SUMMARY
The urothelium is an epithelia barrier lined by a luminal layer of binucleated, octoploid, superficial cells. Superficial cells are critical for production and transport of uroplakins, a family of proteins that assemble into a waterproof crystalline plaque that helps protect against infection and toxic substances. Adult urothelium is nearly quiescent, but rapidly regenerates in response to injury. Yet the mechanism by which binucleated, polyploid, superficial cells are produced remains unclear. Here, we show that superficial cells are likely to be derived from a population of binucleated intermediate cells, which are produced from mononucleated intermediate cells via incomplete cytokinesis. We show that binucleated intermediate and superficial cells increase DNA content via endoreplication, passing through S phase without entering mitosis. The urothelium can be permanently damaged by repetitive or chronic injury or disease. Identification of the mechanism by which superficial cells are produced may be important for developing strategies for urothelial repair.
Graphical Abstract
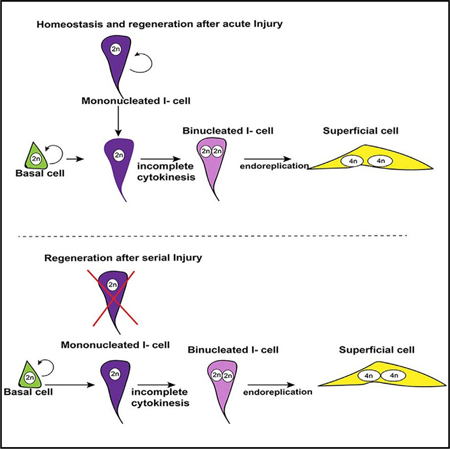
In Brief
Binucleated superficial cells are critical for urothelial barrier function. Wang et al. show that they derive from binucleated intermediate cells that form via incomplete cytokinesis. Both superficial and intermediate cells increase ploidy via endoreplication, a feature likely to be important for repair and response to environmental changes.
INTRODUCTION
The urothelium is an epithelial barrier that extends from the renal pelvis to the bladder neck, protects against pathogens and toxins, and controls the passage of water and ions between the mucosa and underlying tissue. The adult urothelium is nearly quiescent but can quickly regenerate after acute injury from urinary tract infection (UTI) or exposure to toxins, indicating that progenitors exist in adults that are able to repair the urothelium. The mouse urothelium contains two sub-populations of basal cells (K5-basal cells and K14-basal cells), intermediate cells (I-cells), and a luminal layer lined with superficial cells (S-cells; Figure 1). S-cells are binucleated, polyploid, and post-mitotic (Hicks, 1975). They are cellular machines, specialized for synthesis and transport of Uroplakins, a family of integral membrane proteins that assemble into a crystalline apical plaque that covers most of the urothelial apical surface (Lin et al., 1994; Wu et al., 1990, 1994, 2009). S-cells connect to one another via high resistance tight junctions, forming a waterproof barrier that prevents leakage during voiding, which occurs under pressure. These cells, which can be as large as 250 µm, are able to respond to environmental cues in a number of ways. For example, during the filling phase of micturition, S-cells increase their apical surface via exocytosis of specialized fusiform vesicles that shuttle Upks to the surface, where they are assembled into uroplaque crystals. During the emptying phase of micturition, S-cells decrease their surface area via endocytosis, shuttling apical membrane/plaque into the cell for degradation (Carattino et al., 2013; Khandelwal et al., 2009; Wu et al., 2009). These specialized features likely depend on the ability of S-cells to maintain a high rate of metabolism, protein synthesis, and intracellular transport.
Figure 1. Identification of a New Binucleated Intermediate Cell Population Likely to Be Direct Superficial Progenitors.
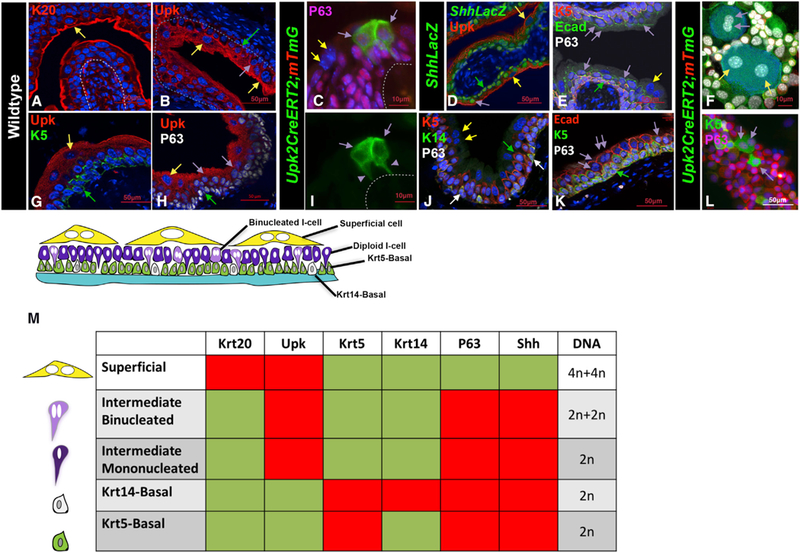
(A) Immunofluorescence staining shows K20 expression in sections of bladder from wild-type adult mice. The yellow arrow points to a K20-positive S-cell. Scale bar, 50 µm.
(B) Immunofluorescence staining for Upk3 in a section of bladder from a wild-type adult mouse. Scale bar, 50 µm.
(C) A cryosection from the urothelium of a Upk2CreERT2;mTmG mouse 10 days after tamoxifen treatment shows expression of membrane-bound Gfp. The purple arrows point to Gfp-labeled mononucleated I-cells that form very close to one another, connected to the basement membrane by thin cytoplasmic extensions (purple arrowheads in I). Scale bar, 10 µm.
(D) Immunofluorescence staining for LacZ, P63, and Upk in sections of a bladder from an adult ShhLacZ reporter mouse (Harfe et al., 2004). The yellow arrow points to a LacZ-negative S-cell. Purple arrows point to LacZ-positive intermediate cells, and the green arrow points to a LacZ-positive basal cell. Scale bar, 50 µm.
(E) Immunostained paraffin section from an adult wild-type mouse shows expression of K5, E-cad, and p63. The yellow arrows points to a binucleated S-cell. The double purple arrows points to binucleated I-cells. The green arrow points to a basal cell. Scale bar, 50 µm.
(F) Urothelial cells isolated from a tamoxifen-induced adult Upk2CreERT2;mTmG mouse. Scale bar, 10 µm.
(G) An immunostained paraffin section from a wild-type mouse shows expression of Upk and K5. The yellow arrow points to an S-cell, and the green arrow points to the K5-labeled basal cell. Scale bar, 50 µm.
(H) An immunostained paraffin section from the urothelium of an adult mouse showing expression of Upk and p63. The yellow arrow points to an S-cell; the purple arrows point to intermediate cells; and the green arrow points to a basal cell. Scale bar, 50 µm.
(I) A cryosection from the urothelium of a Upk2CreERT2;mTmG mouse 10 days after tamoxifen treatment shows expression of membrane-bound Gfp. The purple arrows point to Gfp-labeled mononucleated I-cells that form very close to one another and are connected to the basement membrane by thin cytoplasmic extensions. Purple arrowheads denote the Gfp+ cytoplasmic extensions connecting the I-cell to the basement membrane. Scale bar, 10 µm.
(J) Immunofluorescence staining for K5, K14, and p63 in sections of bladder from a wild-type adult mouse. The white arrows point to K14+ basal cells. Scale bar, 50 µm.
(K) A paraffin section from an adult mouse stained with Ecad, K5, and P63. Double purple arrowheads denote binucleated I-cells. Single purple arrow denotes a mononucleated I-cell. Scale bar, 50 µm.
(L) Cells washed from a Upk2CreERT2;mTmG adult mouse urothelium stained with K5 and P63. Scale bar, 50 µm.
(M) Combinatorial markers used to distinguish different urothelial populations.
Variation in cell ploidy, or sets of chromosomes generated through duplication(s) of the whole genome, is a natural and important part of development in different organisms and tissues across many taxa (Edgar et al., 2014; Fox and Duronio, 2013). Polyploidy has been shown to have positive features, for example, enabling cells to better respond to environmental changes or challenges, as well as negative features, including increased potential for aneuploidy, which is commonly associated with cancer and birth defects due to genome instability (Duncan et al., 2010; Losick et al., 2013; Schoenfelder and Fox, 2015; Storchova and Pellman, 2004). Besides cell-cell fusion, polyploidy can be programmed through non-canonical cell cycles, also known as endoreplication or endocycling, in which DNA replication (S phase) proceeds without intervening mitoses (Frawley and Orr-Weaver, 2015). In this case, genome duplication is uncoupled from daughter cell formation by oscillating G and S phases, as observed in plant trichromes, Drosophila salivary glands, and in many other fly tissues (Edgar and Orr-Weaver, 2001; Hammond and Laird, 1985; Swanhart et al., 2005). Polyploidy can also result from failed cytokinesis in which cells become binucleate (here on referred to as endomitosis), as observed in megakaryocytes, trophoblasts, hepatocytes, and Drosophila male accessory gland cells and larval tissues (Cao et al., 2017; Gentric and Desdouets, 2014; Nguyen and Ravid, 2010; Sarto et al., 1982; Taniguchi et al., 2014). Importantly, endomitosis and endocycling are not mutually exclusive, and both pathways for increasing genome copy number per cell have been found to occur in polyploid cells, for example, in zebrafish epicardium (Taniguchi et al., 2014).
The cellular mechanism by which S-cells achieve polyploidy is largely unexplored. Lineage studies and pulse-chase analyses suggest that both I-cells and basal cells can produce S-cell daughters depending on the conditions (Colopy et al., 2014; Gandhi et al., 2013; Papafotiou et al., 2016; Schäfer et al., 2017; Shin et al., 2011). Yet it remains unclear how binucleated polyploid S-cells are produced from mononucleated diploid populations. Here, we identify a population of binucleated I-cells that reside below the superficial layer of the urothelium that are likely to replace S-cells as they die off during homeostasis and regeneration. Using fate mapping, we show that binucleated I-cells are derived from mononucleated I-cells during urothelial homeostasis and regeneration. Using real-time imaging, we show that binucleated I-cells form via endomitosis in which diploid I-cells enter mitosis but fail to complete cytokinesis. Our studies also suggest that binucleated I- and S-cells can further increase ploidy during differentiation, via endoreplication, passing through S phase without proceeding through mitosis. Interestingly, we found that the pathway to polyploidy is likely to be conserved, regardless of which populations act as progenitors. Binucleated I-cells, are produced from a diploid I-cell population during homeostasis and after acute injury; however, diploid I-cells have a limited capacity for division and are depleted after serial or chronic injury. Under these conditions, basal cells replenish the diploid I-cell population, producing S-cells via endomitosis, thereby preserving the urothelial barrier.
RESULTS
Identification of a Binucleated Intermediate Cell Population Likely to Be Direct Superficial Progenitors
The urothelium is stratified, containing layers of basal cells, I-cells, and S-cells critical for producing and maintaining the crystalline plaque that serves as the urothelial barrier. These different populations can be distinguished by combinatorial markers (Figure 1M). Krt20 marks mature S-cells, which are also positive for Upk and negative for P63, K5, and Shh (Figures 1A–1H, 1J, 1L, and 1M, yellow arrows denote S-cells). I-cells co-express Upk, P63, and Shh and display low or undetectable expression of Krt20 and Krt5 (Figures 1b–1F, 1H, and 1K–1M, purple arrows denote I-cells). Analysis of Upk2CreERT2;mTmG mice, in which Gfp is localized to the cell membrane, reveals that I-cells (Gandhi et al., 2013) form very close to one another and display fine cytoplasmic projections that connect with the basement membrane (Figures 1C and 1I) The presence of these connections is likely to be important for maintaining the I-cell population, since epithelial cells require basement membrane connections in order to undergo cell division (Le Bras and Le Borgne, 2014; Oliferenko et al., 2009; Sambandamoorthy et al., 2015). The basal population contains K5-basal cells that co-express Krt5, P63, and Shh and exhibit low or undetectable expression of K14, Upk, or Krt20 (Figures 1B, 1D, 1E, 1G, 1H, 1J, 1K, and 1M, green arrows denote basal cells), and a second smaller population of basal cells (K14-basal cells) that express K14 as well as K5 and P63 (Figure 1J, K14 basal cells are denoted by white arrows).
Lineage studies suggest that both I-cells and Basal cells can divide and produce S-cell daughters (Colopy et al., 2014; Gandhi et al., 2013; Papafotiou et al., 2016; Schafer et al., 2017; Shin et al., 2011). Regardless of which urothelial populations serve as progenitors, it is still unclear how S-cells, which are post-mitotic, binucleated, and polyploid, are formed from basal or I-cells, which are mononucleated and diploid. Because S-cells are post-mitotic, their binucleation is likely to occur in either basal or I-cell progenitors before they begin to differentiate into S-cells. To begin to address this, we analyzed the adult urothelium during homeostasis, when there is little, if any, proliferation, to determine whether we could detect a binucleated population of I-cells or basal cells that could be S-cell precursors. To distinguish mononucleated cells from binucleated cells, we used E-cadherin, which stains adherens junctions to visualize cell boundaries, and DAPI staining to label nuclei. Combinatorial markers were used to distinguish different urothelial sub-populations (Figure 1M). This analysis revealed a previously uncharacterized population of I-cells that are binucleated and reside just below the S-cell layer (Figures 1E and 1K, double purple arrows denote binucleated I-cells). Analysis of whole-mount preps from Upk2CreERT2;mTmG mice revealed that binucleated I-cells are considerably smaller than S-cells, with smaller nuclei compared to S-cells, suggesting that both cell size and DNA content change during S-cell differentiation (Figures 1F and 1L). Consistent with this, DNA content analysis by flow cytometry and Integrated DAPI fluorescence from adult urothelial samples at homeostasis, indicate that binucleated I-cells have a DNA content of about 4n (2n+2n) (Figure S1), half that compared to S-cells, which generally have a DNA content of 8n (4n+4n) (Figure S1). Based on quantification of cell numbers in paraffin-embedded tissue, binucleated I-cells account for about 11% of the total I-cell population during homeostasis, the remainder of which are mononucleated I-cells. The presence of a binucleated I-cell population that resides below the superficial layer of the urothelium suggests that these cells may be progenitors that can quickly replace S-cells upon injury, thereby preserving the urothelial barrier.
Diploid I-Cells Produce Binucleated I-Cells and S-Cells after Injury
The existence of a newly identified binucleated I-cell sub-population, raises the possibility that these are direct S-cell progenitors. Recent studies suggest that both I-cells and basal cells can contribute to the S-cell population, depending on the extent of urothelial injury (Schafer et al., 2017). To determine which populations produce binucleated I-cells, we performed fate-mapping experiments using the Upk3aGCE;mCherry and Krt5CreERT2;mTmG mice to label I-cells and basal cells, respectively, and then we compared their respective contributions to different urothelial sub-populations after regeneration in response to acute injury. We first used UTI, a well-studied model of acute injury and repair (Hung et al., 2009), to induce regeneration. For these experiments, females received a transurethral inoculation of UTI 89, a strain of Uropathogenic E. coli (UPEC) isolated from a patient with cystitis (Garofalo et al., 2007). UPEC infects S-cells and establishes intracellular bacterial communities 6- and 12-hr post-infection (p.i.) (Figure S2A, white arrows). UPEC infected S-cells subsequently undergo exfoliation, which occurs between 6- and 12-hr post-inoculation (Figure S2B; arrows denote exfoliating S-cells). Basal and I-cells survive UTI and undergo a burst of cell-cycle activity 24 hr p.i., evidenced by expression of Ki67 and EdU, which is taken up during S phase, markers that are rarely detectable in the homeostatic urothelium, which is virtually quiescent (Jost, 1986; Jost and Potten, 1986) (Figures 2A–2E, 2H, 2I, S2D, and S2E). A new S-cell layer is established by 72-hr p.i. and regeneration is complete 2 weeks after infection when Krt20 expression is restored in the S-cell layer (Figure S2C).
Figure 2. Binucleated I-Cells Are Produced by I-Cells after Acute Injury and Give Rise to Superficial Cells.
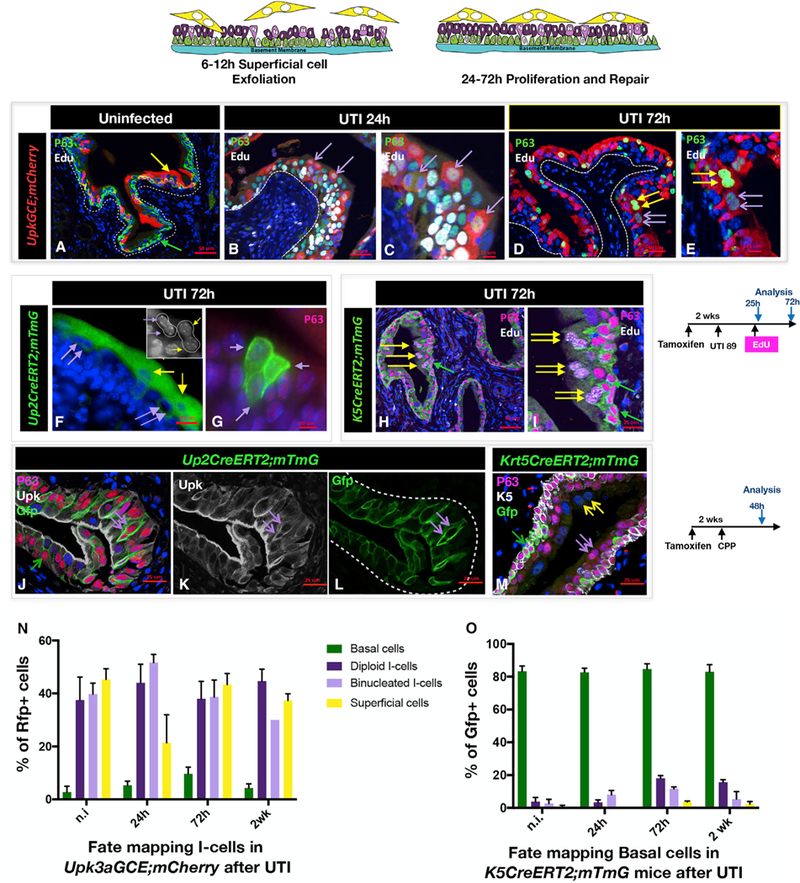
(A) A paraffin section from an uninfected adult Upk3aGCE;mCherry mouse stained for expression of EdU and P63. Scale bar, 50 µm.
(B) A paraffin section from a Upk3aGCE;mCherry mouse 24 hr after UTI, stained for expression of mCherry, p63, and EdU. Purple arrows point to the mCherry-positive mononucleated I-cells. Scale bar, 50 µm.
(C) Same sample used in (B) but at a higher magnification.
(D) A paraffin section from a Upk3aGCE;mCherry mouse 72 hr after UTI stained for expression of mCherry, p63, and EdU. The double yellow arrows point to the mCherry-positive S-cells, which have been generated following UTI. The purple double arrows point to the mCherry-positive binucleated I-cells.
(E) Higher magnification of image in (D). Scale bar, 50 µm.
(F) A cryosection from a Upk2CreERT2;mTmG adult mouse 72 hr after UTI showing lineage-marked binucleated I-cells and S-cells. Scale bar, 25 µm.
(G) A cryosection from a Upk2CreERT2;mTmG adult mouse 72 hr p.i. stained with P63 shows a clone of mononucleated I-cells. Scale bar, 10 µm.
(H) A paraffin section from an adult K5CreER;mTmG mouse 72 hr after UTI stained for expression of Gfp, P63, and EdU. The double yellow arrows point to Gfp-negative S-cells. Scale bar, 50 µm.
(I) Same sample used in (B) but at a higher magnification.
(J) A paraffin section isolated from a CPP-treated adult Upk2CreERT2;mTmG mouse 48 hr after CPP treatment, stained with P63, Upk, and Gfp. Scale bar, 25 µm.
(K) Same sample used in (J) but showing Upk expression. The double purple arrows point to a binucleated I-cell expressing Gfp. Scale bar, 25 µm.
(L) Same sample used in (J) but showing Gfp expression.
(M) A section from a K5CreER;mTmG mouse 48 hr after administration of CPP and stained with P63 and K5. Scale bar, 25 µm.
(N) Quantification of lineage-marked urothelial populations at different stages after UTI in Upk3aGCE;Rfp mice. For quantification, a minimum of three inde-pendent experiments were performed, and numbers are means of percentages ± SEM.
(O) Quantification of lineage-marked urothelial populations at different stages after UTI in K5CreER;mTmG mice.
The Upk3aGCE;mCherry line has a labeling efficiency of about 56%, and Cre-dependent recombination will label I-cells and their daughters with mCherry (Gandhi et al., 2013; Van Batavia et al., 2014). Analysis of Upk3aGCE;mCherry mice between 24 hr and 2 weeks p.i. revealed mCherry expression labeling 44% of mononucleated I -cells and 51% of the binucleated I-cell population 24 hr after infection, 43% of S-cells by 72 hr, as well as a small number (5%) of basal cells (Figures 2A–2E, 2N, and S2F–S2I). Importantly, we identified clones containing lineage-marked binucleated I-cells situated just below a line-age-marked binucleated S-cell, as well as clones containing two diploid I-cell and two likely daughters (Figures 2F and 2G), suggesting that I-cells can produce a binucleated population as well as divide, and produce diploid I-cells. Similar findings were observed using the Cyclophosphamide (CPP) model to induce a single round of regeneration. In this case, we used the Up2CreERT2;mTmG line, which has a labeling efficiency of 89% to mark I-cells and their daughters, and we analyzed the distribution of lineage-marked (Gfp+) diploid (mononucleated) I-cells, binucleated I- and S-cells 48 hr after CPP treatment, when proliferation peaks (Figures 2J, 2K, and S2N–S2U). These studies revealed Gfp labeling in 73% of I-cells and binucleated I-cells, and 87% of S-cells (Figure 5A), supporting the idea that I-cells produce binuclear I- and S-cells after acute injury.
Figure 5. S-Cells Are Generated from Either I or Basal Cells during Regeneration, Depending on the Extent of Injury.
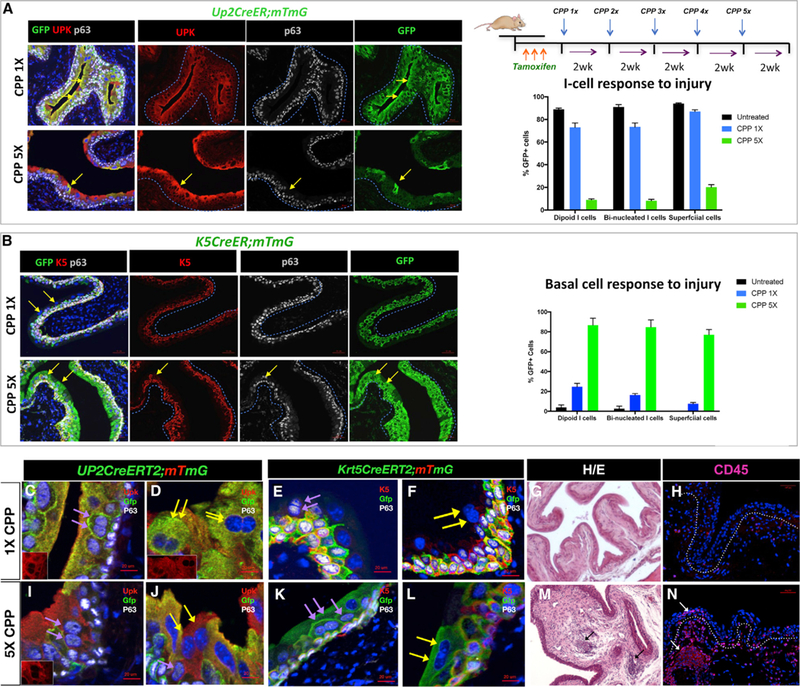
(A) Paraffin sections of bladders isolated from Up2CreERT2;mTmG mice after one or five rounds of CPP-induced injury and regeneration. The yellow arrows point to lineage-marked S-cells. Sections were stained for expression of Gfp, Upk, and P63. Scale bar, 50 µm. The right-top panel shows a schematic description of the injury model. The graph shows the distribution of Gfp-expression in mononucleated I-cells, binucleated I-cells, and S-cells in Up2CreERT2;mTmG that were untreated, treated 13 with CPP, and after 53 CPP treatment.
(B) Paraffin sections from K5CreER;mTmG after one or five rounds of CPP-induced injury and regeneration. Sections were stained for expression of Gfp, K5, and p63. Scale bar, 50 µm. The graph in the right panel shows the distribution of Gfp-expression in mononucleated I-cells, binucleated I-cells, and S-cells in untreated K5CreER;mTmG mice and in K5CreER;mTmG mice after 13 CPP and 53 CPP treatment. Scale bar, 50 µm.
(C) A section from a Up2CreERT2;mTmG mouse treated 13 with CPP showing lineage-marked binucleated I cells (purple arrows). Inset shows Upk expression in a low power magnification of the same section.
(D) A section from a Up2CreERT2;mTmG mouse treated 13 with CPP showing lineage-marked S-cells (yellow arrows). Inset is Upk staining alone in a lower magnification image of the same section.
(E) A section from a Krt5CreERT2mTmG mouse after 1 round of CPP showing lineage-marked basal cells as well as a binucleated I cell that is unlabeled (purple arrows).
(F) A section from a Krt5CreERT2mTmG mouse after 1 round of CPP showing Gfp-labeled basal cells as well as a binucleated S cell that is unlabeled (yellow arrows).
(G) Representative H&E-stained section from the bladder of a mouse treated with a single dose of CPP. Scale bar, 50 µm.
(H) Expression of CD45 in sections from bladders of mice treated with a single dose of CPP. Scale bar, 50 µm.
(I) A section from a Upk2CreERT2mTmG mouse showing lack of Gfp-labeling in binucleated I cells after five cycles of CPP-induced injury and repair (purple arrows). Inset is Upk staining alone in a lower magnification image of the same section.
(J) A section from a Upk2CreERT2mTmG mouse after 5X CPP treatment. Yellow arrows point to b inucleated S-cells, which are unlabeled.
(K) A section from Krt5CreERT2mTmG mouse showing Gfp-labeled binucleated I-cells (purple arrows) after five cycles of CPP-induced regeneration and repair.
(L) A section from Krt5CreERT2mTmG mouse showing Gfp-labeled binucleated S-cells (yellow arrows) after five cycles of CPP-induced regeneration and repair.
(M) Representative H&E-stained section of a bladder from a mouse treated with five doses of CPP. Scale bar, 50 µm.
(N) Expression of CD45 in sections from a mouse treated with five doses of CPP. Scale bar, 50 µm.
To examine whether basal cells also contribute to the I- or S-cell populations, we used the Krt5CreERT2;mTmG line, which has a labeling efficiency of about 84%, to follow the fate of basal cells during regeneration. Analysis of the distribution of Gfp+ cells in Krt5CreERT2;mTmG mice 24 hr after UTI revealed extensive labeling (greater than 80%) in the basal population as well as 3% of mononucleated I-cells and 8% of binucleated I-cells, while analysis at 72 hr when newly formed S-cells are present, revealed labeling in 3% of the S-cell population (Figure S2O). These observations indicate that basal cells contribute to the I-cell population. Analysis of regeneration in Krt5CreERT2; mTmG mice using the CPP model of injury and repair revealed a higher rate of contribution to the I- and S-cell lineages. In this case, about 20% of the diploid I-cell population were lineage marked, as well as, 16% of the binucleated I-cell population and 7.5% of the S-cell population after 1X CPP (Figures 2M and 5B). Together, these studies suggest that I-cells are able to divide and to produce binucleated I- and S-cell daughters after acute injury, while K5-basal cells can contribute to the I-cell population after UTI, but make a more significant contribution to the I-cell population after CPP. These findings also raise the possibility that the UTI and CPP injury models affect the behavior of basal and I-cell progenitors differently.
Binucleated I-Cells Form via Endomitosis
Binucleated cells can be produced by cell fusion of mononucleated cells or by endomitosis, in which cells progress partway through mitosis, but fail to complete cytokinesis and daughter cell separation (Duncan et al., 2010; Fortier et al., 2017; DePamphilis, 2016; Rios et al., 2016). If binucleated I-cells are produced by cell fusion, we would expect nuclei to be derived from two different mononucleated I-cells, and hence distributed randomly. Whereas, if binucleated cells arise via endomitosis, we expect that both nuclei would be derived from the same I-cell of origin. To distinguish between these possibilities, we performed fate mapping using Upk2CreERT2;nTnG mice. In this line, cells that undergo Cre-dependent recombination express nuclear Gfp and in cells that have not undergone recombination, nuclei are Tomato positive (Figure 3A). Upk2CreERT2;nTnG mice were treated with tamoxifen to induce recombination, infected with UPEC 2 weeks later, then analyzed 24- or 72-hr p.i. to evaluate the distribution of nuclear Gfp and Tomato. This analysis revealed 522/522 instances in which both nuclei were Gfp+, Tomato+, or negative for both markers and no instances in which marker expression was in one nucleus, but not the other (Figure 3B), strongly suggesting that binucleation occurs via a mechanism other than cell fusion.
Figure 3. Binucleated I-Cells Form via Endomitosis.
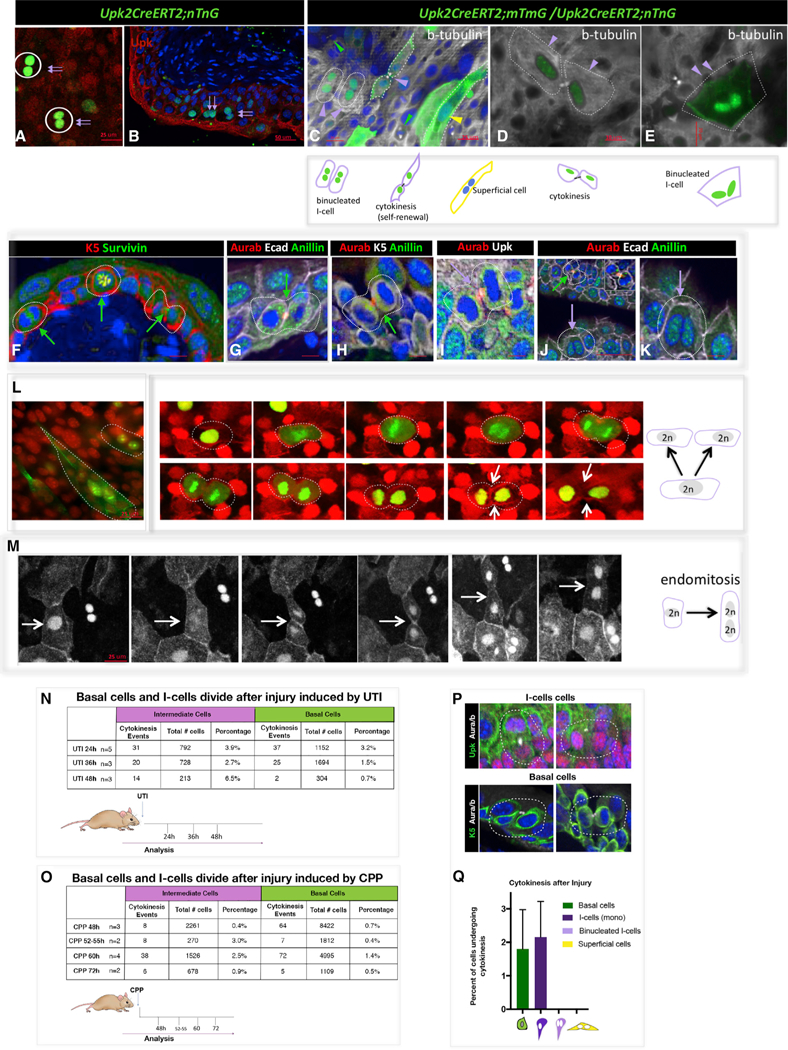
(A) Nuclear Gfp and Tomato expression in a whole-mount preparation from a Up2CreERT2;nTnG mouse. Scale bar, 25 µm.
(B) A section from a Up2CreERT2;nTnG mouse 24 hr after UTI shows binucleated Gfp+ I-cells (purple arrows). Scale bar, 50 µm.
(C) Whole-mount prep of urothelium from an adult Up2CreERT2;mTmG;nTnG mouse isolated 24 hr after UTI and stained with beta-tubulin showing membrane-bound Gfp in an I-cell that has divided and in an S-cell (purple and green arrowheads), as well as an unlabeled basal cell which is dividing.
(D) Whole-mount prep of urothelium from an adult Up2CreERT2;mTmG;nTnG mouse isolated 24 hr after UTI and stained with beta-tubulin showing a pair of I-cells that have undergone cytokinesis.
(E) Whole-mount prep of urothelium from an adult Up2CreERT2;mTmG;nTnG mouse isolated 24 hr after UTI and stained with beta-tubulin showing membrane and nuclear Gfp staining in a binucleated I-cell, which is negative for b-tubulin expression and is therefore likely to be a produce of incomplete cytokinesis. Yellow arrowheads denote S-cells, purple arrowheads deonote I-cells and green arrowheads denote Basal cells.
(F) A paraffin section from a CPP-treated wild-type mouse 48 hr after CPP treatment, stained for K5 and Survivin expression. Scale bar, 25 µm.
(G) A paraffin section from a CPP-treated wild-type mouse 48 hr after CPP treatment, stained for Ecad anillin and Aura/b expression. Scale bar, 25 µm.
(H) A paraffin section from a CPP-treated wild-type mouse 48 hr after CPP treatment, stained for K5, anillin, and Aura/b expression. Scale bar, 25 µm.
(I) A paraffin section from a CPP-treated wild-type mouse 48 hr after CPP treatment, stained for Upk and Aura/b expression. Scale bar, 25 µm.
(J) A paraffin section from a CPP-treated wild-type mouse 48 hr after CPP treatment, stained for Ecad, anillin, and Aura/b expression. Scale 50 µm.
(K) Same sample used in (J) but at a higher magnification. The purple arrow points to a binucleated I-cell that is not expressing mitotic markers. Scale bar, 10 µm.
(L) A whole-mount preparation from a cultured Up2CreERT2;mTmG;nTnG mouse urothelium showing lineage-marked binucleated I-cells and S-cells and a series of images from a real-time video of cultured urothelium and stroma from a Up2CreERT2;mTmG;nTnG mouse showing an I-cell undergoing normal cytokinesis. White arrow points to the cleavage furrow. Scale bar, 25 µm.
(M) A series of images from a real-time move of cultured urothelium and stroma from a Up2CreERT2;mTmG;nTnG mouse undergoing endomitosis. The white arrows point to the cleavage furrow, which forms and then regresses. Scale bar, 25 µm.
(N) Quantitation of cytokinetic events in I-cells and basal cells after UTI.
(O) Quantitation of cytokinetic events in I-cells and basal cells after injury and regeneration induced by CPP.
(P) Images showing examples of I-cells and basal cells stained with Aura/b, showing cells undergoing cytokinesis.
(Q) Comparison of cytokinetic events in basal cells, mononuclear I-cells, binuclear I-cells, and S-cells after injury. For quantification, a minimum of three inde-pendent experiments were performed, and numbers are means of percentages ± SEM.
The failure to observe evidence of mononucleated I-cell fusion after UTI suggests that binucleated I-cells form via endomitosis, entering the cell cycle, and exiting mitosis prior to completing cytokinesis. To begin to investigate how binucleation occurs, we analyzed sections of urothelium from wild-type adult mice 24 hr after infection with UPEC to identify mitotic cells and binucleated cells that are likely products of endomitosis. Urothelial sections were stained for expression of mitotic markers including aurora B kinase, survivin, and anillin. Aurora B and survivin, which are components of the chromosome passenger complex (CPC), localize to the nucleus and centromeres during early mitosis and to the central spindle and cell cortex during cytokinesis (Caldas et al., 2005; Carmena and Earnshaw, 2003). Anillin accumulates at the contractile ring and midbody in late anaphase or telophase during cytokinesis and is localized in the nucleus in late G1, G2, and S phases (Field and Alberts, 1995). These analyses revealed K5-basal cells in all phases of the cell cycle (Figures 3F–3H and 3J, green arrows point to mitotic basal cells). We observed I-cells expressing anillin and aurora B that were in late telophase or had undergone normal cytokinesis (Figures 3I–3K). This analysis also revealed binucleated I-cells with nuclear expression of Anillin and undetectable expression of aurora B/survivin, indicating that these cells are in interphase rather than in mitosis and hence likely to be prod-ucts of endomitosis (Figures 3E, 3J, 3K, 3N, 3R, and 3S). The observation that similar proportions of basal and diploid I-cells were in cytokinesis (1.8% and 2.2%, respectively; Figures 3N– 3P), suggests that both populations divide during regeneration (Figures 3N, 3P, and 3Q).
Taken together, our findings suggest that basal cells divide and produce a diploid I-cells, which differentiate into S-cell daughters after acute injury, whereas diploid I-cells divide and produce binucleated I- and S-cell daughters by endomitosis. To directly address this question, we used lineage tracing to mark I-cells and then we examined their behavior during mitosis using real-time imaging and an organotypic culture system developed in the lab (Batourina et al., 2012). Thick sections (0.3 to 0.5 cm in size) containing urothelium and associated stroma were placed in culture where they undergo a period of regeneration that lasts about 7 days, followed by a period of quiescence, which can be maintained for up to 1 month. In control experiments, we first compared the urothelium in organotypic cultures during the period of proliferation with in vivo samples of regenerating urothelium, using the Upk3aGCE;mCherry line in both cases to lineage mark I-cells (Figures S3A–S3H). For in vivo lineage tracing, Upk3aGCE;mCherry mice were in-jected with tamoxifen to induce recombination, infected with UPEC, then treated with EdU 24 hr after infection to label cells in S phase, which are exceedingly rare in the urothelium during homeostasis. For in vitro analysis, explants from Upk3aGCE; mCherry mice were cultured for 3 days, treated with 4-OHT to induce recombination and analyzed 2 days later to compare the distribution of lineage-marked cells in vitro and in vivo. Analysis of tissue from in vivo samples of urothelium revealed mCherry expression restricted to mononuclear and binucleated I- and S-cells, as expected, whereas basal cells were unlabeled (Figures S3A, S3B, S3E, and S3F). Analysis of cultured urothe-lium revealed remarkable preservation of urothelial stratification and sub-populations. We observed mononucleated and binu-cleated I-cells and S-cells co-labeled with mCherry and EdU (Figures S3C, S3D, S3G, and S3H) and unlabeled K5-basal cells that were EdU positive (Figure S3C). These observations sug-gest that the culture model effectively maintains urothelial prolif-eration, stratification, and differentiation and can be used to analyze urothelial regeneration in vitro.
To visualize the mitotic behavior of I-cells in vitro, we generated a lineage-model in which I-cell nuclei and cell membranes can be simultaneous visualized by intercrossing Upk2CreERT2;mTmG mice with UpkCreERT2;nTnG mice. Cre-dependent recombination induces expression of membrane-bound Gfp in Upk2CreERT2;mTmG mice, and nuclear Gfp in UpkCreERT2;nTnG mice (Figures 3C, 3D, and 3L). Analysis of whole-mount urothelium isolated from Up2CreERT2; mTmG;nTnG mice 24 hr after UTI revealed beta-tubulin expression in Gfp-positive mononucleated I-cells that were undergoing cytokinesis (Figures 3C and 3D, purple arrowheads) as well as in binucleated I- and S-cells, which were negative for beta-tubulin and presumably post-mitotic (Figures 3C–3E, purple arrow-heads denote binucleated I-cells expressing nuclear Gfp, yellow arrowhead denotes a S-cell expressing membrane-bound Gfp). Thus, we distinguished between lineage-marked diploid and binucleated I-cells using this system.
To directly visualize the behavior of I-cells during regeneration, we performed real-time imaging of organotypic cultures. Ex-plants from Up2CreERT2;mTmG;nTnG mice were maintained for 3 days in culture then treated with 4-OHT to induce recombination and placed on a stage-top incubator. Multiphoton confocal images were collected every 5 min for 24 hr to produce real-time videos. Representative image sequences are shown in Figures 3L and 3M. We observed four instances in which I-cells underwent apparently normal cell division, passing through the regular stages of mitosis and cytokinesis (Figure 3L; Video S1). We also observed three instances in which I-cells undergoing endomitosis, progressing through mitosis and forming a cytokinetic furrow that subsequently regressed (Figure 3M; Video S2). Normal cytokinesis lasted 2–3 hr, whereas endomitosis lasted up to 7 hr. Importantly, we did not observe evidence of I- or S-cells fusing in these experiments, nor did we observe binucleated I- or S-cells undergoing cytokinesis after injury (Figure 3Q), suggesting that S-cells, which have twice the DNA content compared to binucleated I-cells can increase their DNA content without entering mitosis.
Binucleated I-Cells Increase Ploidy as They Differentiate into S-Cells via Endoreplication, Entering S Phase without Passing through Mitosis
S-cells have two nuclei, a total DNA content equivalent to 8n (4n+4n) or higher (Stewart, 1986), and are considered to be post-mitotic (Jost, 1986). Next, we sought to address how S-cells attain a DNA content twice that of binucleated (2n+2n) I-cells. To evaluate whether this ploidy increase is associated with cell division of binucleated I or S-cells, we first analyzed the distribution of aurora B, survivin, and anillin, to label dividing cells in wild-type urothelium after UTI. This analysis revealed 0/927 instances of binucleated I-cells in mitosis/cytokinesis and 0/4,163 instances of S-cells in mitosis/cytokinesis, suggesting that the ploidy increase in S-cells is not associated with cell division of either precursor cell type (Figure 4I). Since we have not observed evidence for fusion or expression of cell division markers, these observations suggest that binucleated I and/or S-cells likely undergo endocycling, entering S phase without completing mitosis (Duncan et al., 2010; Fortier et al., 2017; DePamphilis, 2016; Rios et al., 2016).
Figure 4. Binucleated I-Cells and S-Cells Are Likely to Increase Ploidy by Endocycling.
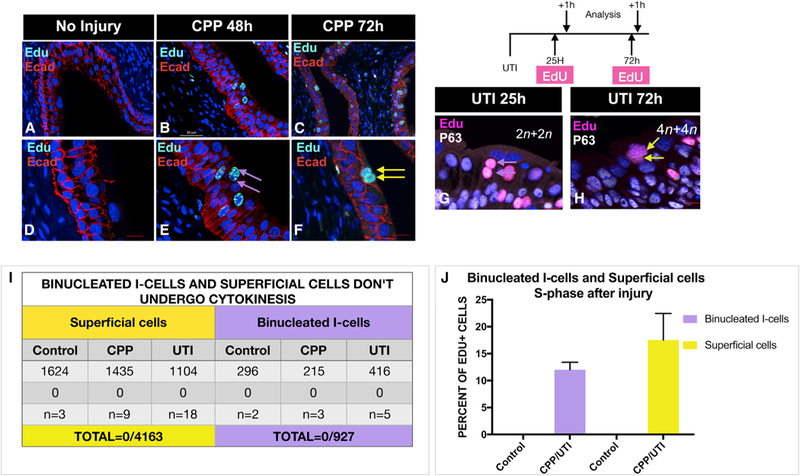
(A) A section from the urothelium of an untreated adult mouse stained for expression of Ecad and EdU. Scale bar, 50 µm.
(B) A section from an adult mouse 48 hr after CPP, given a 1-hr pulse of EdU stained for EdU and Ecad. Note EdU-expressing binucleated I-cells, which are likely to be in S phase. Scale bar, 50 µm.
(C) A section from an adult mouse 72 hr after CPP, given a 1-hr pulse of EdU prior to sacrifice stained for expression of Ecad and EdU. Note EdU-expressing S-cells, which are likely to be in S phase. Scale bar, 50 µm.
(D) Same sample used in (A) but at a higher magnification.
(E) Same sample used in (B) but at a higher magnification.
(F) Same sample used in (C) but at a higher magnification.
(G) A paraffin stained section from an adult mouse 24 hr p.i. analyzed 1 hr after a pulse of EdU and stained for EdU and P63 expression. Scale bar, 25 µm.
(H) A paraffin-stained section from an adult mouse 72 hr p.i. analyzed 1 hr after a pulse of EdU and stained for EdU and P63 expression. Scale bar, 25 µm.
(I) A table showing a summary of quantification of binucleated I-cells and S-cells in cytokinesis.
(J) A bar graph showing the percentage of EdU+ binucleated I-cells and S-cells after a 1-hr pulse, which are likely to be in S phase, in animals 24–72 hr after UTI and CPP-induced injury and repair.
To investigate S-cell endocycling more directly, we per-formed pulse-chase experiments to visualize the ontogeny of 8n S-cells during regeneration induced by CPP and UTI. For CPP-induced regeneration, animals were treated with CPP and exposed to a 1-hr pulse of EdU either at 48 hr, when proliferation peaks, or at 72 hr, when S-cells are forming in the luminal layer. Importantly, we did not detect EdU uptake in the non-injured homeostatic urothelium (Figures 4A, 4D, and 4I), which has an extremely slow rate of cell turnover. Representative samples analyzed 48- or 72-hr post-CPP reveal EdU-positive binucleated I-cells and S-cells (Figures 4B, 4C, 4E, and 4F), suggesting both of these polyploid cells are able to enter S phase. Analysis of the urothelium during regeneration induced by UTI, revealed similar findings: EdU-labeled binucle-ated I-cells and S-cells were observed after a 1-hr EdU pulse at 24 hr, with the peak of proliferation at 72 hr when S-cells are re-plenished in the luminal layer (Figures 4G and 4H), suggesting that these polyploid cells are likely to be in S phase. Quantification after UTI and CPP revealed EdU expression in 12% of binucleated I-cells and 17.5% of S-cells, suggesting that both cell types have the capacity to enter S phase (Figure 4J). Together, the absence of mitotic markers in binucleated I- and S-cells suggests that both cell types increase ploidy through endocycling.
Basal Cells Produce Diploid and Binucleated I-Cell Daughters after Serial Injury and Repair
Our previous studies and results shown here suggest that I-cells undergo normal cytokinesis and produce binucleated I- and S-cells after acute injury. Recently published work, comparing the regenerative potential of I and basal cells in surgical models of bladder augmentation indicates that both I and basal cells contribute to repair after focal injury, whereas only basal cells have the capacity to generate urothelial cells de novo, which populate acellular grafts (Schäfer et al., 2017), suggesting that different types of bladder injury may favor different progenitor populations. Further support for this idea comes from studies indicating that basal cells serve as the major urothelial progenitors both after acute and serial injury induced by high doses of Cyclophosphamide (Papafotiou et al., 2016), a drug used to treat cancer that can cause cystitis (Cox, 1979; Vasconcelos-Nóbrega et al., 2012). Thus, we tested whether serial injury changes the behavior of I or basal cells during regeneration. We used the K5CreERT2;mTmG line to label K5-basal cells and their daughters, and the Up2CreERT2;mTmG line to mark I-cells and their daughters and then compared their respective regenerative capacities after a single round of CPP, or after five rounds of CPP. K5CreERT2;mTmG and Up2CreERT2;mTmG mice were treated with tamoxifen to induce recombination and injected with CPP 2 weeks later. For a single round of injury, animals were treated with CPP and analyzed 2 weeks after CPP administration. For chronic injury, animals were given five doses of CPP with 2 weeks in between each dose and analyzed after the last CPP injection (Figure 5). Analysis of Upk2CreERT2;mTmG mice after one round of CPP revealed Gfp expression confined to the I- and the S-cell compartments, labeling 73% of mononucleated I-cells, 73% of binucleated I-cells, and 87% of S-cells (Figures 5A, 5C, and 5D). After five rounds of CPP, however, Gfp-labeling was present in only 9% of I-cells, 8% of binucleated I-cells and 20% of S-cells (Figure 5A, bottom panel; Figures 5I and 5J), suggesting that the I-cell population was exhausted after chronic injury and no longer contributes to the regenerative process.
Analysis of K5CreERT2;mTmG mice after one round of CPP-induced injury and regeneration revealed Gfp labeling in 24% of I-cells, 16% of binucleated I-cells and 7% of S-cells (Figure 5B, top panel; Figures 5E and 5F), while after five rounds of CPP, 86% of mononucleated I-cell population, 84% of binucleated I-cells and 87% of S-cells expressed the Gfp lineage mark (Figure 5B, bottom panel; Figures 5K and 5L). Together, these findings suggest that I-cells are likely to divide and produce binucleated I-cells and S-cells during homeostasis and after acute injury. On the other hand, basal cells produce a fraction of I-cells during regeneration following acute injury, whereas, after serial injury, basal cells are the progenitor population that repairs the urothelium. Interestingly, histological analysis and analysis for expression of leukocyte markers, reveals significant inflammation in the urothelium after five rounds of CPP, which is not observed after one round of CPP (Figures 5G, 5H, 5M, and 5N). CPP is used to treat leukemia, but is also a carcinogen that causes hemorrhagic cystitis (Cox, 1979; Farsund and Dahl, 1978; Golubeva et al., 2014). Whether serial injury plays a role in carcinogenesis by altering the behavior of urothelial cell types, is an interesting possibility.
DISCUSSION
The urothelium forms a barrier that prevents exchange of water and toxins between the urinary tract and the blood. S-cells, which are binucleated and polyploid, line the apical surface of the urothelium and perform many functions that are critical for maintaining the urothelial barrier (reviewed in Khandelwal et al. [2009]; Wu et al. [2009]). S-cells are long-lived, surviving 40 weeks or longer (Farsund, 1975). They are connected to one another via high-resistance tight junctions, and their apical surface is lined with Upk-containing plaque, features important for protecting against pathogens and toxins, for preventing leakage of urine into underlying tissue, and preventing water loss. S-cells maintain a system of fusiform vesicles that transport Upks from the Golgi to the cell surface where they assemble into plaque, and a second system of endosomal vesicles that transport apical membrane and plaque back into the cells for degradation (Amano et al., 1991; Khandelwal et al., 2009; Wu et al., 2009). Polyploidy and endomitosis may be critical in S-cells for a number of important functions. The ability to adjust DNA concentration without undergoing cell division may enable S-cells to respond to environmental changes without disrupting the urothelial barrier, and the presence of a binucleated I-cell population just below the Superficial layer, provides a population of progenitors that can quickly replace S-cells as they die off, thereby maintaining the urothelial barrier.
S-Cells Form from Binucleated I-Cells and S-Cells via Endomitosis and Endoreplication
Polyploid cells are common in the plant kingdom and are increasingly found in cells and tissues in the animal kingdom. In Drosophila, they include nurse cells, salivary gland cells, ovarian follicle cells, and glia cells (Frawley and Orr-Weaver, 2015; Hammond and Laird, 1985; Unhavaithaya and Orr-Weaver, 2012); in zebrafish, they include epicardium (Cao et al., 2017); and in mammals, they include hepatocytes (Duncan, 2013; Fortier et al., 2017), megakaryocytes (Machlus and Italiano, 2013), and syncytial trophoblasts (Hu and Cross, 2010), among others. They generally arise via successive rounds of cell fusion; via endomitosis, in which cells progress through mitosis but fail to complete cell division, resulting in binucleation; or via endoreplication or endocycling in which cells completely bypass mitosis and instead alternate between G and S phases (Lee et al., 2009; Orr-Weaver, 2015).
To visualize urothelial cell division in real time, we developed an organotypic culture system, in which we culture thick sections of urothelium and associated stroma. Our system maintains the stratification and cell types present in the in vivo urothelium for up to 1 month. We developed a mouse strain (Upk2CreERT2; mTmG;nTnG mice) expressing both membrane-bound Gfp and nuclear Gfp in I- and S-cells, enabling us to simultaneously visualizate of nuclei and cell boundaries during cell division. Real-time imaging of Upk2CreERT2;mTmG;nTnG cultures revealed mononucleated I-cells in the process of cytokinesis, undergoing normal cell division, and mononucleated I-cells entering mitosis without undergoing cytokinesis, a process known as endomitosis. In this case, binucleated cells progress to anaphase and develop a contractile ring and cytokinetic furrow that subsequently regresses, generating a binucleated cell with two diploid (2n+2n) nuclei.
S-cells are post-mitotic and have a DNA content of 8n (4n+4n), twice that of I-cells. Importantly, we have not observed either binucleated I- or S-cells at any stage of mitosis, indicating that this further increase in ploidy is unlikely to occur during cell division. Pulse labeling with EdU during regeneration indicates that binucleated I-cells, which initially have a DNA content of 4n (2n+2n), can enter S phase as they differentiate into newly formed S-cells. Interestingly, these experiments suggest that S-cells can also enter S phase, perhaps enabling them to directly respond to environmental or metabolic changes. The ability of S-cells to endocycle, as opposed to undergo cell division, may also help maintain urothelial impermeability, which depends on high-resistance tight junctions that prevent leakage during micturition (Rickard et al., 2008)
While the function of polyploidy remains unclear, a number of studies suggest that the large amount of DNA and the ability to adjust DNA content without entering mitosis may enable terminally differentiated cells to respond more efficiently to environmental changes (Bergmann et al., 2015; Duncan, 2013; Guidotti et al., 2003; Losick et al., 2013; Pandit et al., 2013). Polyploidy is associated with increased cell size in plants, insects, and animals (Beaulieu et al., 2008; Epstein, 1967; Orr-Weaver, 2015), and polyploid cells are susceptible to aneuploidy and can lead to cancer, since they evade the regular cell cycle checkpoints that would normally induce apoptosis (Gordon et al., 2012). On the other hand, recent studies suggest that polyploid hepatocytes suppress tumorigenesis compared to diploid hepatocytes (Zhang et al., 2018). The urothelium is a source of cells that produce bladder cancers. The extremely slow rate of urothelial turnover (almost 1 year) may be a factor that helps prevent aneuploidy and tumorigenesis. Whether polyploidy in the binucleated I-cell or S-cell populations leads to increased or reduced tendency for bladder cancer is an interesting question.
Binucleated S-Cells Are Generated via Similar Pathways Whether They Arise from I-Cells or Basal Cells
Lineage studies in mice aimed at defining S-cell progenitors during urothelial regeneration yield conflicting results. In some cases, basal cells have been identified as progenitors, but, in others, I-cells have been identified as progenitors (Colopy et al., 2014; Gandhi et al., 2013; Mysorekar et al., 2009; reviewed in Balsara and Li [2017]; Paraskevopoulou et al. [2016]; Wang et al. [2017]; Yamany et al. [2014]). A potential explanation for these disparities comes from recent fate mapping studies comparing the potential of I-cells and basal cells in a surgical model of bladder augmentation (Schäfer et al., 2017). This analysis indicates that both I and basal cells contribute to urothelial repair in response to focal injury, whereas basal cells alone were able to produce urothelial cells de novo, suggesting that differences in the extent or type of injury affect the regenerative potential of I-cells and basal cells (Schäfer et al., 2017). Our results agree with these observations. Using two different models, we show that mononucleated I-cells can produce S-cells in response to acute injury, with a smaller contribution from basal cells, but, after serial injury, I-cells are depleted and basal cells give rise to I- and S-cells, a feature that is likely to be critical for maintaining the urothelial barrier. Whether basal cell lineage capacity or I-cells depletion in chronic or repeated injury are a response to factors secreted from immune cells, is an interesting question. Recurrent UTI, chronic injury, and indwelling catheters can damage the urothelium, leading to abnormal differentiation and disease. Thus, defining the molecular pathways that are altered in the urothelium by chronic injury and inflammation will be important for developing new strategies to promote urothelial regeneration and repair.
STAR★METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| p63 (4A4) antibody | Santa Cruz Biotechnology | SC8431: RRID:AB 628091 |
| p63 antibody [N2C1), Internal | GenTex | GTX102425: RRIDiAB 1952344 |
| Keratin 5 Polyclonal Antibody | Covance | PRB-160P: RRID:AB 10063444 |
| Keratin 5 Polyclonal Chicken Antibody | Covance | SIG-3475; RRID:AB 10720202 |
| Cytokeratin 20 antibody | DAKO | M7019: RRIDiAB 2133718 |
| Ki67 antibody - Proliferation Marker | Abeam | ab15580: RRIDiAB 443209 |
| Green Fluorescent Protein (GFP) Antibody | AVES Lab | GFP-1020. RRIDiAB 10000240 |
| UPIII (M-17) antibody | Santa Cruz Biotechnology | sc-15186: RRIDAB 671435 |
| Survivin (71G4B7) Rabbit mAb antibody | Cell signaling technology | 2808S: RRIDiAB 10691694 |
| Mouse monoclonal Anti-beta-Tubulin antibody | Sigma | T4026: RRIDiAB 477577 |
| Phospho-Histone H3 (Seri 0) (6G3) Mouse monoclonal Antibody | Cell signaling technology | 9706S; RRIDiAB 331748 |
| Rabbit Anti-Cyclin D1 Monoclonal Antibody | Abeam | ab16663: RRIDiAB 443423 |
| Goat Anti-Mouse E-cadherin Polyclonal antibody.Unconjugated | R&D system | AF748; RRIDiAB 355568 |
| Anti-RFP (RABBIT) Polyclonal Antibody | Rockland | 600–401-379; RRIDiAB 2209751 |
| Aurora A/B kinase. Mouse monoclonal | EnCor Biotechnology | MCA-5A12: RRIDiAB 2572231 |
| Anillin Antibody, Rabbit Polyclonal | Nov us | NBP1–31060; RRIDiAB 2058287 |
| Alexa Fluor® 488-AffiniPure Donkey Anti-Rabbit IgG (H+L) antibody | Jackson Immuno-Research Labs | 711–545-152: RRIDiAB 2313584 |
| Alexa Fluor 488-AffiniPure Donkey Anti-Mouse IgG (H+L) antibody | Jackson Immuno-Research Labs | 715–545-150: RRIDiAB 2340846 |
| Cy3-AffiniPure Donkey Anti-Rabbit IgG (H+L) antibody | Jackson Immuno-Research Labs | 711–165-152; RRIDiAB 2307443 |
| Alexa Fluor 594-AffiniPure Donkey Anti-Mouse IgG (H+L) antibody | Jackson Immuno-Research Labs | 715–585-151; RRIDiAB 2340855 |
| Alexa Fluor 647-AffiniPure Donkey Anti-Mouse IgG (H+L) antibody | Jackson Immuno-Research Labs | 715–605-150; RRIDiAB 2340862 |
| Alexa Fluor 647-AffiniPure Donkey Anti-Rabbit IgG (H+L) antibody | Jackson Immuno-Research Labs | 711–605-152: RRIDiAB 2492288 |
| Alexa Fluor 488 AffiniPure Donkey Anti-Chicken IgY(lgG) (H+L) antibody | Jackson Immuno-Research Labs | 703–545-155; RRIDiAB 2340375 |
| Alexa Fluor 594-AffiniPure Donkey Anti-Chicken IgY (IgG) (H+L) antibody | Jackson Immuno-Research Labs | 703–585-155; RRIDiAB 2340377 |
| Bacterial and Virus Strains | ||
| Uropathogenic E.coi Strain UTI 89 | Gift from Scott Hultgren Lab | N/A |
| Biological Samples | ||
| Mouse adult female bladder. UTI infected and control animals. | This paper | N/A |
| Mouse urothelium: dissected tissue for the organotypic culture. | This paper | N/A |
| Mouse urothelium. CPP-treated male/female. | This Paper | N/A |
| Chemicals. Peptides, and Recombinant Proteins | ||
| EdU (ô-ethynyl-^-deoxyuridine). pulse chase | Invitrogen | Cat# A10044 |
| Tamoxifen, for inducing Cre recombinase | Sigma | cat# T5648 |
| 4-OHT. for urothelial culture | Sigma | #H7904 |
| CPP for injury/repair model | Sigma | cat #C7397 |
| KSFM and supplements for tissue culture | GIBCO | #17005042 |
| Critical Commercial Assays | ||
| Click-iT EdU Alexa Fluor 647 Imaging Kit | Invitrogen | Cat #C 10340 |
| Experimental Models: Organisms/Strains | ||
| Mouse line: FVB.Cg-Tg(KRT5-cre/ERT2) 2lpc/JeldJ | Obtained from D. Metzger and P. Cham bon (Indra et al., 1999) |
RRIDJMSR JAX:018394 |
| Mouse line: Upk2CreERT (B6:CBA-Tg (Upk2-icre/ERT2)1 Ccc) | Obtained from the Cord on-Card o lab. (Shen et al.. 2012) |
RRIDJMSR JAX:024768 |
| Mouse line Upk3aGCE (B6:DBA-Tg (Upk3a-GFP/cre/ERT2)26Amc/J | Obtained from the McMahon lab | RRIDJMSR JAX:015855 |
| Mouse line B6;129S6-Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J, (Rosa26:mCheriy) | The Jackson Laboratory | RRIDJMSR JAX:007908 |
| Mouse line Rosa26mTmG *B6.129(Cg) Gt(ROSA)26Sortm4(ACT– tdTomato,–EGFP) Luo/J33) | The Jackson Laboratory (Muzumdar et al.. 2007) | RRIDJMSR JAX:007676 |
| Oligonucleotides | ||
| Forward Upk2CreER: CATTGCCCCTGTTTCACTATC | This paper | N/A |
| Reverse Upk2CreER CAATGATGTTTCACTGGTTATG | This paper | N/A |
| Forward Krt5CreER ATTTGCCTGCATTACCGGTC | This paper | N/A |
| Reverse Krt5CreER ATCAACGTTTTGTTTTCGGA | This paper | N/A |
| Forward R26mGmT/R26nTnG CTCTGCTGCCTC CTGGCTTCT | This paper | N/A |
| Reverse R26mGmT/R26nTnG TCAATGGGCGG GGGTCGTT | This paper | N/A |
| Forward Upk3aGCE AGGAGCTTTGCAGAAGACCA | This paper | N/A |
| Reverse Upk3aGCE GAACTTCAGGGTCAGCTTGC | This papier | N/A |
| Forward Rosa26:mCherry GGCATTAAAGCAGC GTATCC | This paper | N/A |
| Reverse Rosa26:mCherry CTGTTCCTGTACGG CATGG | This paper | N/A |
| Software and Algorithms | ||
| NIS-Elements | Nikon | RRID:SCR_014329; https://www.nikon.com/products/microscopesolutions/support/download/software/imgsfw/nis_v41100mac.htm |
| Fiji package ImageJ | ImageJ | https://fiji.se/ |
| Prism | GraphPad | PRISM, RRID:SCR_005375 RRID:SCR_000306; https://www.graphpad.com/scientificsoftware/prism/ |
| Other | ||
| Zeiss Axiovert 200M microscope with an Apotome | Zeiss | N/A |
| Nikon Eclipse TE200 microscope | Nikon | N/A |
| Confocal microscopy. A1R MP confocal microscope | Nikon | N/A |
CONTACT FOR REAGENT AND RESOURCE SHARING
Requests for reagents or resources should be directed to the Lead Contact, Cathy Mendelsohn (clm20@cumc.columbia.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
In Vitro Studies Organotypic culture and time lapse recording
Bladder tissue (urothelium and associated stroma) was isolated from male and female UPCreER;mTmG/UPCreER,nTnG mice and dissected into small pieces, approximately 0.3 to 0.5 cm in size, then cultured in KFSM medium with 10%FBS, EGF 5ng/ml, BPE 50ng/ml (supplements and KSFM, GIBCO #17005042 on collagen coated Millicell 0.4um 30mm filters, (Millipore) in glass-bottom microwell dishes (Mat-Tek Corporation, Ashland, MA, P35GCOL-0–14-C). 4-OHT was added to culture medium to induce Credependent recombination, then tissue was incubated for an additional 24h, medium was replaced, and cultures were transferred to a stage-top incubator maintaining 37 C and 5% CO2 (Tokai Hit, Shizuoka-ken, Japan) on a confocal microscope (A1R MP, Nikon Instruments, Melville, NY). Time-lapse multiphoton confocal images were captured using a 25x/1.1 Apochromat water-immersion objective. Excitation was at 920 nm, and emission was collected through standard GFP and RFP filters using a non-descanned detector. Time-lapse images were collected from 1–3 positions in the tissue every 4–6 min for 24hr. Z series were collected at each time point with a z interval of 3–4 0.3–0.4 µm Z sections and total stack size up to 50 µm.
In Vivo Animal Studies Mice
K5CreERT2 mice (FVB.Cg–Tg(KRT5–cre/ERT2)2Ipc/JeldJ)32 were obtained from D. Metzger and P. Chambon at the IGBMC, France (Indra et al., 1999). This line was used in lineage tracing experiments in conjunction with Rosa26mTmG reporter mice Gt(ROSA) 26Sortm4(ACTB– tdTomato,–EGFP) Luo/J33. Controls for UTI and CPP experiments were uninfected/untreated Krt5CreERT2;Rosa26mTmG littermates, and Rosa26mTmG littermates lacking the Cre-recombinase. Upk2CreERT2 mice (TgUICBAC mice) were generated in the Cordon-Cardo lab (Shen et al., 2012). This line was used in lineage/regenerative studies with the UTI and CPP models, and in organotypic culture with the B6;129S6-Gt(ROSA)26Sortm1(CAG-tdTomato*,-EGFP*)Ees/J reporter line (Rosa26;nTnG mice; Prigge et al., 2013). Upk3aGCE mice (Tg(Upk3a-GFP/cre/ERT2)26Amc) were from obtained from the McMahon lab, and are available from Jackson Labs. This line was used in conjunction with the mCherry line (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J), in lineage tracing experiments with the UTI model. Controls were uninfected Upk3aGCE females, and littermates (Upk3a-GFP/cre) without the tdTomato reporter strain. All experiments were carried out in triplicate (n = 3 experimental and control animals). All work with mice was approved by and performed under the regulations of the Columbia University Institutional Animal Care and Use Committee. Animals were housed in the animal facility of Irving Cancer Research Center, Columbia University. Protocol numbers and title: IUCUC #AC-AAAT7487. Lineage analysis of the urothelium; IUCUC #AC-AAAR0419: Analysis of the regenerative response of the urothelium. Bacterial suspensions were administered via transurethral catheter, hence UTI experiments were performed only on females. In all other experiments, females and male littermates or age matched animals were used for the experimental and controls in groups of n = 3 or more.
UTI with Uropathogenic
E. coli: Adult female mice (8–14 weeks) were anesthetized with isoflurane, and inoculated via transurethral catheterization with 75ml of bacterial suspension (107 cfu/ml) in phosphate-buffered saline (PBS) or 75µl sterile PBS according to the published protocol from the Hultgren Lab (Hung et al., 2009). EdU was administered to animals 24h after infection (0.1mg/20 g body weight) to label cells in S-phase. Urine was collected 4–6h p.i. assayed for bacterial counts and analyzed using the Cytospin and Hema3 staining Kit (Fisher Scientific). Titers of 106-107 CFU/ml were considered to be a robust infection. Sulfatrim (240mg/kg) was administered 30h after inoculation with UTI 89 to avoid reinfection. At the indicated times, mice were sacrificed, their bladders were aseptically removed and processed for microscopy and histology. Experiments were performed on females, due to anatomical constraints, and analysis was performed on n = 3 or greater bladder sample for each time-point/condition.
Bacterial strain UTI
E. coli UTI 89 was obtained from the Scott Hultgren lab (Chen et al., 2006). This strain was isolated from a patient with acute bladder infection (Chen et al., 2006). Cultures were prepared from freshly plated bacterial colonies.
Chemical Injury
For chemical injury, CPP (Sigma-Aldrich; catalog #C7397) was dissolved in PBS (15 mg/ml) and given to adult mice at a dose of 150 mg/kg by IP injection. In the case of chronic injury, CPP was administered 5X, with 2 weeks between each round of injury and repair, and bladders were analyzed 2 weeks after the last injection. Each group of animals included males and females, and analysis was performed on at least n = 3 control and experimental animals for each time point or condition. All work with mice was approved by and performed under the regulations of the Columbia University Institutional Animal Care and Use Committee. Animals were housed in the SPF animal facility of Irving Cancer Research Center, Columbia University. Protocol numbers and titles: IUCUC # AC-AAAT7487. Lineage analysis of the urothelium; IUCUC #AC-AAAR0419: Analysis of the regenerative response of the urothelium.
Pulse-Chase experiments
EdU was administered to animals during infection or after CPP injection (0.1mg/20 g body weight) to label cells in S-phase, animals were sacrificed 1h later, and analyzed to assess the distribution of Edu in the urothelium. All experiments were performed in triplicate.
METHOD DETAILS
Genotyping
Genotyping was done by PCR analysis of tail DNA. Primers for genotyping Upk2CreERT2 mice were 5ʹ -CATTGCCCCTGTTTCAC TATC-3ʹ (forward) and 5ʹ -CAATGATGTTTCACTGGTTATG-3ʹ (reverse), generating a 600 bp product. Primers for Krt5CreERT mice were 5ʹ -ATTTGCCTGCATTACCGGTC-3ʹ (forward) and 5ʹ -ATCAACGTTTTGTTTTCGGA-3ʹ (reverse), generating a 350 bp product. Primers for genotyping the R26mGmT and R26nTnG reporters were 5ʹ -CTCTGC TGCCTCCTGGCTTCT-3ʹ (forward) and 5ʹ -TCA ATGGGCGGGGGTCGTT-3ʹ (reverse), generating a 250 bp product. Primers for genotyping Upk3aGCE mice were: 5ʹ -AGG AGCTTTGCAGAAGACCA (forward) and 5ʹ -GAACTTCAGGGTCAGCTTGC (reverse), generating a 371 bp product. Primers for genotyping mCherry were: 5ʹ -GGCATTAAAGCAGCGTATCC (forward) and CTGTTCCTGTACGGCATGG (reverse), generating a 196 bp product.
Tamoxifen and 4-OHT administration
Male and female adult mice (3 months of age) were injected with tamoxifen Sigma, cat# T5648, intraperitoneally, (5 mg per 30 g body weight) three times over a period of 7 days. For inducing Cre-dependent recombination in bladder explant cultures, 4-OHT (Sigma #H7904) was added to culture medium at a concentration of 200nM.
Immunostaining
Bladders were embedded in paraffin and serial sections were generated. For immunohistochemistry, sections were deparaffinized using HistoClear and rehydrated through a series of ethanol and 1XPBS washes. Antigen retrieval was performed by boiling slides for 30 min in buffer. Primary antibodies in 1% horse serum were incubated overnight at 4◦C. The next day, slides were washed with TBST 3 times for 10 min each and secondary antibodies were applied for 2h at room temperature. The following primary antibodies and concentrations were used in these studies: p63 mouse IgG (clone 4A4, Santa Cruz Biotechnology, sc8431, 1:100) or rabbit IgG, (GenTex, GTX102425, 1:300). CK5 rabbit IgG (Covance, AF-138, PRB- 160P, 1:300) or chicken IgY (Covance, SIG-3475, 1:300). CK20 antibody was mouse IgG2a, Kappa, clone Ks20.8 (DAKO, M7019, 1:250). Ki67 staining was with rabbit IgG (Abcam, ab15580, 1:300), GFP chicken IgY (AVES, GFP-1020, 1:300). UPK3a goat (Santa Cruz, 1:300), Survivin, rabbit IgG (Cell signaling, 2808S, 1:300), b-tubulin mouse (Sigma, T4026, 1:300), P-H3 (S-10), mouse (Cell signaling, 9706S, 1:200), Cyclin D1 rabbit (Abcam, ab16663, 1:300), E-cad goat (R&D system, AF748, 1:300), RFP rabbit (Rockland, #600–401-379,1:1000). Aurora A/B mouse (Encore, MCA-5A12, 1:200), Anillin (anti-rabbit; Novus, NBP1–31060, 1:300). The following secondary antibodies and concentrations were used in our studies: Jackson ImmunoResearch Alexa Fluor 488 donkey anti-rabbit IgG (711–545-152; 1:600), Alexa Fluor 488 donkey anti- mouse (715–545-150;1:600), CY3-conjugated donkey anti-rabbit IgG (711–165-152; 1:600), Alexa Fluor 594 donkey anti-mouse IgG (715–585-151; 1:500), Alexa Fluor 647-conjugated donkey anti-mouse IgG (715–605-150; 1:300), Alexa Fluor 647-conjugated donkey anti-rabbit IgG (711–605-152; 1:300), Alexa Fluor 488 donkey anti-chicken (703–545-155, 1:500), Alexa Fluor 594 donkey anti-chicken (703–585-155, 1:500). DAPI was either applied as part of the secondary antibodies cocktail or for 10 min, for nuclear staining, and then the slides were sealed with coverslips. Controls for antibody staining were either without primary antibody (nega-tive control) or using tissue where the protein is known to be expressed (positive controls).
Microscopy
Immuno-fluorescence images were collected using a Zeiss Axiovert 200M microscope with an Apotome (Zeiss). Bright-field images were collected using a Nikon Eclipse TE200 microscope. Confocal microscopy was performed on an A1R MP confocal microscope (Nikon Instruments) and data was analyzed and rendered using NIS Elements (Nikon) and the Fiji package of ImageJ.
QUANTIFICATION AND STATISTICAL ANALYSIS
DNA content analysis
Stacks of images were obtained using confocal microscopy with 0.02 mm steps and 3D reconstructions were analyzed with the Fiji distribution of ImageJ (Schindelin et al., 2012; Schneider et al., 2012). Nuclei were segmented and incomplete nuclei were disregarded. The integrated density and volume of each nucleus were calculated. The ploidy of cells was determined by quantifying the intensity of DAPI within the nucleus. The average DAPI intensity of Basal cells was set as 2n. As shown in Figure S1, a total of 183 cells from three adult mice were quantified using Basal cells as a reference. Based on these measurements, mononuclear I-cells are assigned a ploidy of 2n, binucleated I-cells 4n (2n+2n), and S-cells 8n or larger (4n+4n).
Statistical Analysis
All quantification was performed on at least three independent biological samples, using the ImageJ software. Data presented are mean values ± s.e.m. Statistical analysis was performed using the GraphPad Prism software v6. In two group comparisons, statistical significance was determined using a two-tailed Student’s t test, considering a value of p < 0.05 as significant. Multiple comparisons were performed using the Kruskal–Wallis statistical test. All sample sizes met the minimum requirements of the respective statistical test used.
Supplementary Material
Highlights.
Binucleated S-cells are critical for maintaining the urothelial barrier
S-cells derive from tetraploid I-cells that form via incomplete cytokinesis
Binucleated I- and S-cells increase ploidy via endoreplication
Polyploidy may enable the urothelium to efficiently adjust to environmental changes
ACKNOWLEDGMENTS
We thank Daniel Metzger and Pierre Chambon for the K5CreERT2 line, Ben Ohlstein for discussions and comments, Carlos Cordon-Cardo for the Up2CreERT2 line, and Andy McMahon for the Upk3aGCE line. We thank the Scott Hultgren lab for the gift of UTI strain 89 and Henry Sun and Xue-Rue Wu for antibodies and helpful discussions. Confocal and multiphoton images were collected and analyzed in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, which is supported by an NIH grant (P30 CA013696) (National Cancer Institute). This work was supported by NIH grants (R01 DK95044 to C.L.M.; R01GM117407 to J.C.C.; and R01 DK100644 to I.U.M.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Amano O, Kataoka S, and Yamamoto TY (1991). Turnover of asymmetric unit membranes in the transitional epithelial superficial cells of the rat urinary bladder. Anat. Rec 229, 9–15. [DOI] [PubMed] [Google Scholar]
- Balsara ZR, and Li X (2017). Sleeping beauty: awakening urothelium from its slumber. Am. J. Physiol. Renal Physiol 312, F732–F743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batourina E, Gandhi D, Mendelsohn CL, and Molotkov A (2012). Organotypic culture of the urogenital tract. Methods Mol. Biol 886, 45–53. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, and Knight CA (2008). Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179, 975–986. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. (2015). Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575. [DOI] [PubMed] [Google Scholar]
- Caldas H, Jiang Y, Holloway MP, Fangusaro J, Mahotka C, Conway EM, and Altura RA (2005). Survivin splice variants regulate the balance between proliferation and cell death. Oncogene 24, 1994–2007. [DOI] [PubMed] [Google Scholar]
- Cao J, Wang J, Jackman CP, Cox AH, Trembley MA, Balowski JJ, Cox BD, De Simone A, Dickson AL, Di Talia S, et al. (2017). Tension creates an endoreplication wavefront that leads regeneration of epicardial tissue. Dev. Cell 42, 600–615.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino MD, Prakasam HS, Ruiz WG, Clayton DR, McGuire M, Gallo LI, and Apodaca G (2013). Bladder filling and voiding affect umbrella cell tight junction organization and function. Am. J. Physiol. Renal Physiol 305, F1158–F1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, and Earnshaw WC (2003). The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol 4, 842–854. [DOI] [PubMed] [Google Scholar]
- Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, et al. (2006). Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103, 5977–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colopy SA, Bjorling DE, Mulligan WA, and Bushman W (2014). A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev. Dyn 243, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PJ (1979). Cyclophosphamide cystitis–identification of acrolein as the causative agent. Biochem. Pharmacol 28, 2045–2049. [DOI] [PubMed] [Google Scholar]
- DePamphilis ML (2016). Genome duplication: the heartbeat of developing organisms. Curr. Top. Dev. Biol 116, 201–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW (2013). Aneuploidy, polyploidy and ploidy reversal in the liver. Semin. Cell Dev. Biol 24, 347–356. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ, and Grompe M (2010). The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 467, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, and Orr-Weaver TL (2001). Endoreplication cell cycles: more for less. Cell 105, 297–306. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Zielke N, and Gutierrez C (2014). Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat. Rev. Mol. Cell Biol 15, 197–210. [DOI] [PubMed] [Google Scholar]
- Epstein CJ (1967). Cell size, nuclear content, and the development of poly-ploidy in the Mammalian liver. Proc. Natl. Acad. Sci. USA 57, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsund T (1975). Cell kinetics of mouse urinary bladder epithelium. I. Circadian and age variations in cell proliferation and nuclear DNA content. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol 18, 35–49. [DOI] [PubMed] [Google Scholar]
- Farsund T, and Dahl E (1978). Cell kinetics of mouse urinary bladder epithelium. III. A histologic and ultrastructural study of bladder epithelium during regeneration after a single dose of cyclophosphamide, with special reference to the mechanism by which polyploid cells are formed. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol 26, 215–223. [PubMed] [Google Scholar]
- Field CM, and Alberts BM (1995). Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol 131, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier M, Celton-Morizur S, and Desdouets C (2017). Incomplete cytokinesis/binucleation in mammals: The powerful system of hepatocytes. Methods Cell Biol 137, 119–142. [DOI] [PubMed] [Google Scholar]
- Fox DT, and Duronio RJ (2013). Endoreplication and polyploidy: insights into development and disease. Development 140, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley LE, and Orr-Weaver TL (2015). Polyploidy. Curr. Biol 25, R353– R358. [DOI] [PubMed] [Google Scholar]
- Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, et al. (2013). Retinoid signaling in pro-genitors controls specification and regeneration of the urothelium. Dev. Cell 26, 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, and Hultgren SJ (2007). Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect. Immun 75, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentric G, and Desdouets C (2014). Polyploidization in liver tissue. Am. J. Pathol 184, 322–331. [DOI] [PubMed] [Google Scholar]
- Golubeva AV, Zhdanov AV, Mallel G, Dinan TG, and Cryan JF (2014). The mouse cyclophosphamide model of bladder pain syndrome: tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol. Rep 2, e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, and Pellman D (2012). Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet 13, 189–203. [DOI] [PubMed] [Google Scholar]
- Guidotti JE, Brégerie O, Robert A, Debey P, Brechot C, and Desdouets C (2003). Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J. Biol. Chem 278, 19095–19101. [DOI] [PubMed] [Google Scholar]
- Hammond MP, and Laird CD (1985). Chromosome structure and DNA replication in nurse and follicle cells of Drosophila melanogaster. Chromosoma 91, 267–278. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, and Tabin CJ (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528. [DOI] [PubMed] [Google Scholar]
- Hicks RM (1975). The mammalian urinary bladder: an accommodating organ. Biol. Rev. Camb. Philos. Soc 50, 215–246. [DOI] [PubMed] [Google Scholar]
- Hu D, and Cross JC (2010). Development and function of trophoblast giant cells in the rodent placenta. Int. J. Dev. Biol 54, 341–354. [DOI] [PubMed] [Google Scholar]
- Hung CS, Dodson KW, and Hultgren SJ (2009). A murine model of urinary tract infection. Nat. Protoc 4, 1230–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, and Metzger D (1999). Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res 27, 4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost SP (1986). Renewal of normal urothelium in adult mice. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol 51, 65–70. [DOI] [PubMed] [Google Scholar]
- Jost SP, and Potten CS (1986). Urothelial proliferation in growing mice. Cell Tissue Kinet 19, 155–160. [DOI] [PubMed] [Google Scholar]
- Khandelwal P, Abraham SN, and Apodaca G (2009). Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol 297, F1477– F1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S, and Le Borgne R (2014). Epithelial cell division - multiplying without losing touch. J. Cell Sci 127, 5127–5137. [DOI] [PubMed] [Google Scholar]
- Lee HO, Davidson JM, and Duronio RJ (2009). Endoreplication: poly-ploidy with purpose. Genes Dev 23, 2461–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Wu XR, Kreibich G, and Sun TT (1994). Precursor sequence, processing, and urothelium-specific expression of a major 15-kDa protein subunit of asymmetric unit membrane. J. Biol. Chem 269, 1775–1784. [PubMed] [Google Scholar]
- Losick VP, Fox DT, and Spradling AC (2013). Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr. Biol 23, 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlus KR, and Italiano JE Jr. (2013). The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol 201, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. [DOI] [PubMed] [Google Scholar]
- Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, and Hultgren SJ (2009). Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HG, and Ravid K (2010). Polyploidy: mechanisms and cancer promotion in hematopoietic and other cells. Adv. Exp. Med. Biol 676, 105–122. [DOI] [PubMed] [Google Scholar]
- Oliferenko S, Chew TG, and Balasubramanian MK (2009). Positioning cytokinesis. Genes Dev 23, 660–674. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver TL (2015). When bigger is better: the role of polyploidy in organogenesis. Trends Genet 31, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, and de Bruin A (2013). Physiological significance of polyploidization in mammalian cells. Trends Cell Biol 23, 556–566. [DOI] [PubMed] [Google Scholar]
- Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, and Klinakis A (2016). KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun 7, 11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou V, Papafotiou G, and Klinakis A (2016). KRT14 marks bladder progenitors. Cell Cycle 15, 3161–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge JR, Wiley JA, Talago EA, Young EM, Johns LL, Kundert JA, Sonsteng KM, Halford WP, Capecchi MR, and Schmidt EE (2013). Nuclear double-fluorescent reporter for in vivo and ex vivo analyses of biological transitions in mouse nuclei. Mamm. Genome, Published online September 11, 2013. 10.1007/s00335-013-9469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard A, Dorokhov N, Ryerse J, Klumpp DJ, and McHowat J (2008). Characterization of tight junction proteins in cultured human urothelial cells. In Vitro Cell. Dev. Biol. Anim 44, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Jamieson PR, Pal B, Whitehead L, Nicholas KR, Lindeman GJ, and Visvader JE (2016). Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun 7, 11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandamoorthy S, Mathew-Steiner S, Varney S, Zuidema JM, Gilbert RJ, Van De Water L, and LaFlamme SE (2015). Matrix compliance and the regulation of cytokinesis. Biol. Open 4, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarto GE, Stubblefield PA, and Therman E (1982). Endomitosis in human trophoblast. Hum. Genet 62, 228–232. [DOI] [PubMed] [Google Scholar]
- Schafer FM, Algarrahi K, Savarino A, Yang X, Seager C, Franck D, Costa K, Liu S, Logvinenko T, Adam R, and Mauney JR (2017). Mode of surgical injury influences the source of urothelial progenitors during bladder defect repair. Stem Cell Reports 9, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder KP, and Fox DT (2015). The expanding implications of poly-ploidy. J. Cell Biol 209, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TH, Gladoun N, Castillo-Martin M, Bonal D, Domingo-Domenech J, Charytonowicz D, and Cordon-Cardo C (2012). A BAC-based transgenic mouse specifically expresses an inducible Cre in the urothelium. PLoS ONE 7, e35243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, and Beachy PA (2011). Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FA (1986). Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br. J. Cancer Suppl 7, 280–291. [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, and Pellman D (2004). From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol 5, 45–54. [DOI] [PubMed] [Google Scholar]
- Swanhart L, Kupsco J, and Duronio RJ (2005). Developmental control of growth and cell cycle progression in Drosophila. Methods Mol. Biol 296, 69–94. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kokuryo A, Imano T, Minami R, Nakagoshi H, and Adachi-Yamada T (2014). Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophila male accessory gland cells. BMC Dev. Biol 14, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unhavaithaya Y, and Orr-Weaver TL (2012). Polyploidization of glia in neural development links tissue growth to blood-brain barrier integrity. Genes Dev 26, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Batavia J, Yamany T, Molotkov A, Dan H, Mansukhani M, Batourina E, Schneider K, Oyon D, Dunlop M, Wu XR, et al. (2014). Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol 16, 982–991. [DOI] [PubMed] [Google Scholar]
- Vasconcelos-Nóbrega C, Colaço A, Lopes C, and Oliveira PA (2012). Review: BBN as an urothelial carcinogen. In Vivo 26, 727–739. [PubMed] [Google Scholar]
- Wang C, Ross WT, and Mysorekar IU (2017). Urothelial generation and regeneration in development, injury, and cancer. Dev. Dyn 246, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XR, Manabe M, Yu J, and Sun TT (1990). Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J. Biol. Chem 265, 19170–19179. [PubMed] [Google Scholar]
- Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, and Sun TT (1994). Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J. Biol. Chem 269, 13716–13724. [PubMed] [Google Scholar]
- Wu XR, Kong XP, Pellicer A, Kreibich G, and Sun TT (2009). Uroplakins in urothelial biology, function, and disease. Kidney Int 75, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamany T, Van Batavia J, and Mendelsohn C (2014). Formation and regeneration of the urothelium. Curr. Opin. Organ Transplant 19, 323–330. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou K, Luo X, Li L, Tu HC, Sehgal A, Nguyen LH, Zhang Y, Gopal P, Tarlow BD, et al. (2018). The polyploid state plays a tumor-suppressive role in the liver. Dev. Cell 44, 447–459.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


