ABSTRACT
Background
Interventions are needed to address iron deficiency in low-income settings.
Objective
This secondary outcome analysis aimed to compare the hemoglobin (Hb) and iron status [zinc protoporphyrin (ZPP)] of children born to women enrolled in the iLiNS-DYAD trial in Ghana.
Methods
Women ≤20 wk pregnant (n = 1320) were assigned to receive 60 mg Fe/d and 400 µg folic acid/d until delivery and placebo thereafter, and no supplementation for infants (IFA group); or multiple micronutrients containing 20 mg Fe/d until 6 mo postpartum and no supplementation for infants (MMN); or small-quantity lipid-based nutrient supplements (SQ-LNSs) containing 20 mg Fe/d until 6 mo postpartum, and SQ-LNSs for infants from 6 to 18 mo of age (LNS). We compared infants’ Hb (g/L) and ZPP (µmol/mol heme) at 6 and 18 mo of age.
Results
At 6 mo of age, groups did not differ in mean ± SD Hb (overall: 113 ± 9.9 g/L) or geometric mean (95% CI) ZPP [overall: 62.6 (60.6, 64.7)]. At 18 mo of age, mean ± SD Hb (overall: 112 ± 10.4 g/L) did not differ significantly between groups, whereas geometric mean (95% CI) ZPP was lower (P = 0.031) in the LNS group [53.9 (50.7, 57.3)] than the IFA [60.4 (56.7, 64.3)] but not the MMN [58.8 (55.6, 62.2)] group. Further, the LNS group, compared with the IFA and MMN groups combined, had a lower prevalence of elevated (>70) ZPP (27.5% compared with 35%; P = 0.02) and a marginally lower prevalence of anemia (38.7% compared with 44.9%; P = 0.06). These results generally remained unchanged when controlling for prespecified covariates or correcting for inflammation.
Conclusions
In this setting, providing SQ-LNSs or multiple micronutrients with 20 mg Fe/d, compared with iron (60 mg/d) and folic acid, to pregnant women does not affect their infants’ Hb or iron status at 6 mo of age, but maternal and infant supplementation with SQ-LNSs increases infants’ iron status at 18 mo of age.
This trial was registered at clinicaltrials.gov as NCT00970866.
Keywords: micronutrient supplementation, iron and folic acid, multiple micronutrient supplements, small-quantity lipid-based nutrient supplements, infants
Introduction
Inadequate micronutrient intake is common in low-income countries (1) and has been associated with increased risk of perinatal morbidity and mortality (2), low birth weight (3), and poor child growth and development (4). Consequently, addressing the micronutrient needs of women and children especially during the “first 1000 d” is a global priority (5–7).
As part of the International Lipid-based Nutrient Supplements (iLiNS) Project, we developed small-quantity lipid-based nutrient supplements (SQ-LNSs), which can be used to enrich home-prepared foods for women and children (8), and thereby increase the intakes of essential nutrients without causing substantial changes in usual dietary practices. During the last several years, similar or different formulations of SQ-LNS products (9) have been used in many intervention trials and programs worldwide (10).
In the iLiNS-DYAD randomized, controlled supplementation trial in Ghana, we evaluated the efficacy of SQ-LNSs given to women during pregnancy and the first 6 mo postpartum, and to their offspring from 6 to 18 mo of age, based on evidence that child malnutrition in developing countries often begins in utero and continues after birth (11). We previously reported the primary growth outcomes (12, 13) as well as several other secondary outcomes (14–18) of the trial and showed that maternal–infant supplementation with SQ-LNSs, compared with maternal-only supplementation with iron and folic acid or multiple micronutrients, promoted infant and child growth in our trial setting.
In West Africa, an estimated 80% of children <5 y of age are anemic (19), up to one-half of which are estimated to be due to iron deficiency (20). Possible consequences of iron deficiency anemia (IDA) include cognitive impairment in children (21) and lower school performance (22). In this present analysis of secondary outcomes of the trial, we aimed to determine the impact of the iLiNS-DYAD intervention on children's blood hemoglobin (Hb) and iron status and inflammation biomarkers at 6 and 18 mo of age.
Methods
Study setting, design, and participants
The iLiNS-DYAD Ghana trial (NCT00970866) has been described in detail previously (12–14). Briefly, the trial was conducted in the Somanya–Odumasi–Kpong area, a semiurban setting ∼70 km north of Accra, and was designed as a partially double-blind, individually randomized, controlled trial with 3 equal-size groups. Women ≥18 y old and ≤20 wk pregnant identified from antenatal clinics were recruited after obtaining informed consent for themselves and for their infants upon delivery. Exclusion criteria were: not considered as a resident of the area, intention to move out of the area, milk or peanut allergy, participation in another trial, HIV infection, asthma, epilepsy, tuberculosis, any malignancy, or unwillingness to receive fieldworkers or take the study supplement.
The trial was approved by 3 ethics committees (University of California, Davis; Ghana Health Service; and Noguchi Memorial Institute for Medical Research) and monitored by a Data and Safety Monitoring Board.
Group assignments and blinding
As reported previously (12–14), recruited women who remained eligible were, after baseline assessments, randomly assigned to consume 60 mg Fe + 400 µg folic acid/d (IFA supplement or group), multiple micronutrients containing 18 vitamins and minerals (MMNs supplement or group), or 20 g SQ-LNS/d (LNS group) during pregnancy. In the first 6 mo postpartum, women in the IFA group were assigned 200 mg Ca/d as placebo, and those in the MMN and LNS groups assigned the same supplements as during pregnancy. From 6 to 18 mo of age, infants born to women in the IFA and MMN groups were assigned to receive no micronutrient supplementation, whereas those of women in the LNS group were assigned to consume SQ-LNSs designed for infants. The IFA and MMN supplements were provided as capsules in blister packs of 10, whereas the SQ-LNS for women was in 20-g sachets, and that for infants in 10-g sachets (given 2/d).
The following individuals completed the group assignments (12, 13, 17): 1) the Study Statistician at UC Davis, Janet M Peerson, developed the allocations in blocks of 9 (SAS for Windows version 9.4); 2) someone at the University of Ghana not involved in the recruitment of subjects prepared the envelopes containing the assignments, which were numbered and stacked by block number; and 3) the Study Nurse at the field site performed the random assignment. At each enrollment, the Study Nurse shuffled 9 envelopes taken from the top of the stack and asked the participant to make a pick to reveal the assignment. The Nurse then returned the unused envelopes to the top of the stack. When there were <9 women left to be enrolled, the Nurse shuffled whatever number of envelopes remained. Any allocation information was kept securely by the Field Supervisor in Ghana and the Study Statistician at UC Davis only.
At enrollment, the Study Nurse gave women a 2-wk supply of supplements, advice to take 1 capsule/d with water after a meal, or one 20-g SQ-LNS sachet/d mixed with food, and a standard nutrition message about the need to “eat meat, fish, eggs, fruits, and vegetables” whenever possible (12, 13). The IFA and MMN capsules were known to the study team and participants only by their color codes (3 colors for IFA and 3 for MMN). It was not possible to blind study workers and participants to the capsules and the LNSs owing to their apparent differences, but laboratory staff and data analysts had no knowledge of the group assignments.
Follow-up
During pregnancy, fieldworkers visited women in their homes biweekly to deliver supplements. All live-born singleton infants delivered by the women were enrolled into the study. In cases of multiple births, only 1 infant was selected randomly, and the selected infant was enrolled if he or she was born alive. After women gave birth, fieldworkers visited them and their infants weekly, but delivered the women's supplements or placebo biweekly as before.
Women exited the study at 6 mo postpartum, but the weekly visits for the infants continued. At 6 mo of age, the Study Nurse delivered the “minimum message” on complementary feeding to all mothers at the laboratory after the infants’ first laboratory assessment: “Breastfeed your baby as you did before 6 mo of age; do not forget to feed your baby meat, fish, eggs, fruits and vegetables whenever you can.” During the next usual weekly home visit, fieldworkers delivered the first supply of SQ-LNSs for infants in the LNS group, advised those mothers or caregivers on how to feed the supplements (i.e., mix the entire content of 1 sachet with 2–3 tablespoons of food for the infant before feeding additional foods if the infant desires, 2 times/d), and repeated the “minimum message” on complementary feeding given by the Study Nurse. For infants in the IFA and MMN groups who were not assigned to any supplements, fieldworkers also repeated the “minimum message” to their mothers or caregivers. Fieldworkers delivered a fresh supply of infants’ SQ-LNSs during the usual weekly visits, but the “minimum message” was not repeated again thereafter. Infants exited the study at 18 mo of age.
Trial supplements
As previously reported (8, 12–14, 16), the IFA reflected the Ghana Health Service's standard micronutrient supplementation for pregnant women in Ghana at the time of the study (23). The daily dose of MMN contained 18 vitamins and minerals at 1 or 2 times the RDA for pregnancy, except iron. For iron, we used 20 mg/d instead of the 30 mg/d in the UNICEF/WHO/United Nations University International Multiple Micronutrient Preparation (UNIMMAP) formulation (24) or the 30–60 mg/d in the WHO guideline (23), because of previous evidence (25) showing that 20 mg Fe/d was just as effective as 40 mg/d or 80 mg/d in treating anemia during pregnancy and was likely to cause fewer gastrointestinal side effects compared with a 30–60 mg/d dosage. The SQ-LNS for women had similar micronutrient contents to the MMN, plus energy, protein, and essential fatty acids as well as the maximum amounts of calcium, magnesium, phosphorus, and potassium that could be included given technical and organoleptic constraints. Including 20 mg Fe/d in the SQ-LNS for women made it possible to have just one product for both pregnancy and lactation, assuming that this amount, in addition to iron from the usual diet, would give a total daily intake that was close to the amount in the UNIMMAP formulation (24) and would therefore meet the RDA of 27 mg Fe for pregnancy, while at the same time not greatly exceeding the RDA (9 mg/d) for lactation (8).
The SQ-LNS for infants was designed to generally supply the WHO/FAO Recommended Nutrient Intake for key micronutrients for infants 7–12 mo of age, with a few exceptions (8). We used an iron dosage (6 mg/d) that was approximately the WHO/FAO Recommended Nutrient Intake for infants 7–12 mo of age when assuming high bioavailability (6.2 mg/d), and 33% lower than the dosage previously used in Ghana (26), because of concerns about increasing the risk of malaria and infections in an area of high malaria endemicity (27). Zinc content (8 mg/d) was kept higher (8, 28) than the 4 mg/d used in our previous study (26) because zinc absorption may be decreased in the study area's predominantly maize-based diet high in phytate.
Data collection
Women's baseline assessments included sociodemographic characteristics; gestational age (by using ultrasound biometry, Aloka SSD 500); anthropometric status [by using standard procedures (29)]; venous blood Hb concentration (HemoCue Hb301; Hemocue AG); zinc protoporphyrin concentration (ZPP) (Hematofluorometer; Aviv Biomedical Co.); and peripheral malaria parasitemia (Vision Biotech) (13). Hb concentration was measured within 2 min after the blood draw. We used the original Aviv cover-slides and 3-level control material for the ZPP measurements, after red blood cells were washed 3 times with normal saline. Women's supplement intakes were monitored biweekly by collecting unused blister packs or SQ-LNS sachets at each visit. In addition, during the biweekly interviews, women were asked on how many days since the last visit they had consumed the supplements, reasons (if any) they did not consume the supplements, and, for those in the LNS group, whether someone else had consumed some of the supplements. Any discrepancies between the responses to these questions and the number of capsules or sachets remaining since the last visit were resolved during the interview.
For infants in the LNS group, supplement intakes were monitored weekly by training mothers to use a calendar grid to indicate, on a daily basis, whether infants consumed the supplement. Calendar grids were checked at each weekly visit. Mothers unable to use the calendar grid were asked to recall the children's supplement intakes for each day since the last visit. In addition, fieldworkers collected any unused SQ-LNS sachets at each visit, and reconciled the number of sachets remaining since the last visit with the consumption information recorded on the calendar grid or provided by the mother. At 6 and 18 mo of age, infants were brought to the laboratory, where we collected venous blood, and measured Hb concentration within 2 min, as well as measuring ZPP concentrations and peripheral malaria parasitemia for all infants using the same techniques used for the women. The infants’ plasma samples obtained after centrifuging the blood at 1252 × g for 15 min at a room temperature of ∼23°C were stored in Ghana at −33°C, before being air-freighted on dry ice to the USDA Western Human Nutrition Research Center (WHNRC) in Davis, CA, where we determined the C-reactive protein (CRP) and α-1 glycoprotein (AGP) concentrations in a randomly selected subsample of infants by using a Cobas Integra 400 plus Automatic Analyzer (Roche Diagnostic Corp.).
The secondary outcomes evaluated herein were infants’ Hb (grams per liter), ZPP (micromoles per mole of heme), CRP (milligrams per liter), and AGP (grams per liter) concentrations at 6 and 18 mo of age, and the percentages of infants with anemia (indicated by low Hb), iron deficiency (indicated by elevated ZPP), and IDA (low Hb plus elevated ZPP) at 6 and 18 mo of age.
Sample size and data analysis
As described previously (12, 13), the sample size for the iLiNS-DYAD-Ghana study was based on detecting an effect size or Cohen's d (30) of 0.3 between any 2 groups for any continuous variables, with a 2-sided 5% test and 80% power. We previously reported the temporary mislabeling of some IFA and MMN supplements (12, 13), as a result of which 170 women in the IFA group inadvertently received MMN capsules either throughout (n = 85) or during part of (n = 85) their pregnancy before receiving the intended IFA capsules, and 170 women in the MMN group received IFA capsules either throughout (n = 78) or during part of (n = 92) their pregnancy before receiving the intended MMN capsules. In total, 1320 women were enrolled.
This present analysis includes all of the children for whom data were available, consistent with our analysis of the primary growth outcomes (12). For CRP and AGP analyses (as well as several other biochemical outcomes in the trial), however, the target sample size was based on detecting an effect size of ≥0.5 between any 2 groups, with a 2-sided 5% test and 80% power. This required 105 subjects/group, or 315 subjects for the 3 groups, after taking into account ≤25% attrition. The subsample for the CRP and AGP analyses was selected from the children whose mothers had not been pregnant during the period when the temporary mislabeling occurred.
The analysis reported herein was part of the iLiNS-DYAD-Ghana statistical analysis plan, which we posted at our website (www.ilins.org) before data analysis began. Both the statistical analysis plan and the trial protocol at clinicaltrials.org listed children's blood Hb and iron status as secondary outcomes to be analyzed separately from the primary outcomes. We used Hb <100 g/L to define anemia in women (14, 31, 32). For children, we defined anemia by using the cutoff of Hb <110 g/L according to the WHO (32, 33), as well as by the cutoff values of Hb <105 g/L for 6 mo of age described by Domellöf et al. (34), and Hb <100 g/L for 18 mo of age (31) described by the International Nutritional Anemia Consultative Group (INACG), because of concerns that the WHO cutoff may be set too high. We defined elevated ZPP as ZPP >70 µmol/mol heme (35–37); elevated CRP as CRP >5.0 mg/L (38); and elevated AGP as AGP >1.0 g/L (38). Infants’ Hb values were considered to be normally distributed, but the ZPP, CRP, and AGP values (Shapiro–Wilk W <0.95 in all cases) were ln-transformed before analysis.
We analyzed data on an intention-to-treat basis (by including children regardless of adherence to treatment), using SAS for Windows version 9.4 (SAS Institute). Background maternal and household variables were summarized by intervention groups based on supplements women were intended to receive when enrolled. Because of the protocol violation associated with the consumption of mislabeled IFA or MMN capsules, we analyzed our data by using 2 scenarios, as done previously (12): in the first, intervention groups were based on the supplements women were intended to receive when enrolled; in the second, groups were based on the supplements women actually received when enrolled. In addition, we performed a secondary analysis by using a 2-group comparison in which the IFA and MMN groups were combined and compared with the LNS group (12).
We analyzed continuous and binary outcomes at 6 and 18 mo of age, by using linear and logistic regression models (SAS, PROC GLIMMIX), with Tukey–Kramer adjustment for multiple comparisons. We compared group means ± SDs (or SEs) or geometric means (95% CIs) for continuous outcome measures, and group percentages (95% CIs) for binary outcomes. Along with group comparisons, we estimated contrasts between groups (effect size) and their 95% CIs (39), including difference in means and ratio of log-transformed means for continuous outcomes, and RRs (40) for binary outcomes.
Both unadjusted and adjusted analyses were performed. In the adjusted analyses, only prespecified potential covariates (maternal age, height, BMI, education, gestational age at enrollment, primiparity, season at enrollment, baseline anemia status, and proxy indicators for household socioeconomic status and child sex) significantly associated with the outcome at α = 0.1 in bivariate analyses were included in the final models. For binary outcomes, the adjusted percentages were generated by using the technique described by Kleinman and Norton (41).
In the subsample of children for whom there were CRP and AGP data, we performed a sensitivity analysis as done previously (14), by correcting for the effect of inflammation (CRP and AGP) on the Hb and iron status outcomes (38) and repeating the above analyses. We used 3 inflammation categories, namely: reference (normal CRP and AGP), incubation (raised CRP and normal AGP), and early (raised CRP and AGP) or late (normal CRP and raised AGP) convalescence, to calculate the corrected Hb and ZPP values (44).
We have presented statistics in the text as means ± SDs or geometric means (95% CIs) for continuous outcomes, and percentages for binary outcomes. As we published previously, the self-reported adherence to supplement intake for women (pregnancy/lactation) was 88.1%/85.7% for the IFA group, 87.0%/85.0% for the MMN group, and 83.7%/80.0% for the LNS group (45), and that for the infants in the LNS group was 73.5% (12).
Results
Data were collected between December 2009 and March 2014. In total, 1320 women were enrolled. At baseline, the women were ∼27 y of age on average, had ∼8 y of formal education, and were at ∼16 weeks of gestation (Table 1); 10% tested positive in the rapid diagnostic test for malaria, and 14% had Hb <100 g/L.
TABLE 1.
Characteristics of women (n = 1320) enrolled in the iLiNS-DYAD-Ghana nutrient supplementation trial, by group according to the supplements women were intended to receive when enrolled1
| Background characteristics | IFA (n = 441) | MMN (n = 439) | LNS (n = 440) |
|---|---|---|---|
| Age, y | 27 ± 5 (441) | 27 ± 6 (439) | 27 ± 6 (440) |
| Formal education, y | 8 ± 4 (441) | 8 ± 4 (439) | 8 ± 4 (440) |
| Gestational age at enrollment, wk | 16.2 ± 3.3 (438) | 16.0 ± 3.2 (438) | 16.1 ± 3.3 (435) |
| Asset index2 | 0.05 ± 1.01 (433) | 0.05 ± 0.99 (431) | –0.09 ± 1.00 (432) |
| Housing index2 | 0.05 ± 0.98 (433) | –0.03 ± 1.02 (431) | –0.01 ± 1.00 (432) |
| HFIAS score3 | 2.8 ± 4.6 (436) | 2.4 ± 4.1 (429) | 2.6 ± 4.0 (432) |
| Married or cohabiting, n/N (%) | 406/441 (92.1) | 413/439 (94.1) | 405/440 (92.0) |
| Primiparous women, n/N (%) | 162/441 (36.7) | 137/439 (31.2) | 147/440 (33.4) |
| Positive for malaria at baseline,4n/N (%) | 40/441 (9.1) | 39/438 (8.9) | 54/440 (12.3) |
| Positive for malaria at 36 wk,4n/N (%) | 30/348 (8.6) | 34/362 (9.4) | 38/336 (11.3) |
| Anemic at baseline,5n/N (%) | 55/441 (12.5) | 70/438 (16.0) | 60/440 (13.6) |
| Anemic at 36 wk,5n/N (%) | 13/349 (3.7) | 23/362 (6.4) | 27/338 (8.0) |
Values are means ± SDs (N) or n/N (%). HFIAS, Household Food Insecurity Access Scale; IFA, Iron and Folic Acid group randomly assigned to receive 60 mg Fe/d and 400 mg folic acid/d during pregnancy and 200 mg Ca/d as placebo during the first 6 mo postpartum; iLiNS, International Lipid-based Nutrient Supplements; LNS, Lipid-based Nutrient Supplement group randomly assigned to receive 20 g small-quantity Lipid-based Nutrient Supplements/d with the same micronutrients as the MMN group, plus 4 more minerals (calcium, phosphorus, potassium, and magnesium) and macronutrients until 6 mo postpartum; MMN, Multiple Micronutrient group randomly assigned to receive 18 vitamins and minerals, including 20 mg Fe, daily until 6 mo postpartum; n, number of participants identified as “yes” for the variable in question; N, total number of participants in the group in question.
Proxy indicators for household socioeconomic status; higher values represent higher socioeconomic status.
HFIAS score is a proxy indicator for household food insecurity (42); higher values represent higher food insecurity.
Rapid diagnostic test (Clearview Malarial Combo; Vision Biotech).
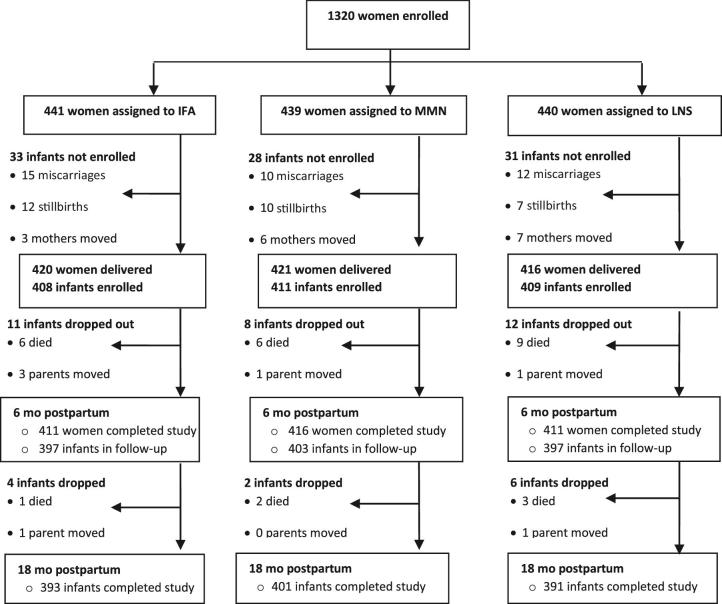
We enrolled 1228 infants at birth out of 1257 deliveries; infants from 29 deliveries who were stillborn could not be enrolled (Figure 1). In all, 1197 infants attended the laboratory visit at 6 mo of age, and 1185 of the 1228 enrolled completed the study at 18 mo of age. The reasons some infants did not complete the study were death (n = 27), parental refusal (n = 9), and relocation from the study site (n = 7). Only 1.4% of infants at 6 mo of age and 1.1% at 18 mo of age had positive results in the rapid diagnostic test for malaria; these results did not differ by group at either of the 2 time points.
FIGURE 1.
Study profile showing infants whose mothers were enrolled into the trial, and the reasons some infants were lost to follow-up. IFA, Iron and Folic Acid group: infants were assigned to receive no supplements, their mothers were assigned to receive 60 mg Fe/d and 400 µg folic acid/d during pregnancy and 200 mg Ca/d as placebo during the first 6 mo postpartum; LNS, Lipid-based Nutrient Supplement group: infants were assigned to receive 20 g Lipid-based Nutrient Supplement/d (designed for infants) containing 6 mg Fe/d from 6 to 18 mo of age, their mothers received 20 g Lipid-based Nutrient Supplement/d (designed for women) with the same micronutrients as the MMN group during pregnancy and the first 6 mo postpartum—both Lipid-based Nutrient Supplement products contained 4 additional minerals (calcium, phosphorus, potassium, and magnesium) as well as macronutrients; MMN, Multiple Micronutrient group: infants were assigned to receive no supplements, their mothers were assigned to receive a multiple micronutrient capsule containing 18 vitamins and minerals, including 20 mg Fe, daily during pregnancy and the first 6 mo postpartum.
Overall, the children's mean ± SD Hb concentration was 113 ± 9.9 g/L at 6 mo of age and 112 ± 10.4 g/L at 18 mo of age, whereas the geometric mean (95% CI) ZPP concentration was 62.6 µmol/mol heme (60.6, 64.7 µmol/mol heme) at 6 mo of age and 57.6 µmol/mol heme (55.7, 59.7 µmol/mol heme) at 18 mo of age. When using the WHO cutoff (32, 33), the prevalence of anemia was 35.3% at 6 mo of age and 42.9% at 18 mo of age, whereas the prevalence of IDA was 19.1% at 6 mo of age and 21.0% at 18 mo of age. When using the cutoffs described by Domellöf et al. (34) and the INACG (31), however, the overall prevalences of anemia (18.6% at 6 mo of age; 5.2% at 18 mo of age) and IDA (11.9% at 6 mo of age; 3.9% at 18 mo of age) were much lower.
Group comparisons
In the unadjusted analyses of continuous outcomes (Table 2), mean Hb and geometric mean ZPP concentrations at 6 mo of age did not differ by intervention group, but geometric mean CRP and AGP were significantly lower in the IFA and LNS groups than in the MMN group. At 18 mo of age, mean Hb and geometric mean CRP and AGP concentrations did not differ by intervention group, but geometric mean ZPP concentration was lower in the LNS group compared with the IFA, but not the MMN group, whereas the IFA and MMN groups did not differ in any of these outcomes. In the secondary analysis in which the IFA and MMN groups were combined (Supplemental Table 1), geometric mean (95% CI) ZPP concentration at 18 mo of age was significantly lower (P = 0.008) in the LNS group [53.9 µmol/mol heme (50.7, 57.3 µmol/mol heme)] than in the IFA + MMN group [59.6 µmol/mol heme (57.1, 62.1 µmol/mol heme)].
TABLE 2.
Unadjusted continuous outcome measures (hemoglobin and biomarkers of iron status and inflammation) for infants in the iLiNS-DYAD randomized trial of daily nutrient supplementation in a semiurban setting in Ghana, by intervention group1
| Intervention group based on supplements mothers were intended to receive when enrolled | Intervention group based on supplements mothers actually received when enrolled | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome variable | IFA (n = 441) | MMN (n = 439) | LNS (n = 440) | P 2 | IFA (n = 441) | MMN (n = 439) | LNS (n = 440) | P 2 |
| Hemoglobin, g/L | ||||||||
| 6 mo | 113 ± 11 (310) | 113 ± 10 (325) | 114 ± 10 (313) | 0.72 | 114 ± 10 (308) | 112 ± 10 (327) | 114 ± 10 (313) | 0.17 |
| 18 mo | 112 ± 11 (333) | 112 ± 11 (328) | 113 ± 10 (328) | 0.21 | 112 ± 11 (321) | 112 ± 10 (340) | 113 ± 10 (328) | 0.19 |
| ZPP, µmol/mol heme | ||||||||
| 6 mo | 62.8 (59.1, 66.7) [300] | 61.5 (58.3, 65.0) [316] | 63.5 (60.0, 67.1) [299] | 0.49 | 60.8 (57.3, 64.5) [301] | 63.4 (60.0, 67.1) [315] | 63.5 (60.0, 67.1) [299] | 0.74 |
| 18 mo | 60.4 (56.7, 64.3)b [329] | 58.8 (55.6, 62.2)ab [323] | 53.9 (50.7, 57.3)a [320] | 0.031 | 59.6 (56.2, 63.3)b [317] | 59.5 (56.0, 63.1)ab [335] | 53.9 (50.7, 57.3)a [320] | 0.026 |
| CRP, mg/L | ||||||||
| 6 mo | 0.32 (0.22, 0.46)a [102] | 0.65 (0.43, 0.99)b [100] | 0.41 (0.28, 0.60)ab [101] | 0.033 | — | — | — | |
| 18 mo | 0.58 (0.39, 0.84) [101] | 0.71 (0.50, 1.01) [101] | 0.94 (0.63, 1.42) [100] | 0.19 | — | — | — | |
| AGP, g/L | ||||||||
| 6 mo | 0.80 (0.75, 0.86)ab [102] | 0.90 (0.84, 0.97)b [100] | 0.80 (0.74, 0.86)a [101] | 0.027 | — | — | — | |
| 18 mo | 0.96 (0.89, 1.04) [101] | 0.94 (0.88, 1.00) [101] | 1.03 (0.96, 1.11) [100] | 0.17 | — | — | — | |
All supplements were intended for daily consumption. The subsample for the CRP and AGP analyses was selected from the children whose mothers were not pregnant during the period when the temporary mislabeling occurred. Results are based on ANOVA (SAS PROC GLIMMIX). Values for hemoglobin are means ± SDs (number of participants analyzed for the outcome); values for ZPP, CRP, and AGP are geometric means (95% CIs) [number of participants analyzed for the outcome in question]. For each analysis scenario, values in the same row without a common superscript letter are significantly different at α = 0.05. AGP, α-1 acid glycoprotein; CRP, C-reactive protein; IFA, Iron and Folic Acid group: infants were assigned to no supplements, their mothers were assigned to receive 60 mg Fe/d and 400 µg folic acid/d during pregnancy and 200 mg Ca/d as placebo during the first 6 mo postpartum; iLiNS, International Lipid-based Nutrient Supplements; LNS, Lipid-based Nutrient Supplement group: infants were assigned to receive 20 g Lipid-based Nutrient Supplement/d (designed for infants) containing 6 mg Fe/d from 6 to 18 mo of age, their mothers received 20 g Lipid-based Nutrient Supplement/d (designed for women) with the same micronutrients as the MMN group during pregnancy and the first 6 mo postpartum—both Lipid-based Nutrient Supplement products contained 4 additional minerals (calcium, phosphorus, potassium, and magnesium) as well as macronutrients; MMN, Multiple Micronutrient group: infants were assigned to receive no supplements, their mothers were assigned to receive a multiple micronutrient capsule containing 18 vitamins and minerals, including 20 mg Fe, daily during pregnancy and the first 6 mo postpartum; ZPP, zinc protoporphyrin.
P values compare the means or geometric means of 3 groups, with Tukey–Kramer adjustment for pairwise comparisons.
In the unadjusted analyses of binary outcomes (Table 3), there was a trend (P = 0.07) towards a lower prevalence of elevated ZPP in the LNS group than in the other 2 groups at 18 mo of age when groups were based on supplements women were intended to receive when enrolled; when groups were based on supplements women actually received when enrolled, the prevalence of elevated ZPP at 18 mo of age was significantly lower in the LNS group than in the other 2 groups, which did not differ in this outcome regardless of how groups were analyzed. The intervention groups did not differ in any of the other binary outcomes. In the secondary analysis (Supplemental Table 2), children in the LNS group had a marginally lower prevalence of anemia (38.7% compared with 44.9%; P = 0.06) based on the WHO cutoff, and a significantly lower RR (95% CI) of elevated ZPP [0.79 µmol/mol heme (0.64, 0.97 µmol/mol heme); P = 0.02] at 18 mo of age compared with the IFA and MMN groups combined.
TABLE 3.
Unadjusted binary outcome measures (anemia and biomarkers of iron status) for infants in the iLiNS-DYAD randomized trial of daily nutrient supplementation in a semiurban setting in Ghana, by intervention group1
| Intervention groups based on supplements mothers were intended to receive when enrolled | Intervention groups based on supplements mothers actually received when enrolled | |||||||
|---|---|---|---|---|---|---|---|---|
| IFA (n = 441) | MMN (n = 439) | LNS (n = 440) | P 2 | IFA (n = 441) | MMN (n = 439) | LNS (n = 440) | P 2 | |
| Anemia3 | ||||||||
| 6 mo | 37.1 (31.9, 42.6) [310] | 35.4 (30.4, 40.7) [325] | 33.5 (28.5, 39.0) [313] | 0.65 | 33.4 (28.4, 38.9) [308] | 38.8 (33.7, 44.2) [327] | 33.5 (28.5, 39.0) [313] | 0.26 |
| 18 mo | 42.6 (37.4, 48.0) [333] | 47.3 (41.9, 52.7) [328] | 38.7 (33.6, 44.1) [328] | 0.09 | 43.9 (38.6, 49.4) [321] | 45.9 (40.6, 51.2) [340] | 38.7 (33.6, 44.1) [328] | 0.16 |
| Anemia4 | ||||||||
| 6 mo | 19.7 (15.6, 24.5) [310] | 19.4 (15.4, 24.1) [325] | 16.6 (12.9, 21.2) [313] | 0.55 | 19.2 (15.1, 23.9) [308] | 19.9 (15.9, 24.6) [327] | 16.6 (12.9, 21.2) [313] | 0.54 |
| 18 mo | 6.0 (3.9, 9.1) [333] | 4.9 (3.0, 7.8) [328] | 4.6 (2.8, 7.5) [328] | 0.68 | 5.6 (3.6, 8.7) [321] | 5.3 (3.4, 8.2) [340] | 4.6 (2.8, 7.5) [328] | 0.83 |
| Elevated ZPP5 | ||||||||
| 6 mo | 31.7 (26.6, 37.2) [300] | 37.7 (32.5, 43.1) [316] | 35.8 (30.5, 41.4) [299] | 0.28 | 34.6 (29.4, 40.1) [301] | 34.9 (29.8, 40.4) [315] | 35.8 (30.5, 41.4) [299] | 0.95 |
| 18 mo | 35.3 (30.3, 40.6) [329] | 34.7 (29.7, 40.0) [323] | 27.5 (22.9, 32.7) [320] | 0.07 | 36.6 (31.5, 42.0)b [317] | 33.4 (28.6, 38.7)ab [335] | 27.5 (22.9, 32.7)a [320] | 0.046 |
| IDA6 | ||||||||
| 6 mo | 19.0 (14.9, 23.8) [300] | 18.7 (14.7, 23.4) [316] | 19.7 (15.6, 24.6) [299] | 0.94 | 17.9 (14.0, 22.7) [301] | 19.7 (15.7, 24.5) [315] | 19.7 (15.6, 24.6) [299] | 0.82 |
| 18 mo | 23.4 (19.1, 28.3) [329] | 21.4 (17.2, 26.2) [323] | 18.1 (14.3, 22.7) [320] | 0.25 | 23.3 (19.0, 28.3) [317] | 21.5 (17.4, 26.2) [335] | 18.1 (14.3, 22.7) [320] | 0.26 |
| IDA7 | ||||||||
| 6 mo | 12.0 (8.8, 16.2) [300] | 11.7 (8.6, 15.8) [316] | 12.0 (8.8, 16.2) [299] | 0.99 | 11.6 (8.5, 15.8) [301] | 12.1 (8.9, 16.2) [315] | 12.0 (8.8, 16.2) [299] | 0.98 |
| 18 mo | 4.9 (3.0, 7.8) [329] | 3.4 (1.9, 6.0) [323] | 3.4 (1.9, 6.1) [320] | 0.55 | 4.4 (2.6, 7.3) [317] | 3.9 (2.3, 6.6) [335] | 3.4 (1.9, 6.1) [320] | 0.82 |
All supplements were intended for daily consumption. Results are based on logistic regression models (SAS PROC GLIMMIX). Values are percentages (95% CIs) of participants identified as “yes” for the outcome in question [number of participants analyzed for the outcome in question]. Values in the same row without a common superscript letter are significantly different at α = 0.05. IDA, iron deficiency anemia; IFA, Iron and Folic Acid group: infants were assigned to receive no supplements, their mothers were assigned to receive 60 mg Fe/d and 400 µg folic acid/d during pregnancy and 200 mg Ca/d as placebo during the first 6 mo postpartum; iLiNS, International Lipid-based Nutrient Supplements; LNS, Lipid-based Nutrient Supplement group: infants were assigned to receive 20 g Lipid-based Nutrient Supplement/d (designed for infants) containing 6 mg Fe/d from 6 to 18 mo of age, their mothers received 20 g Lipid-based Nutrient Supplement/d (designed for women) with the same micronutrients as the MMN group during pregnancy and the first 6 mo postpartum—both LNS products contained 4 additional minerals (calcium, phosphorus, potassium, and magnesium) as well as macronutrients; MMN, Multiple Micronutrient group: infants were assigned to receive no supplements, their mothers were assigned to receive a multiple micronutrient capsule containing 18 vitamins and minerals, including 20 mg Fe, daily during pregnancy and the first 6 mo postpartum; ZPP, zinc protoporphyrin.
P values compare all 3 groups, with Tukey–Kramer adjustment for pairwise comparisons.
Anemia defined as blood hemoglobin <110 g/L (33).
Anemia defined as blood hemoglobin <105 g/L for children at 6 mo of age (34), and blood hemoglobin <100 g/L for children at 18 mo of age (31, 34).
Elevated ZPP considered indicative of iron deficiency was defined as ZPP >70 µmol/mol heme. This (moderate) cutoff is consistent with ZPP concentration >10th percentile for preschool children (35–37).
The results were unchanged in the adjusted analyses (data not shown), except for the prevalence of elevated ZPP, in which case the difference between the LNS group and the other 2 groups became nonsignificant, although the point estimates were consistent with the unadjusted results. Finally, in the sensitivity analysis in which Hb and ZPP values were corrected for inflammation in a subsample (Supplemental Table 3), the geometric mean (95% CI) ZPP concentration at 18 mo of age was significantly lower (P = 0.044) in the LNS group [53.9 µmol/mol heme (50.7, 57.3 µmol/mol heme)] than in the IFA [59.6 µmol/mol heme (56.2, 63.3 µmol/mol heme)] or the MMN [59.5 µmol/mol heme (56.0, 63.1 µmol/mol heme)] group, whereas the IFA and MMN groups did not differ in this outcome. None of the binary outcome measures generated from the inflammation-corrected Hb and ZPP values (Supplemental Table 4) differed between the 2 intervention groups.
Discussion
We found that supplementation of women's diet with 60 mg Fe/d and 400 µg folic acid/d during pregnancy and placebo in the first 6 mo postpartum, or multiple micronutrient supplements or SQ-LNSs containing 20 mg Fe/d during both periods, did not result in differences in infant Hb or iron status at 6 mo of age. However, infants who consumed SQ-LNSs from 6 to 18 mo of age, after their mothers had been given SQ-LNSs during pregnancy and lactation, had greater iron supply for Hb synthesis and marginally lower prevalence of anemia than their counterparts who did not consume any supplements from 6 to 18 mo of age and whose mothers consumed iron and folic acid during pregnancy only, or multiple micronutrients during pregnancy and lactation.
We previously reported the main weaknesses of our study, including the inability to blind the study women and fieldworkers to who was in the LNS group (in which women and children received SQ-LNSs) and who was in the non-LNS groups (in which women received IFA or MMN capsules and children received no supplementation), assessment of adherence to supplement intakes via maternal reporting rather than through direct observation, and the exposure of 340 women in the IFA and MMN groups to unintended supplements during all or part of pregnancy (12, 13). Further, determining CRP and AGP in a subsample may have limited our ability to detect significant differences among groups in the sensitivity analysis in which we corrected for the effect of inflammation on the Hb and iron status outcomes.
The study, however, had several strengths, such as using a fully randomized design, having active control groups, and complete blinding of the laboratory workers involved in sample collection and analysis. Our approach of measuring anemia by using various Hb cutoffs was an added strength of the study. As previously reported (12), the unintended exposure to IFA or MMN supplements occurred on only 13% of follow-up days, and no women in the LNS group were exposed to any other supplement apart from the intended SQ-LNSs. We compared several secondary outcomes simultaneously, and it is possible that some of our findings may be due to chance because of multiple testing (46). However, these outcomes were prespecified, measured at the same time, and highly correlated, and it was logical that they would be analyzed together (47). Under these circumstances, correcting for multiplicity may be unnecessary and counterproductive (46).
We chose ZPP concentration (i.e., ZPP:H ratio, expressed as µmol/mol heme) as the indicator of children's iron status because it measures the adequacy of iron supply to the bone marrow for Hb synthesis (48), and is able to detect iron deficiency in the bone marrow at early stages (49, 50). In infants, iron stores are often low or depleted (48) because of the high iron requirement (51) for growth and development, and in addition, iron stores may be depleted before absorption is sufficiently upregulated.
It is noteworthy that infants in the 3 groups did not differ in mean Hb concentration or iron status at 6 mo of age. We previously reported (14) that mothers of infants in the MMN and LNS groups, who consumed supplements with a lower iron content (20 mg/d) than did those in the IFA group (60 mg/d), had a lower mean Hb concentration, lower iron status (higher mean ZPP and plasma transferrin receptor), and a higher prevalence of anemia at 36 weeks of gestation. Therefore, the infants in the MMN and LNS groups were expected to be more likely than those in the IFA group to be anemic and/or to have lower iron status (e.g., higher mean ZPP concentration) by 6 mo of age, because of evidence that an infant's risk of developing anemia after birth is related to the mother's anemia and iron status at delivery (52–54), whereas maternal iron intake postpartum does not affect breast-milk iron content (55). On the other hand, infants in the LNS group were previously reported to have a greater mean birth weight than those in the IFA group (13), and thus may have had greater iron stores at birth (52, 56). Our findings suggest that any risk of developing anemia among children in the LNS group (and possibly the MMN group) during the first 6 mo after delivery as a result of their mothers consuming supplements with lower iron content during pregnancy may have been offset through greater iron stores resulting from increased birth weight. Few randomized controlled trials have examined the impact of prenatal iron supplementation on the Hb concentration or iron status of the offspring during the first 6 mo of life (57). Our study provides evidence that in this setting where iron deficiency in infancy is common (58), the consumption of SQ-LNSs during pregnancy (in comparison with the standard dose of iron and folic acid) does not compromise the Hb production or iron status of infants at 6 mo of age. The extent to which the increased birth weight in the LNS group contributed to the Hb concentration and iron status at 6 mo of age requires further investigation.
From 6 to 18 mo of age, the iron dosage (6 mg/d) given to infants in the LNS group was lower than that used in most home fortification trials (59) including our previous study in Ghana (26). However, a similar dosage was used in SQ-LNS products in a trial in Burkina Faso (60), where infants consuming these products from 9 to 18 mo of age along with malaria and diarrhea treatment had lower prevalence of anemia and iron deficiency than did children who received no intervention. In China, the consumption of a fortified food supplement containing 6 mg Fe/d by infants 4–12 mo of age increased the children's mean Hb concentration compared with the consumption of an unfortified food supplement (61). Thus, it is likely that the iron dosage used in the present study was adequate.
Our results on infants’ iron status at 18 mo of age are consistent with those from Burkina Faso (60) and our previous study in Ghana (62) showing that SQ-LNS supplementation reduced the prevalence of iron deficiency when compared with no supplementation for infants. In those 2 previous studies, the percentage of children with low ferritin or elevated serum transferrin receptor was lower among those who consumed SQ-LNSs than among children who did not consume any supplements, although the geometric mean ZPP concentration did not differ between the intervention and nonintervention groups in Burkina Faso (60), and ZPP was not measured in Ghana (62). A previous meta-analysis demonstrated that home fortification of complementary foods, including the use of SQ-LNSs, is effective for the prevention of IDA (59). In the current study, there were no significant differences in mean Hb concentration or prevalence of anemia, which appears to contradict previous results (60, 62). However, there was a trend for a difference in anemia prevalence at 18 mo of age when comparing the LNS group with the other 2 groups combined, equivalent to a 14% reduction in the risk of anemia. A possible reason for the lack of significant group differences could be that in the current trial, children's mean Hb concentration at 6 mo of age (113 g/L) was relatively high, compared with that (107 g/L) in the previous study in Ghana (62) or that (88 g/L) of the 9-mo-old children in Burkina Faso (60). Thus, infants in this study may have been less likely to demonstrate a response to SQ-LNSs than infants in the other 2 studies (60, 62).
According to the Ghana Demographic and Health Survey (GDHS) 2014 report (63), 78% of children 12–17 mo of age and 74% of children 18–23 mo of age in Ghana were anemic when using the WHO cutoff (Hb <110 g/L) to define anemia. The relatively low anemia prevalence at 18 mo of age observed in our trial (42.9%) therefore conflicts with the GDHS 2014 report, perhaps because of regional and/or subregional variations in the distribution of anemia prevalence in Ghana, or methodological differences such as collection of capillary as opposed to venous blood, use of different models of the Hemocue photometer, or different protocols for handling samples for measuring Hb concentration. It is unlikely that the discrepancy is due to a decreasing secular trend of anemia prevalence in Ghana, because the GDHS and our trial were conducted around the same period of time.
Our observation that nearly 39% of the children in the LNS group were anemic at 18 mo of age based on the WHO cutoff of 110 g/L, despite the SQ-LNS supplementation, warrants further discussion. In West Africa including Ghana, the main causes of anemia in children <5 y of age are iron deficiency, malaria, schistosomiasis, sickle cell disorders, and hookworm infection (in decreasing order of importance) (64). We do not have data on sickle cell disorders, but given the low percentage (∼1% at 6 or 18 mo of age) of children who tested positive for malaria, as well as the children's young age of 18 mo, it is unlikely that malaria, schistosomiasis, and hookworm infections contributed substantially to anemia in our sample. Thus, nonresponsive anemia in the LNS group might be due to poor iron absorption or utilization attributable to factors including: antinutritional compounds, e.g., phenolic compounds in the predominantly plant-based diet reducing iron absorption (65–68); gastric acid hyposecretion (69, 70) reducing iron absorption (71, 72); and hepcidin-induced inhibition of iron absorption and transport due to inflammation (73, 74). Alternatively, the WHO cutoff may be too high, leading to an overestimation of anemia prevalence. When using a lower cutoff of <100 g/L, as recommended by INACG (31), anemia prevalence in the LNS group at 18 mo of age was only 4.6%. In Ghana, anemia prevalence in children (using the standard cutoff) has remained high (63) and supposedly nearly unresponsive to various malaria and helminth reduction interventions (75).
We conclude that maternal and infant supplementation with SQ-LNSs increases infants’ iron status in this semiurban setting in Ghana. More research is needed to investigate the lack of Hb response to micronutrient supplementation among children in this and similar settings, and to identify appropriate cutoffs for defining anemia in children (76).
Supplementary Material
Acknowledgments
We thank the laboratory staff, Seth Antwi, Ronnie Osei-Boateng, and Francis Maunger, for their roles in the blood collection as well as Hb and ZPP determination; Lacey Baldiviez and Setti Shahab-Ferdows at the WHNRC for the CRP and AGP analyses; Janet M Peerson and Charles D Arnold for contributing to SAS programming; Elizabeth L Prado for suggestions; iLiNS Project Steering Committee members Kenneth H Brown, Mamane Zeilani, Stephen A Vosti, Kenneth Maleta, and Jean Bosco Ouedraogo for advice in trial conceptualization; and Lindsay Allen for helping to define the SQ-LNS formulation. The authors’ responsibilities were as follows—SA-A, AL, PA, and KGD: designed the research; SA-A, AL, and HO: conducted the research; SA-A and RTY: performed the statistical analysis; SA-A and KGD: wrote the manuscript; RTY, AL, HO, PA, UA, BMO, and MA: reviewed the draft manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by Bill & Melinda Gates Foundation grant OPP49817 to the University of California, Davis (to KGD).
Author disclosures: SA-A, RTY, AL, HO, PA, UA, BMO, MA, and KGD, no conflicts of interest.
The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AGP, α-1 glycoprotein; CRP, C-reactive protein; GDHS, Ghana Demographic and Health Survey; Hb, hemoglobin; IDA, iron deficiency anemia; IFA, iron and folic acid; iLiNS, International Lipid-based Nutrient Supplements; INACG, International Nutritional Anemia Consultative Group; LNS, lipid-based nutrient supplement; MMN, multiple micronutrient; SQ-LNS, small-quantity lipid-based nutrient supplement; ZPP, zinc protoporphyrin.
References
- 1. Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 2015;66(Suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 2. Gernand AD, Schulze KJ, Stewart CP, West KP Jr, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol 2016;12:274–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2017;4:CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 2006;84:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Bank Scaling up Nutrition: A Framework for Action. Washington (DC): The World Bank; 2010. [cited 2016 Jul 22]. Available from: http://siteresources.worldbank.org/NUTRITION/Resources/281846-1131636806329/PolicyBriefNutrition.pdf. [Google Scholar]
- 6. World Health Organization Resolution WHA 65.6. Comprehensive implementation plan on maternal, infant and young child nutrition. In: Sixty-fifth World Health Assembly. Geneva, 21–26 May, 2012. Resolutions and decisions, annexes [Internet]. Geneva, Switzerland: World Health Organization; 2012. [cited 2014 Dec 6]. Available from: http://www.who.int/nutrition/topics/WHA65.6_resolution_en.pdf?ua=1. [Google Scholar]
- 7. World Health Organization Global targets 2025. To improve maternal, infant and young child nutrition [Internet]. Geneva: World Health Organization; 2012. [cited 2014 Dec 1]. Available from: http://www.who.int/nutrition/topics/English_Poster_B_Global_Target_2025.pdf?ua=1. [Google Scholar]
- 8. Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, Dewey KG. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) project. Matern Child Nutr 2015;11(Suppl 4):31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young Child Nutrition Working Group: Formulation Subgroup Formulations for fortified complementary foods and supplements: review of successful products for improving the nutritional status of infants and young children. Food Nutr Bull 2009;30(2 Suppl):S239–55. [PubMed] [Google Scholar]
- 10. Das JK, Salam RA, Weise Prinzo Z, Sadiq Sheikh S, Bhutta ZA. Provision of preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes [Internet]. Cochrane Database Syst Rev 2017;3:CD012611 [cited 2017 Dec 1]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD012611/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125:e473–80. [DOI] [PubMed] [Google Scholar]
- 12. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, Ashorn U, Zeilani M, Vosti S, Dewey KG. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr 2016;104:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, Arimond M, Vosti S, Dewey KG. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr 2015;101:835–46. [DOI] [PubMed] [Google Scholar]
- 14. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Baldiviez LM, Oaks BM, Vosti S, Dewey KG. Impact of small-quantity lipid-based nutrient supplement on hemoglobin, iron status and biomarkers of inflammation in pregnant Ghanaian women. Matern Child Nutr 2017;13:e12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, Arimond M, Vosti SA, Dewey KG. Maternal supplementation with small-quantity lipid-based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi-urban Ghana: a randomized controlled trial. J Nutr 2017;147:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okronipa H, Adu-Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RR, Dewey KG. Maternal supplementation with small-quantity lipid-based nutrient supplements during pregnancy and lactation does not reduce depressive symptoms at 6 months postpartum in Ghanaian women: a randomized controlled trial. Arch Womens Ment Health 2018;21:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adu-Afarwuah S, Young RT, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, Dewey KG. Supplementation during pregnancy with small-quantity lipid-based nutrient supplements or multiple micronutrients, compared with iron and folic acid, increases women's urinary iodine concentration in semiurban Ghana: a randomized controlled trial. Matern Child Nutr 2018;14:e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oaks BM, Laugero KD, Stewart CP, Adu-Afarwuah S, Lartey A, Ashorn P, Vosti SA, Dewey KG. Late-pregnancy salivary cortisol concentrations of Ghanaian women participating in a randomized controlled trial of prenatal lipid-based nutrient supplements. J Nutr 2016;146:343–52. [DOI] [PubMed] [Google Scholar]
- 19. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M, Nutrition Impact Model Study Group (Anaemia) Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull 2003;24:S99–103. [DOI] [PubMed] [Google Scholar]
- 21. Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 2005;8:117–32. [DOI] [PubMed] [Google Scholar]
- 22. Cook JD, Skikne BS, Baynes RD. Iron deficiency: the global perspective. Adv Exp Med Biol 1994;356:219–28. [DOI] [PubMed] [Google Scholar]
- 23. WHO Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women [Internet]. Geneva: World Health Organization; 2012. [cited 2014 Jul 13]. Available from: http://apps.who.int/iris/bitstream/10665/77770/1/9789241501996_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 24. UNICEF/WHO/UNU Composition of a Multi-Micronutrient Supplement to be Used in Pilot Programmes Among Pregnant Women in Developing Countries [Internet]. New York: UNICEF; 1999. [cited 2015 Jan 18]. Available from: http://apps.who.int/iris/bitstream/10665/75358/1/UNICEF-WHO-multi-micronutrients.pdf?ua=1. [Google Scholar]
- 25. Zhou SJ, Gibson RA, Crowther CA, Makrides M. Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose-response trial. Eur J Clin Nutr 2009;63:183–90. [DOI] [PubMed] [Google Scholar]
- 26. Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr 2007;86:412–20. [DOI] [PubMed] [Google Scholar]
- 27. WHO/UNICEF Iron Supplementation of Young Children in Regions where Malaria Transmission is Intense and Infectious Disease Highly Prevalent [Internet]. Geneva, Switzerland: World Health Organization; 2006. [cited 2017 Nov 14]. Available from: http://www.who.int/malaria/publications/atoz/who_statement_iron/en/. [Google Scholar]
- 28. Adu-Afarwuah S, Lartey A, Zeilani M, Dewey KG. Acceptability of lipid-based nutrient supplements (LNS) among Ghanaian infants and pregnant or lactating women. Matern Child Nutr 2011;7:344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cogill B. Anthropometric Indicators Measurement Guide [Internet]. Washington (DC): Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2001. [cited 2018 Feb 17]. Available from: ftp://200.235.128.138/dns/sylvia/anthro.pdf. [Google Scholar]
- 30. Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 31. Nestel P, INACG Steering Committee Adjusting Hemoglobin Values in Program Surveys [Internet]. Washington (DC): INACG; 2002. [cited 2014 Jan 10]. Available from: http://pdf.usaid.gov/pdf_docs/PNACQ927.pdf. [Google Scholar]
- 32. WHO Assessing the Iron Status of Populations: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. 2nd ed Geneva: World Health Organization; 2007. [cited 2015 Oct 22]. Available from: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107.pdf. [Google Scholar]
- 33. WHO Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity [Internet]. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1). Geneva: World Health Organization; 2011. [cited 2017 Jul 6]. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 34. Domellöf M, Dewey KG, Lonnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr 2002;132:3680–6. [DOI] [PubMed] [Google Scholar]
- 35. Crowell R, Ferris AM, Wood RJ, Joyce P, Slivka H. Comparative effectiveness of zinc protoporphyrin and hemoglobin concentrations in identifying iron deficiency in a group of low-income, preschool-aged children: practical implications of recent illness. Pediatrics 2006;118:224–32. [DOI] [PubMed] [Google Scholar]
- 36. Rettmer RL, Carlson TH, Origenes ML, Jack RM, Labb RF. Zinc protoporphyrin/heme ratio for diagnosis of preanemic iron deficiency. Pediatrics 1999;104:e37. [DOI] [PubMed] [Google Scholar]
- 37. Soldin OP, Pezzullo JC, Hanak B, Miller M, Soldin SJ. Changing trends in the epidemiology of pediatric lead exposure: interrelationship of blood lead and ZPP concentrations and a comparison to the US population. Ther Drug Monit 2003;25:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010. Geneva: World Health Organization; 2012. [cited 2014 Nov 14]. Available from: http://www.who.int/nutrition/publications/micronutrients/background_paper4_report_assessment_vitAandIron_status.pdf. [Google Scholar]
- 39. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 41. Kleinman LC, Norton EC. What's the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res 2009;44:288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide (version 3). Washington (DC): Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [cited 2013 Aug 12]. Available from: http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf. [Google Scholar]
- 43. WHO/UNICEF/UNU Iron Deficiency Anaemia Assessment, Prevention, and Control: A Guide for Programme Managers. WHO/NHD/01.3. Geneva: World Health Organization; 2001. [cited 2014 Dec 1]. Available from: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. [Google Scholar]
- 44. Grant FK, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr 2012;142:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klevor MK, Adu-Afarwuah S, Ashorn P, Arimond M, Dewey KG, Lartey A, Maleta K, Phiri N, Pyykko J, Zeilani M et al.. A mixed method study exploring adherence to and acceptability of small quantity lipid-based nutrient supplements (SQ-LNS) among pregnant and lactating women in Ghana and Malawi. BMC Pregnancy Childbirth 2016;16:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity—whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102:721–8. [DOI] [PubMed] [Google Scholar]
- 47. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance [Internet]. Swindon: Medical Research Council, UK; 2006. [cited 2017 Jan 5]. Available from: http://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/. [Google Scholar]
- 48. Lynch S. The rationale for selecting and standardizing iron status indicators. In: World Health Organization Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010 [Internet]. Geneva: World Health Organization; 2012. [cited 2018 Feb 2]. Available from: http://apps.who.int/nutrition/publications/micronutrients/background_paper3_report_assessment_vitAandIron_status.pdf. [Google Scholar]
- 49. Labbe RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: a metabolite with a mission. Clin Chem 1999;45:2060–72. [PubMed] [Google Scholar]
- 50. Labbe RF, Dewanji A. Iron assessment tests: transferrin receptor vis-a-vis zinc protoporphyrin. Clin Biochem 2004;37:165–74. [DOI] [PubMed] [Google Scholar]
- 51. Adu-Afarwuah S, Lartey A, Dewey KG. Meeting nutritional needs in the first 1000 days: a place for small-quantity lipid-based nutrient supplements. Ann N Y Acad Sci 2017;1392:18–29. [DOI] [PubMed] [Google Scholar]
- 52. Scholl TO. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev 2011;69(Suppl 1):S23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, Donat J, Fernandez-Delgado R, Donat F, Alvarez-Dardet C. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol 1990;4:196–204. [DOI] [PubMed] [Google Scholar]
- 54. De Pee S, Bloem MW, Sari M, Kiess L, Yip R, Kosen S. The high prevalence of low hemoglobin concentration among Indonesian infants aged 3–5 months is related to maternal anemia. J Nutr 2002;132:2215–21. [DOI] [PubMed] [Google Scholar]
- 55. Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am J Clin Nutr 2005;81:1206S–12S. [DOI] [PubMed] [Google Scholar]
- 56. Faldella G, Corvaglia L, Lanari M, Salvioli GP. Iron balance and iron nutrition in infancy. Acta Paediatr Suppl 2003;91:82–5. [DOI] [PubMed] [Google Scholar]
- 57. Pena-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015;Jul 22(7):CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71:1280S–4S. [DOI] [PubMed] [Google Scholar]
- 59. Dewey KG, Yang Z, Boy E. Systematic review and meta-analysis of home fortification of complementary foods. Matern Child Nutr 2009;5:283–321. [Google Scholar]
- 60. Abbeddou S, Yakes Jimenez E, Some JW, Ouedraogo JB, Brown KH, Hess SY. Small-quantity lipid-based nutrient supplements containing different amounts of zinc along with diarrhea and malaria treatment increase iron and vitamin A status and reduce anemia prevalence, but do not affect zinc status in young Burkinabe children: a cluster-randomized trial. BMC Pediatr 2017;17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Chen C, Wang F, Wang K. [Effects of nutrient fortified complementary food supplements on growth of infants and young children in poor rural area in Gansu Province]. Wei Sheng Yan Jiu 2007;36:78–81. [PubMed] [Google Scholar]
- 62. Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. Am J Clin Nutr 2008;87:929–38. [DOI] [PubMed] [Google Scholar]
- 63. GSS/GHS/ICFI Ghana Demographic and Health Survey 2014. Rockville, MD: Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF International; 2015. [cited 2016 Oct 7]. Available from: https://dhsprogram.com/pubs/pdf/FR307/FR307.pdf. [Google Scholar]
- 64. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP et al.. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glahn RP, Wortley GM, South PK, Miller DD. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: studies using an in vitro digestion/Caco-2 cell model. J Agric Food Chem 2002;50:390–5. [DOI] [PubMed] [Google Scholar]
- 66. Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hulten L, Pleehachinda R, Suwanik R, Hallberg L. Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am J Clin Nutr 1991;53:554–7. [DOI] [PubMed] [Google Scholar]
- 67. Afsana K, Shiga K, Ishizuka S, Hara H. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Biosci Biotechnol Biochem 2004;68:584–92. [DOI] [PubMed] [Google Scholar]
- 68. Roos N, Sorensen JC, Sorensen H, Rasmussen SK, Briend A, Yang Z, Huffman SL. Screening for anti-nutritional compounds in complementary foods and food aid products for infants and young children. Matern Child Nutr 2013;9 (Suppl 1):47–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sarker SA, Davidsson L, Mahmud H, Walczyk T, Hurrell RF, Gyr N, Fuchs GJ. Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children. Am J Clin Nutr 2004;80:149–53. [DOI] [PubMed] [Google Scholar]
- 70. Bardhan PK. Epidemiological features of Helicobacter pylori infection in developing countries. Clin Infect Dis 1997;25:973–8. [DOI] [PubMed] [Google Scholar]
- 71. Kordas K, Stoltzfus RJ. New evidence of iron and zinc interplay at the enterocyte and neural tissues. J Nutr 2004;134:1295–8. [DOI] [PubMed] [Google Scholar]
- 72. DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol 2005;100:453–9. [DOI] [PubMed] [Google Scholar]
- 73. Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003;102:783–8. [DOI] [PubMed] [Google Scholar]
- 74. Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest 2004;113:1251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. SPRING, Ghana Health Service Ghana: Landscape Analysis of Anemia and Anemia Programming [Internet]. Arlington, VA: Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project; 2016. [cited 2018 Feb 2]. Available from: https://www.spring-nutrition.org/sites/default/files/publications/reports/ghana_anemia_landscape_analysis_final.pdf. [Google Scholar]
- 76. WHO Use and Interpretation of Haemoglobin Concentrations for Assessing Anaemia status in Individuals and Populations. Geneva, Switerzerland: World Health Organization; 2017. [cited 2018 Feb 7]. Available from: http://www.who.int/nutrition/callforauthors_anaemia_status/en/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.