ABSTRACT
This study investigates the association of PD-L1 expression and immune cell infiltrates and their impact on clinical outcome, in addition to their overlap with microsatellite instability (MSI), HER2 and ATM molecular subgroups of gastric cancer (GC). PD-L1 membrane expression on tumour cells (TC) and infiltrating immune cells (IC), CD3 + T-lymphocytes, CD8+ cytotoxic T-cells, ATM and HER2 were assessed by immunohistochemistry (IHC) in the ACRG (Asian Cancer Research Group) GC cohort (N = 380). EBV status was determined using in situ hybridization and MSI status was performed using PCR and MLH1 IHC. The PD-L1 segment was associated with increased T-cell infiltrates, while the MSI-high segment was enriched for PD-L1, CD3, and CD8. Multivariate analysis confirmed PD-L1 positivity, high CD3 and high CD8 as independent prognostic factors for both disease-free survival and overall survival (all p < 0.05). Patients with MSI-high tumours had better overall survival by both univariate and multivariate analysis. The ATM-low and HER2-high subgroups differed markedly in their immune profile; the ATM-low subgroups enriched for MSI, PD-L1 positivity and CD8 + T-cells, while the HER2 segment was enriched for MSS, with no enrichment for immune markers. Hence, we demonstrate a molecular profiling approach that can divide GC into four molecular subgroups, namely ATM-low, HER2-high, PD-L1 positive and MSI-high with differing levels of immune infiltrates and prognostic significance which may help to stratify patients for response to targeted therapies.
KEYWORDS: PD-L1, immune infiltrates, gastric cancer, ATM, HER2
Introduction
Gastric cancer (GC) is the fifth most common malignancy worldwide, the third leading cause of cancer mortality, and occurs with a high incidence in East Asia.1 GC is often diagnosed at an advanced stage, for which therapeutic options are largely limited to cytotoxic chemotherapy and five-year survival is less than 20%.2 Despite the availability of Trastuzumab for use in human epidermal growth factor receptor 2 (HER2)-positive disease and the anti-VEGFR2 antibody ramucirumab for use as a second line therapy, prognosis remains poor, with an urgent need for effective targeted therapies and an understanding of those patients most likely to benefit.2–4
The interaction of programmed cell death-1 (PD-1) on T-cells with its ligand, programmed cell death ligand-1 (PD-L1) on immune and tumour cells, limits T-cell mediated responses.3 Immune checkpoint blockade with anti-PD1 or anti-PD-L1 antibodies is emerging as a promising therapeutic approach for several cancer types, including non-small cell lung cancer (NSCLC),4–6 melanoma,7 bladder8 and renal cancer.9 In early clinical studies anti-PD-1 therapies, including pembrolizumab10 have reported promising efficacy in metastatic GC and recently, a phase III trial comparing nivolumab versus best supportive care in the salvage setting demonstrated survival benefit in GC.11 Tumour PD-L1 expression is being explored as a predictive biomarker for response to these agents,10,12 however not all PD-L1 positive patients respond and responses occur in patients with PD-L1 negative tumours, hence alternative markers are required. Microsatellite instability (MSI) predicts response to PD-1/PD-L1 blockade,13 potentially related to the high mutational burden and neo-antigen generation associated with MSI-high tumours14 and approximately 22% gastric tumours are MSI-H.13,15 Another pathway, with the potential to modulate the response to immune checkpoint inhibition, is the cellular DNA damage repair pathway, which is necessary to maintain genome stability and in which the ataxia-telangiectasia-mutated (ATM) kinase plays an essential role.16 Loss of function variants in ATM confer an increased risk of GC15 and approximately 10% to 20% of GCs have low or undetectable ATM expression as assessed by immunohistochemistry (IHC) and this subtype is enriched with MSI.17,18 Based on the association with the cellular DNA damage response and mismatch repair, we hypothesized that the ATM low could be an immunologically primed subtype.
Here we have assessed the association of PD-L1 expression and immune cell infiltrates with clinicopathological features and outcome and investigated the overlap of these with MSI-high, ATM low and HER2-high segments. In addition, we have correlated the peritumoral immune cell infiltrates according to gene expression based molecular classifications in GC.19
Results
Patient and tumour characteristics
We procured 380 primary GC specimens at the time of total or subtotal gastrectomy from Samsung Medical Center. We selected the cases based on > 60% histological purity and sufficient tumor blocks for additional immunohistochemistry (IHC). Of 380 specimens, 300 samples were previously profiled with gene expression signatures.19 The remaining 80 specimens were not qualified for gene expression profiling but qualified for further IHC staining, thus included in this study (Sup. Figure 1). Patient and tumour characteristics of this Korean GC cohort (n = 380) are summarized in Table 1. Of 380 specimens, there was a good balance of tumours representative of the two major Lauren subgroups, with 45.5% (n = 173) intestinal and 51.1% (n = 194) diffuse type and the remainder comprised of 2.9% mixed subtype and 0.5% indeterminate subgroups. The frequency of EBV positive tumours was 6.6% (n = 25). Tumours with high HER2 expression assessed by immunohistochemistry (IHC 3+) occurred with a frequency of 7.2% (n = 27).
Table 1.
Cohort clinicopathological characteristics.
| Characteristic | N | % |
|---|---|---|
| Age (years) | ||
| Median Range |
63 24–86 |
|
| Gender | ||
| Male Female |
258 122 |
67.9 32.1 |
| Lauren subtype | ||
| Intestinal Diffuse Mixed Unknown |
173 194 11 2 |
45.5 51.1 2.9 0.5 |
| Stage | ||
| I II III IV Unknown |
31 103 135 107 4 |
8.2 27.1 35.5 28.2 1.0 |
| Depth of invasion (pT) | ||
| T1 T2 T3 T4 Unknown |
0 223 123 30 4 |
0.0 58.7 32.4 7.9 1.0 |
| Lymph node status (pN) | ||
| N0 N1 N2 N3 |
41 155 116 68 |
10.8 40.8 30.5 17.9 |
| Distant metastasis (pM) | ||
| M0 M1 |
344 36 |
90.5 9.5 |
| EBV | ||
| Positive Negative Unknown |
25 352 3 |
6.6 92.6 0.8 |
| MSI | ||
| MSI* MSS |
72 308 |
18.9 81.1 |
| HER2 | ||
| High (3+) Low (0,1+,2+) Unknown |
27 349 4 |
7.2 91.8 1.1 |
* MSI includes 10 patients which have NR24 only modifications
Prevalence of PD-L1, CD3 and CD8 T-cells in GC
PD-L1 expression on both the tumour cell (TC) membrane compartment and infiltrating immune cells (IC) was assessed by IHC analysis and PD-L1 positivity was defined as ≥ 1% of cells staining positive. This showed that 16.4% of tumours (62/379) scored positive for PD-L1 TC expression, while 90.2% (339/376) were positive for PD-L1 IC expression (Table 2). CD3 expression was assessed as a marker of global T-lymphocytes and CD8 expression as a marker of cytotoxic T-cells. Cut-offs for defining CD3 and CD8 high versus low were identified by investigating the associations between outcome and CD3 or CD8 positive cell count, where the cut-off giving the largest significant survival hazard ratio was selected (in silico validation Sup. Figure 2). Using these cut-offs of 500 cells/mm2 to define low versus high CD3 and 600 cells/mm2 for low versus high CD8 expression, high CD3 expression was observed in 69.7% (242/347) tumours, while high CD8 expression was observed in 25.6% (89/347) of tumours (Table 2). A positive correlation was observed between CD3 high and CD8 high tumours (p < 0.01; data not shown). Increased TC or IC PD-L1 staining were significantly associated with increased CD3+ lymphocyte infiltration (PD-L1 TC Spearman correlation 0.34, p < 0.01; PD-L1 IC Spearman correlation 0.37, p < 0.01) and with CD8+ cytotoxic T-cell density (PD-L1 TC Spearman correlation 0.41; p < 0.01; PD-L1 IC Spearman correlation 0.42, p < 0.01).
Table 2.
Biomarker prevalence.
| Characteristic | N | % |
|---|---|---|
| PD-L1 Tumour Cells (n379) | ||
| 0 1 ≤ 5 6 ≤ 25 26 ≤ 50 51 ≤ 100 |
317 26 27 4 5 |
83.6 6.9 7.1 1.1 1.3 |
| PD-L1 Immune Cells (n376) | ||
| 0 1 ≤ 5 6 ≤ 25 26 ≤ 50 51 ≤ 100 |
37 127 116 64 32 |
9.8 33.8 30.9 17.0 8.5 |
| ATM (n373) | ||
| Low (0) High (1+, 2+, 3+) |
43 330 |
11.5 88.5 |
| CD3 (n347) | ||
| Low (≤ 500) High (> 500) |
105 242 |
30.3 69.7 |
| CD8 (n347) | ||
| Low (≤ 600) High (> 600) |
258 89 |
74.6 25.6 |
Association of PD-L1, CD3 and CD8 expression with clinicopathological features
Tumours with MSI were associated with increased TC and IC PD-L1 staining (Figure 1(a,c): PD-L1 TC P < 0.01; PD-L1 IC P < 0.001) and with increased CD3 and CD8 immune cell densities (Figure 1(a,c): CD3 P < 0.05; CD8 P < 0.01). Statistical analysis indicated that, whilst there was no evidence for a significant association between Lauren subtype and PD-L1 TC or CD8, there was a significant association between Lauren subtype and PD-L1 IC and CD3, where diffuse type GC had significantly lower PD-L1 IC expression (p < 0.01) and significantly higher CD3 cell infiltrates (p = 0.027), compared to intestinal type GC (Figure 1d). Of note, EBV+ GC demonstrated higher percentage of PD-L1 positivity on tumour and immune cells when compared to non-EBV GC tumours (p < 0.001, Figure 1e) and also had a higher incidence of both CD3 and CD8 positive immune infiltrates (p < 0.01, Figure 1e).
Figure 1.
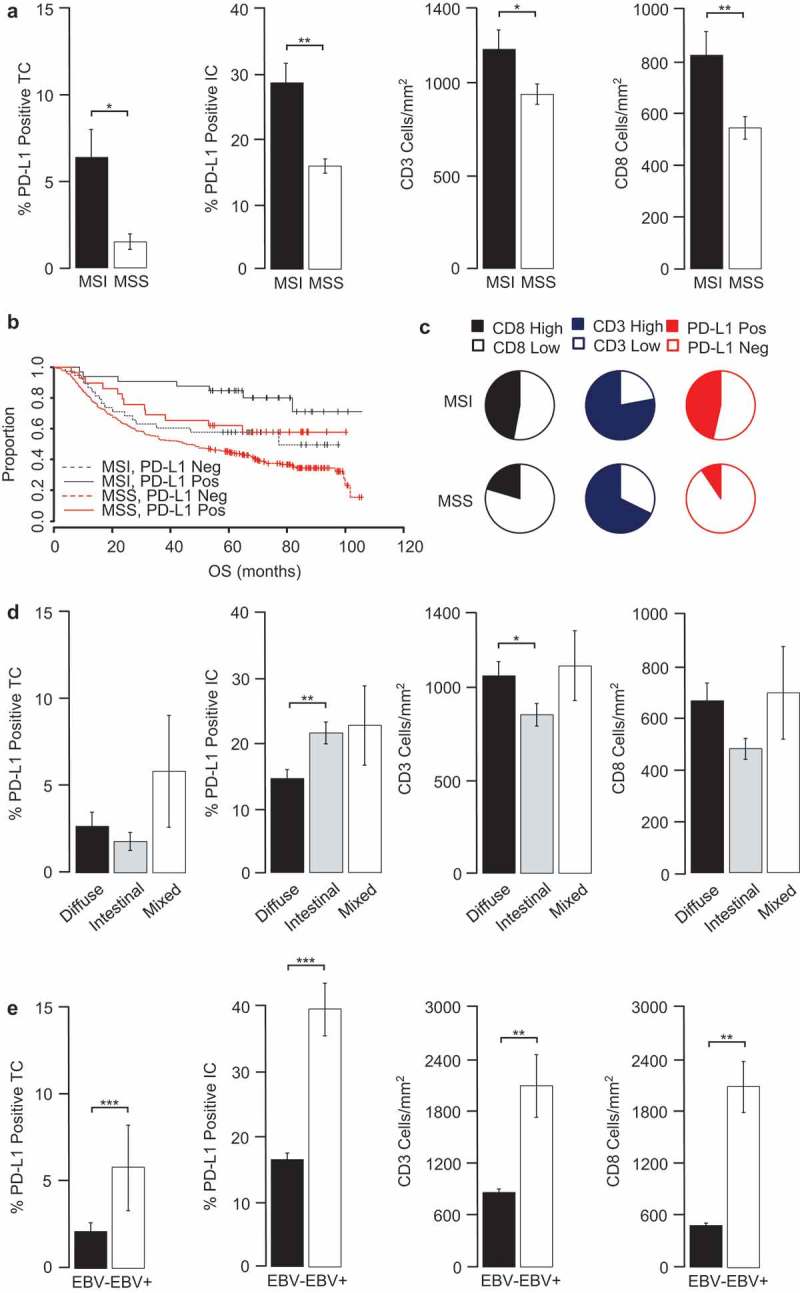
PD-L1, CD3 and CD8 significantly associate with MSI-high and EBV+ samples.
GC tissues were examined for changes in PD-L1 expression and immune prevalence. Shown are (a) PD-L1, CD3 and CD8 in MSI and MSS subgroup, (b) Kaplan-Meier estimates of overall survival (OS) according to PD-L1 TC and MSI stratification, (c) Pie charts depict the frequency of patients with high and low CD8, CD3 and with positive and negative PD-L1 TC densities in MSI and MSS populations. The cut-offs used were PD-L1 TC and IC (≥ 1%), CD3 (500 cells/mm2) and CD8 (600 cells/mm2). Also shown are PD-L1, CD3 and CD8 expression in (d) Lauren subgroups and (e) by EBV status. Data were statistically analysed by Mann-Whitney test (MSI, EBV) or KruskalWallis test (Lauren) (*P < 0.05, **p < 0.01, ***p < 0.001, error bars depict ± 1 s.e, TC; tumour cells, IC; immune cells, MSI; microsatellite instable, MSS; microsatellite stable)
Association of PD-L1, CD8 and CD3 immune markers with survival
The median disease-free survival (DFS) and overall survival (OS) in this patient cohort were 57.2 (38.9–75.4) and 64.0 (47.7–77.2) months, respectively (95% confidence intervals). Both DFS and OS were significantly longer in GC patients with high CD3 (p < 0.01) or CD8 (p < 0.01) immune infiltrates, compared with patients with low CD3 or CD8 GCs (Figure 2(c,d)). DFS and OS were significantly improved for patients with positive (≥ 1%) TC PD-L1 expression (OS p < 0.01) compared to negative TC PD-L1 expression (Figure 2a). In contrast, PD-L1 IC positivity was not associated with survival (Figure 2b). However, when the cut-off for defining PD-L1 IC positivity was raised to ≥ 5%, a significant association with both DFS and OS was observed (P < 0.01, Sup. Figure 3). Using this higher cut-off, 56% (212/379) tumours were PD-L1 IC positive (Table 2). Multivariate analysis, adjusting for age, gender, Lauren subtype, TNM stage and metastasis, confirmed positive PD-L1 tumour membrane status (P < 0.01) and both high CD3 (OS P < 0.01; DFS p = 0.021) and high CD8 (OS P < 0.01; DFS p = 0.027) immune infiltrates as independent prognostic factors to predict better OS and DFS following surgery (Table 3).
Table 3B.
Univariate and multivariate model analysis.
| Univariate |
Multivariate |
Univariate |
Multivariate |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | Median OS | HR | P-value | HR | p-value | Median DFS | HR | P-value | HR | p-value | |
| PD-L1 TC |
-ve | 48.5 (35.9 - 67.8) | 0.387 (0.241 - 0.620) | <0.01 | 0.394 (0.243 - 0.639) | <0.01 | 42.5 (25.8 - 60.2) | 0.482 (0.298 - 0.776) | <0.01 | 0.506 (0.311 - 0.824) | <0.01 |
| +ve | N/C | N/C | |||||||||
| PD-L1 IC |
-ve | 35.9 (19.2 - N/C) | 0.766 (0.507 - 1.189) | 0.245 | 0.773 (0.494 - 1.207) | 0.258 | 30.0 (12.0 - N/C) | 0.713 (0.456 - 1.118) | 0.141 | 0.665 (0.419 - 1.056) | 0.083 |
| +ve | 67.5 (53.0 - 82.2) | 59.5 (42.4 - (N/C) | |||||||||
| CD3 | Low | 28.3 (23.4 - 49.8) | 0.623 (0.461 - 0.838) | <0.01 | 0.648 (0.473 - 0.890) | <0.01 | 15.6 (11.6 - 43.8) | 0.629 (0.455 - 0.868) | <0.01 | 0.665 (0.471 - 0.939) | 0.021 |
| High | 71.2 (57.1 - N/C) | 60.2 (43.4 - N/C) | |||||||||
| CD8 | Low | 37.9 (30.9 - 59.7) | 0.517 (0.358 - 0.746) | <0.01 | 0.516 (0.347 - 0.770) | <0.01 | 30.0 (18.6 - 57.2) | 0.560 (0.374 - 0.837) | <0.01 | 0.619 (0.405 - 0.947) | 0.027 |
| High | N/C | 79.8 (73.7 - N/C) | |||||||||
| ATM | Low | 81.8 (71.2 - N/C) | 1.469 (.905 - 2.385) | 0.119 | 1.273 (0.770 - 2.103) | 0.346 | N/C | 2.129 (1.112 - 4.031) | 0.02 | 1.713 (0.887 - 3.309) | 0.109 |
| High | 57.6 (44.6 - 72.4) | 43.8 (31.4 - 73.7) | |||||||||
| MSI/S | MSI | N/C | 2.094 (1.379 - 3.180) | <0.01 | 1.570 (1.012 - 2.435) | 0.044 | N/C | 2.105 (1.321 - 3.352) | <0.01 | 1.385 (0.851 - 2.252) | 0.189 |
| MSS | 48.7 (35.9 - 67.5) | 42.5 (25.8 - 59.6) | |||||||||
| HER2 | Low | 64.0 (48.5 – 77.5) | 0.969 (0.579 - 1.640) | 0.907 | 1.014 (0.578 – 1.777) | 0.961 | 57.3 (39.4 - 79.8) | 1.091 (0.620 – 1.921) | 0.762 | 1.101 (0.597 – 2.029) | 0.758 |
| High | 51.8 (24.9 - N/C) | 21.6 (11.6 - N/C) | |||||||||
Figure 2.
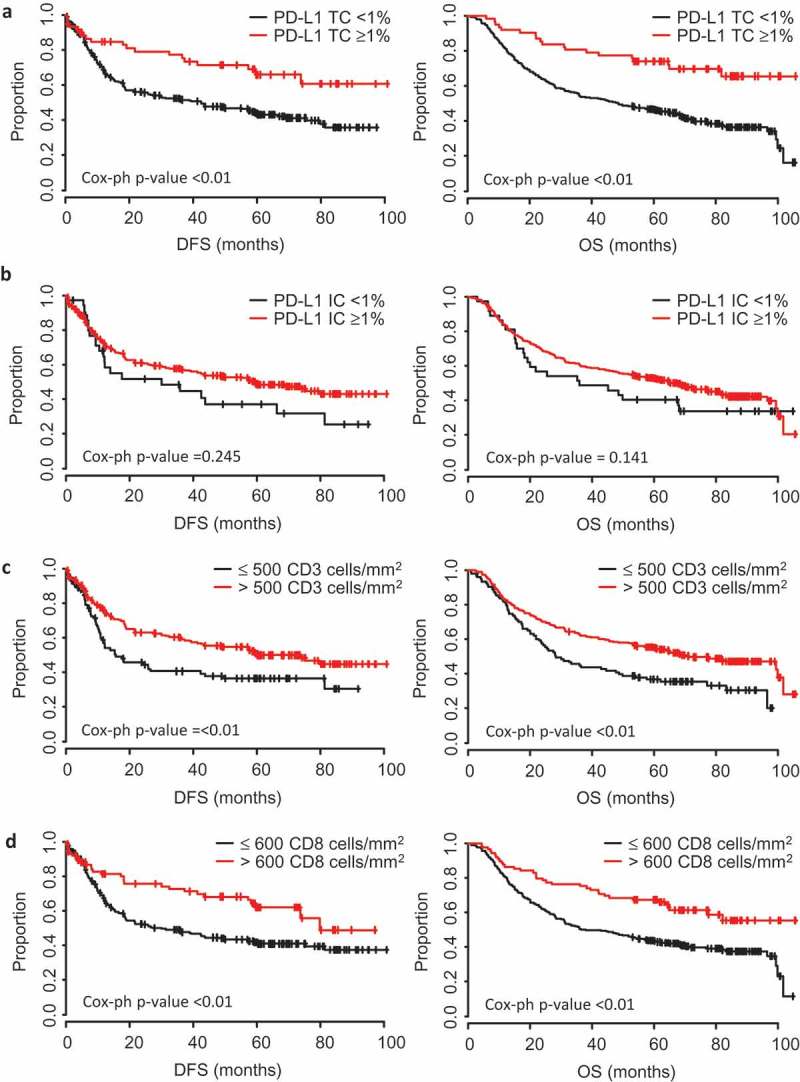
Tumour PD-L1, CD3 and CD8 marker expression are prognostic for improved survival.
Kaplan-Meier estimates of overall survival (OS) and disease-free survival (DFS) according to (a) PD-L1 tumour cell (TC) positivity, (b) PD-L1 immune cell (IC) positivity, (c) CD3 + T cell densities, and (d) CD8+ cytotoxic T cell densities. The cut-offs used were PD-L1 TC and IC (≥ 1%), CD3 (500 cells/mm2) and CD8 (600 cells/mm2). Data were statistically analysed by Cox Proportional Hazards (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 3.
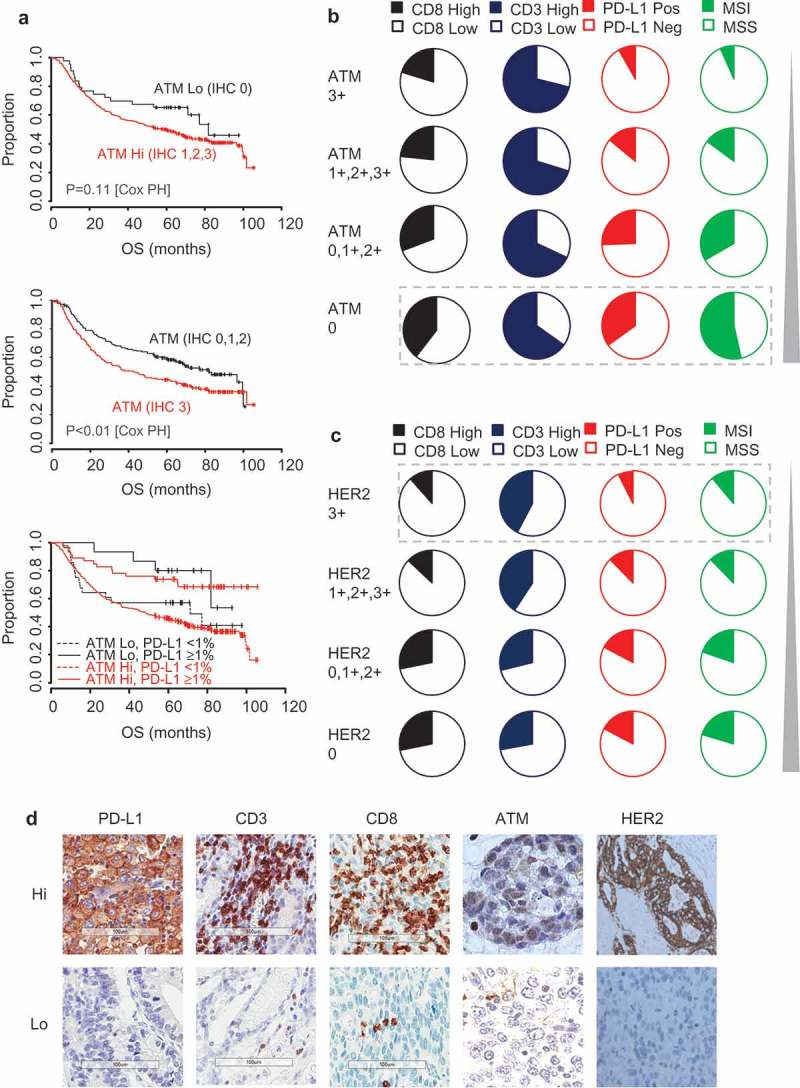
ATM-low and HER2-high segments are mutually exclusive and differ in their immune profile.
(a) Kaplan-Meier estimates of overall survival (OS) according to ATM and PD-L1 TC stratification. Pie charts depict the frequency of patients with High and Low CD8, CD3 and PD-L1 TC densities and MSI status in (b) ATM and (c) HER2 populations. (d) Representative images for high and low examples of PD-L1, CD3, CD8, ATM and HER2. The cut-offs used were PD-L1 TC (≥ 1%), CD3 (500 cells/mm2) and CD8 (600 cells/mm2). Data were statistically analysed by Cox Proportional Hazards (*P < 0.05, **P < 0.01, ***P < 0.001).
Table 3A.
Univariate and multivariate model analysis.
| Univariate p-value |
||
|---|---|---|
| Variable | OS | DFS |
| age | 0.214 | 0.641 |
| Sex | 0.260 | 0.658 |
| Lauren | 0.021 | 0.041 |
| WHO Stage | <0.01 | <0.01 |
| T stage | <0.01 | <0.01 |
| N stage | <0.01 | <0.01 |
| Metastasis | <0.01 | <0.01 |
| Number of positive nodes | <0.01 | <0.01 |
As we previously reported,19 patients with MSI-high tumours had better overall survival by both univariate and multivariate analysis (Table 3, p < 0.01 univariate and P < 0.05 multivariate). Moreover, when we divided the MSI or MSS cohort into sub-groups of either PD-L1 TC positive or negative tumours, within each cohort PD-L1 positive tumours had an improved OS (MSI p = 0.031; MSS p = 0.039), with the best outcome observed for patients with MSI-high/PD-L1 positive tumours and those with MSS/PD-L1 negative tumours having the worst outcome (Figure 1b). Hence, MSI status and PD-L1 status in TC combine to influence the outcome of GC patients.
HER2-high and ATM-low are mutually exclusive segments, which differ in MSI and immune marker status
As previously reported,19 the majority of HER2 positive (IHC 3+) GCs were of the intestinal subtype (24/27, 88.9%) and were MSS (24/27, 88.9%). When the HER2-high (3+) segment was compared to the HER2 low (0) segment, there was a trend towards a lower incidence of TC PD-L1 (7.4% vs 17%; HER2 3+ vs 0, respectively) and CD8 (11.5% vs 21%; HER2 3+ vs 0, respectively) positivity (Figures 3c and 5) without statistical significance (Table 3). Consistent with previous reports,17,18 we observed that 11.5% (43/373) of gastric tumours had ATM low expression by IHC (Table 2). This ATM low subtype was largely mutually exclusive with the HER2-high segment (only 1/27 HER2 positive tumours were ATM low). Of the ATM low tumours, 51.2% (22/43) were intestinal, 37.2% (16/43) were diffuse and 11.6% (5/43) were mixed subtype in Lauren classification. In agreement with published literature,18 an increased incidence of MSI was observed within the ATM low GC compared to ATM high GC (23/43, 53.5% vs 49/330, 14.8%; p < 0.01) (Figure 5). A significantly increased incidence of PD-L1 TC positivity (15/43, 34.9% vs 46/329 14.0%; p < 0.01) and of CD8+ cytotoxic immune infiltrates (17/43, 39.5% vs 70/299, 23.4%; p = 0.033) was also observed for ATM low GC compared to ATM high GC (Figures 3b and 5). Although a significantly longer DFS (p = 0.02) was observed for patients with ATM low compared to ATM high GCs, this was not significant by multivariate analysis and no association was observed with OS (Table 3, Figure 3a). Interestingly, within the ATM positive GCs, the subset with very high expressing ATM tumours (IHC 3+, 196/373) had a significantly worse outcome (Figure 3a; P < 0.01; HR = 1.464 p = 0.077 multivariate analysis) together with a significantly lower prevalence of PD-L1 TC positivity (16/196 vs 46/183; p < 0.001) and lower prevalence of MSI-high tumours (13/196 vs 59/184; p < 0.001). Representative example images are illustrated in Figure 3d.
Figure 5.
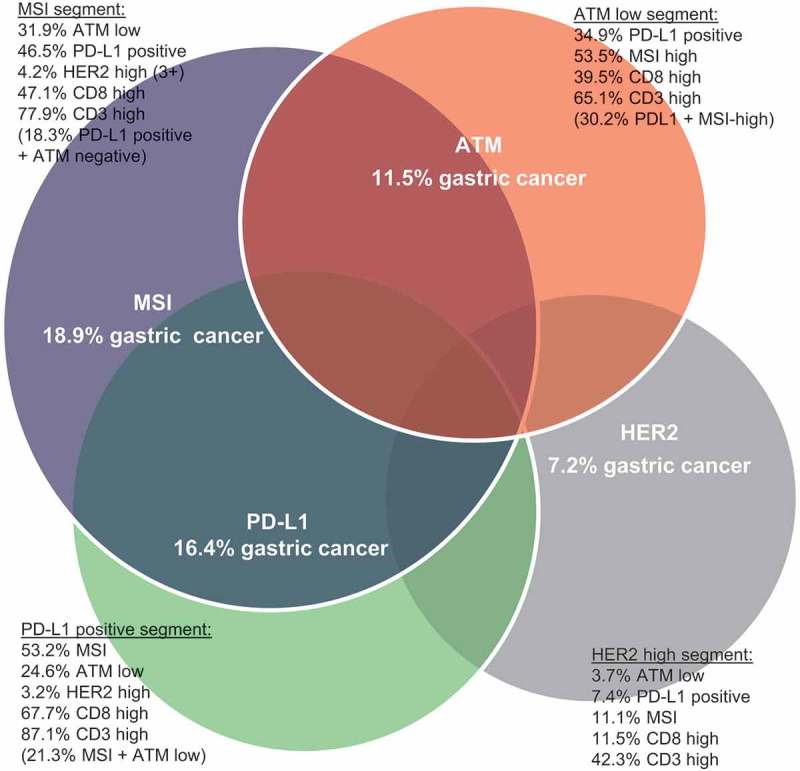
Segmentation of gastric cancers into four distinct molecular subtypes.
Illustrative representation of the overlap observed across the different molecular subtypes of gastric cancer (MSI, ATM, PD-L1 and HER2), encompassing the biomarker breakdown for each subtype.
Molecular subgroups and PD-L1, CD3 and CD8 T-cells
Finally, we have evaluated the prevalence of PD-L1 and immune infiltrates across previously published GC subgroups19 (Figure 4). Based on gene expression profiling, we previously defined 4 molecular subgroups; MSI, MSS/EMT (epithelial-to-mesenchymal transition), MSS/P53 active, and MSS/P53 inactive.19 In the analysis, we included 300 GC specimens with molecular profiling available. The proportion of CD8 high was highest in MSI subtype (41.2%) followed by MSS/TP53 active (29.1%), EMT (23.9%) and MSS/TP53 inactive (11.2%). Distribution of CD3 + T cells were similar between MSS/TP53 active (74.7%), MSI (69.1%), EMT (60.9%) and lowest in MSS/TP53 inactive (44.9%). PDL-1 TC positivity was highest in the MSI subtype (44.1%), followed by MSS/TP53 active (12.7%), MSS/TP53 inactive (8.4%) and EMT (8.7%). This gene expression based classifier confirms the association of CD8 + T-cells and TC PD-L1 with MSI tumours.
Figure 4.
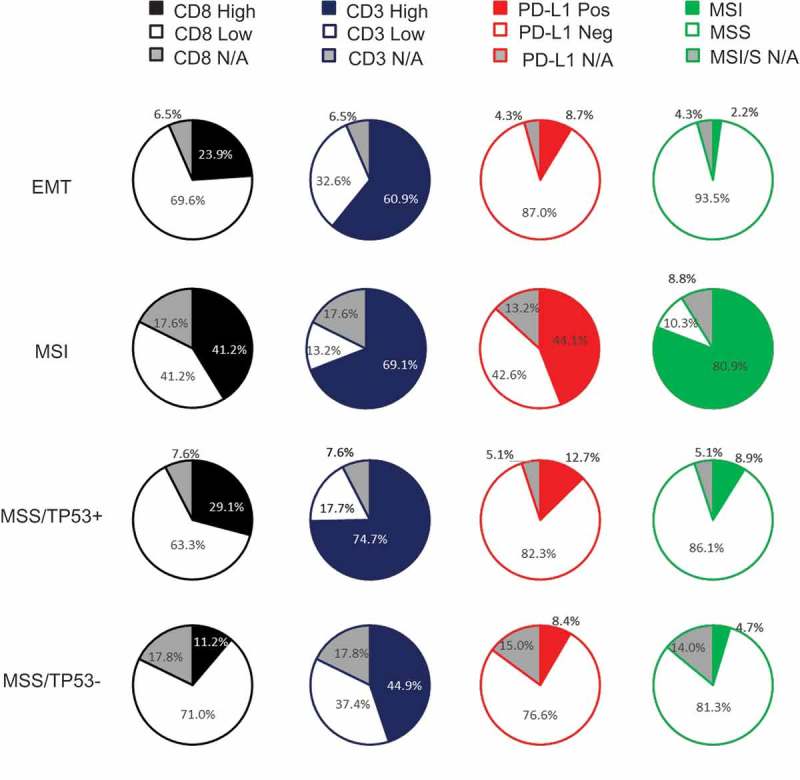
Distribution of CD8, CD3, PD-L1 according to subtypes.
Pie charts depict the frequency of patients with CD8, CD3, PD-L1 and MSI. The cut-offs used were PD-L1 TC (≥ 1%), CD3 (500 cells/mm2) and CD8 (600 cells/mm2).
Discussion
Here we have explored the association of the immune markers PD-L1, CD3 and CD8 with outcome and with other marker defined subgroups, namely ATM, HER2 and MSI, in a large Asian GC cohort. We show that TC PD-L1 expression is an independent prognostic factor predictive for better outcome in Asian GC patients. Furthermore, TC PD-L1 correlates with increased T-lymphocytes and cytotoxic T-cell infiltrates, as assessed by CD3 and CD8 marker expression and these markers are also prognostic for improved survival. PD-L1 status has been correlated with both favorable and unfavorable outcome in different cancers20-26 and increased immune prevalence has also been reported to associate with improved clinical outcome.27,28 Previous GC studies have been contradictory, reporting that PD-L1 is associated with both good29-32 and poor prognosis33-35 or has no association with survival.36 The reason for these contradictory findings are uncertain, but could be attributable to use of different PD-L1 antibodies, assays and scoring systems or alternatively represent differences between GC cohorts.
Overall this study shows that in a Korean cohort of patients with immuno-competent gastric tumours have a better outcome and supports exploration of blockade of the PD-1/PD-L1 checkpoint as a potential therapeutic strategy for GC. Furthermore, PD-L1 staining and CD3/CD8 immune infiltrates are significantly associated with MSI-high and these observations are consistent with reports in GC37 and CRC14 and clinical data has shown that mismatch repair deficiency predicts for response to PD1 blockade.13 Our data are also in agreement with several recent studies that demonstrated an association between PD-L1 and EBV-associated malignancies.37,38 Overall our findings suggest that EBV and MSI-high tumours are well placed to benefit from immune therapy. In contrast, the lack of association between immune markers and Lauren subtype suggests that patient stratification by Lauren classification alone may not be sufficient for PD-L1 therapy selection in immuno-competent GC. The cut-off value for PD-L1 positivity and comparison to other available PD-L1 antibodies for IHC should be evaluated in future studies.
Trastuzumab is the only targeted therapy currently approved for GC and high tumour HER2 expression by IHC is used to select patients for this therapy.2,39,40 Another molecular subtype of GC, identifiable by IHC analysis, are ATM low expressing tumours but no approved targeted therapy exists for this molecular subtype. In the current study, it is clear that the ATM low and HER2 high segments are largely mutually exclusive with contrasting immune profiles. The ATM low segment is enriched for PD-L1, CD8 positive cytotoxic T-cells and MSI-high status, while the majority of HER2 high tumours are MSS with no enrichment for PD-L1 positivity nor for CD8 immune infiltrates. This suggests that the ATM-low segment is well placed to benefit from anti-PD-L1/PD1 therapy. Interestingly within the subset of ATM positive tumours those cancers with the highest level of ATM expression (IHC 3+) had a worse outcome, together with a lower prevalence of both MSI-high and PD-L1 expression. ATM plays an essential role in the cellular DNA damage response necessary to maintain genome stability and clinically ATM mutations are associated with microsatellite mutations in the ATM gene.18,41,42 The genomic instability induced by low/loss of ATM expression could result in the generation of neoantigens and an immunologically “hot” tumour microenvironment. This ATM low segment could benefit from combination therapy with immunotherapy and DDR inhibitors. Although in a Phase III study, the PARP inhibitor olaparib failed to deliver the expected benefit (Bang et al., ESMO 2016), other DDR inhibitors such as ATR inhibitors should be tested in GC, especially in those tumours that are ATM-low and PD-L1 positive. In contrast, it could be argued that the HER2 high segment is less likely to benefit from PD-L1/PD1 checkpoint therapy alone. In this regard it will be of interest to understand the effects of trastuzumab therapy on the tumour immune environment.
Here we have assessed the overlap and immune marker expression of GCs divided into four distinct molecular subgroups, namely ATM-low, HER2-high, PD-L1 positive and MSI-high (Figure 5). Significant overlap exists between the ATM low, PD-L1 and MSI-high subgroups. Notably the ATM low and HER2 high segments are mutually exclusive and differ markedly in their immune profile, the “immunologically hot” ATM low segment being enriched for MSI-high, PD-L1 and CD8 while the HER2 segment is enriched for MSS, with no enrichment for immune markers. The PD-L1 segment is associated with T-cell infiltrates as assessed by CD3 and CD8 expression and is prognostic by multivariate analysis for better outcome, while the MSI-high segment is enriched for immune markers including PD-L1 and has significant overlap with the ATM-low but not HER2 segments. Recently pembrolizumab was approved by the U.S. FDA for use in any solid tumour with MSI-high (https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm). In our study, when overlaid with the known ACRG molecular subgroups, PD-L1 was highly enriched in MSI subtype.
Limitations of this study include that it is a retrospective analysis conducted on a GC cohort collected at a single Korean institution. While these observations do need to be confirmed in Western GC, it has similarly been reported that PDL1 is associated with EBV+, MSI-high and is an independent survival prognosticator in a Western cohort of 465 GC (Boger et al). In the current study tumour samples are from patients undergoing primary resection and further studies are required to determine whether the observations apply to metastatic cancers.
In conclusion, this study adds to the body of evidence illustrating that in GC PDL1, CD3/CD8 and MSI-high are prognostic markers and highlights the potential for molecular subsets of GC to respond differently to immune therapy.
Materials and methods
For full details please see online supplementary materials and methods
Cohort
Samsung Medical Centre, Seoul, Korea, procured the n = 380 primary independent GC specimens at the time of total or subtotal gastrectomy from 2004–2007.19 Of the initial 380 patients, 347 met the full study inclusion criteria (Sup. Fig. 1). All of the tissue specimens were at chemo-naïve state during primary resection of gastric cancer. Samsung Medical Centre Institutional Review Board (IRB no. 2010–12-088) approved the protocol and informed consent was obtained according to the IRB protocol.
Immunohistochemistry
CD3 and CD8 IHC was carried out as previously described.43 In brief, anti-CD3 2GV6 (Ventana, 790–4341) and anti-CD8 C8/144B (Dako, M7103) were quantified using image analysis. Anti-PD-L1 E1L3N (Cell Signaling Technologies, 13684) was evaluated by a pathologist determining frequency and staining intensity on the membrane of tumour cells and the percentage of PD-L1+ immune cells (macrophages, dendritic cells, and lymphocytes).44 Anti-ATM antibody Y170 (Abcam, ab32420), anti-HER2 (PATHWAY® HER-2/neu (4B5 (Ventana Medical Systems) and anti-NCL-L-MLH1 (Novocastra, Leica) were quantified via pathology (scoring (please see supplementary methods).
Ebv-encoded RNA in situ hybridization
The in-situ hybridization procedure was performed on the fully automated BOND-MAX system, with an EBV-encoded RNA probe (Leica Biosystem) following the manufacturer’s instructions. Only cases with a strong signal within more than 95% of tumour cell nuclei were considered to be positive.
Microsatellite instability
The MSI test was performed using multiplex PCR comprising five quasimonomorphic mononucleotide repeat markers (NR27, NR21, NR24, BAT25, and BAT26).45 Samples with no allelic size variations in any of the microsatellites were classified as microsatellite stable (MSS). Tumours with allelic size variations in one (NR24) or more of the microsatellites were considered MSI-high.
Statistical analysis
Associations between categorical variables were assessed for significance by Fisher’s exact test. Associations between numerical and categorical markers were assessed by Kruskal-Wallis test and\or Mann-whitney test. Median survival times and confidence intervals were calculated by Kaplan Meier estimation. Hazard ratios were calculated by Cox proportional hazards modelling, with each marker modelled by a univariate model and also by a multivariate model incorporating age, gender, Lauren subtype, TNM stage and metastasis as covariates. For all statistical analysis p < 0.05 was considered statistically significant. Differences in survival were also considered significant at p < 0.05. No consideration was given to multiplicity of testing due to the exploratory nature of this study. In silico validation of the CD3 and CD8 data were achieved, where optimal cut-points were determined using the k-folds validation approach with bootstrapping. Agreement was seen with those determined from a visual assessment of hazard ratio and associated confidence intervals (Sup. Figure 2).
Funding Statement
This work was supported by AstraZeneca. This study was supported partly by a grant from the 20 by 20 project of Samsung Medical Centre (GF01140111). This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI16C1990, HI14C3418). The funders had no role in the design and conduct of the study.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed at here.
Conflict of interest statement
The authors declare no potential conflicts of interest
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Price TJ, Shapiro JD, Segelov E, Karapetis CS, Pavlakis N, Van Cutsem E, Shah MA, Kang Y-K, Tebbutt NC. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol. 2012;6:199–208. quiz 209. doi: 10.1586/egh.11.103. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 5.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 8.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S-L, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, Brahmer JR, Carvajal RD, Hammers HJ, Puzanov I, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013–2020. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 11.Kang YK. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol. 2017:35. doi: 10.1200/JCO.2017.35.4_suppl.2 Journal of Clinical Oncology 35(4_suppl):2–2 February 2017 [DOI] [Google Scholar]
- 12.Ilie M, Hofman V, Dietel M, Soria J-C, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016. doi: 10.1007/s00428-016-1910-4. [DOI] [PubMed] [Google Scholar]
- 13.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Helgason H, Rafnar T, Olafsdottir HS, Jonasson JG, Sigurdsson A, Stacey SN, Jonasdottir A, Tryggvadottir L, Alexiusdottir K, Haraldsson A, et al. Loss-of-function variants in ATM confer risk of gastric cancer. Nat Genet. 2015;47:906–910. doi: 10.1038/ng.3342. [DOI] [PubMed] [Google Scholar]
- 16.Cremona CA, Behrens A. ATM signalling and cancer. Oncogene. 2014;33:3351–3360. doi: 10.1038/onc.2013.275. [DOI] [PubMed] [Google Scholar]
- 17.Bang YJ, Im SA, Lee KW, Cho JY, Song E-K, Lee KH, Kim YH, Park JO, Chun HG, Zang DY, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33:3858–3865. doi: 10.1200/JCO.2014.60.0320. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Im SA, Kim MA, Cho HJ, Lee DW, Lee K-H, Kim T-Y, Han S-W, Oh D-Y, Lee H-J, et al. Ataxia-telangiectasia-mutated protein expression with microsatellite instability in gastric cancer as prognostic marker. Int J Cancer. 2014;134:72–80. doi: 10.1002/ijc.28245. [DOI] [PubMed] [Google Scholar]
- 19.Cristescu R, Lee J, Nebozhyn M, Kim K-M, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama S, Miyoshi H, Nakashima K, Shimono J, Hashiguchi T, Mitsuoka M, Takamori S, Akagi Y, Ohshima K. Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res. 2016. doi: 10.1158/1078-0432.CCR-16-0434. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Xu L, Wang Q, An G, Feng G, Liu F. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med. 2015;8:14595–14603. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, Fang YC, Chen XF, Liu GT. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Sun JM, Zhou W, Choi YL, Choi S-J, Kim SE, Wang Z, Dolled-Filhart M, Emancipator K, Wu D, Weiner R, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–1011. doi: 10.1016/j.jtho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Zhang SD, McCrudden C, Chan K-W, Lin Y, Kwok H-F. The prognostic significance of PD-L1 in bladder cancer. Oncol Rep. 2015;33:3075–3084. doi: 10.3892/or.2015.3933. [DOI] [PubMed] [Google Scholar]
- 25.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Kim EK, Yoon SO, Jung WY, Lee H, Kang Y, Jang Y-J, Hong SW, Choi SH, Yang WI. Implications of NOVA1 suppression within the microenvironment of gastric cancer: association with immune cell dysregulation. Gastric Cancer. 2016;20(3):438–447. doi: 10.1007/s10120-016-0623-3. [DOI] [PubMed] [Google Scholar]
- 30.Boger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–24283. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 32.Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8:64066–64082. doi: 10.18632/oncotarget.19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakhmilevich AL, Baldeshwiler MJ, Van De Voort TJ, Felder MAR, Yang RK, Kalogriopoulos NA, Koslov DS, Van Rooijen N, Sondel PM. Tumor-associated myeloid cells can be activated in vitro and in vivo to mediate antitumor effects. Cancer Immunol Immunother. 2012;61:1683–1697. doi: 10.1007/s00262-012-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2015;20:273–281. doi: 10.1007/s10147-014-0701-7. [DOI] [PubMed] [Google Scholar]
- 35.Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu G-L, Luo H, Yang Y-X, Dai X-Y, Zhou S-F, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901–909. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong M, Wang HY, Zhao XX, Chen J-N, Zhang Y-W, Huang Y, Xue L, Li H-G, Du H, Wu X-Y, et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2016;53:25–34. doi: 10.1016/j.humpath.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016. doi: 10.18632/oncotarget.v7i22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research N Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692–700. doi: 10.1016/j.ctrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Bang YJ. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46:637–648. doi: 10.1097/MCG.0b013e3182557307. [DOI] [PubMed] [Google Scholar]
- 41.Goodarzi AA, Jeggo PA. The heterochromatic barrier to DNA double strand break repair: how to get the entry visa. Int J Mol Sci. 2012;13:11844–11860. doi: 10.3390/ijms130911844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurz EU, Lees-Miller SP. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst). 2004;3:889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Galon J, Pages F, Marincola FM, Lathelize H, Page O, Valagier A, Badet L, Hauet T. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rebelatto M, Mistry A, Sabalos C, Walker J, Midha A, Steele K, Robins PB, Li X, Shi L, Blake-Haskins JA, et al. Development of a PD-L1 companion diagnostic assay for treatment with MEDI4736 in NSCLC and SCCHN patients. J Clin Oncol. 2015;33(suppl):abstr 8033. [Google Scholar]
- 45.Buhard O, Suraweera N, Lectard A, Duval A, Hamelin R. Quasimonomorphic mononucleotide repeats for high-level microsatellite instability analysis. Dis Markers. 2004;20:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


