the LV apical mass. Management of the atrial flutter was changed to a rate control approach. The patient was loaded with IV digoxin. As calcium channel or beta blockers were no longer viable options due to hypotension, amiodarone was also initiated to further optimize rate control. The heart rate was reasonably controlled (76 BPM at rest) at the time of discharge on oral amiodarone, metoprolol, and digoxin. The patient was bridged to warfarin for anticoagulation. An angiotensin-converting enzyme inhibitor was also initiated for LV systolic dysfunction. As the patient did not have chest pain, and the troponin elevation was disproportionate to the degree of left ventricular dysfunction, no further evaluation for coronary artery disease was performed as an inpatient.
At 6-week follow-up, transthoracic echocardiography demonstrated persistent LV apical thrombus with an improved LVEF of 34%. ECG demonstrated continued atrial flutter with a heart rate of 81 BPM. At 12-week follow-up, the thrombus was no longer present (Movie S3) and LVEF had improved to 50%. Through physician–patient shared decision making, the patient subsequently underwent ECV due to symptoms of fatigue with successful restoration of sinus rhythm and improvement of his symptoms. A myocardial positron emission tomography (PET) study was normal and negative for ischemia. The patient has remained in sinus rhythm without any documented recurrences of atrial flutter on amiodarone therapy over 5 years of follow-up. Cessation of amiodarone was discussed with the patient, but the patient preferred to continue therapy due to the clinical response and lack of adverse reactions. The LV systolic function has since normalized without evidence of LV apical thrombus.
2 |. DISCUSSION
This case report highlights the therapeutic dilemma that clinicians face when encountering patients with LV dysfunction undergoing TEE-guided cardioversion for acute atrial fibrillation (AF) with the unusual, but important condition of LV apical thrombus.
3 |. ELECTRICAL CARDIOVERSION IN THE PRESENCE OF LEFT VENTRICULAR THROMBUS
Although there is concordance across current AF guidelines against performing cardioversion for AF in the presence of LAA thrombus,1 whether the same recommendations should be applied in the setting of LV apical thrombus is unclear. It is unclear whether the mechanical impact of cardioversion or potential myocardial stunning following cardioversion could result in systemic embolization from a LV thrombus. Most of the data reporting the embolic potential of LV thrombi come from the postmyocardial infarction literature. The reported risk of embolic events from a LV thrombus in this setting ranges from 6.6% to 86% and seems to be greatest in the first three months after myocardial infarction.2–7 Anticoagulation decreases the probability of development of LV thrombus and systemic embolism in at-risk patients as well as those with established thrombus.2 In keeping with these observations, current guidelines suggest anticoagulation for 3 months in patients with postmyocardial infarction LV thrombus.8
A retrospective study reported results of cardioversion in 21 patients with known LV apical thrombus, of whom, the procedure was indicated for AF in 38% and ventricular tachyarrhythmia (VT) in 62%.9 None of the patients had clinically apparent embolic events after cardioversion. However, the majority of the LV thrombi were described as laminated (71%). None of the patients had mobile thrombi, which were present in our case.
Recently, VT ablation in a cohort of 8 patients with LV thrombus was described.10 All of these patients had thrombi described as mural and laminated. A majority of these patients had sustained multiple ICD shocks and also underwent multiple intra-procedural cardioversions. One patient out of 8 suffered an ischemic stroke on postprocedure day 9. A left atrial appendage thrombus was detected on TEE in this patient. These are the only studies to our knowledge addressing the safety of ECV in the setting of LV thrombus. Neither of these studies included patients with echocardiographic features of acute LV thrombus, as in our case.
Echocardiographic features can predict embolic potential and guide management of LV thrombi. Multiple studies have demonstrated that thrombus protrusion into the LV cavity is a risk factor for systemic embolism.4,5,11 An additional risk factor for systemic embolism is thrombus mobility independent of the myocardium.4,5,11 Thrombi described as laminated are immobile and less likely to embolize. Calcified thrombi appear as bright and echo dense structures and are more likely to be chronic. The embolic potential of calcified thrombi might be lower, due to their chronic nature. However, systemic embolism has been reported even with calcified thrombi.12
In our case, the LV thrombus was protruding freely mobile and minimally echo dense suggestive of being acute. In the presence of features of acute LV thrombus, we felt that the safest approach in our case was to continue anticoagulation and proceed with a rate control strategy in lieu of cardioversion. As the patient did not tolerate beta blockers and other AV nodal blocking agents, amiodarone was utilized for rate control.1 Although there is a paucity of data, cardio-version should be avoided in patients with echocardiographic features of protruding and mobile LV thrombi, which have been reported to be associated with a high risk of embolism.4,5,11,13
While TEE is the most sensitive and reliable technique for identifying left atrial appendage thrombi before cardioversion of AF, it is a semi-invasive procedure and carries associated risks. A focused transthoracic echocardiogram (TTE) prior to cardioversion can provide clinically useful diagnostic information that may obviate the need for a TEE, as in the present case. Irrespective of the imaging modality utilized, if LV function is found to be depressed, special emphasis should be placed on assessing the LV for possible thrombus.
4 |. CONCLUSION
In summary, this case highlights management of AF in the setting an acute LV apical thrombus. While further data are needed to support the safety of ECV in patients with acute LV apical thrombus, routine ECV should be deferred in patients with echocardiographic features of acute LV apical thrombus.
Supplementary Material
TEE and TTE appearance of mobile and protruding echo density in the left ventricular apex.
TEE and TTE appearance of mobile and protruding echo density in the left ventricular apex.
TTE apical four-chamber view zoomed in on the LV apex with contrast agent demonstrates resolution of thrombus.
Acknowledgments
Funding information
Dr Melduni is supported by National Institutes of Health K01 (HL 135288)
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22:1004–1009. [DOI] [PubMed] [Google Scholar]
- 3.Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol. 1990;15:790–800. [DOI] [PubMed] [Google Scholar]
- 4.Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J. Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol. 1985;5:1276–1280. [DOI] [PubMed] [Google Scholar]
- 5.Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75:1004–1011. [DOI] [PubMed] [Google Scholar]
- 6.Kupper AJ, Verheugt FW, Peels CH, Galema TW, Roos JP. Left ventricular thrombus incidence and behavior studied by serial two-dimensional echocardiography in acute anterior myocardial infarction: left ventricular wall motion, systemic embolism and oral anticoagulation. J Am Coll Cardiol. 1989;13:1514–1520. [DOI] [PubMed] [Google Scholar]
- 7.Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echo-cardiography. Am J Med. 1983;74:989–995. [DOI] [PubMed] [Google Scholar]
- 8.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e637S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangalore S, Petre L, Herweg B, et al. Cardioversion in patients with left ventricular thrombus is not associated with increased thromboembolic risk. J Am Soc Echocardiogr. 2006;19:438–440. [DOI] [PubMed] [Google Scholar]
- 10.Rao HB, Yu R, Chitnis N, et al. Ventricular tachycardia ablation in the presence of left ventricular thrombus: safety and efficacy. J Cardiovasc Electrophysiol. 2016;27:453–459. [DOI] [PubMed] [Google Scholar]
- 11.Haugland JM, Asinger RW, Mikell FL, Elsperger J, Hodges M. Embolic potential of left ventricular thrombi detected by two-dimensional echocardiography. Circulation. 1984;70:588–598. [DOI] [PubMed] [Google Scholar]
- 12.Cullen JG, Korcuska K, Musser G, Schiller NB, Clark RD. Calcified left ventricular thrombus causing repeated retinal arterial emboli: clinical, echocardiographic, and pathologic features. Chest. 1981;79:708–710. [DOI] [PubMed] [Google Scholar]
- 13.Kinney EL. The significance of left ventricular thrombi in patients with coronary heart disease: a retrospective analysis of pooled data. Am Heart J. 1985;109:191–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEE and TTE appearance of mobile and protruding echo density in the left ventricular apex.
TEE and TTE appearance of mobile and protruding echo density in the left ventricular apex.
TTE apical four-chamber view zoomed in on the LV apex with contrast agent demonstrates resolution of thrombus.