Abstract
Background
Multidetector computed tomography (MDCT) scanning technology has increased the ease with which pulmonary emboli (PE) are evaluated. Our aim was to determine whether the incidence and severity of postoperative PE have changed since the adoption of MDCT.
Study Design
A prospective postoperative morbidity and mortality database from a single institution was used to identify all cancer patients who experienced a PE within 30 days of thoracic, abdominal, or pelvic surgery. The incidence, type (central, segmental, and subsegmental), and severity of PE were examined.
Results
A total of 295 PE were documented among 47,601 postoperative cancer patients. The incidence of PE increased yearly from 2.3 per 1000 patients in 2000 to 9.3 in 2005 (p < 0.0001). This corresponded to an increasing number of CT scans of the chest performed (6.6 CT scans per 1000 postoperative patients in 2000 vs. 45 in 2005, p < 0.0001). The increased incidence was due to a 7.8% (confidence interval [CI] 4.0–11.7) and 5.4% (CI 4.1–6.7) average annual increase in segmental and subsegmental PE, respectively. There is no change in the number of central (0.1%, CI −1.0–1.12) PE. The overall incidence of fatal PE was 0.4 and did not change over the time period (p = 0.3). A central PE was more commonly associated with hypoxia, intensive care unit (ICU) admission, and 30-day mortality (33% vs 5% for peripheral, p = 0.02).
Conclusions
Chest CT scans are being performed more frequently on postoperative cancer patients and have resulted in an increased diagnosis of peripheral PE. The clinical significance of and the optimal treatment for the diagnosed subsegmental PE are incompletely defined.
Keywords: postoperative complication, pulmonary embolism, surgical complication, computed tomography, X-ray
Introduction
Cancer patients undergoing surgery have more than twice the risk of a postoperative deep vein thrombosis (DVT) and more than three times the risk of fatal pulmonary embolism (PE) compared with patients with benign disease who are undergoing similar procedures.1 A venous thromboembolic event (VTE) is the most common cause of postoperative death in cancer patients undergoing surgery, underscoring the importance of detection and treatment of postoperative PE in these patients.2 Postoperative PE is more difficult to diagnose compared with a spontaneous PE since clinical symptoms and signs suggestive of PE, including chest pain, shortness of breath, tachycardia, and oxygen desaturation, can be explained by the effects of surgery, such as incisional pain, hypovolemia, and atelectasis, or may be masked by analgesics, including epidural anesthetics. The index of suspicion must be particularly high in postoperative cancer patients who, even in the absence of any symptoms, are considered to have a moderate clinical probability of having a PE.3
The introduction of multidetector computed tomography (MDCT) scans has improved the visualization of the pulmonary vasculature in the middle and peripheral lung zones4, and has improved our ability to diagnose PE by cross-sectional imaging. Conventional single detector CT misses one-third of peripheral PE.5, 6 This improved sensitivity for detection of PE by MDCT scan has also increase the incidence of nonfatal PE that were not suspected clinically by the ordering physician.7–9 In these studies, malignancy and surgery in the previous two months were risk factors for the development of an unsuspected PE.10 The clinical relevance of the incidentally discovered PE and the requirement for therapeutic anticoagulation in these patients is unclear, with one study suggesting that therapeutic anticoagulation was associated with a significantly higher one-year mortality in patients compared with those who received none or only prophylactic anticoagulation.11
The first MDCT scan was obtained at our institution (Memorial Sloan-Kettering Cancer Center [MSKCC]) in the year 2000. The introduction of this technology has resulted in an increase in the incidence of postoperative PE, as recorded in a prospective 30-day perioperative mortality database. In the current study we sought to identify the factors responsible for the increased detection of PE from the years 2000 to 2005. In addition, we sought to determine whether the clinical presentation, anatomic location within the pulmonary vasculature, clinical significance, and outcome associated with these PE have changed during the same time period.
Methods
Incidence of PE and Fatal PE
Approval to conduct the study was obtained from the Institutional Review Board of MSKCC. The institutional surgery database was queried to identify 47,601 patients who underwent abdominal, pelvic, thoracic, or soft-tissue (for truncal and extremity soft tissue sarcoma) surgery between January 1st, 2000 and December 31st, 2000. Among this cohort, 311 patients were identified as having a postoperative PE as recorded in a Perioperative Complications Database. This database is prospectively maintained through Morbidity and Mortality Rounds and the outcome for each patient is recorded for a minimum of 30 days. Each complication is graded for severity, defined as follows: grade 1 (no treatment or bedside management); grade 2 (intravenous medication required); grade 3 (invasive intervention required, including surgery); grade 4 (life threatening or permanent deficit); and grade 5 (complication resulted in death). The annual incidence of postoperative PE was determined by dividing the number of patients with a postoperative PE by the total number of patient surgeries for a given year and is reported as the rate per 1000 surgeries.
Incidence of MDCT Scans with PE protocol
The prospective QuadRIS radiology database was used to identify all patients who underwent a CT angiogram to rule out PE from January 1st, 2000 to December 31st, 2005. The PE protocol CT scan was performed on a 16-slice MDCT scanner (GE Medical Systems, Milwaukee, WI) following injection with 100 mL of contrast at approximately 4 mL per second with a reconstruction of 1.25 mm × 0.8 mm from 2 cm below the diaphragm to the aortic arch. This cohort of patients was cross referenced with the institutional surgery database to identify all patients who underwent a CT scan of the chest with a PE protocol in the postoperative period (defined as the first 30 days after the surgical procedure). This cohort included 1441 postoperative patients who underwent a CT scan of the chest with PE protocol. The annual incidence of postoperative PE studies was determined by dividing the number of postoperative chest CT scans to rule out PE by the total number of patient surgeries for a given year and is reported as the number per 1000 surgeries.
Postoperative PE Study Population
From the cohort 311 patients who experienced a postoperative PE between 2000 and 2005, 17 were excluded because they did not have a diagnosis of malignancy while the remaining 295 patients, with both a diagnosis of malignancy and a postoperative PE, formed the study population. The medical record, including clinic notes, radiology, pathology, and operative reports were reviewed to confirm demographic and pathologic data including patient age, gender, site, stage, and histologic type of cancer, comorbidities (history of DVT or PE), and type of surgery. The presenting symptoms prior to the diagnosis of PE (including shortness of breath, chest pain, tachycardia, oxygen saturation, hypotension, admission to intensive care unit (ICU), and cardiopulmonary arrest) were also recorded, as were the length of stay or requirement for readmission. The diagnostic modality used to document a PE (MDCT, ventilation-perfusion scan, or clinical/autopsy), the location of the PE within the pulmonary vasculature (central, segmental, or subsegmental), the number and the laterality of the PE, as well as documentation of a concurrent DVT, were also recorded. The use of unfractionated heparin (UF), low molecular weight heparin (LMWH), or oral anticoagulation (OAC) for prophylactic or therapeutic treatment and the placement of an inferior vena cava (IVC) filter were also recorded. Follow-up data were obtained by review of medical records and included the occurrence of a second VTE, complications related to anticoagulation, and the date and cause of death or last follow-up.
Statistical Analysis
In univariate analysis, statistical comparison between groups was performed using the Student t test for continuous variables or the χ2 test for discrete variables. Overall survival (OS) was calculated from the date of the PE to death or last follow-up and estimated using the Kaplan-Meier method. A p value ≤ .05 was considered statistically significant in all analyses. Statistical analyses were performed using the SAS (SAS Institute, Cary, NC) software package.
Results
Annual Incidence of Postoperative Evaluation and Detection of PE
A total of 295 postoperative PE were documented among 47,601 cancer surgery patients (6.2 per 1000 surgeries). The annual incidence of PE increased significantly from 2.6 in 2000 to 9.3 in 2005 (p < .001) and occurred at a steady increase each year (Table 1, Figure 1). This increase corresponded to a yearly increase in the number of contrast-enhanced high resolution CT scans performed in surgical patients in the postoperative period. In 2000, the year a MDCT scan was introduced at our institution, the incidence of postoperative CT to rule out PE was 6.6 per 1000 postoperative patients and increased steadily each year to 44.7 by 2005 (p < .0001) (Table 1). However, the rate of detection of PE among patients who underwent MDCT to rule out PE remained the same throughout the study period (20%–26%). The location of the PE in the pulmonary vasculature was recorded as central or peripheral (segmental or subsegmental) in 271 patients. The increased incidence was due to a 7.8% (confidence interval (CI) 4.0–11.7) and 5.4% (CI 4.1–6.7) average annual increase in segmental and subsegmental PE, respectively, from 2000 to 2005. There was no change in the number of central (0.1%, CI −1.0–1.12) PE during this time period (Figure 2).
Table 1.
Annual Number and Incidence (per 1,000 Surgeries) of 295 Patients with a Postoperative Pulmonary Embolism by Central or Peripheral (Segmental and Subsegmental) Location in the Pulmonary Vasculature
| Year (no. of operations) | Type of pulmonary embolism n (%) | Total incidence | |||
|---|---|---|---|---|---|
| Central | Segmental | Subsegmental | Unknown | ||
| 2000 (n = 6,808) | 5 (0.73) | 12 (1.76) | 1 (0.15) | 0 (0) | 18 (2.64) |
| 2001 (n = 7,313) | 7 (0.96) | 11 (1.50) | 3 (0.41) | 5 (0.68) | 26 (3.56) |
| 2002 (n = 7,604) | 8 (1.05) | 19 (2.50) | 7 (0.92) | 6 (0.79) | 40 (5.26) |
| 2003 (n = 8,239) | 2 (0.24) | 42 (5.10) | 13 (1.58) | 1 (0.12) | 58 (7.04) |
| 2004 (n = 8,622) | 6 (0.70) | 32 (3.71) | 23 (2.67) | 6 (0.70) | 67 (7.77) |
| 2005 (n = 9,015) | 5 (0.55) | 48 (5.32) | 27 (3.00) | 6 (0.67) | 86 (9.32) |
| Total (n = 47,601) | 33 (6.93) | 164 (3.45) | 74 (1.55) | 24 (0.50) | 295 (6.19) |
Figure 1.
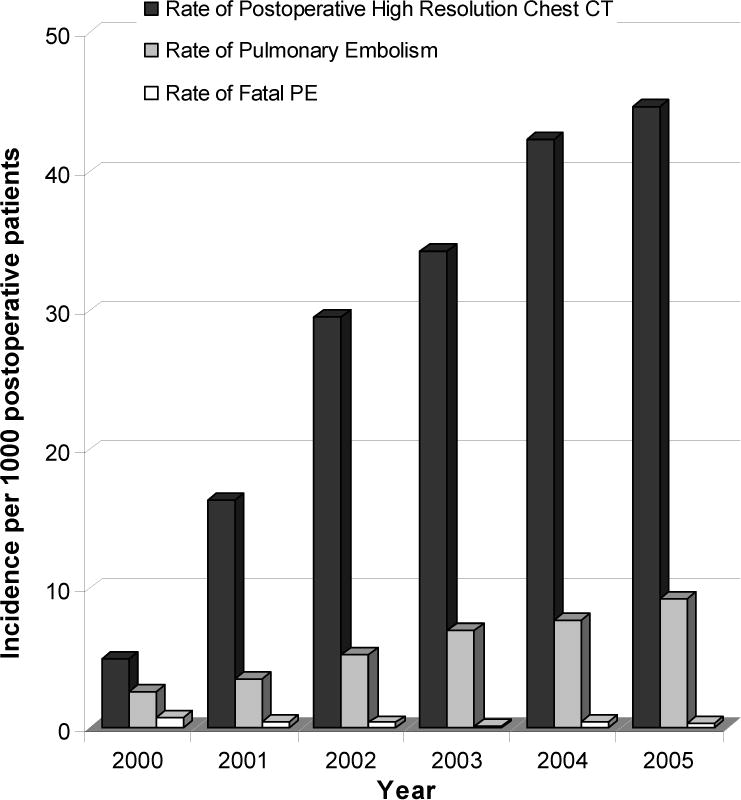
Annual postoperative incidence of multidetector computed tomography (MDCT) scan of the chest, pulmonary embolism (PE), and fatal PE. The number (incidence per 1,000 postoperative patients) of MDCT of the chest performed to evaluate patients for a PE in the postoperative period (black bar) and the number of postoperative PEs (gray bar) detected have increased from 2000 to 2005, while the incidence of a fatal PE among surgical patients in the postoperative period has remained unchanged (white bar).
Figure 2.
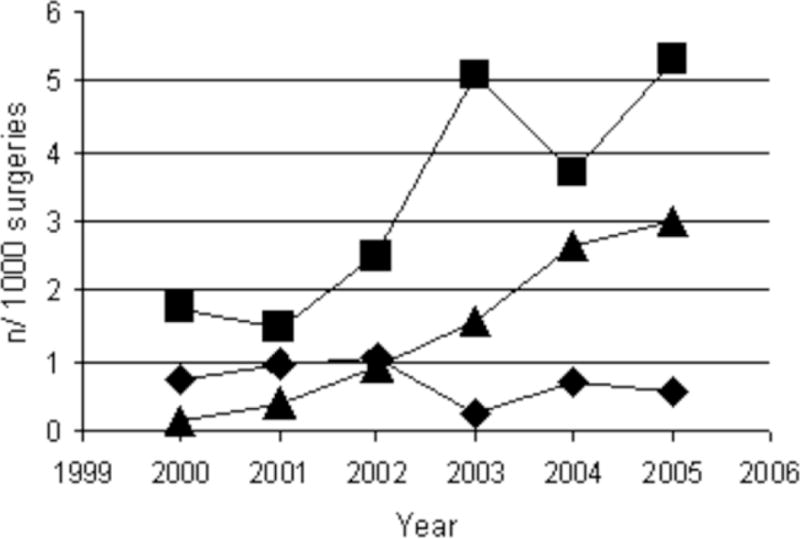
Annual incidence of central (diamond) and peripheral (segmental [square] and subsegmental [triangle]) pulmonary embolism during the 2000–2005 study periods.
Characterization of Postoperative PE Study Population
The characteristics of the 271 patients with postoperative PE of known location in the pulmonary vasculature are presented in Table 2. The average age was 63.2 years and 37.2% of patients were male. The majority of patients underwent a laparotomy with pelvic dissection (60.6%) followed by abdominal laparotomy (23.1%). The most common cancer sites were gynecologic (35.1%), followed by genitourinary (22.1%), and colorectal (11.8%). Almost 25% had stage IV disease at the time of surgery. Pharmacologic VTE prophylaxis was administered to 56.7% of patients, including dalteparin in 27.5%, enoxaparin in 11.2%, and UF in 15.3%. The number of patients receiving pharmacologic VTE prophylaxis did not differ between those who developed a central versus peripheral PE or between surgical years (data not shown).
Table 2.
Demographic Characteristics for Patients with a Postoperative Pulmonary Embolism of Known Location (n = 271) in the Pulmonary Vasculature
| Variable | Location of PE | Total (n = 271) | Central versus peripheral PE, p value | ||
|---|---|---|---|---|---|
| Central (n = 33) | Segmental (n = 164) | Subsegmental (n = 74) | |||
| Clinical and pathologic characteristics | |||||
| Age at PE (y) | 65.1 ± 12.2 | 63.4 ± 12.8 | 62.1 ± 13.4 | 63.2 ± 12.9 | NS* |
| Male | 46.7% | 36.2% | 35.5% | 37.2% | NS |
| BMI | 28.1 ± 5.2 | 27.1 ± 5.7 | 28.9 ± 8.9 | 27.7 ± 6.8 | |
| Type of surgery, % | |||||
| Thoracotomy | 15.2 | 12.8 | 13.5 | 13.3 | |
| Laparotomy-abdominal | 57.6 | 26.8 | 31.1 | 31.7 | |
| Laparotomy-pelvic | 21.2 | 54.3 | 44.6 | 47.6 | |
| Laparoscopy/thoracoscopy | 0 | 4.3 | 10.8 | 5.5 | |
| Soft tissue resection | 6.1 | 1.8 | 0 | 1.8 | |
| Cancer site, % | |||||
| Thoracic | 12.1 | 9.1 | 14.9 | 11.1 | |
| Colorectal | 12.1 | 13.4 | 8.1 | 11.8 | |
| GI (other) | 9.1 | 6.7 | 8.1 | 7.4 | |
| Hepatobiliary/pancreatic | 0.0 | 0.6 | 0.0 | 0.4 | |
| Genitourinary | 24.2 | 21.3 | 23.0 | 22.1 | |
| Gynecologic | 21.2 | 36.0 | 39.2 | 35.1 | |
| Soft tissue tumor | 6.1 | 3.7 | 1.4 | 3.3 | |
| Other | 0.0 | 3.7 | 0.0 | 2.2 | |
| Pathologic stage, % | NS* | ||||
| 0 | 5.3 | 0.0 | 2.4 | 1.1 | |
| I | 21.1 | 26.2 | 20.4 | 24.0 | |
| II | 21.1 | 19.6 | 18.4 | 19.4 | |
| III | 42.1 | 27.1 | 34.7 | 30.9 | |
| IV | 10.5 | 27.1 | 24.5 | 24.6 | |
| PE symptoms and severity | |||||
| Complication grade, % | < .001 | ||||
| 1 | 0.0 | 0.6 | 6.6 | 2.2 | |
| 2 | 53.3 | 84.1 | 85.5 | 81.0 | |
| 3 | 13.3 | 12.9 | 6.6 | 11.2 | |
| 4 | 0.0 | 1.2 | 0.0 | 0.7 | |
| 5 | 33.3 | 1.2 | 1.3 | 4.8 | |
| Clinical symptoms, % | |||||
| Shortness of breath | 67.9 | 48.5 | 58.7 | 53.4 | .041 |
| SaO2 < 92% | 74.1 | 36.7 | 40.5 | 41.6 | < .001 |
| Heart rate > 100 bpm | 85.2 | 61.7 | 61.3 | 64.0 | .007 |
| Chest pain | 14.3 | 8.7 | 10.8 | 9.9 | NS |
| Hemoptysis | 0.0 | 0.0 | 1.3 | 0.4 | NS |
| Clinical DVT | 17.9 | 10.5 | 6.8 | 10.2 | NS |
| ICU admission | 39.3 | 19.3 | 20.0 | 21.6 | .018 |
| Cardiopulmonary arrest | 27.6 | 5.6 | 8.0 | 8.7 | .013 |
| Concurrent DVT** | 56.3 | 37.5 | 22.2 | 34.8 | NS |
| Anticoagulation. % | |||||
| Pharmacologic prophylaxis | 60.7 | 56.6 | 55.3 | 56.7 | NS |
| Bleeding complications | 13.8 | 14.5 | 14.5 | 14.4 | NS |
| Followup. % | |||||
| Second VTE event | 6.9 | 11.0 | 9.2 | 10.1 | NS |
| 30-d PE mortality | 33.3 | 4.9 | 5.4 | 8.5 | < .001 |
Continuous variables compared with t-test for independent samples.
BMI, body mass index; DVT, deep vein thrombosis; GI, gastrointestinal; ICU, intensive care unit; VTE, venous thromboembolic event.
Clinical Presentation of PE by Year of Surgery and Pulmonary Location
The clinical symptoms and signs, including shortness of breath, chest pain, tachycardia (hazard ratio [HR] > 100), hypoxia (oxygen saturation < 92%), clinical evidence of DVT, and hemoptysis were recorded in 282 patients (95.6%). Only 5.3% of PE patients were asymptomatic, while 71.6% had 1 or 2 clinical findings and 23% had 3 or more. There was a progressive decrease in the number of clinical symptoms and signs identified in patients with a postoperative PE from 2000 to 2005 (Figure 3). No patients diagnosed with a postoperative PE were asymptomatic in 2000, while 55.6% had 3 or more clinical findings, compared with 10.7% asymptomatic patients and 13.1% with 3 or more clinical findings in 2005 (p < .0001). There was no significant difference between the number of patients with a postoperative PE who presented with cardiopulmonary arrest or were admitted to the ICU between the years 2000 and 2005 (data not shown).
Figure 3.
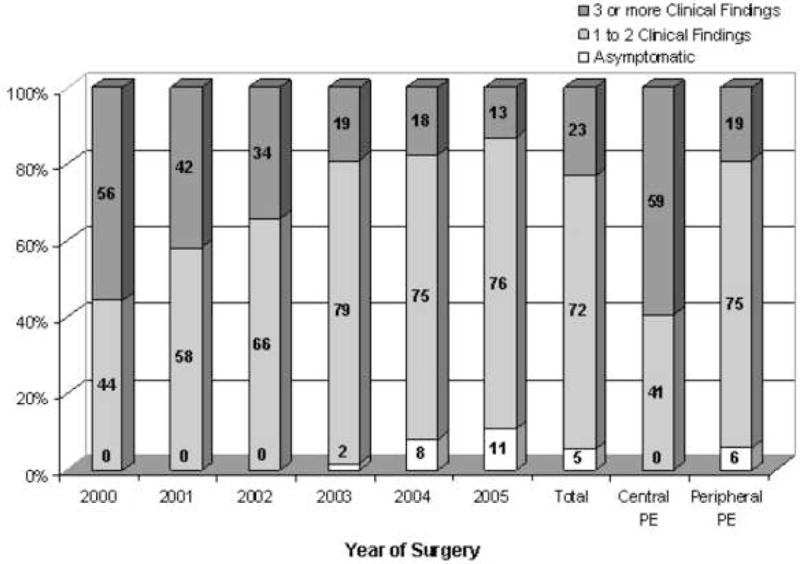
Clinical symptoms and signs among patients with a postoperative pulmonary embolism (PE) by surgery year and central versus peripheral location in the pulmonary vasculature. (Clinical findings include shortness of breath, chest pain, tachycardia, hypoxia, clinical evidence of deep vein thrombosis, or hemoptysis.) PE, pulmonary embolism.
Location of the PE within the pulmonary vasculature was also associated with clinical presentation (Table 2, Figure 3). A central PE was significantly more likely to be associated with tachycardia (89.2% vs 61.6%, p = 0.019), hypoxia (74.1% vs 37.9%, p = 0.001), ICU admission (39.3% vs 19.4%, p = 0.026), and cardiopulmonary arrest (27.6% vs 6.4%, p = 0.001) compared with a peripheral PE. The difference between central and peripheral PE causing shortness of breath, clinical symptoms of a DVT, and chest pain was 16.2%, 8.6%, and 5.1%, respectively, but these differences did not reach statistical significance. Central PE were asymptomatic in 0%, and associated with 1 or 2 clinical symptoms in 40.6% and 3 or more clinical symptoms in 59.4% of patients, versus data for peripheral PE, where 5.9%, 75.2%, and 18.9% were asymptomatic, with 1–2 symptoms, or with 3 or more symptoms respectively (Figure 3).
The Wells criteria are a widely used clinical assessment tool for predicting the probability of a PE.3 The criteria, including clinical symptoms and signs, as well as other risk factors for a PE. Patients are given a score and the combined score separates patients into low, moderate, and high clinical probability of having a PE. The patients in this study all had a malignancy and were all in the postoperative period and therefore had a baseline score of 2.5, defining them as having at least moderate probability for a PE. In a retrospective review it was not possible to evaluate the criterion of “other diagnosis less likely than PE” and therefore a score was calculated without this variable included. The mean Wells score in 2000 was 4.50 ± 1.26 SD compared with 3.58 ± 1.06 SD in 2005 (p = .002). The mean Wells score was also significantly higher in patients with a central PE (4.63, ± 1.33 SD) compared with patients with a peripheral PE (3.79 ± 1.13 SD, p < .0001).
Postoperative Morbidity and Mortality and Overall Survival Following Diagnosis of PE by Year of Surgery and Pulmonary Location
A total of 19 patients died of a VTE, 15 from the initial PE and 4 from a subsequent PE. Among the 280 patients who did not die of the initial PE, 29 developed a second PE (10.1%); this did not differ between patients with central and peripheral PE (p = .8). A total of 30 patients developed a complication on therapeutic anticoagulation (14.4%), including a gastrointestinal (GI) bleed in 14 patients, hematuria in 5 patients, a retroperitoneal or intraabdominal bleed in 5 patients, hemoptysis in 4 patients, a central nervous system (CNS) bleed in one patient, and heparin-induced thrombocytopenia (HIT) in one patient.
The grade of complication is an indicator of the severity of the adverse event (defined in the Methods section). The mean grade of postoperative PE was 2.23 ± 1.25 SD in 2000 and decreased to 2.20 ± .72 SD in 2005 (p = .004). The proportion of patients with a PE graded 3 or higher was 29.8% in the first 3 years of the study (2000–2002), while only 13.3% had a grade 3 or higher PE in the latter half of the study (2003–2005) (p = .001). The overall incidence of a fatal PE postoperatively was 0.4 and did not change over the 2000–2005 time period (p = 0.26) (Figure 1). However, due to the increased detection of peripheral PE, the chance of dying from a PE among all patients diagnosed with a postoperative PE decreased from 27.8% in 2000 to 3.6% in 2005 (p = .003). Thirty day postoperative mortality was also significantly different between patients with central (33.3%) versus peripheral (5.0%) PE (p = 0.02) (Figure 4).
Figure 4.
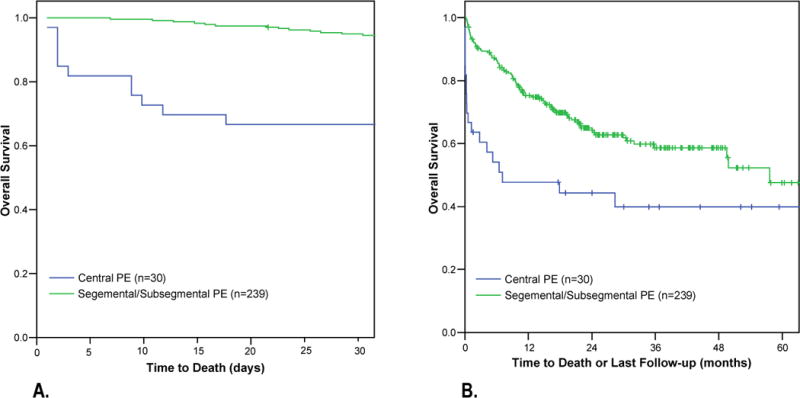
Kaplan-Meier survival curves among patients with postoperative central versus peripheral pulmonary embolism (PE). (A) Thirty-day mortality among cancer patients who were found to have a central PE (n = 33) was significantly worse compared with those who were found to have a peripheral PE (n = 237). (B) The median survival among cancer patients with a postoperative central PE was 7.1 months compared with 57.6 months among cancer patients with a postoperative peripheral PE (p = 0.003).
Discussion
Postoperative PE is a dreaded complication following cancer surgery, with a mortality rate of up to 45% in previous studies.2 The present study demonstrates than an increased awareness of VTE as a serious postoperative complication combined with more sensitive and available imaging modalities to confirm the diagnosis have led to a change in the clinical presentation and severity of postoperative PE. Specifically, advances in CT technology, including the advent of the MDCT scan, have enabled acquisition of thinner slices in a shorter time period, resulting in improved visualization of the pulmonary vasculature in the middle and peripheral lung zones, facilitating routine detection of the segmental and subsegmental arteries.3, 4 When compared with the gold standard imaging modality, pulmonary angiography, the sensitivity of MDCT for the diagnosis of PE was 100% and, in fact, detected 9% more patients with PE that, based on consensus review, were felt to represent false negatives for pulmonary angiography.12 This represents a significant improvement over conventional single slice CT scans where rates of 30% and higher have been reported for missing a peripheral pulmonary embolism.13–15
An MDCT was introduced at our tertiary care cancer center in 2000. The present study has demonstrated that the number (incidence per 1000 postoperative patients) of MDCT scans performed in the 30-day postoperative period to evaluate cancer patients for a PE has increased significantly between 2000 and 2005. Furthermore, the incidence of PE has increased during this time period, and can be attributed to a higher number of segmental and subsegmental PE diagnosed, with no change in the diagnosis of central PE. Despite this increased detection of postoperative PE, the incidence of a fatal PE among surgical patients in the postoperative period has remained unchanged. Taken together, this suggests that the prognosis following the diagnosis of a postoperative PE has changed since the introduction of MDCT scan technology.
The clinical need for a meticulous analysis of the peripheral pulmonary vessels is subject to debate. It has been shown that 6%–30% of patients with documented PE have clots in only subsegmental and smaller arteries.16, 17 In the absence of central emboli, however, the clinical relevance of these small peripheral clots is uncertain. Smaller clots have been previously shown to have less dramatic clinical manifestations and lower morbidity and mortality rates.18, 19 A cohort study of patients with a suspected PE and a negative CT scan compared 98 patients with a negative single-detector CT and 100 patients with a negative MDCT. This study demonstrated similar rates of death and subsequent thromboembolic disease on follow-up, despite the documented inferiority of single-detector CT at identifying peripheral emboli.6 Similarly, a systematic review of patients with suspected PE and a negative CT scan demonstrated that both single-detector and MDCT have a greater than 99% negative predictive value for the development of a subsequent VTE and that there was no difference in the subsequent development of a VTE based on CT modality used.5 These studies, as well as the current study, suggest that peripheral emboli may not impact adversely on patient outcome, even if left untreated.
The necessity for anticoagulation in patients with PE detected on MDCT has also been questioned. In a retrospective review of MDCT of the chest, a PE was detected in 117 patients. In 47 patients therapeutic anticoagulation was not given as the PE was diagnosed only during a retrospective review of the CT scan. Despite the lack of therapeutic anticoagulation, the one-year survival and subsequent PE-related morbidity were similar in the treated and untreated groups.11 By contrast, recent evidence suggests that LMWH may provide a survival benefit to cancer patients, which is unrelated to the VTE incidence and risk.20 In a meta-analysis of cancer patients without documented VTE, prophylactic anticoagulation with LMWH appeared to improve cancer-specific and overall survival.21 It is also possible that LMWH could similarly improve survival in cancer patients undergoing surgery,22, 23 providing further rationale to anticoagulate these patients, even in the presence of an asymptomatic, peripheral PE.
Anticoagulation therapy is not without risk, particularly in postoperative cancer patients. Previous studies have suggested an increased susceptibility to hemorrhagic complications in patients with malignancy, which are particularly relevant in the perioperative period. In the present study, a total of 14.4% of patients developed a complication related to the anticoagulation, with 2 patients dying from the complication. This rate is comparable to other studies of postoperative therapeutic anticoagulation for VTE.24 While there is currently no evidence to suggest that these patients should have anticoagulation withheld, clearly the risk benefit ratio must be considered when deciding to initiate therapeutic anticoagulation for postoperative cancer patients with subsegmental pulmonary emboli.
The present study suggests that the prognosis of a postoperative PE in a cancer patient is changing. The historical figure suggesting a 30% or higher mortality rate among patients with an untreated or misdiagnosed PE25, 26 may no longer be applicable. In the present study, an increased index of suspicion for a postoperative PE, coupled with the ease and availability of MDCT scanning technology and the increased ability to detect subsegmental and segmental PE using this imaging modality, have led to an increased incidence of postoperative PE in this group of patients with a reduction in the mortality rate associated with a postoperative PE. Whether this change in prognosis should influence our management of cancer patients diagnosed with a postoperative PE remains uncertain but is an interesting area for future investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Dr Schulman was supported in part by a fellowship grant from the Sarnoff Cardiovascular Research Foundation.
References
- 1.Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost. 2005;94:867–871. doi: 10.1160/TH04-03-0189. [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–179. doi: 10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 4.Raptopoulos V, Boiselle PM. Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. Radiology. 2001;221:606–613. doi: 10.1148/radiol.2213010473. [DOI] [PubMed] [Google Scholar]
- 5.Quiroz R, Kucher N, Zou KH, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293:2012–2017. doi: 10.1001/jama.293.16.2012. [DOI] [PubMed] [Google Scholar]
- 6.Prologo JD, Gilkeson RC, Diaz M, Cummings M. The effect of single-detector CT versus MDCT on clinical outcomes in patients with suspected acute pulmonary embolism and negative results on CT pulmonary angiography. AJR Am J Roentgenol. 2005;184:1231–1235. doi: 10.2214/ajr.184.4.01841231. [DOI] [PubMed] [Google Scholar]
- 7.Storto ML, Di Credico A, Guido F, et al. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184):264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 8.Gosselin MV, Rubin GD, Leung AN, et al. Unsuspected pulmonary embolism: prospective detection on routine helical CT scans. Radiology. 1998;208:209–215. doi: 10.1148/radiology.208.1.9646815. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian AJ, Paddon AJ. Clinically unsuspected pulmonary embolism—an important secondary finding in oncology CT. Clin Radiol. 2006;61:81–85. doi: 10.1016/j.crad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell CL, Boswell WD, Duddalwar V, et al. Unsuspected pulmonary emboli in cancer patients: clinical correlates and relevance. J Clin Oncol. 2006;24:4928–4932. doi: 10.1200/JCO.2006.06.5870. [DOI] [PubMed] [Google Scholar]
- 11.Engelke C, Rummeny EJ, Marten K. Pulmonary embolism at multi-detector row CT of chest: one-year survival of treated and untreated patients. Radiology. 2006;239:563–575. doi: 10.1148/radiol.2392050118. [DOI] [PubMed] [Google Scholar]
- 12.Winer-Muram HT, Rydberg J, Johnson MS, et al. Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology. 2004;233:806–815. doi: 10.1148/radiol.2333031744. [DOI] [PubMed] [Google Scholar]
- 13.Perrier A, Howarth N, Didier D, et al. Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism. Ann Intern Med. 2001;135:88–97. doi: 10.7326/0003-4819-135-2-200107170-00008. [DOI] [PubMed] [Google Scholar]
- 14.Drucker EA, Rivitz SM, Shepard JA, et al. Acute pulmonary embolism: assessment of helical CT for diagnosis. Radiology. 1998;209:235–241. doi: 10.1148/radiology.209.1.9769837. [DOI] [PubMed] [Google Scholar]
- 15.Goodman LR, Curtin JJ, Mewissen MW, et al. Detection of pulmonary embolism in patients with unresolved clinical and scintigraphic diagnosis: helical CT versus angiography. AJR Am J Roentgenol. 1995;164:1369–1374. doi: 10.2214/ajr.164.6.7754875. [DOI] [PubMed] [Google Scholar]
- 16.Oser RF, Zuckerman DA, Gutierrez FR, Brink JA. Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology. 1996;199:31–35. doi: 10.1148/radiology.199.1.8633168. [DOI] [PubMed] [Google Scholar]
- 17.Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 18.Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 20.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, et al. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost. 1999;82:947–952. [PubMed] [Google Scholar]
- 21.Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 22.Kingston RD, Fielding JW, Palmer MK. Peri-operative heparin: a possible adjuvant to surgery in colo-rectal cancer? Int J Colorectal Dis. 1993;8:111–115. doi: 10.1007/BF00299339. [DOI] [PubMed] [Google Scholar]
- 23.von Tempelhoff GF, Harenberg J, Niemann F, et al. Effect of low molecular weight heparin (Certoparin) versus unfractionated heparin on cancer survival following breast and pelvic cancer surgery: A prospective randomized double-blind trial. Int J Oncol. 2000;16:815–824. doi: 10.3892/ijo.16.4.815. [DOI] [PubMed] [Google Scholar]
- 24.Della Valle CJ, Jazrawi LM, Idjadi J, et al. Anticoagulant treatment of thromboembolism with intravenous heparin therapy in the early postoperative period following total joint arthroplasty. J Bone Joint Surg Am. 2000;82:207–212. doi: 10.2106/00004623-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 26.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]


