ABSTRACT
Microspore embryogenesis is a powerful biotechnological tool that is very useful in crop breeding for the rapid production of haploid and double-haploid embryos and plants. In this in vitro system, the haploid microspore is reprogrammed by the application of specific stress treatments. A high level of cell death after the stress is a major factor that greatly reduces embryogenesis yield at its initial stages. Autophagy is a degradation pathway that is present in all eukaryotes and plays key roles in a range of processes, including stress responses. Many proteases participate in autophagy and cell death; among them, cathepsins are the most abundant enzymes with a role in plant senescence and programmed cell death (PCD). Moreover, although plant genomes do not contain homologues of caspases, caspase 3-like activity (main executioner protease of animal cell death) has been detected in many plant PCD processes. Recent studies by our group in barley microspore cultures reported that the stress treatment required for inducing microspore embryogenesis (cold treatment), also produced reactive oxygen species (ROS) and cell death, concomitantly with the induction of autophagy, as well as cathepsin-like and caspase 3-like proteolytic activities. In the present study, we report new data on microspore embryogenesis of rapeseed that indicate, as in barley, activation of cell death and autophagy processes after the inductive stress. The results revealed that treatments modulating autophagy and proteases produced the same effect in the two plant systems, regardless of the stress applied, cold in barley or heat in rapeseed. Pharmacological treatments with small bioactive compounds that inhibit ROS, autophagy and specific cell death-proteases led to reduced cell death and an increased embryogenesis initiation rate in both, barley and rapeseed. Taken together, these findings open up new intervention pathways by modulating autophagy and proteases, which are very promising in terms of increasing the efficiency of in vitro microspore embryogenesis systems for biotechnological applications in crop breeding.
KEYWORDS: Cell death, cathepsins, autophagy, microspore embryogenesis, stress, small compounds
Introduction
Stress-induced microspore embryogenesis is an in vitro system in which the haploid microspore is reprogrammed by the application of specific stress treatments and, as a totipotent cell, enters into an embryogenesis pathway, producing haploid and doubled-haploid embryos and plants. This in vitro process is a very useful biotechnological tool in plant breeding as source of new genetic variability, fixed in homozygous plants in only one generation.1 Moreover, stress-induced microspore embryogenesis is an excellent system to study stress response, cell reprogramming, totipotency acquisition, and embryogenesis. The system is widely used by plant nursery and seed companies in their breeding programs but it still presents low efficiency in many crop and forest species of economic and environmental interest. The occurrence of cell death2–5 and the low rates of reprogramming efficiency1,6 are major factors that greatly reduce the yield of the process, at its initial stages.
Autophagy, the major degradation and self-quality control pathway in all eukaryotes including plants, recycles cell materials upon stress conditions or during specific developmental processes. In plants, autophagy plays key roles in stress resistance, nutrient remobilization, defense, and senescence.7 Together with a pro-survival role, autophagy has also been reported as being a cell death initiator and/or executioner.8–11 Activation of autophagy involves induction of AuTophaGy-related genes (ATGs) and activation of specific proteases.7 In plants, degradation of cellular components by autophagy begins with the engulfment of subcellular components into a double membrane structure, the autophagosome, which can fuse with either the central vacuole or with smaller vacuoles or lysosome-like organelles, where degradation takes place by lytic enzymes.12
In plants, Papain-like C1A Cys-Proteases, cathepsins, are the most abundant enzymes with proteolytic activity. These enzymes play a role in plant senescence, programmed cell death (PCD), and proteolysis mediated by abiotic stress, among other processes.13,14 In animals, cathepsins are well-known lysosomal proteases with a role in autophagy and cell death,15 in which they have a critical role in the degradation of proteins within autolysosomes, after autophagosome fusion.16–18 However, the role of plant cathepsins in autophagy is still unresolved. Other important family of cell death proteases is that of caspases. Plant genomes do not contain structural homologues of caspases, but caspase-like activities have been detected in many plant species and they are required for PCD in several plant systems. A recent report has demonstrated in Arabidopsis that cathepsin B protease has caspase-3-like activity and is inhibited by caspase 3-specific inhibitors.19
Recent studies carried out by our group focusing on stress-induced microspore embryogenesis of barley,2,20 a process induced by cold stress at 4°C,2 have shown the occurrence of cell death, and the activation of autophagy and proteolytic activities (cathepsin-like and caspase 3-like activities) after the induction of microspore embryogenesis. In the present study, we have analyzed microspore embryogenesis in rapeseed, a eudicot species in which embryogenesis is induced by heat stress, and have compared the occurrence of cell death and autophagy processes in both plant species. Interestingly, the integration of the data reported here with the findings recently published by us has revealed that treatments modulating autophagy and proteases had the same effects in the two plant systems, independently of the stress applied, cold or heat. Pharmacological treatments with small compounds that inhibit autophagy and protease activities result in a reduction in cell death levels and an increase in the embryogenesis initiation rate, in both plant species.
Stress-induced microspore cell death is accompanied by ROS production and activation of autophagy and specific proteases
Two model crop species are mainly used to study microspore embryogenesis, the monocot Hordeum vulgare (barley) and the eudicot Brassica napus (rapeseed), both of which are species in which efficient and well established in vitro systems of stress-induced microspore embryogenesis have been developed, through isolated microspores cultures.2,4,21 After isolation, vacuolated microspores (Figure 1A, D), the most responsive developmental stage for embryogenesis induction in both monocot and dicot species,22–25 were subjected to the corresponding inductive stress treatment for each system, i.e. 32ºC for B. napus and 4°C for H. vulgare. Around four days after induction and culture initiation, responsive microspores divided and produced multicellular structures or proembryos, still surrounded by the microspore wall (Figure 1B, E); these structures are considered as the earliest sign of embryogenesis initiation. At this time point of microspore culture, proembryos were accompanied by non-responsive and dead microspores. As microspore embryogenesis progressed, the exine broke down, and embryos developed and followed a similar pathway to zygotic embryogenesis in monocot and dicot species. In the case of barley, globular, transitional, scutellar and coleoptilar monocot embryos (Figure 1C) developed,2 while in rapeseed microspore cultures, globular, heart, torpedo and cotyledonary embryos (Figure 1F) were formed.4
Figure 1.
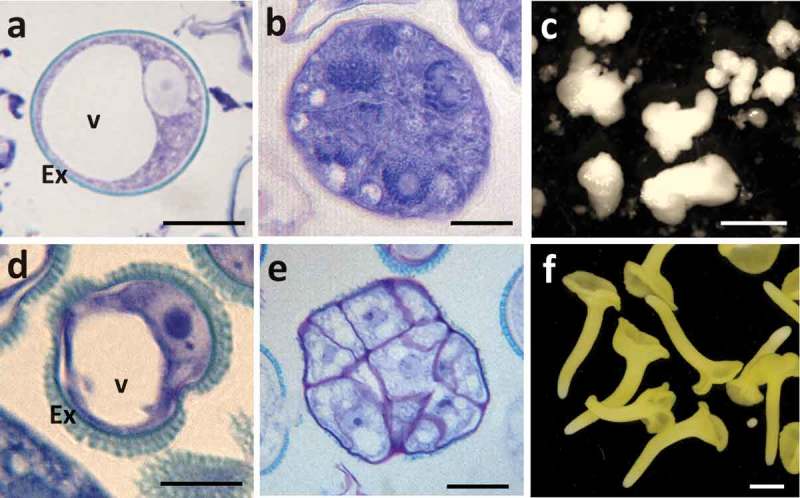
Main stages of stress-induced microspore embryogenesis in Hordeum vulgare, barley (A–C) and Brassica napus, rapeseed (D–F). A, D: Vacuolated microspores at the beginning of culture. B, E: Proembryos formed after stress treatment to induce embryogenesis, after around 4 days in culture. C, F: Cultures of 30 days showing microspore-derived embryos. C: Barley developing and coleoptilar embryos. F: Rapeseed cotyledonary embryos. A, B, D, E: Toluidine blue-stained semithin sections of Technovit resin-embedded samples, observed under bright field microscopy. C, F: Developed microspore-derived embryos, observed under stereomicroscope. Bars in A, D: 10 μm, in B, E: 20 μm, in C: 0.5 mm, in F: 1 mm.
Analyses of cell death have shown that there was a marked increase in cell death levels after stress treatment, in both microspore embryogenesis systems, B. napus and H. vulgare,2,26 as revealed by Evan’s blue staining (Figure 2). In rapeseed the mean percentage of cell death after microspore isolation from anthers (before stress) was 46.84 ± 1.45, and it significantly increased (Student t-test, p ≤ 0.05) after stress to 55.64 ± 2.49. In barley, the inductive stress also led to an increase in the percentage of cell death, from 41.92 ± 1.03 (after cell isolation) to 66.45 ± 1.07. Several studies have shown that the inductive stress for microspore embryogenesis also leads to oxidative stress with the production of reactive oxygen species (ROS).2,27 After stress, concomitantly with cell death, a high level of ROS production has been detected in microspores of barley, using specific fluorescent probes and the quantification of fluorescence intensity.2 Increasing evidence has connected ROS and autophagy in plants and algae28; furthermore, autophagy has been shown to play a role in removing damaged proteins and organelles that can be the result of ROS accumulation. In a recent report, together with ROS production, we have demonstrated activation of autophagy after the inductive stress of microspore embryogenesis in barley.20 Interestingly, key autophagy genes, particularly HvATG5 and HvATG6, were up-regulated in microspore cultures after the stress treatment to induce embryogenesis.20
Figure 2.
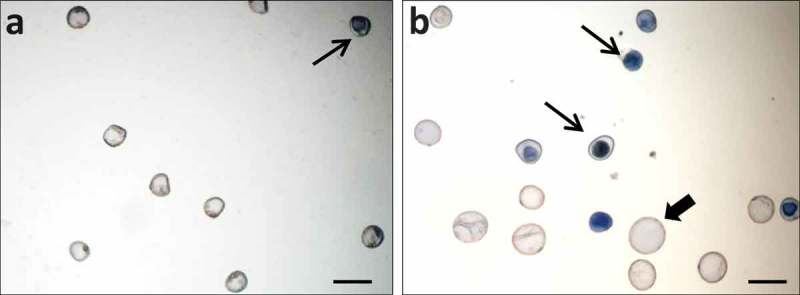
Detection of cell death in microspore cultures by Evan’s blue staining. A: Barley isolated microspores before the stress treatment. B: Barley microspore culture after the stress treatment and 4 days in culture. Dead cells are stained by Evan’s blue (thin arrows). Proembryos (thick arrows) are formed in 4 day-old cultures, together with dead microspores (thin arrows). Bars: 50 µm.
Our results showed that barley stress-treated microspores showed an increase in the presence of autophagosomes and autophagic bodies, which exhibited specific immunofluorescence labeling with antibodies against ATG520 and ATG829,30 (provided by M.F. Suárez, University of Málaga, Spain, and J.L. Crespo, IBVF-CSIC, Seville, Spain, respectively) (Figure 3A–D). Ultrastructural studies with electron microscopy revealed autophagosomes in the cytoplasm of stress-treated microspores (Figure 3E, F), with the autophagosomes showing typical organization with engulfed cytoplasmic material and/or organelles at their interior (Figure 3E, F).
Figure 3.
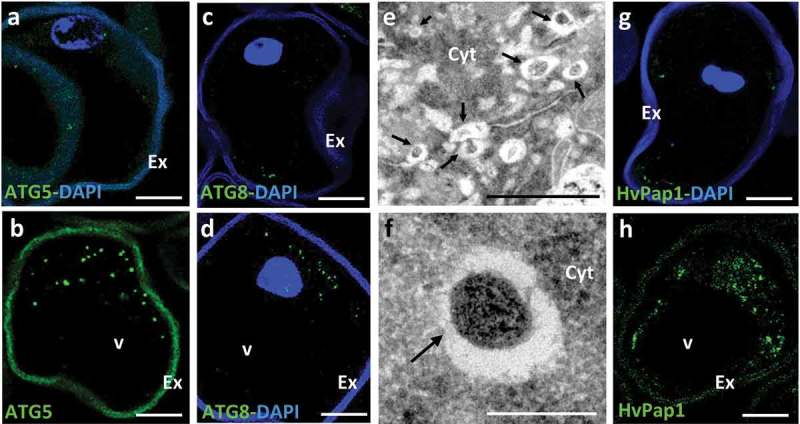
Localization of autophagy ATG5 and ATG8 proteins, autophagic structures and cathepsin HvPap1 in microspores after the stress treatment to induce embryogenesis. A, C, G: Isolated microspores, before stress treatment. B, D, E, F, H: Stress-treated microspores. A, B, C, D, G, H: Confocal microscopy images of immunofluorescence assays (green signal) localizing the autophagy proteins ATG5 (A, B) and ATG8 (C, D), and cathepsin HvPap1 (G, H), in barley microspores; some of them are merged images with DAPI staining (blue signal) to reveal nuclei (A, C, D, G). E, F: Transmission electron microscopy images of cytoplasmic regions of stress-treated microspores of rapeseed; E: Low magnification EM image showing various autophagic structures (arrows); F: Detail of an early autophagosome (arrow) with cytoplasmic material in its interior. The exine (Ex), the microspore wall, shows unspecific autofluorescence in green and blue channels. Cyt, cytoplasm; v, vacuole. Bars in A-D, G-H: 20 µm, in E: 1 µm, in F: 0.2 µm.
Previous reports that analyzed the possible role of certain cell death-related proteases in the stress response of the microspores have revealed that cathepsin L/F-, B- and H-like activities were induced after stress; concomitantly, cathepsin-like genes HvPap-1 and HvPap-6 were up-regulated.20 The use of specific antibodies against HvPap-1 and HvPap-6 cysteine-proteases in western and immunofluorescence assays has shown not only that cathepsin gene expression and enzymatic activity increased but also that the proteins increased in microspores after the stress.20 Our results using anti-HvPap-120 antibodies (provided by I. Díaz, CBGP-UAM, Montegancedo, Spain) in immunofluorescence assays analyzed by confocal microscopy, showed that cathepsins increased in stress-treated microspores and localized in small cytoplasmic rounded areas (Figure 3G, H) which would correspond to small lytic organelles and/or vacuoles. Caspase 3-like activity was also analyzed in microspore cultures and results showed similar kinetics to that of C1 cysteine proteases, with a marked increase in proteolytic activity after the stress treatment to induce embryogenesis.20 Taken together, these data indicated that the inductive stress for embryogenesis led to ROS production and cell death in a proportion of the microspores, concomitantly with activation of autophagy, cathepsin-like and caspase 3-like proteolytic activities.
Pharmacological treatments with inhibitors of ROS, autophagy and several proteolytic activities reduce cell death and improve microspore embryogenesis initiation
To assess the possible involvement of ROS, autophagy, and proteases in the cell death of microspores, functional analyses with specific scavengers and inhibitors were performed. Small molecules (low molecular weight (<1000 Daltons) organic compounds) are very useful in pharmacology to study biological processes due to their properties of cell permeability, specific binding to biological macromolecules and capacity to alter target activity. At present, several ROS, autophagy, and protease inhibitors are commercially available for in vitro assays; many of which have proved their efficiency in plants.7,31 In our recent publication, pharmacological treatments were carried out in barley microspore embryogenesis using various small compounds that were added to the microspore culture media (in the range of concentrations reported for plant suspensions and microspore cultures2,7,15,16), and their effects on cell death levels were evaluated in comparison with untreated microspore cultures.20 In the present study, we have performed treatments in rapeseed microspore cultures with some of the bioactive compounds previously tested in barley, as well as with other molecules that were applied to microspore cultures in the two species. Results showed analogous effects for every small compound in barley and rapeseed microspore embryogenesis cultures, regardless of whether the stress treatment used in each in vitro system was cold or heat.
Previous reports in barley microspore embryogenesis demonstrated that scavenging of ROS by MnCl2, which specifically eliminates superoxide anions, significantly reduced the percentage of dead microspores after the stress20, an effect that was also observed by scavenging hydrogen peroxide with ascorbate.2 Similar reduction in cell death levels has been found in rapeseed microspore cultures treated with ROS scavengers (Figure 4A). The proportion of proembryos produced in rapeseed microspore cultures treated with MnCl2 was also significantly higher compared to control cultures, indicating a positive effect of ROS scavengers on embryogenesis initiation (Figure 4B), probably as a direct consequence of the reduction of cell death. Concanamycin A and E64 are small compounds commonly used to inhibit autophagy in plants by different mechanisms. Concanamicyn A inhibits autophagy cargo degradation through alkalinization of the vacuole and inactivation of its lytic enzymes32, whereas E64 is a known inhibitor of cysteine proteases that reduces autophagosome content degradation by these enzymes in vacuoles12,33,34. Inhibition of autophagy by either concanamycin A or E64 has been reported to decrease cell death proportion after stress, and to increase proembryo percentage at initial stages of microspore embryogenesis, in barley20. The present analyses in rapeseed microspore cultures treated with concanamycin A and E64 showed that both treatments also led to a decrease in the proportion of cell death after stress, which resulted in higher percentage of proembryos. Treatments with other protease inhibitor, leupeptin, which inhibits serine and cysteine proteolytic activities, showed similar effect in microspore cultures to that observed with E64 (Figure 4C, D). Leupeptin-treated cultures exhibited significant changes (Student t-test, p ≤ 0.05) in the proportion of cell death and proembryos in comparison with untreated cultures; the mean percentage reduction of cell death was 12.00 ± 3.17 in rapeseed and 30.20 ± 1.05 in barley, while the mean percentage increase in embryogenesis initiation (proembryos) was 20.00 ± 2.57 and 27.53 ± 2.87 in rapeseed and barley, respectively.
Figure 4.
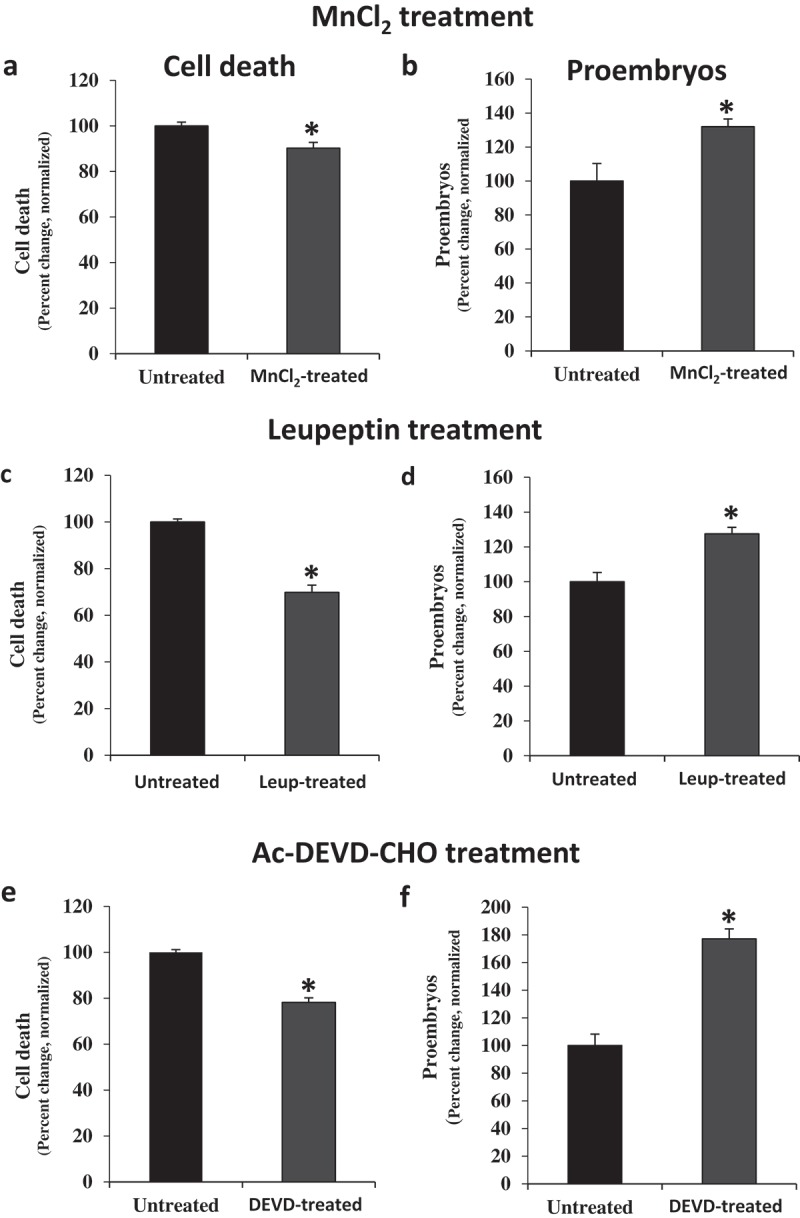
Effects of treatments with MnCl2 (O2− scavenger), leupeptin (Ser- and Cys-protease inhibitor) and Ac-DEVD-CHO (caspase 3 inhibitor) in stress-induced microspore embryogenesis. A, B, E, F: Rapeseed microspore cultures. C, D: Barley microspore cultures. Quantification of the percentage of cell death (A, C, E) and proembryos (B, D, F) in microspore cultures 4 days after stress in untreated cultures and cultures treated with MnCl2 (A, B), leupeptin (C, D) and Ac-DEVD-CHO (E, F). In ordinates, results are expressed as percentages (percent change) and referred to normalized mean percentage of dead cells or proembryos in untreated cultures (100%). Bars indicate the SEM (standard error of the mean). Asterisks indicate significant differences between treated and untreated cultures, within each treatment, assessed by Student’s t-test, at P< 0.05.
Proteases with caspase 3-like activity are also activated by stress treatments to induce embryogenesis in the two species, rapeseed and barley.2,26 Ac-DEVD-CHO (N-Acetyl-Asp-Glu-Val-Asp-al) is a synthetic tetrapeptide which has been proved as a highly specific competitive inhibitor for Caspase 3 in animals.35 In plants, several reports have shown that treatments with Ac-DEVD-CHO reduced enzymatic caspase three-like activity during PCD of various plant systems.19,36 In microspore cultures of barley, pharmacological treatments with Ac-DEVD-CHO-containing media significantly reduced caspase 3-like activity2 and cell death levels, after inductive stress.20 Our analyses demonstrated a similar effect of the inhibition of caspase 3-like activity by Ac-DEVD-CHO in rapeseed microspore cultures, being cell death level reduced (Figure 4E) and proembryo percentage increased (Figure 4F) in cultures treated with this inhibitor.
Conclusions
The results reported here demonstrate the positive effects on microspore viability and embryogenesis initiation of several small compounds that modulate autophagy, ROS and various protease activities, like concanamycin A, MnCl2, E64, and Ac-DEVD-CHO, in stress-induced microspore embryogenesis of rapeseed. These modulators have recently shown the same effects in stress-induced microspore embryogenesis cultures of a different plant, barley. Furthermore, we have tested here other protease inhibitor, leupeptin, which also improved microspore embryogenesis initiation in these two different crop species, B. napus and H. vulgare, regardless of the stress treatment applied to induce embryogenesis, cold (4°C) in barley, and heat (32°C) in rapeseed. The reduction of ROS, autophagy or proteolytic activities (cathepsin-like, caspase 3-like or Ser- and Cys-proteases) produced a decrease in stress-induced cell death and an increase in embryogenesis initiation rate, in both species (Figure 5).
Figure 5.
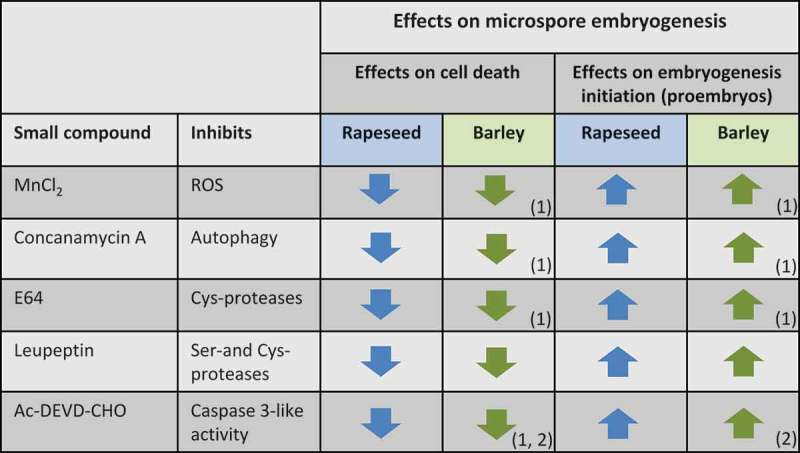
Summary of the effects on microspore embryogenesis of treatments with small compounds inhibiting ROS, autophagy and different proteases, in rapeseed (blue arrows) and barley (green arrows). “Up” and “down” arrows indicate significant “increase” and “decrease” of cell death and embryogenesis initiation (proembryos) in microspore cultures treated with each compound in comparison with control cultures. (1) Reported in Bárány et al. 2018. (2) Reported in Rodríguez-Serrano et al. 2012.
Early cell death is one of the major factors that greatly affects the efficiency of microspore embryogenesis cultures; with such cell death being mainly the result of the inductive stress, with the participation of autophagy and several proteases, as indicated by recent reports. The data reported here on pharmacological treatments with autophagy and protease modulators are very promising with regard to enhancing in vitro microspore viability. The comparative study of the new results reported here and the data recently published by us, clearly shows similar results for stress-induced microspore death in two different species, a monocot and a dicot plant, which suggests that there are common death mechanisms in both plant groups. These findings also indicate that similar strategies, using small molecules targeting autophagy and proteases, could be extended to other species and stress-induced embryogenesis systems. Taken together, these results are opening up new intervention pathways with small bioactive compounds that modulate ROS accumulation, autophagy, and proteolytic activities to increase the efficiency of in vitro microspore embryogenesis systems, by reducing cell death, thus benefiting biotechnological applications in crop breeding and conservation programs.
Funding Statement
This work was supported by the Spanish "Agencia Estatal de Investigación (AEI)" and European Regional Funds (ERF/FEDER) [AGL2017-82447-R]; "Comunidad de Madrid" [PEJ15/BIO/AI-01S8]; Horizon 2020 [COST-CA15138]; Spanish "Ministerio de Economía, Industria y Competitividad (MINECO)" and ERF/FEDER [AGL2014-52028-R].
Acknowledgments
The authors thank Dr. M. F. Suarez (University of Málaga, Málaga, Spain), Dr. J.L. Crespo (IBVF, CSIC, Seville, Spain) and Dr. I. Díaz (CBGP, UPM, Montegancedo, Spain) for their generous gift of anti-ATG5, anti-ATG8, and anti-HvPap1 antibodies, respectively. Work supported by projects (AGL2014-52028-R and AGL2017-82447-R) funded by Spanish National Research Agency (AEI), Ministry of Economy and Competitiveness (MINECO) and European Regional Development Fund (ERDF/FEDER). Thanks are due to COST action TRANSAUTOPHAGY (CA15138), European Network of Multidisciplinary Research and Translation of Autophagy Knowledge. YPP was the recipient of a grant (PEJ15/BIO/AI-01S8) funded by Comunidad de Madrid and European Commission through ERDF/FEDER.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Maluszynski M, Kasha K, Forster B, Szarejko I.. Doubled haploid production in crop plants: a manual. Dordrecht: Kluwer;2003 [Google Scholar]
- 2.Rodríguez-Serrano M, Bárány I, Prem D, Coronado MJ, Risueño MC, Testillano PS.. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot. 2012;63(5):2007–2024. doi: 10.1093/jxb/err400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satpute GK, Long H, Seguí-Simarro JM, Risueño MC, Testillano PS. Cell architecture during gametophytic and embryogenic microspore development in Brassica napus L. Acta Physiol Plant. 2005;27(4B):665–674. doi: 10.1007/s11738-005-0070-y [DOI] [Google Scholar]
- 4.Prem D, Solís MT, Bárány I, Rodríguez-Sánz H, Risueño MC, Testillano PS. A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol. 2012;12:127. doi: 10.1186/1471-2229-12-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maraschin SF, de Priester W, Spaink HP, Wang M.. Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot. 2005;56(417):1711–1726. doi: 10.1093/jxb/eri190 [DOI] [PubMed] [Google Scholar]
- 6.Ferrie AMR, Caswell KL. Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tiss Organ Cult. 2011;104(3):301–309. doi: 10.1007/s11240-010-9800-y [DOI] [Google Scholar]
- 7.Avin-Wittenberg T, Baluska F, Bozhkov PV, Elander PH, Fernie AR, Galili G, Hassan A, Hofius D, Isono E, Le Bars R, et al. Autophagy-related approaches for improving nutrient use efficiency and crop yield protection. J Exp Bot. 2018;69(6):1335–1353. doi: 10.1093/jxb/ery069 [DOI] [PubMed] [Google Scholar]
- 8.Masclaux-Daubresse C, Chen Q, Have M.. Regulation of nutrient recycling via autophagy. Curr Opin Plant Biol. 2017;39:8–17. doi: 10.1016/j.pbi.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Hofius D, Li L, Hafren A and Coll NS. Autophagy as an emerging arena for plant-pathogen interactions. Curr Opin Plant Biol. 2017;38:117–123. doi: 10.1016/j.pbi.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 10.Minina EA, Filonova LH, Fukada K, Savenkov EI, Gogvadze V, Clapham D, Sanchez-Vera V, Suarez MF, Zhivotovsky B, Daniel G, et al. Autophagy and metacaspase determine the mode of cell death in plants. J Cell Biol. 2013;203(6):917–927. doi: 10.1083/jcb.201307082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minina EA, Bozhkov PV. Hofius D.. Autophagy as initiator or executioner of cell death. Trends Plant Sci. 2014;19(11):692–697. doi: 10.1016/j.tplants.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Bassham DC. Plant autophagy–more than a starvation response. Curr Opin Plant Biol. 2007;10(6):587–593. doi: 10.1016/j.pbi.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Mendoza M, Dominguez-Figueroa JD, Velasco-Arroyo B, Cambra I, Gonzalez-Melendi P, Lopez-Gonzalvez A, Garcia A, Hensel G, Kumlehn J, Diaz I. HvPap-1 C1A protease and HvCPI-2 cystatin contribute to barley grain filling and germination. Plant Physiol. 2016;170(4):2511–2524. doi: 10.1104/pp.15.01944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco-Arroyo B, Diaz-Mendoza M, Gandullo J, Gonzalez-Melendi P, Santamaria ME, Dominguez-Figueroa JD, Hensel G, Martinez M, Kumlehn J, Diaz I. HvPap-1 C1A protease actively participates in barley proteolysis mediated by abiotic stresses. J Exp Bot. 2016;67(14):4297–4310. doi: 10.1093/jxb/erw212 [DOI] [PubMed] [Google Scholar]
- 15.Turk B, Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett. 2007;581(15):2761–2767. doi: 10.1016/j.febslet.2007.05.038 [DOI] [PubMed] [Google Scholar]
- 16.Jung M, Lee J, Seo HY, Lim JS, Kim EK. Cathepsin inhibition-induced lysosomal dysfunction enhances pancreatic beta-cell apoptosis in high glucose. PLoS One. 2015;10(1):e0116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5(11):886–897. doi: 10.1038/nrc1738 [DOI] [PubMed] [Google Scholar]
- 18.Man SM, Kanneganti TD. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy. 2016;12(12):2504–2505. doi: 10.1080/15548627.2016.1239679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Y, Cai YM, Bonneau L, Rotari V Danon A, McKenzie EA, McLellan H, Mach L, Gallois P. Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death Differ. 2016;23(9):1493–1501. doi: 10.1038/cdd.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bárány I, Berenguer E, Solís MT., Pérez-Pérez Y, Santamaría ME, Crespo JL, Risueño MC, Díaz I, Testillano PS. Autophagy is activated and involved in cell death with participation of cathepsins during stress-induced microspore embryogenesis in barley. J Exp Bot. 2018;69(6):1387–1402. doi: 10.1093/jxb/erx455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pechan PM, Keller WA, Mandy F, Bergeron M.. Selection of Brassica napus L. embryogenic microspores by flow sorting. Plant Cell Rep. 1988;7(6):396–398. doi: 10.1007/BF00269521 [DOI] [PubMed] [Google Scholar]
- 22.González-Melendi P, Testillano PS, Ahmadian P, Fadón B, Vicente O, Risueño MC.. In situ characterization of the late vacuolate microspore as a convenient stage to induce embryogenesis in Capsicum. Protoplasma. 1995;187(1–4):60–71. doi: 10.1007/BF01280233 [DOI] [Google Scholar]
- 23.Testillano PS, Ramírez C, Domenech J, Coronado MJ, Vergne P, Matthys-Rochon E, Risueño MC. Young microspore-derived maize embryos show two domains with defined features also present in zygotic embryogenesis. Int J Dev Biol. 2002;46(8):1035–1047. [PubMed] [Google Scholar]
- 24.Testillano PS, González-Melendi P, Coronado MJ, Seguí-Simarro JM, Moreno-Risueño MA, Risueño MC. Differentiating plant cells switched to proliferation remodel the functional organization of nuclear domains. Cytogenet Genome Res. 2005;109(1–3):166–174. doi: 10.1159/000082396 [DOI] [PubMed] [Google Scholar]
- 25.Bárány I, González-Melendi P, Fadón B, Mitykó J, Risueño MC, Testillano PS.. Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biol Cell. 2005;97(9):709–722. doi: 10.1042/BC20040142 [DOI] [PubMed] [Google Scholar]
- 26.Solís MT. Pollen reprogramming to embryogenesis induced by stress: cell identity, programmed cell death and role of the methylation of DNA [PhD thesis, Complutense]. Madrid: University of Madrid; 2012. [Google Scholar]
- 27.Zur I, Dubas E, Golemiec E, Szechynska-Hebda M, Golebiowska G, Wedzony M.. Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (x Triticosecale Wittm.). Plant Cell Rep. 2009;28(8):1279–1287. doi: 10.1007/s00299-009-0730-2 [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Pérez ME, Lemaire SD, Crespo JL.. Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 2012;160(1):156–164. doi: 10.1104/pp.112.199992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez C, Garcia I, Moreno I, Pérez-Pérez ME, Crespo JL, Romero LC, Gotor C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell. 2012;24(11):4621–4634. doi: 10.1105/tpc.112.105403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Pérez ME, Florencio FJ, Crespo JL.. Inhibition of target of rapamycin signaling and stress activate autophagy in Chlamydomonas reinhardtii. Plant Physiol. 2010;152(4):1874–1888. doi: 10.1104/pp.109.152520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueño MC, Luis A, Sandalio LM. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150(1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuoka K, Higuchi T, Maeshima M, Nakamura K. A Vacuolar-Type H+-ATPase in a nonvacuolar organelle is required for the sorting of soluble vacuolar protein precursors in tobacco cells. Plant Cell. 1997;9(4):533–546. doi: 10.1105/tpc.9.4.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassham DC. Methods for analysis of autophagy in plants. Methods. 2015;75:181–188. doi: 10.1016/j.ymeth.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 34.Moriyasu Y, Inoue Y. Use of protease inhibitors for detecting autophagy in plants. Methods Enzymol. 2008;451:557–580. doi: 10.1016/S0076-6879(08)03232-1 [DOI] [PubMed] [Google Scholar]
- 35.Swe M, Sit KH. zVAD-fmk and DEVD-cho induced late mitosis arrest and apoptotic expressions. Apoptosis. 2000;5(1):29–36. [DOI] [PubMed] [Google Scholar]
- 36.Sinha RK, Pospisil P, Maheshwari P, Eudes F. Bcl-2 big up tri, open21 and Ac-DEVD-CHO Inhibit death of wheat microspores. Front Plant Sci. 2016;7:1931. doi: 10.3389/fpls.2016.01931 [DOI] [PMC free article] [PubMed] [Google Scholar]


