Abstract
Disclosed is a five-step synthesis of (±)-vibralactone, a biologically active terpenoid natural product. A key photochemical valence isomerization of 3-prenyl-pyran-2-one forges both the all-carbon quaternary stereocenter and the β-lactone at an early stage. Cyclopropanation of the resulting bicyclic β-lactone furnishes a strained housane structure that is converted to the natural product through a sequential ring expansion and reduction strategy. Our concise and modular route to the natural product provides the shortest total synthesis of (±)-vibralactone reported to date.
Keywords: total synthesis, photochemistry, cyclopropanation, ring expansion, housane
First isolated by Liu and co-workers in 2006 from the basidiomycete fungus, Boreostereum vibrans, vibralactone (1) is a terpenoid natural product that has attracted the attention of both the synthetic chemistry and chemical biology communities in recent years.1–7 The interest in this natural product stems not only from its unique structural architecture consisting of a β-lactone-fused bicycle, but also its compelling biological activity.1–4 Vibralactone is an inhibitor of pancreatic lipase with an IC50 value comparable to the FDA approved anti-obesity drug Orlistat (IC50 vibralactone = 0.4 μg mL–1; IC50 Orlistat = 0.18 μg mL–1).1,8 Furthermore, it was found that synthetic elaboration of the isolated natural product at the C13 position to furnish analogs resulted in a 3,000-fold increase in inhibitory potency against pancreatic lipase in vitro.2 In addition to its lipase inhibition activity, vibralactone was also recently identified as a potent covalent inhibitor of caseinolytic peptidase ClpP, a highly conserved bacterial enzyme responsible for virulence in Listeria monocytogenes.3,4 Interestingly, unlike most β-lactone-containing molecules that target solely the ClpP1 isoform of the ClpP complex, vibralactone is particularly unique in its ability to bind both the ClpP1 and ClpP2 isoforms.3 This bioactivity renders vibralactone an attractive lead for the development of antibiotics.
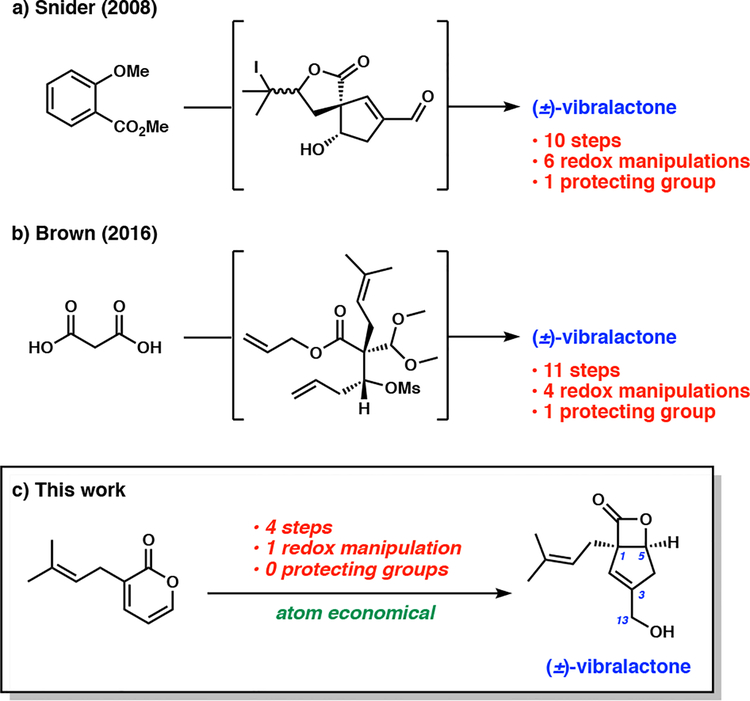
The discovery of the potent bioactivity of vibralactone has been driven by access to vibralactone derivatives, wherein simple functionalization has been utilized to prepare compounds with enhanced activity and handles for chemical biology studies.2,3 These advances were enabled by culture growth of B. vibrans and isolation of natural vibralactone, as well as by synthetic vibralactone prepared using the methods described in existing total syntheses.3,5 A 10-step total synthesis of (±)-vibralactone was first reported by Snider and co-workers in 2008, which required six redox manipulations and one complex protecting group strategy to furnish the natural product in 9% overall yield (Scheme 1a).5 Their synthesis was also extended toward accessing (–)-vibralactone through the utilization of a chiral auxiliary-guided diastereoselective Birch reductive alkylation (4.8% yield over 11 steps).6 In 2016, Brown and coworkers reported the synthesis of (±)-vibralactone in 11 steps (16% overall yield) which contained four redox manipulations and one protecting group strategy (Scheme 1b).7
Scheme 1.
Total synthesis of (±)-vibralactone by (a) Snider and co-workers, (b) Brown and co-workers. (c) This work.
Given the complexity of current synthetic routes to accessing a seemingly simple molecule, we became interested in developing a more concise synthesis of (±)-vibralactone. Herein, we describe a four-step synthesis of (±)-vibralactone (1) from known 3-prenyl-pyran-2-one, which can be readily accessed in one step from commercial materials using a reported protocol (Scheme 1C).9 The synthesis is highly atom economical, protecting group-free, and requires only one redox manipulation.
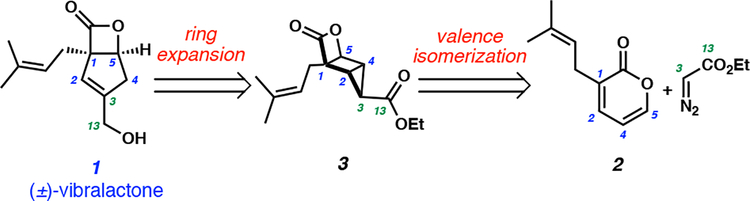
We envisioned that we could access the core of (±)-vibralactone (1) from housane 3 through a sequential ring expansion and reduction strategy (Scheme 2). We hypothesized that cyclopropanation of the Dewar isomer of prenyl pyrone 2 would generate highly strained tricyclic ester 3. Importantly, the photochemical valence isomerization of pyrone 2 would install the sterically congested β-lactone moiety and the key all-carbon quaternary stereocenter, constructing the key topological features of the natural product at an early stage. We reasoned that the labile β-lactone would remain intact through the duration of the synthesis given the congested environment imparted by the adjacent quaternary C1 carbon.
Scheme 2.
Retrosynthetic analysis of (±)-vibralactone (1).
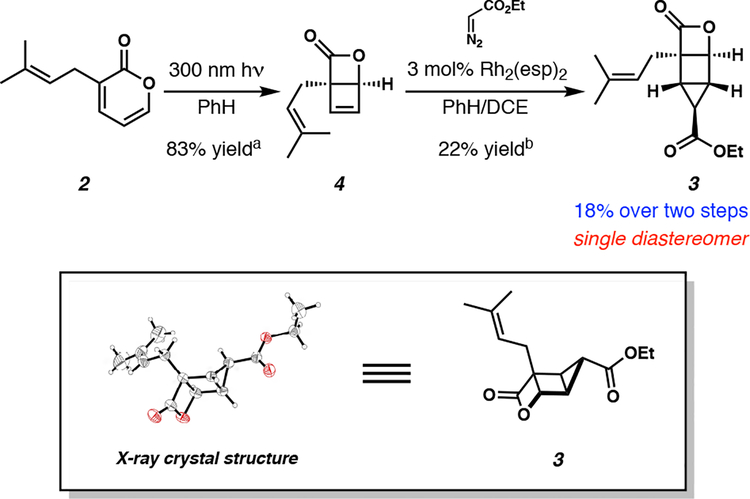
Our synthesis of (±)-vibralactone commenced with the valence isomerization of known prenyl pyrone 2, which was prepared in one step through the prenylation of commercially available 3-bromo-pyran-2-one using a previously reported procedure.9 Compound 2 was irradiated with 300 nm light resulting in a smooth photochemical valence isomerization to afford the oxabicyclo[2.2.0]hexenone 4 in 83% yield, with little to no detectable side products (Scheme 3).
Scheme 3.
Synthesis and X-ray crystal structure of advanced housane intermediate 3. aYield determined by 1H NMR. b30% yield determined by 1 NMR.
Next, we investigated the cyclopropanation of the cyclobutenyl olefin of bicycle 4 (Scheme 3). After screening several transition metal-mediated carbene transfer reactions, we discovered that bis[rhodium(α,α,α’,α’-tetramethyl-1,3-benzenedipropionic acid)] (Rh2(esp)2) afforded the desired cyclopropane 3 in 22% yield.10 We attribute the poor reactivity of the [2.2.0]bicycle to the sterically congested environment surrounding the reactive olefin. Furthermore, due to the notorious instability of the oxabicyclo[2.2.0]hexenone scaffold, we believe that in situ decomposition of the starting material is competing with the kinetics of the cyclopropanation, which contributes to the diminished yield of the housane product.11 Nevertheless, we were gratified to install the final two carbons of the natural product, furnishing the highly strained and structurally unique housane 3 as a single diastereomer. The structure of intermediate 3 was confirmed unambiguously by X-ray crystallography (Scheme 3).
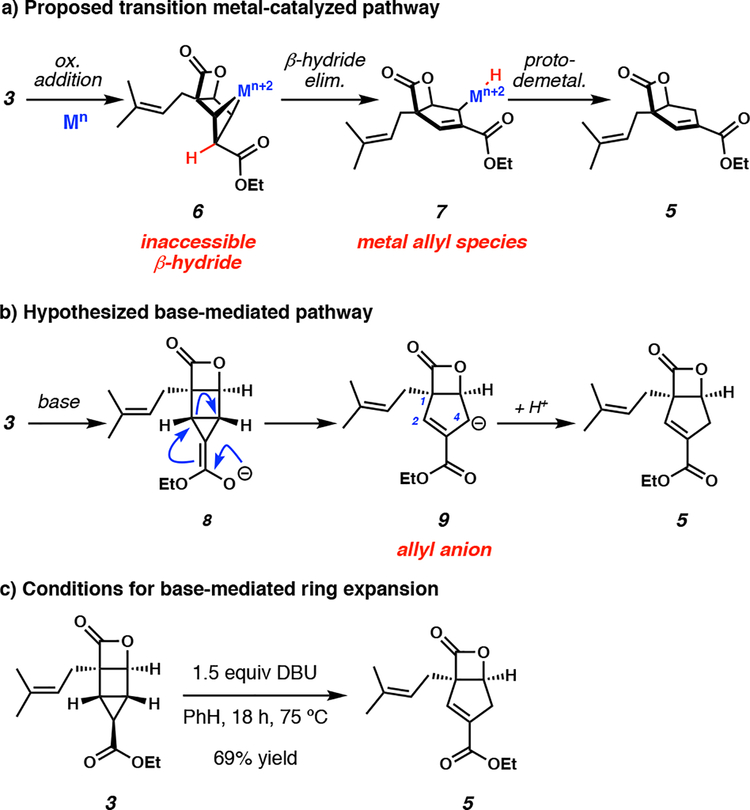
Having rapidly synthesized housane 3 in a mere three steps, we found ourselves one chemical manipulation away from arriving at the carbocyclic core of the natural product (1). Subsequently, our attention turned to early reports by Bishop and co-workers wherein similar bicyclo[2.1.0]pentane scaffolds were converted to cyclopentenes by transition metal catalysis.12 Analogous to Bishop’s work, we imagined the transition metal-mediated mechanism for ring expansion of housane 3 to cyclopentenyl ester 5 in our system to occur via oxidative addition of the strained cyclopropyl C–C bond to the metal affording metallocycle 6. Following β-hydride elimination to allyl species 7, protodemetalation would furnish cyclopentene product 5 (Scheme 4a).
Scheme 4.
Ring expansion strategies of housane intermediate 3 via (a) transition metal catalysis, and (b) base-mediated fragmentation. (c) Successful base-mediated ring expansion of housane 3 to cyclopentene 5.
It was observed by Bishop, however, that the success of the transformation was dependent on the syn-relationship of the β-hydrogen to the metal center in an intermediate akin to 6.12 In our system, we recognized that oxidative addition would likely lead to an anti-configuration between the metal center and β-hydride. Nevertheless, we doggedly attempted this transformation despite our mechanistic intuition, in the hope that outer-sphere elimination could be achieved. Unfortunately, we were unable to observe ring expansion of housane 3 in the presence of a variety of transition metal complexes (see Supporting Information). No reactivity was observed at lower temperatures, while decomposition to complex product mixtures was observed at elevated temperatures. While this attempt was unsuccessful, the putative mechanism of the transition metal-mediated pathway highlighted a strategic opportunity to exploit an analogous allyl intermediate. We sought to harness the natural reactivity of the molecule and recognized that the acidic α-proton of the exocyclic ester could be deprotonated to afford ester enolate 8, arriving at key allylic anion 9 through cleavage of the bridging C–C bond in an E1cB-type fashion. Protonation of this anion would then furnish enoate 5, thereby completing the carbocyclic core of the natural product in only four steps (Scheme 4b). To our delight, we successfully achieved ring expansion of housane 3 under mild, basic conditions using 1.5 equivalents of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in benzene solvent, affording α,β-unsaturated ester 5 as a single allyl isomer in 69% yield (Scheme 4c). We attribute selective formation of the desired isomer to the kinetic protonation of allyl anion 9 at the C4 carbon distal to the sterically congested C1 quaternary center.
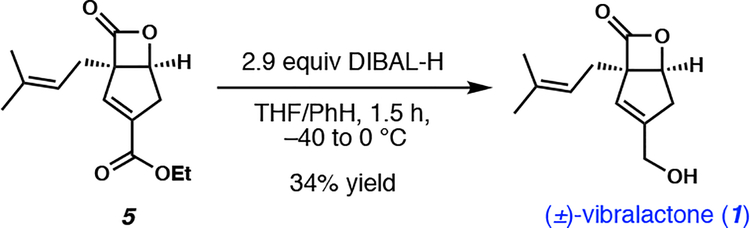
In previous syntheses of (±)-vibralactone, the chemical instability of the β-lactone moiety necessitated several protecting group and redox manipulations prior to its installation. Here, we were faced with the daunting endeavor of selective reduction of the exocyclic ester in the presence of the highly strained β-lactone moiety installed early on in the synthesis. Despite our trepidation with this selective reduction, we were enticed by the prospect of exploiting kinetic control to facilitate the selective reduction of the less sterically encumbered exocyclic ester. Pleasingly, reduction of the ethyl ester was achieved in 34% yield through the use of diisobutylaluminum hydride (DIBAL-H), completing our synthesis of (±)-vibralactone in only four steps from known prenyl pyrone 2 (Scheme 5), and five steps from commercially available materials. In addition to the desired product, 32% starting material was recovered, which could be recycled. Trace amounts of other reduction side products were observed under these conditions, though in relatively low yields.
Scheme 5.
Reduction of ester 5 and completion of synthesis.
In conclusion, (±)-vibralactone was prepared in a concise four steps (4.3% overall yield) from known prenyl pyrone 2 (five steps from commercially available materials) in an atom economical, protecting group-free fashion with only a single redox manipulation. Through a key photochemical valence isomerization, the all-carbon quaternary center and β-lactone moiety were installed at an early stage. In the subsequent two steps, the carbocyclic core of the natural product was forged through a sequential cyclopropanation and ring expansion strategy. In the final step of the synthesis, a selective reduction of the exocyclic ester over the strained β-lactone completed the natural product. Early installation of the C1 quaternary center proved critical in obtaining kinetic selectivity in both the ring expansion and reduction steps. We envision that our dramatically shortened synthetic route to the natural product reported here will facilitate further exploration of the biological activity of vibralactone.
Supplementary Material
Acknowledgements
We thank the David and Lucile Packard Foundation (to H.M.N.) and the Alfred P. Sloan Foundation (to H.M.N.) for generous support. S.K.N. acknowledges the USPHS National Research Service Award (T32GM008496). L.A.B. acknowledges the USPHS National Research Service Award (5T32GM008496). We thank Dr. S. Khan and the UCLA Molecular Instrumentation Center for NMR instrumentation, X-ray crystallography (S. Khan), and mass spectrometry. This material is based upon work supported by the National Institutes of Health under instrumentation grant no. 1S10OD016387–01. We also thank the mass spectrometry facility at the University of California, Irvine for mass spectrometry data. We thank the group of M. A. Garcia-Garibay (UCLA) for the use of their Hanovia lamps during initial photochemical experiments. We thank N. K. Garg (UCLA) and P. G. Harran (UCLA) for useful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Liu D-Z; Wang F; Liao T-G; Tang J-G; Steglich W; Zhu H-J;Liu J-K Org. Lett 2006, 8, 5749–5752. [DOI] [PubMed] [Google Scholar]
- [2].Wei K, Wang GQ, Bai X et al. Nat. Prod. Bioprospect 2015, 5, 129–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zeiler E; Braun N; Böttcher T; Kastenmuller A; Weinkauf S; Sieber SA Angew. Chem. Int. Ed 2011, 50, 11001–11004. [DOI] [PubMed] [Google Scholar]
- [4].Böttcher T; Sieber SA J. Am. Chem. Soc 2008, 130, 14400–14401. [DOI] [PubMed] [Google Scholar]
- [5].Zhou Q; Snider BB Org. Lett 2008, 10, 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou Q; Snider BB J. Org. Chem 2008, 73, 8049–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leeder AJ; Heap RJ; Brown LJ; Franck X; Brown RC D. Org. Lett 2016, 18, 5971–5973. [DOI] [PubMed] [Google Scholar]
- [8].Hochuli E; Kupfer E; Maurer R; Meister W; Mercadal Y;Schmidt KJ Antibiot. 1987, 40, 1086–1091. [DOI] [PubMed] [Google Scholar]
- [9].Posner GH; Harrison W; Wettlaufer DG J. Org. Chem 1985, 2, 5041–5044. [Google Scholar]
- [10].Espino CG; Fiori KW; Kim M; Du Bois JJ Am. Chem. Soc 2004, 126, 15378–15379. [DOI] [PubMed] [Google Scholar]
- [11].Corey EJ; Streith JJ Am. Chem. Soc, 1964, 86, 950–951. [Google Scholar]
- [12].Wiberg KB; Bishop WC Tetrahedron Lett. 1973, 14, 2727.–. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.