ABSTRACT
Background
Phylloquinone is the primary form of vitamin K in the diet and circulation. Large intra- and interindividual variances in circulating phylloquinone have been partially attributed to age. However, little is known about the nondietary factors that influence phylloquinone absorption and metabolism. Similarly, it is not known if phylloquinone absorption is altered by the individual's existing vitamin K status.
Objective
The purpose of this secondary substudy was to compare plasma response with deuterium-labeled phylloquinone intake in older and younger adults after dietary phylloquinone depletion and repletion.
Methods
Forty-two older [mean ± SD age: 67.2 ± 8.0 y; body mass index (BMI; in kg/m2): 25.4 ± 4.6; n = 12 men, 9 women] and younger (mean ± SEM age: 31.8 ± 6.6 y; BMI: 25.5 ± 3.3; n = 9 men, 12 women) adults were maintained on sequential 28-d phylloquinone depletion (∼10 µg phylloquinone/d) and 28-d phylloquinone repletion (∼500 µg phylloquinone/d) diets. On the 23rd d of each diet phase, participants consumed deuterated phylloquinone-rich collard greens (2H-phylloquinone). Plasma and urinary outcome measures over 72 h were compared by age group, sex, and dietary phase via 2-factor repeated-measures ANOVA.
Results
The plasma 2H-phylloquinone area under the curve (AUC) did not differ in response to phylloquinone depletion or repletion, but was 34% higher in older than in younger adults (P = 0.02). However, plasma 2H-phylloquinone AUC was highly correlated with the serum triglyceride (TG) AUC (r2 = 0.45). After adjustment for serum TG response, the age effect on the plasma 2H-phylloquinone AUC was no longer significant.
Conclusions
Plasma 2H-phylloquinone response did not differ between phylloquinone depletion and repletion in older and younger adults. The age effect observed was explained by the serum TG response and was completely attenuated after adjustment. Plasma response to phylloquinone intake, therefore, seems to be a predominantly lipid-driven effect and not dependent on existing vitamin K status. More research is required to differentiate the effect of endogenous compared with exogenous lipids on phylloquinone absorption.
This trial was registered at clinicaltrials.gov as NCT00336232.
Keywords: age, phylloquinone, stable isotope, triglycerides, vitamin K
Introduction
Vitamin K is a fat-soluble micronutrient, essential as a cofactor in the γ-carboxylation of vitamin K–dependent proteins (1). Although its most established role is in blood coagulation, additional roles for vitamin K have been proposed, such as in regulation of soft tissue calcification (2). To understand emerging roles for vitamin K, further progress in understanding its absorption and metabolism is needed, including knowledge of the factors influencing the physiologic response to dietary vitamin K.
Phylloquinone is the primary form of vitamin K in the diet and circulation (3). Large intra- and interindividual variances in circulating phylloquinone exist, and little is known about the dietary and nondietary factors that influence phylloquinone metabolism. Menadione is a metabolite of phylloquinone, and is formed upon removal of the phytyl side chain from the naphthoquinone ring (4). Menaquinones are additional forms of vitamin K with varying side-chain lengths (MK-n) and are found in body tissues. Menadione has been demonstrated to be a precursor to MK-4 (5). Menadione is excreted in the urine and may be reflective of vitamin K metabolism.
The data reported here are a report on a stable isotope substudy conducted within a larger metabolic study investigating factors that influence the response of vitamin K status biomarkers to dietary phylloquinone depletion and repletion, the main results of which have been reported (6). In the main study, we reported that serum phylloquinone concentrations responded to dietary intake, but did not differ by age, sex, adiposity measures, or fasting serum TGs. However, excretion of urinary menadione was significantly greater among older adults than among younger adults during dietary vitamin K depletion.
Whereas there are suggestions that older adults have better vitamin K status than younger adults, based on current vitamin K status biomarkers, the data have been inconsistent (7–9). One of the challenges has been that many early studies did not consider the role of serum lipids. In one of the first metabolic studies of vitamin K metabolism (9), phylloquinone supplementation among healthy younger and older participants previously fed a low–vitamin K diet failed to bring plasma phylloquinone concentrations of young participants back to normal levels. It is possible that lower relative TG levels characteristic of younger adults, together with relatively modest stepwise supplementation dosages (reaching only 45 μg/d, compared with the supplementation dosage of 500 μg/d in the current study), may have in part explained the insufficient repletion of young adults over the observed time period.
The stable isotope substudy reported here utilized deuterium-labeled phylloquinone (2H-phylloquinone) in the form of collard greens grown in deuterated water to measure phylloquinone absorption and excretion under conditions of dietary vitamin K depletion and repletion. This substudy expands upon the knowledge generated by the parent study (6), because its design enabled the dynamic tracing of a single dose of 2H-phylloquinone delivered through food, including kinetic characterization of its residency in plasma and capture of the urinary metabolite 2H-menadione. Furthermore, given known dynamics in circulating TGs associated with meals and circadian rhythms (10), serum TG response over the nonfasted 72-h study period rather than baseline fasting TG concentration (as traditionally has been used) was considered in this study in order to more accurately assess plasma response to 2H-phylloquinone intake.
Therefore, the objective of this study was to compare measures of plasma 2H-phylloquinone response and urinary excretion of 2H-menadione in older and younger adults after dietary phylloquinone depletion and repletion.
Methods
Materials
HPLC-grade solvents (Fisher Scientific Inc.) were used for extraction and chromatography procedures. Vitamin K-1(25) (GL Synthesis) was used as an internal standard and was prepared as previously described (11). Commercial enzymatic reagents (Beckman Coulter, Inc., Diagnostics Division Headquarters) were used for cholesterol and TG analysis, as specified in AU400 Clinical Chemistry Analyzer procedural inserts.
Deuterated collard greens
The cultivation of 2H-collard greens and their preparation for consumption have been described in detail elsewhere (12), and this approach has been used successfully in previous metabolic studies by this team (12, 13). Collard greens are a rich dietary source of phylloquinone and are easy to cultivate, and thus were chosen as the source of 2H-phylloquinone. The collard greens were grown hydroponically with the use of deuterated water (31 atom% 2H2O). After harvest and shipment, leaves were steamed, pureed, portioned, and stored at –80°C until use.
Participants
Twenty-one younger (18–40 y of age) and 21 older (55–80 y of age) healthy, community-dwelling men and women were recruited from the greater Boston area for a metabolic study on the determinants of vitamin K absorption and metabolism (Supplemental Figure 1), the primary outcomes of which have been reported (6). The study was conducted in the Metabolic Research Unit (MRU) at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University. All participants had normal function of heart, kidney, liver, and thyroid. Older women were postmenopausal for ≥3 y.
Written informed consent for this trial was obtained from all participants. This study (NCT00336232) was approved by the Institutional Review Board at Tufts University.
Study design
The parent metabolic study was designed to determine the dietary (phylloquinone depletion and repletion) and nondietary (age, sex, BMI, percentage body fat, and fasting serum TGs) determinants of phylloquinone absorption and metabolism. The study was split into 3 phases (Supplemental Figure 1), the first being a 6-d run-in period to establish baseline vitamin K intake (days 0–5; 200 μg phylloquinone/d through the diet), followed by a 28-d vitamin K depletion phase (days 6–33; 10 μg/d), and then a 28-d vitamin K repletion phase (days 34–61; 510 μg/d). During the vitamin K depletion and repletion phases, a low-phylloquinone (10 µg/d) diet was consumed by participants, with a daily dietary supplement taken with breakfast providing additional phylloquinone (500 µg/d) in the repletion phase. Phylloquinone was supplemented rather than obtained through phylloquinone-rich foods to control for natural phylloquinone variation in food, as well as to control for dietary influences on absorption and utilization when comparing the depletion and repletion periods. During all phases, the diets contained identical amounts of calcium, vitamin D, phosphorus, and protein, and ∼30% of daily energy came from fat. Direct analyses of phylloquinone from aliquots of the diets were performed randomly throughout the study to confirm actual dietary phylloquinone concentrations. All other nutrients were calculated with the use of the Minnesota Nutrient Data Software (version 4.04_32). All meals were provided by the MRU and adjusted for energy intake to maintain individual body weight.
The data reported here represent secondary outcomes from the stable isotope substudy examining the absorption and excretion of 2H-phylloquinone obtained from 2H-collard greens. On days 28–30 and 56–58 (during the depletion and repletion phases, respectively), participants were housed in the MRU for a 72-h residency period. At 0 h, 2H-collard greens were administered along with a breakfast of yogurt (130 g) with orange (100 g), banana (100 g), and wheat germ (13 g), a toasted English muffin (50 g) with butter (5 g) and honey (10 g), and skim milk (25 g). The breakfast contained 14% energy from fat, and over the course of the day 30.1% of total energy was from fat [as has been previously reported (14)]. Dietary TGs were held constant by design. Meals were provided at 0800, 1200, and 1800 on all study days (corresponding to 0, 4, 10, 24, 28, 34, 48, 52, and 58 h). Plasma 2H-phylloquinone and urinary 2H-menadione were monitored over the 72-h study period. Supplements included in the parent study were withheld during these days.
Biochemical measurements
Serial blood samples were collected as follows: on days 28 and 56 (depletion and repletion phases, respectively) of the parent metabolic study, the draw at 0 h was a fasted sample on the first residency day before administration of the 2H-collard greens at breakfast. Draws at 4, 5, 6, 7, 9, 12, and 16 h were nonfasted draws. Samples obtained at 24, 48, and 72 h represent fasted samples obtained on the subsequent days of the residency period. Plasma was separated from blood by centrifugation, and ultracentrifugation was used to further isolate the TG-rich lipoprotein (TRL) fraction as previously described (12).
Unlabeled and 2H-phylloquinone were purified from plasma and TRL fractions (15), and measured simultaneously via LC-atmospheric pressure chemical ionization (APCI)/MS as described previously (11). Phylloquinone was similarly measured in 2H-collard greens. The ratio of abundance of predominant isotopomers to all labeled isotopomers in 2H-collard greens was determined, and used to correct the abundance of 2H-phylloquinone in the plasma and TRL samples, as previously described (12). Enrichment of 2H-phylloquinone in the plasma and TRL fractions is defined as 2H-phylloquinone/total phylloquinone. Standard phylloquinone solution was run daily as a control to monitor peak areas and elution times. Plasma 25-hydroxyvitamin D [25(OH)D] was quantified by radioimmunoassay (DiaSorin).
Further fractionation was conducted to examine 2H-phylloquinone distribution across all the lipoprotein fractions. However, owing to the large number of samples with nondetectable amounts of 2H-phylloquinone in individual HDL and LDL fractions (LC-APCI/MS limit of detection: 0.05 nmol/L), complete measures were only obtained from n = 20 participants and the data were therefore not considered statistically. Total cholesterol and TGs were analyzed on the AU400 Clinical Chemistry Analyzer (Beckman Coulter, Inc.) as specified in procedural inserts.
Twenty-four-hour urine collections were obtained at 24, 48, and 72 h. Total menadione in urine was measured by HPLC, as described elsewhere (16). Percentage enrichment of menadione was measured via an adapted LC-APCI/MS method, as previously described (4). All plasma and urine samples were de-identified before laboratory analyses.
Statistical analyses
Sample size calculations for the parent metabolic study were based on differences between older and younger adults in changes in plasma phylloquinone concentration from phylloquinone depletion to repletion, and have been previously reported (6).
Data are presented as geometric means ± SEMs unless otherwise indicated. Parallel analyses were conducted with the use of: 1) 2H-phylloquinone in plasma; and 2) 2H-phylloquinone in the TRL fraction only. Results from the analyses in the TRL fraction are included in Supplemental Table 1. 2H-phylloquinone in the plasma and TRL fractions was summarized by total AUC via the linear trapezoidal rule on the arithmetic means at serial time points during the 72-h absorption periods. Serum TG response over the 72-h periods was similarly summarized by AUC. Total excretion of 2H-menadione was summed from three, 24-h urine collections during the 72-h absorption periods in both the depletion and repletion phases, separately. Owing to incomplete urine collection, data from 2 participants were excluded from menadione analysis during the depletion phase (40 of 42), and 1 during the repletion phase (41 of 42).
Maximal concentration (Cmax) of 2H-phylloquinone and the corresponding time (Tmax) for each individual participant were visually identified from the data (14). Kinetic curves of the natural log of plasma 2H-phylloquinone as a function of time were individually fitted for each subject from Tmax to time of 2H-phylloquinone clearance from the plasma (defined as at or below the detection limit of 0.05 nmol/L). The β coefficient of the linear regression model was used to estimate the disappearance rate (k) (17), and half-life was calculated according to first-order kinetics, ln(2)/k. The y-intercept of the fitted curve corresponded to the predicted Cmax.
Variables with skewed distributions were ln-transformed for normality before analyses. Linear models were used to investigate univariate relations between dietary phase and the main outcomes of interest [plasma 2H-phylloquinone AUC and total (72-h) 2H-menadione excretion], and of age and sex with the main outcomes within each dietary phase. Plasma and urinary responses were then assessed via 2-factor repeated-measures ANOVA with “age” and “sex” as the between-participant factors and “dietary vitamin K depletion/repletion” as the within-participant factor. An interaction term between “age” and “sex” was also included in the model. Comparisons were adjusted for BMI and percentage body fat (Model 1), and BMI, percentage body fat, and serum TG AUC (Model 2). Although dietary fat is important for the absorption of fat-soluble vitamins such as phylloquinone, dietary fat intake was controlled by design and therefore comparisons were not adjusted for dietary TGs. Significance testing was done through the use of 2-tailed tests at an α level of 0.05. If an interaction term was significant, pairwise comparisons were conducted within the fully adjusted model (Model 2) with the use of Tukey's honestly significant difference test to account for multiple comparisons. All analysis was done in R version 3.2.3.
Results
Baseline characteristics
Baseline characteristics have been previously reported (6), but are briefly summarized here. The mean ± SEM age (in years) was 30.4 ± 3.1 for younger men (n = 9), 32.9 ± 3.0 for younger women (n = 12), 66.3 ± 2.9 for older men (n = 12), and 68.6 ± 3.6 for older women (n = 9). Body fat percentage was higher in women than in men (P = 0.005). Plasma 25-hydroxyvitamin D [25(OH)D] was higher in older adults than in younger adults (P = 0.02). BMI and baseline phylloquinone did not differ significantly by age or sex.
2H-phylloquinone in collard greens
A 120-g serving of collard greens (containing a mean ± SD of 131 ± 24 µg phylloquinone/100 g collard greens; the provided dose equaled 157 µg 2H-phylloquinone) was given at breakfast during each of the absorption residency periods during the depletion and repletion phases. 2H-phylloquinone isotopomers were similarly distributed in collard greens and in plasma (Supplemental Figure 2).
2H-phylloquinone absorption and transport
The majority of 2H-phylloquinone absorbed into plasma was found in the TRL fractions (Supplemental Figure 3, n = 20). During the depletion phase, concentrations of 2H-phylloquinone peaked in non-TRL fractions at 6 h, during which 11.3% of total 2H-phylloquinone was found in LDL and 6.2% in HDL, with the remainder in TRL. No 2H-phylloquinone was found in HDL or LDL fractions by 48 h. During the repletion phase, concentrations of 2H-phylloquinone peaked in non-TRL fractions at 6 h, during which 10.1% was found in LDL and 6.7% in HDL, with the remainder in TRL. No 2H-phylloquinone was found in HDL or LDL fractions by 24 h. It is important to note that there were no intermediate time points between 24 h and 48 h, therefore clearance of 2H-phylloquinone from the non-TRL fractions may have occurred at ∼24 h in both phases.
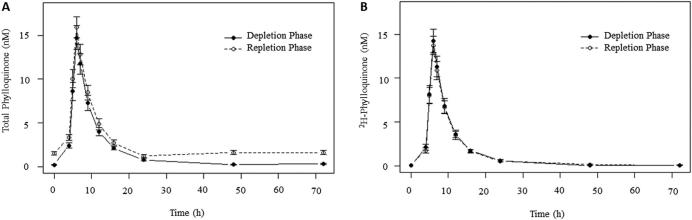
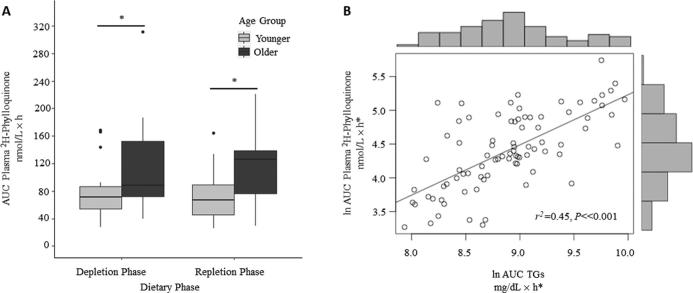
As the findings of the analyses conducted in the TRL fraction largely paralleled those conducted in plasma, all data reported here refer to plasma 2H-phylloquinone, with the analysis of 2H-phylloquinone within the TRL included in the Supplemental Table 1. Total plasma phylloquinone (unlabeled and 2H-phylloquinone) and 2H-phylloquinone over the 72-h absorption periods are shown in Figure 1. In univariate analyses, the plasma 2H-phylloquinone AUC did not differ between vitamin K depletion and repletion, but was found to be significantly higher in older adults than in younger adults during both the depletion (r2 = 0.12, P = 0.02) and repletion (r2 = 0.15, P = 0.01) periods (Figure 2A). In the repeated-measures multivariate model adjusted for BMI and percentage body fat, age remained a significant predictor of the plasma 2H-phylloquinone AUC (P = 0.01, Table 1, Model 1). Despite a constant dietary TG intake, the serum TG AUC over the 72-h absorption periods (serum TG response) was significantly higher in men than in women (P < 0.001), and in older than in younger adults (P = 0.008, Table 1). The plasma 2H-phylloquinone AUC was correlated with the serum TG response (r2 = 0.45, P < 0.001, Figure 2B). After adjustment for the TG response, the age effect on the plasma 2H-phylloquinone AUC was no longer significant (Table 1, Model 2 as compared with Model 1). The same was observed of the 2H-phylloquinone AUC in the TRL fraction (Supplementary Table 1).
FIGURE 1.
Concentration of total phylloquinone (A) and 2H-phylloquinone (B) in plasma over time by phase. Sexes within age groups are combined (n = 21 older, n = 21 younger). Points indicate arithmetic means and bars indicate 1 SEM.
FIGURE 2.
Box and whisker plot of plasma 2H-phylloquinone AUC by dietary phase and age group (A); scatterplot and corresponding histograms of 2H-phylloquinone AUC and TG AUC (B). (A) Plasma 2H-phylloquinone AUC was not significantly different by dietary phase, but was significantly higher in older adults during both the depletion phase (r2 = 0.12, P = 0.02) and repletion phase (r2 = 0.15, P = 0.01). Bold central lines represent the median for each age group; lower and upper hinges represent the first and third quartiles, respectively. Whiskers extend to the furthest point within 1.5 × IQR, and dots represent data points beyond 1.5 × IQR. Sexes within age groups are combined (n = 21 older, n = 21 younger). *P ≤ 0.02. (B) Fitted line indicates ordinary least squares regression slope (r2 = 0.45, P < 0.001, n = 42, all participants combined). *ln-transformed for normality.
TABLE 1.
Kinetic variables of 2H-phylloquinone in total plasma of older and younger adults fed 2H-collard greens1
| Depletion diet | Repletion diet | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger, 20–40 y | Older, 55–80 y | Younger, 20–40 y | Older, 55–80 y | Two-factor repeated-measures ANOVA P values | |||||||||
| Variable | Men n = 9 | Women n = 12 | Men n = 12 | Women n = 9 | Men n = 9 | Women n = 12 | Men n = 12 | Women n = 9 | Model | Phase | Sex | Age | Sex × Age |
| AUC 2H-phylloquinone, nmol/L × h | 87.1 ± 12.5 | 58.1 ± 11.1 | 101 ± 22.6 | 99.8 ± 14.9 | 69.1 ± 10.7 | 59.9 ± 11.4 | 99.8 ± 17.7 | 102 ± 15.9 | Model 1 | 0.59 | 0.11 | 0.012 | 0.32 |
| Model 2 | 0.35 | 0.12 | 0.44 | 0.90 | |||||||||
| Tmax, h | 6.11 ± 0.43 | 6.07 ± 0.54 | 6.43 ± 0.29 | 6.21 ± 0.15 | 5.94 ± 0.29 | 5.97 ± 0.17 | 6.23 ± 0.13 | 6.36 ± 0.38 | Model 1 | 0.59 | 0.22 | 0.29 | 0.94 |
| Model 2 | 0.57 | 0.60 | 0.54 | 0.88 | |||||||||
| Cmax, nmol/L | 15.1 ± 2.84 | 10.6 ± 2.37 | 14.1 ± 2.96 | 16.0 ± 3.02 | 12.7 ± 1.97 | 9.88 ± 1.59 | 16.5 ± 2.05 | 15.6 ± 2.49 | Model 1 | 0.84 | 0.16 | 0.032 | 0.23 |
| Model 2 | 0.66 | 0.38 | 0.52 | 0.71 | |||||||||
| Cmax, % | 96.2 ± 0.54 | 97.4 ± 0.41 | 96.5 ± 0.54 | 96.0 ± 1.11 | 83.5 ± 2.49 | 89.0 ± 1.63 | 86.3 ± 0.97 | 85.4 ± 3.02 | Model 1 | <0.001 | 0.01 | 0.16 | 0.09 |
| Model 2 | <0.001 | 0.01 | 0.11 | 0.07 | |||||||||
| Predicted Cmax, nmol/L | 12.4 ± 1.86 | 5.91 ± 1.07 | 12.6 ± 2.43 | 12.8 ± 2.72 | 11.7 ± 1.76 | 6.20 ± 1.35 | 12.5 ± 2.22 | 12.2 ± 1.73 | Model 1 | 0.86 | 0.002 | <0.0012 | 0.02 |
| Model 2 | 0.63 | 0.49 | 0.05 | 0.12 | |||||||||
| Disappearance rate, –ln(nmol/L)/h | 0.25 ± 0.04 | 0.18 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.03 | 0.32 ± 0.07 | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.21 ± 0.06 | Model 1 | 0.71 | 0.11 | 0.59 | 0.10 |
| Model 2 | 0.65 | 0.02 | 0.24 | 0.043 | |||||||||
| Half-life, h | 3.08 ± 0.56 | 4.16 ± 0.52 | 3.93 ± 0.40 | 3.64 ± 0.49 | 2.59 ± 0.63 | 4.14 ± 0.54 | 3.85 ± 0.55 | 3.89 ± 0.52 | Model 1 | 0.71 | 0.11 | 0.59 | 0.10 |
| Model 2 | 0.65 | 0.02 | 0.24 | 0.043 | |||||||||
| r 2 | 0.93 ± 0.02 | 0.92 ± 0.03 | 0.95 ± 0.01 | 0.95 ± 0.01 | 0.92 ± 0.02 | 0.92 ± 0.01 | 0.96 ± 0.01 | 0.80 ± 0.08 | Model 1 | 0.37 | 0.82 | 0.33 | 0.27 |
| Model 2 | 0.33 | 0.43 | 0.10 | 0.13 | |||||||||
| AUC TGs, mg/dL × h | 8380 ± 806 | 4660 ± 632 | 9370 ± 1623 | 7450 ± 1300 | 8230 ± 723 | 4980 ± 883 | 9260 ± 1680 | 7980 ± 1910 | Model 1 | 0.24 | <0.001 | 0.008 | 0.14 |
1Values are geometric means ± SEMs. Model 1 adjusts for BMI and percentage body fat. Model 2 adjusts for BMI, percentage body fat, and AUC TGs. Cmax, maximum plasma concentration of 2H-phylloquinone expressed both as 2H-phylloquinone concentration and as a percentage of total phylloquinone (2H and unlabeled); predicted Cmax, estimated concentration at Tmax derived from the y-intercept of the fitted kinetic curve; r2, correlation coefficient for the fitted curve; Tmax, time of maximal plasma concentration.
2Age effect nonsignificant after adjustment for TG response (Model 2).
3Interaction nonsignificant after pairwise comparisons with Tukey's honestly significant difference test.
2H-phylloquinone kinetics
There were no significant differences in Cmax or Tmax by age or sex, or between phylloquinone depletion and repletion. Percentage enrichment at Tmax (Cmax %) was significantly higher in the depletion phase than in the repletion phase (P < 0.001), and higher in women than in men (P = 0.01) (Table 1). A significant age × sex interaction was found in the disappearance rate (and corresponding half-life) of 2H-phylloquinone (P = 0.04) (Table 1). However, pairwise comparisons did not reach significance after adjustment (all P > 0.11). This interaction was not observed in the TRL fraction (Supplementary Table 1). Therefore, all findings were consistent between total plasma and the TRL fraction in the fully adjusted models (Model 2).
2H-menadione excretion
Complete 24-h urine collections over the 72-h absorption period were obtained from 40 of 42 participants. Total 72-h 2H-menadione urinary excretion did not vary by age, sex, or dietary vitamin K depletion or repletion (Figure 3) in the unadjusted or adjusted models (all P > 0.05).
FIGURE 3.
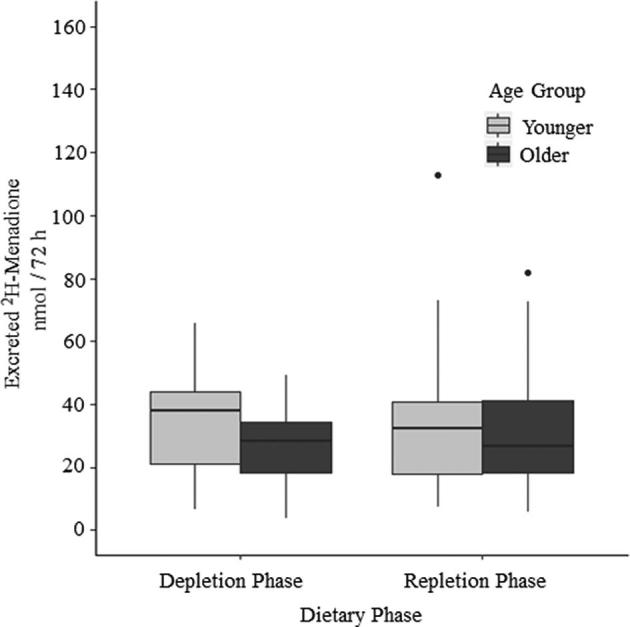
Box and whisker plot of total 2H-menadione excretion (nmol/72 h) by dietary phase and age group. Excretion of 2H-menadione in urine was not significantly different by phase or age group. Bold central lines represent the median for each age group; lower and upper hinges represent the first and third quartiles, respectively. Whiskers extend to the furthest point within 1.5 × IQR, and dots represent data points beyond 1.5 × IQR. Sexes within age groups are combined (depletion phase: n = 19 older, n = 21 younger; repletion phase: n = 20 older, n = 21 younger).
Discussion
This study demonstrated that the plasma response to dietary phylloquinone is largely driven by the serum TG response. We defined the serum TG response as the AUC of plasma TGs over the full 72-h period, including the postprandial period immediately after intake of the meal containing the 2H-collards. Whereas fasting serum TG concentrations explained 22% of the variance in 2H-phylloquinone AUC (r2 = 0.22, P < 0.001), the serum TG response accounted for 45% (r2 = 0.45, P < 0.001). Although age is a significant predictor of 2H-phylloquinone AUC in univariate analyses, after adjustment for the TG response (which varied despite controlled dietary fat intake) no age effect was observed. Dietary phylloquinone status, sex, BMI, and percentage body fat did not predict plasma 2H-phylloquinone AUC, and urinary 2H-menadione excretion did not vary by any of the factors explored.
The prior 23 d of vitamin K intake, be it low (i.e., 23 d at 10 µg phylloquinone/d) or high (23 d at 500 µg phylloquinone/d), did not influence the plasma response to a bolus of 2H-phylloquinone. For a lipid-soluble vitamin, this finding was unexpected. In the parent study, fasting plasma phylloquinone concentrations and the biomarkers of vitamin K function percentage uncarboxylated osteocalcin and Protein Induced by Vitamin Absence or Antagonist-II declined and then increased in response to the manipulation of dietary vitamin K over the 28-d depletion and repletion phases, respectively (6). This is in agreement with observational studies that report plasma vitamin K concentrations are significantly predicted by dietary intake (18). The results presented here expand upon the findings of the parent study. In response to intake of the same amount of 2H-phylloquinone in the form of collards, there was no difference in the response of plasma 2H-phylloquinone between the vitamin K depletion and repletion phases. Similarly, there was no difference in the excretion response as measured by urinary 2H-menadione. Collectively, these data suggest little to no regulation of phylloquinone absorption or excretion.
Consistent with the results presented here, studies examining vitamin K in both men and women have reported no significant sex effect (6, 9, 19). In the current study, although age appeared to be a significant predictor of the plasma 2H-phylloquinone AUC, the difference between age groups was explained primarily by the greater simultaneous TG response in older adults. In a hypothesis-generating analysis of vitamin E in the current study, Traber et al. (14) described a similar finding, in which plasma α-tocopherol concentrations did not vary by age or sex, but were instead related to circulating lipids. We previously reported that plasma concentrations of phylloquinone were significantly higher in older adults than in younger adults at both 0 h and 24 h after phylloquinone administration, even after adjustment for TGs (19). However, when the plasma phylloquinone response over the 24-h period was summarized by AUC and adjusted for TGs, no age effect remained. These results are consistent with the results presented here, and also suggest that analysis of the TG response over time (including the postprandial period) is more informative than individual time points.
Age effects in the excretion of vitamin K urinary metabolites have also been suggested. Younger adults had reduced excretion of γ-carboxyglutamic acid during dietary vitamin K depletion, whereas excretion was unchanged in the older participants (9). Harrington et al. (20) also reported on the responsiveness of excretion of 5-carbon and 7-carbon metabolites to manipulation of dietary phylloquinone in younger adults. The published results of the parent study report a significantly lower excretion of urinary menadione in younger adults than in older adults during the depletion phase, and a greater change in excretion between phylloquinone depletion and repletion (6). Unfortunately, no assay currently exists that can distinguish 2H-labeled from total 5-carbon and 7-carbon metabolites, and thus these metabolites could not be analyzed in the substudy reported here. Although no differences in excretion of 2H-menadione by age or phase were observed, 2H-menadione was still present in the urine at the end of collections for the substudy and therefore incomplete capture may have influenced this analysis. These collective findings are not conclusive but are suggestive. Reduced excretion of vitamin K metabolites in younger adults in states of vitamin K depletion suggests there may be regulation or recycling of vitamin K when intake is low. Unchanged excretion from baseline in older adults with depletion (or higher excretion as compared with younger adults) may be indicative of age-related blunting of these processes. Although these results may also be indicative of underlying renal function, all of the aforementioned studies recruited healthy participants. Therefore, although there do not appear to be differences in phylloquinone absorption from the diet, there may be an association between altered vitamin K metabolism and age.
This study has several strengths. The use of a stable isotope enabled the parsing apart of a dose of 2H-phylloquinone (single intake) from total circulating levels (in which fasting concentrations are reflective of longer-term nutritional status). In addition, the administration of the 2H-phylloquinone in the form of 2H-collard greens allowed its study at a relevant dietary level and under normal physiologic conditions, including digestion from a food matrix (3). This investigation builds upon previous studies that utilized deuterium- or 13C-labeled vegetables to study phylloquinone absorption, kinetics, and transport (12, 13, 21, 22), but was the first to study under controlled, successive vitamin K depletion and repletion conditions. The ∼2- to 4-h half-life of phylloquinone reported here is in agreement with the findings of several previous studies (21, 23, 24).
There are also several limitations to this study. Because the most rapid increase in the circulating deuterium label occurred in the hours immediately after administration of the 2H-collard greens (peaking at Tmax ∼6 h, Table 1), it is assumed that the lipid-driven effect noted in the experiment is primarily driven by the TGs transported in chylomicrons, reflecting differences in dietary lipid absorption and metabolism despite controlled dietary fat intake. However, this was not determined in this study. The individual lipid measures contributing to the TG AUC response over the 72-h absorption periods were obtained at both fasted (0, 24, 48, and 72 h) and nonfasted time points. Therefore, the respective contributions of chylomicrons and VLDL to the TRL fraction were differential by time point, and the influences of dietary and endogenous lipids were not parsed apart. This distinction will be the focus of future studies. Although about half of the variance in plasma 2H-phylloquinone AUC was explained solely by serum TG AUC (r2 = 0.45), the remaining variance was largely unexplained by the other factors (dietary status, age, sex, BMI, and percentage body fat) examined here. The participants in this study were primarily Caucasian, although race and ethnicity have been shown to be a predictor of vitamin K status (25). Although BMI and percentage body fat were not significant predictors of plasma 2H-phylloquinone response in this study, most participants were normal weight or overweight. There may not have been an adequate range of body composition in our study to observe these effects, or a relation may only exist in a state of obesity. There may exist genetic differences in vitamin K metabolism that were not considered here, indirectly suggested by evidence from studies on warfarin, a vitamin K antagonist. Polymorphisms in VKORC1 (vitamin K epoxide reductase complex 1 gene) have been consistently demonstrated to be associated with warfarin dose requirements (26).
In conclusion, plasma response to dietary phylloquinone is a predominantly lipid-driven effect. There may be an association of altered vitamin K metabolism with age, but understanding of factors influencing the physiologic response to dietary vitamin K and more complete measures to capture tissue uptake and metabolism are needed for further interpretation.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SLB and MJS: conceptualized and designed the clinical trial; SLB, XF, AAR, and ES: conducted the research; MAG: provided essential materials; JLE: performed statistical data analysis and wrote the manuscript; ENN and KB: provided statistical support; SLB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, NIH grant 5R01DK69341 (to SLB), and NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant T32 DK062032.
Author disclosures: JLE, XF, AAR, MAG, MJS, ENN, ES, KB, and SLB, no conflicts of interest.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Supplemental Figures 1–3 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Present address for AAR: Alberta Health Services, Calgary, Canada. Present address for MAG: USDA/Agricultural Research Service Red River Valley Agricultural Research Center, Fargo, ND.
Abbreviations used: APCI, atmospheric pressure chemical ionization; Cmax, maximum plasma concentration; MRU, Metabolic Research Unit; Tmax, time of maximum plasma concentration; TRL, TG-rich lipoprotein.
References
- 1. Suttie JW. Synthesis of vitamin K-dependent proteins. FASEB J 1993;7:445–52. [DOI] [PubMed] [Google Scholar]
- 2. Booth SL. Roles for Vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89–110. [DOI] [PubMed] [Google Scholar]
- 3. Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 2012;3:182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr 2012;142:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008;283:11270–9. [DOI] [PubMed] [Google Scholar]
- 6. Truong JT, Fu X, Saltzman E, Al Rajabi A, Dallal GE, Gundberg CM, Booth SL. Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J Nutr 2012;142:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadowski JA, Hood SJ, Dallal GE, Garry PJ. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. Am J Clin Nutr 1989;50:100–8. [DOI] [PubMed] [Google Scholar]
- 8. Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr 1996;63(4):566–73. [DOI] [PubMed] [Google Scholar]
- 9. Ferland G, Sadowski JA, O'Brien ME. Dietary induced subclinical vitamin K deficiency in normal human subjects. J Clin Invest 1993;91:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gnocchi D, Pedrelli M, Hurt-Camejo E, Parini P. Lipids around the clock: focus on circadian rhythms and lipid metabolism. Biology (Basel) 2015;4:104–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem 2009;81:5421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erkkilä AT, Lichtenstein AH, Dolnikowski GG, Grusak MA, Jalbert SM, Aquino KA, Peterson JW, Booth SL. Plasma transport of vitamin K in men using deuterium-labeled collard greens. Metabolism 2004;53:215–21. [DOI] [PubMed] [Google Scholar]
- 13. Dolnikowski GG, Sun Z, Grusak MA, Peterson JW, Booth SL. HPLC and GC/MS determination of deuterated vitamin K (phylloquinone) in human serum after ingestion of deuterium-labeled broccoli. J Nutr Biochem 2002;13:168–74. [DOI] [PubMed] [Google Scholar]
- 14. Traber MG, Leonard SW, Bobe G, Fu X, Saltzman E, Grusak MA, Booth SL. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr 2015;101:752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 16. Al Rajabi A, Peterson J, Choi S-W, Suttie J, Barakat S, Booth SL. Measurement of menadione in urine by HPLC. J Chromatogr B 2010;878:2457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leonard SW, Good CK, Gugger ET, Traber MG. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am J Clin Nutr 2004;79:86–92. [DOI] [PubMed] [Google Scholar]
- 18. Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE, Sadowski JA. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr 1997;127:587–92. [DOI] [PubMed] [Google Scholar]
- 19. Booth SL, Lichtenstein AH, Dallal GE. Phylloquinone absorption from phylloquinone-fortified oil is greater than from a vegetable in younger and older men and women. J Nutr 2002;132:2609–12. [DOI] [PubMed] [Google Scholar]
- 20. Harrington DJ, Booth SL, Card DJ, Shearer MJ. Excretion of the urinary 5C- and 7C-aglycone metabolites of vitamin K by young adults responds to changes in dietary phylloquinone and dihydrophylloquinone intakes. J Nutr 2007;137:1763–8. [DOI] [PubMed] [Google Scholar]
- 21. Jones KS, Bluck LJC, Wang LY, Coward WA. A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. Eur J Clin Nutr 2008;62:1273–81. [DOI] [PubMed] [Google Scholar]
- 22. Novotny JA, Kurilich AC, Britz SJ, Baer DJ, Clevidence BA. Vitamin K absorption and kinetics in human subjects after consumption of 13C-labelled phylloquinone from kale. Br J Nutr 2010;104:858–62. [DOI] [PubMed] [Google Scholar]
- 23. Shearer MJ, Mallinson CN, Webster GR, Barkhan P. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K 1 in man. Br J Haematol 1972;22:579–88. [DOI] [PubMed] [Google Scholar]
- 24. Bjornsson TD, Meffin PJ, Swezey SE, Blaschke F. Effects of clofibrate and warfarin alone and in combination on the disposition of vitamin K1. J Pharmacol Exp Ther 1979;210:322–6. [PubMed] [Google Scholar]
- 25. Shea MK, Booth SL, Nettleton JA, Burke GL, Chen H, Kritchevsky SB. Circulating phylloquinone concentrations of adults in the United States differ according to race and ethnicity. J Nutr 2012;142:1060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Limdi NA, Veenstra DL. Warfarin pharmacogenetics. Pharmacotherapy 2008;28:1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.