ABSTRACT
Methionine restriction, i.e., a partial depletion of the essential sulfur amino acid methionine from nutrition, extends lifespan in model organisms including yeast, nematodes, mice and rats. Recent results indicate that this strategy also prolongs health span and longevity in 2 short-lived strains of mice (with the LmnaG609G/G609G or zmpste24−/- genotypes) that represent animal models of Hutchinson-Gilford progeria syndrome (HGPS). The beneficial effects of methionine restriction on HGPS could be linked to reduced inflammation, and improved DNA stability, as well as the normalization of lipid and bile acid metabolism. Previous work has established that behavioral, nutritional, pharmacological and genetic manipulations that extend longevity in model organisms are only efficient if they induce increased autophagic flux. Methionine restriction extends lifespan in Saccharomyces cerevisiae in an Atg5- and Atg7-dependent fashion, supporting the notion that methionine restriction may indeed mediate its antiaging effects through the induction of macroautophagy/autophagy as well. Based on these findings, we speculate that autophagy might constitute an actionable therapeutic target to treat progeroid syndromes.
KEYWORDS: Caloric restriction, cholic acid, DNA damage response, inflammation, lipid metabolism
There is overwhelming evidence that nutritional interventions (such as caloric restriction and spermidine supplementation), behavioral alterations (such as time-restricted feeding and exercise), pharmacological treatments (such as continuous or intermittent treatment with rapamycin) or genetic interventions (such as transgene-enforced overexpression of ATG5 or a gain-of-function mutation of Becn1 in mice) increase the longevity of model organisms through the induction of macroautophagy (hereafter autophagy). Although the demonstration has not been performed for all model organisms in each of these conditions, it appears that yeast (Saccharomyces cerevisiae), nematodes (Caenorhabditis elegans), flies (Drosophila melanogaster) and rodents (Mus musculus) abide to the rule that longevity extension by the aforementioned manipulations is accompanied by an increase in autophagic flux, and that blockade of autophagy reverses the extension of health span and lifespan. This apparently also applies to methionine restriction, i.e. a partial reduction in the nutritional supply of the essential sulfur amino acid methionine. In yeast, auxotrophic strains that cannot generate methionine are long lived, and this effect is reversed by excess methionine supply, as well as by inhibiting autophagy or lysosomal function.
HGPS is an extremely rare, genetically determined disease in which a de novo C-to-T mutation in position 1824 of the LMNA gene (lamin A/C) affects gene splicing, causing the premature accumulation of an abnormal protein called progerin that also accumulates in normal individuals at old age. This accumulation is linked to alterations in the micro-anatomy of the nuclear envelope that adopts an abnormal shape, as well as in alterations in chromatin organization, thus affecting cell division, genomic stability and the regulation of gene expression, giving rise to premature aging (progeria) and a dramatically reduced life expectance (median 13 years). In mice, the human disease can be mimicked by introducing a similar mutation in the Lmna gene (LmnaG609G) or by knocking out the gene coding for the LMNA-processing metalloprotease ZMPSTE24. In this latter mouse model of HGPS, autophagic flux is increased, giving room to the speculation that this particular type of progeria would constitute an exception rather than a confirmation of the rule enounced above.
In an attempt to extend their health span and lifespan, progeroid mice (with either the LmnaG609G/G609G or zmpste24−/- genotypes) were subjected to lifelong methionine restriction from weaning to death [1]. Although this dietary intervention reduces the growth rate of the mice, it does prolong their median and maximum longevity by approximately 20%, commensurate with the observation that methionine restriction also attenuates signs of premature aging such as skeletal aberrations (lordokyphosis and osteoporosis) or fibrosis of the aorta and skeleton muscles. Of note, methionine restriction leads to an attenuation of signs of inflammation and DNA damage (as determined by analyzing the liver transcriptome) and normalizes the liver metabolome (as determined by mass spectrometric metabolomics), while it enhances cholic and deoxycholic acid levels in the liver and increases the abundancy of some secondary bile acids (especially glycine-conjugated bile acids) in the ileum. Importantly, feeding a diet enriched in cholic acid is sufficient to extend the health span and lifespan of zmpste24−/- mice, implying causality in the metabolic alterations at the level of the abundancy of bile acids. However, the lifespan extension of zmpste24−/- mice induced by feeding cholic acid was only in the range of 5–10%, contrasting with that induced by methionine restriction, which was in the range of 20–30%. Thus, additional mechanisms (beyond effects on bile acids) may be involved in the beneficial effects of methionine restriction.
Indeed, at the biochemical level, methionine restriction tends to reduce the phosphorylation of the MTORC1 (mechanistic target of rapamycin kinase complex 1) substrate AKT/protein kinase B (on Ser471) in the liver of LmnaG609G/G609G mice. Knowing that MTORC1 is an important repressor of autophagy, this may constitute a hint in favor of the hypothesis that methionine restriction causes autophagy in this model. Previous work demonstrated that the accumulation of progerin (the abnormal splice product of LMNA) in fibroblasts from patients with HGPS can be reversed by treating the cells with the MTORC1 inhibitor rapamycin. This effect occurs in an autophagy-dependent fashion, meaning that shRNA-mediated depletion of ATG7 or pharmacological inhibition of autophagy (with bafilomycin A1 or 3-methyladenine) negates the effects of rapamycin on progerin levels. Thus, it appears plausible that methionine restriction stimulates autophagy to improve the phenotype of the murine HGPS. One possible scenario would be that HGPS is associated with increased, but insufficient, autophagic flux (at baseline) and that additional stimulation of this flux helps to delay the disease manifestations.
Future mechanistic studies must disentangle the likely complex interplay (Figure 1) between alterations in nuclear lamina (the primary cause of HGPS), alterations in cell-autonomous stress responses including autophagy, and system-wide metabolic dysregulations including at the level of primary bile acids, as well as the possible implication of the gut microbiota, which determines the abundance of secondary bile acids. Hence, more research is necessary to resolve the puzzle of progeroid disease with the perspective to yield information that may be harnessed for slowing the pace of the ‘normal’ aging process as well. Indeed, on an optimistic note, we may speculate that the time gained by studying progeria in suitable mouse models may accelerate the path toward discovery of generally applicable antiaging strategies.
Figure 1.
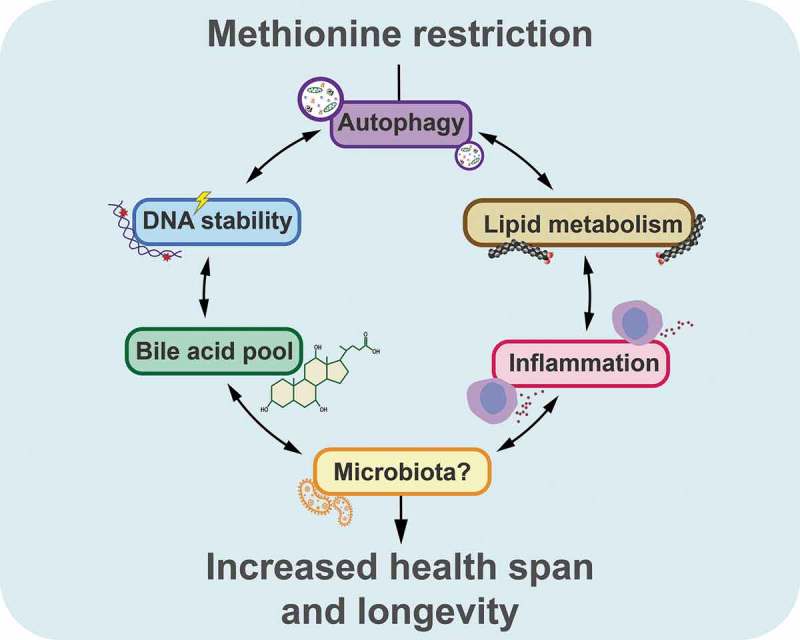
Schematic overview on the effects of methionine restriction in mouse models of Hutchinson-Gilford progeria syndrome.
Funding Statement
This work was supported by the European Research Council [ERC].
Acknowledgments
CLO is supported by grants from European Research Council (DeAge, ERC Advanced Grant), Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (Ciberonc) and Progeria Research Foundation. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Bárcena C, Quirós PM, Durand S, et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep. 2018. August 28;24(9):2392–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]


