ABSTRACT
Autophagy delivers cytosolic components to lysosomes and the vacuole for degradation. This pathway prevents starvation through bulk degradation and recycling of cytoplasmic components, and maintains cellular homeostasis through selective elimination of damaged proteins and organelles. Autophagic delivery processes are categorized into three types: macroautophagy, microautophagy, and chaperone-mediated autophagy. During macroautophagy, nascent, double membrane–bound vesicles termed autophagosomes sequester a portion of cytoplasm and deliver it to the vacuole/lysosomes. Molecular genetic studies in budding yeasts have identified a set of AUTOPHAGY (ATG) genes required for autophagosome formation. Although microautophagy involves the direct lysosomal/vacuolar engulfment and incorporation of a target into the lumen rather than the formation of autophagosomes, the membrane dynamics and possible roles of ATGs during microautophagy are under investigation. Our recent study revealed an ATG-dependent microautophagy process in plants, during which chloroplasts damaged by high visible light (HL) are selectively eliminated. Here, we discuss the membrane dynamics of the plant microautophagy that enables the transport of whole chloroplasts into the vacuole.
KEYWORDS: Autophagy, chlorophagy, chloroplast, microautophagy, photodamage
Text
Autophagy refers to the process in which bulk cytoplasm and entire organelles are transported to lysosomes or the vacuole for degradation in eukaryotes.1 This process is important for eliminating cellular components damaged by stress and for nutrient recycling under starvation conditions. Molecular and cellular studies performed in the budding yeast Saccharomyces cerevisiae identified a type of autophagy called macroautophagy.2 This process involves the formation of specialized double-membrane vesicles, called autophagosomes,3,4 which engulf cytoplasmic components and deliver their cargo to the vacuole for degradation. Over 40 AUTOPHAGY (ATG) genes have been identified in the budding yeast, and a set of genes necessary for autophagosome formation (ATG1–10, ATG12–14, ATG16, ATG18), termed ‘core’ ATGs, are conserved in plants.5,6 One of these core ATG genes, the ubiquitin-like ATG8 protein, conjugates to a lipid, phosphatidylethanolamine (PE), and forms nascent autophagosomal membranes.7 ATG3–5, ATG7, ATG10, ATG12 and ATG16 activate this ATG8-PE conjugation pathway.7
Direct uptake of cytoplasmic components into the vacuole/lysosomes also occurs by invagination or protrusion of the lysosomal/vacuolar membrane in a process known as microautophagy (Figure 1(a)).8,9 Electron microscopy studies show arm- or flap-like protrusions of lysosomal membranes surrounding a portion of the cytoplasm in mouse and rat liver cells;10,11 however, the molecular mechanism for these processes remains unknown.9 In S. cerevisiae, numerous studies report vacuolar invagination-mediated sequestering of cellular components such as cytoplasm,12 endoplasmic reticulum,13 portions of the nucleus (piecemeal microautophagy of the nucleus, or PMN),14 mitochondria,15 and lipid droplets.16,17 The degradation of peroxisomes in the methylotrophic yeast Komagataella phaffii (previously known as Pichia pastoris) is another well-characterized example of microautophagy. This process is activated when the cell’s energy source switches from methanol to glucose.18,19 During this micropexophagy, a micropexophagy-specific membrane apparatus (MIPA) is formed by the action of core ATG proteins, which also produce the autophagosomal membrane in macroautophagy.20 The MIPA is a double-membraned, cap-like structure that is deposited at the far end of an enclosed peroxisome, where it purportedly mediates the final vacuolar-membrane fusion that leads to the release of the microautophagic vesicle into the vacuole (Figure 1(b)). Therefore, the requirement of core ATGs for micropexophagy is clearly established, although the resultant membrane morphology is distinct from that of macroautophagy.21
Figure 1.

A schematic model of microautophagy in mammalian cells, yeast, and plant cells.
(a) Non-selective microautophagy is generally achieved by invagination or protrusion of the lysosomal or vacuolar membrane to incorporate various organelles and proteins. (b) Micropexophagy-specific membrane apparatus (MIPA) structures enclose peroxisomes during micropexophagy in the methylotrophic yeast Komagataella phaffii. (c) High light-damaged chloroplasts exhibiting a swollen shape are selectively eliminated via microautophagy in Arabidopsis leaves. (d) Extension of the vacuolar membrane itself engulfs an anthocyanin aggregate to incorporate it into the vacuole and form anthocyanin vacuolar inclusions (AVIs).
Studies of ATG mutants in Arabidopsis thaliana indicate that the core autophagy machinery for autophagosome production is similarly conserved in plants.5,6 Since chloroplasts accumulate a great deal of photooxidative damage, we investigated the role of autophagy in chloroplast degradation during photooxidative stress. We revealed that after exposure to ultraviolet-B, strong visible light or natural sunlight, entire chloroplasts are digested in the vacuole through an autophagy process that we termed chlorophagy.22,23
In a follow-up study, we monitored how chlorophagy is induced and executed in Arabidopsis leaves with confocal microscopy and transmission electron microscopy.24 When transgenic plants expressing both the stroma-targeted small subunit of Rubisco fused to red fluorescent protein (RBCS-RFP) and the delta tonoplast intrinsic protein fused to green fluorescent protein (δTIP-GFP) were grown under normal conditions, all chloroplasts exhibited chlorophyll auto-fluorescence and stroma-targeted RFP signal located outside of the δTIP-GFP-labeled vacuolar membrane (Figure 2(a)). After 2 h of high light exposure (HL; 2,000 µmol m−2 s−1), abnormally swollen chloroplasts appear and are engulfed by the vacuolar membrane, similar to what occurs during microautophagy, as described above (Figure 2(b), arrowheads).24 In plants expressing the autophagosome marker GFP-ATG8, GFP-ATG8-labeled structures did not completely engulf HL-induced swollen chloroplasts unlike what occurs during macroautophagy, but covered only a part of those chloroplasts.24 These observations suggest that chlorophagy is accomplished via a microautophagy-like process, which we term microchlorophagy. The damaged chloroplasts are not transported into the vacuole in the atg5 or atg7 knockout mutants,24 suggesting that the production of ATG8-containing structures by the action of ATG5 and ATG7 is required during this microchlorophagy process (Figure 1(c)).24
Figure 2.
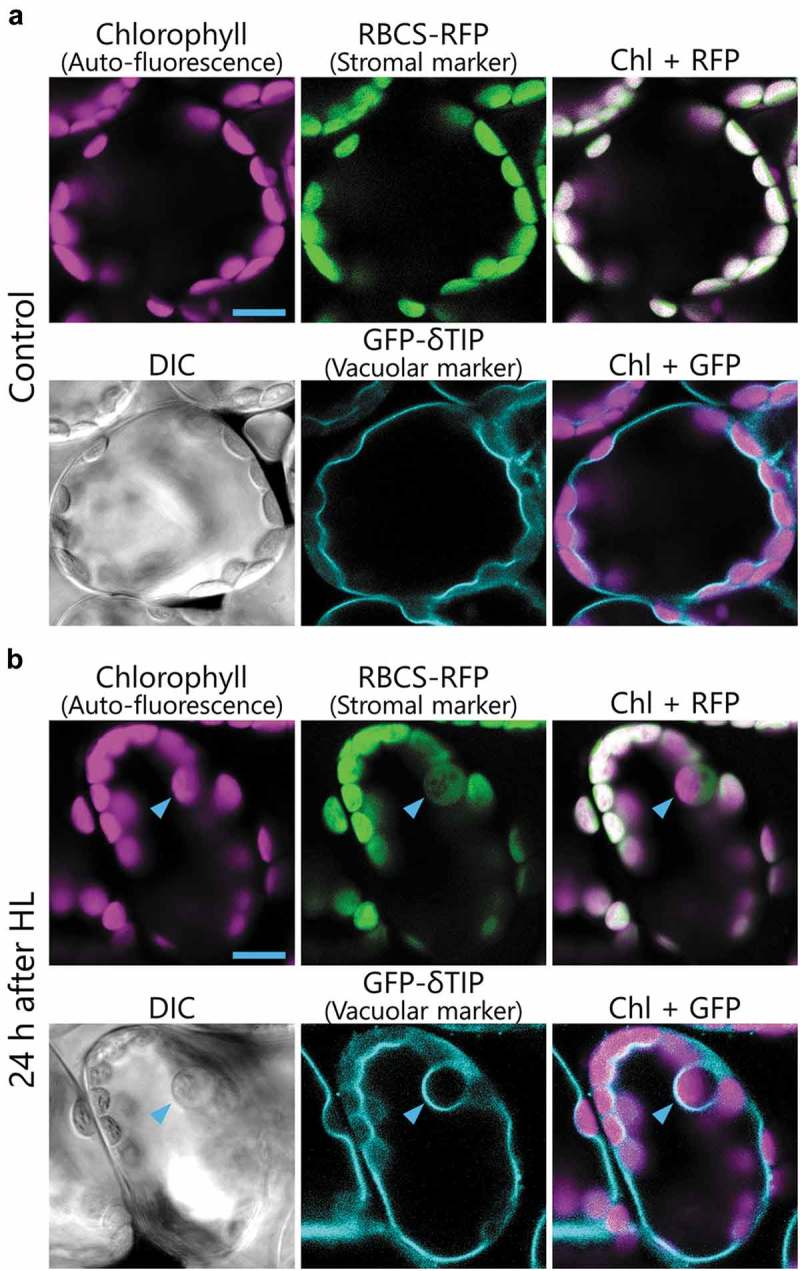
Images of HL damage-induced swollen chloroplasts in Arabidopsis leaves.
(a and b) Confocal images of mesophyll cells expressing stroma-targeted RBCS-RFP under the control of the RBCS promoter along with the vacuolar membrane-targeted GFP-δ-tonoplast intrinsic protein (TIP) under the control of the 35S promoter. The second rosette leaves of non-treated control plants (a) or plants 1 d after exposure to 2-h high visible light (HL; 2,000 µmol m−2 s−1) at 10℃ (b) were observed. Arrowheads indicate HL-induced swollen chloroplasts. Chlorophyll auto-fluorescence, RFP, and GFP signals appear magenta, green, and turquoise, respectively. DIC images are also shown. Scale Bars = 10 µm.
A previous study using electron microscopy showed a microautophagy-like phenomenon involved in degrading starch granules in Vigna mungo cotyledon cells,25 but molecular genetics studies have yet to be performed in this plant species. Thus, the requirement for core ATGs in this process has not been addressed. A more recent study demonstrated that microautophagy-like incorporation of cytoplasmic anthocyanin aggregates forms anthocyanin vacuolar inclusions (AVIs) in Arabidopsis and lisianthus (Eustoma grandiorum) (Figure 1(d)).26 However, AVI formation is independent of ATG5, suggesting that both ATG5-independent and ATG5-dependent types of microautophagy can function in Arabidopsis. The transport of damaged chloroplasts by the vacuolar membrane described above in Arabidopsis is likely the first genetic evidence indicating the occurrence of a core ATG-dependent microautophagy process in plants.
The membrane dynamics during chlorophagy have not been elucidated. Given the available information regarding membrane dynamics of microautophagy in yeasts and mammals (Figure 1(a,b)), we propose two possible methods for chloroplast sequestering during microchlorophagy: i) ATG8-containing membranes recognize damaged chloroplasts and the vacuolar membrane invaginates or protrudes to engulf damaged chloroplasts, or ii) ATG8-containing membranes form a MIPA-like structure to sequester damaged chloroplasts (Figure 1(c)). Chloroplasts are 20–30 µm2, which is much larger than the typical autophagosome (around 1 µm2). Therefore, microchlorophagy may involve specific morphology and machineries that allow the vacuole to incorporate such a huge organelle. The further investigation of these membrane dynamics and regulatory mechanisms would expand our understanding of ATG-dependent microautophagy in general and in the context of a different eukaryotic system.
Funding Statement
This work was supported, in part, by Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 17H05050 and 18H04852 to M.I., 16J03408 to S.N.], the JSPS Research Fellowship for Young Scientists (to S.N.), Japan Science and Technology Agency (JST) PRESTO (Grant Number JPMJPR16Q1 to M.I.), and the Program for Creation of Interdisciplinary Research at Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, Japan (to M.I.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Bio. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 2.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y.. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto K, Ohsumi Y. Unveiling the molecular mechanisms of plant autophagy - from autophagosomes to vacuoles in plants. Plant Cell Physiol. 2018;59:1337–1344. doi: 10.1093/pcp/pcy112. [DOI] [PubMed] [Google Scholar]
- 6.Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annu Rev Plant Biol. 2018;69:173–208. doi: 10.1146/annurev-arplant-042817-040606. [DOI] [PubMed] [Google Scholar]
- 7.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 8.Li WW, Li J, Bao JK. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oku M, Sakai Y. Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays. 2018;40:e1800008. doi: 10.1002/bies.v40.6. [DOI] [PubMed] [Google Scholar]
- 10.de Waal EJ, Vreeling-Sindelarova H, Schellens JP, Houtkooper JM, James J. Quantitative changes in the lysosomal vacuolar system of rat hepatocytes during short-term starvation. A morphometric analysis with special reference to macro- and microautophagy. Cell Tissue Res. 1986;243:641–648. [DOI] [PubMed] [Google Scholar]
- 11.Mortimore GE, Hutson NJ, Surmacz CA. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci U S A. 1983;80:2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller O, Sattler T, Flotenmeyer M, Schwarz H, Plattner H, Mayer A. Autophagic tubes: vacuolar invaginations involved in lateral membrane sorting and inverse vesicle budding. J Cell Biol. 2000;151:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuck S, Gallagher CM, Walter P. ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J Cell Sci. 2014;127:4078–4088. doi: 10.1242/jcs.154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts P, Moshitch-Moshkovitz S, Kvam E, O’Toole E, Winey M, Goldfarb DS. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:129–141. doi: 10.1091/mbc.e02-08-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissova I, Salin B, Schaeffer J, Bhatia S, Manon S, Camougrand N. Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy. 2007;3:329–336. [DOI] [PubMed] [Google Scholar]
- 16.Moeller CH, Thomson WW. Uptake of lipid bodies by the yeast vacuole involving areas of the tonoplast depleted of intramembranous particles. J Ultrastruct Res. 1979;68:38–45. [DOI] [PubMed] [Google Scholar]
- 17.van Zutphen T, Todde V, de Boer R, Kreim M, Hofbauer HF, Wolinski H, Veenhuis M, van der Klei IJ, Kohlwein SD. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2014;25:290–301. doi: 10.1091/mbc.E13-08-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn WA Jr., Cregg JM, Kiel JA, van der Klei IJ, Oku M, Sakai Y, Sibirny AA, Stasyk OV, Veenhuis M. Pexophagy: the selective autophagy of peroxisomes. Autophagy. 2005;1:75–83. [DOI] [PubMed] [Google Scholar]
- 19.Oku M, Sakai Y. Pexophagy in yeasts. Biochim Biophys Acta. 2016;1863:992–998. doi: 10.1016/j.bbamcr.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Mukaiyama H, Oku M, Baba M, Samizo T, Hammond AT, Glick BS, Kato N, Sakai Y. Paz2 and 13 other PAZ gene products regulate vacuolar engulfment of peroxisomes during micropexophagy. Genes Cells. 2002;7:75–90. [DOI] [PubMed] [Google Scholar]
- 21.Mukaiyama H, Baba M, Osumi M, Aoyagi S, Kato N, Ohsumi Y, Sakai Y. Modification of a ubiquitin-like protein Paz2 conducted micropexophagy through formation of a novel membrane structure. Mol Biol Cell. 2004;15:58–70. doi: 10.1091/mbc.e03-05-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izumi M, Ishida H, Nakamura S, Hidema J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell. 2017;29:377–394. doi: 10.1105/tpc.16.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura S, Izumi M. Regulation of Chlorophagy during photoinhibition and senescence: lessons from mitophagy. Plant Cell Physiol. 2018;59:1135–1143. doi: 10.1093/pcp/pcy096. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Hidema J, Sakamoto W, Ishida H, Izumi M. Selective elimination of membrane-damaged chloroplasts via microautophagy. Plant Physiol. 2018;177:1007–1026. doi: 10.1104/pp.18.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyooka K, Okamoto T, Minamikawa T. Cotyledon cells of Vigna mungo seedlings use at least two distinct autophagic machineries for degradation of starch granules and cellular components. J Cell Biol. 2001;154:973–982. doi: 10.1083/jcb.200105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanoca A, Kovinich N, Burkel B, Stecha S, Bohorquez-Restrepo A, Ueda T, Eliceiri KW, Grotewold E, Otegui MS. Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell. 2015;27:2545–2559. doi: 10.1105/tpc.15.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]


