Abstract
Objective
To compare neurodevelopmental outcomes in linear growth-restricted (LGR) infants born <29 weeks with and without weight gain out of proportion to linear growth
Study Design
We compared 2-year neurodevelopmental outcomes between infants with and without LGR and between LGR infants with and without weight gain out of proportion to linear growth. The outcomes were Bayley-III cognitive, motor, and language scores, cerebral palsy, Gross Motor Function Classification System (GMFCS) level ≥ 2, and neurodevelopmental impairment.
Result
1227 infants were analyzed. LGR infants were smaller and less mature at birth, had higher BMI, and had lower Bayley-III language scores (82.3 vs 85.0, p<0.05). Among infants with LGR, infants with high BMI had lower language scores compared to those with low-to-normal BMI (80.8 vs 83.3, p<0.05), and were more likely to have GMFCS level ≥ 2 and neurodevelopmental impairment.
Conclusion
Among infants with LGR, weight gain out of proportion to linear growth was associated with poorer neurodevelopmental outcomes.
Introduction
The association of postnatal growth with neurodevelopmental outcomes in preterm infants is well established. Greater gains in weight, length, head circumference (HC), and body-mass index (BMI) during infancy have all been associated with improved neurodevelopmental scores measured during childhood.1, 2, 3, 4, 5 Greater attention has been paid to promoting weight gain and head growth in premature infants, although some data exist suggesting that linear growth is also important when considering long-term outcomes. For example, in very low birthweight infants, greater length z-score at discharge correlated with higher Bayley-III language score at 24 months6, and more rapid linear growth between term and 4 months’ corrected age was associated with lower odds of IQ < 85 at 18 months of age in preterm infants.7
In preterm infants, postnatal linear growth restriction (LGR), represented by a decline in length z-score from birth to discharge, is common.5, 6 Consequently, it is not uncommon for these infants to gain weight out of proportion to their gain in length, with a resulting increase in BMI. Although an increase in BMI between one week of age and term-equivalent age has been associated with improved neurodevelopmental outcomes in moderately preterm infants, it is unclear whether weight gain out of proportion to linear growth (i.e. high BMI), particularly in the setting of linear growth restriction, confers benefit for extremely preterm infants. The importance of following linear growth is that this measurement is a surrogate for lean body mass accrual and thus represents organ growth and differentiation.8 Thus, linear growth, rather than weight gain, may better represent brain growth and development in premature infants. The objective of this study was to compare neurodevelopmental outcomes among extremely preterm infants with and without LGR (measured from birth to discharge/120 days of age) and with high versus low-to-normal BMI (determined at discharge/120 days of age) in a population experiencing LGR. We hypothesized that, among infants with LGR, weight gain out of proportion to linear growth (i.e. high BMI) would not be associated with improved neurodevelopmental outcomes assessed at 2 years of age.
Methods
This is an observational, retrospective study of prospectively collected data as part of the National Institute for Child Health and Human Development’s Neonatal Research Network Generic Database (GDB) and Follow-Up studies. Infants 23 0/7 to 28 6/7 weeks gestation or with a birthweight of 401 to 1000 grams born 7/1/2012 to 6/30/2014 at participating NICHD Neonatal Research Network sites and who had neurodevelopmental assessment at 22–26 months’ corrected age (CA) were considered for inclusion. Infants were excluded if they carried a diagnosis affecting linear growth (ex. skeletal dysplasia), had length z-score < −2 (which may reflect an unknown inherited condition affecting linear growth) or HC z-score > 2 at birth, or had missing growth or outcome data. All data collection for the birth hospitalization continued until infants reached “status”, defined as hospital discharge or 120 days, whichever came first.
Length, weight and head circumference were measured according to the local practice in each neonatal intensive care unit (NICU); there is no standard practice or required method for measuring length among NRN sites. Although the pattern of linear growth restriction has been previously described, there is no well-accepted definition. Standard deviation scores, or z-scores, have been used to describe patterns of growth in extremely preterm infants, and may be better suited to do so when compared with traditional growth percentiles.9 Therefore, we chose to define LGR as being present when the length z-score at status was more than one point below the length z-score at birth, i.e.
LGR = Length z-score at status – length z-score at birth < −1
The Fenton growth chart10 served as the reference for growth percentiles and corresponding z-scores, which were determined using the bulk calculator provided at http://www.ucalgary.ca/fenton/2013chart. BMI at status was used to determine high (≥ 75th percentile) versus low-to-normal (< 75th percentile) weight gain relative to linear growth. BMI percentile was determined using the Olsen11 (CA ≤ 41 weeks) and WHO (CA > 41 weeks) BMI curves. Data collected included maternal/demographic characteristics, time to first feed and full feeds (120 mL/kg/day), duration of parenteral nutrition and postnatal steroid use. Additional data for in-hospital morbidities included rates of culture-positive sepsis (both early and late), necrotizing enterocolitis requiring surgery, grade 3–4 intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), severe bronchopulmonary dysplasia (BPD, defined as receiving supplemental O2 or positive-pressure ventilation for the first 28 days of life and receiving supplemental O2 ≥ 0.30 or positive-pressure ventilation at 36 weeks PMA or discharge if discharged before 36 weeks), retinopathy of prematurity (ROP) requiring surgery, and patent ductus arteriosus (PDA) requiring surgery. Standardized neurodevelopmental examinations were performed at 22–26 months CA by certified examiners at each NRN center.12 Gross motor function was assessed with the Gross Motor Function Classification System (GMFCS) in all children. CP was defined as abnormal tone or reflexes in at least 1 extremity and abnormal control of movement or posture to a degree that interferes with age-appropriate activity. Children with CP were defined as having moderate-to-severe CP if they had a GMFCS level >2. The Bayley Scales of Infant Development, Third Edition13 were performed by trained, certified examiners.
The primary outcomes for this study were Bayley-III composite cognitive, motor, and language scores in LGR infants with high and low-normal BMI. Additional outcomes compared included the incidence of moderate/severe cerebral palsy, gross motor functional classification scale (GMFCS)14 level ≥ 2 and neurodevelopmental impairment (NDI), defined as Bayley-III composite cognitive score < 70, Bayley-III composite motor score < 70, GMFCS level ≥ 2, bilateral blindness (< 20–200), or hearing impairment (permanent hearing loss that does not permit the child to understand directions of the examiner and communicate ± amplification with cochlear implants or hearing aids). These same outcomes were also compared between infants with and without LGR.
Bivariate analyses were used to compare demographic and clinical characteristics between LGR infants with excessive versus low-normal BMI, as well as between LGR and non-LGR infants using t-tests or Mann Whitney-U for continuous variables and exact tests for categorical variables. Generalized linear models and logistic regression were used to assess the association of neurodevelopmental outcomes with LGR and with BMI among infants with LGR. Models were adjusted for maternal socioeconomic status (using maternal insurance status), infant gender, gestational age at birth, birth weight, small-for-gestational age, and center (model 1). A secondary analysis (model 2) also corrected for postnatal head growth, assessed as postnatal head-sparing, which has been previously defined as: HC z-score at status – HC z-score at birth ≥ −1.9 All analyses were performed using SAS version 9.4 with a p-value < 0.05 indicating statistical significance.
Results
Of the 2257 infants born 7/1/12 through 6/30/14, 595 (26%) died and an additional 435 infants were excluded (Supplemental Figure). The most common reason for exclusion was missing growth or outcome data. From the remaining 1227 infants, 912 (74%) met the definition for LGR. For infants with LGR, 353 (39%) had a BMI ≥ 75th percentile at status.
Maternal and infant characteristics are compared between infants with and without LGR, as well as among LGR infants with high versus low-normal BMI as shown in Table 1. Maternal characteristics were similar between infants with and without LGR, as well as among LGR infants with high and low-normal BMI. Compared with infants without linear growth restriction, LGR infants had lower GA, BW, HC and BMI, but higher length, at birth and received more days of parenteral nutrition. Among infants with LGR, those with BMI at status ≥ 75th percentile were younger, had a higher BW and BMI at birth, and were less likely to be small for gestational age when compared with those with BMI < 75th percentile. LGR infants with a high BMI at status reached full feeds sooner and received fewer days of parenteral nutrition compared with LGR infants with a low-normal BMI.
Table 1.
Maternal and Infant Characteristics
| Variable | No LGR | LGR | Bivariate Analyses | |||
|---|---|---|---|---|---|---|
| N = 315 | All N = 912 | BMI at status ≥ 75th centile N = 353 (39%) | BMI at status < 75th centile N = 559 (61%) | p, no LGR vs. LGR | p, high vs. low BMI, LGR | |
| Maternal race, n (%) | 0.423 | 0.422 | ||||
| African American | 150 (48.4) | 396 (44.5) | 154 (45.2) | 242 (44.2) | ||
| Caucasian | 143 (46.1) | 448 (50.4) | 174 (51.0) | 274 (50.0) | ||
| Other | 17 (5.5) | 45 (5.1) | 13 (3.8) | 32 (5.8) | ||
| Maternal education, n (%) | 0.162 | 0.506 | ||||
| Less than high school | 66 (21.0) | 170 (18.6) | 70 (19.8) | 100 (17.9) | ||
| High school grad | 94 (29.8) | 305 (33.4) | 112 (31.7) | 193 (34.5) | ||
| College | 154 (49.0) | 422 (46.3) | 163 (46.2) | 259 (46.3) | ||
| Private insurance, n (%) | 108 (34.3) | 307 (33.7) | 112 (31.7) | 195 (34.9) | 0.836 | 0.350 |
| PIH, n (%) | 32 (10.2) | 102 (11.2) | 42 (11.9) | 60 (10.7) | 0.676 | 0.591 |
| Maternal Diabetes, n (%) | 16 (5.1) | 38 (4.2) | 14 (4.0) | 24 (4.3) | 0.524 | 0.866 |
| Chorioamnionitis, n (%) | 163 (58.2) | 494 (57.1) | 194 (58.1) | 300 (56.5) | 0.781 | 0.672 |
| Male, n (%) | 153 (48.6) | 481 (52.7) | 197 (55.8) | 284 (50.8) | 0.214 | 0.153 |
| GA at birth, weeks | 25.9 ± 1.2 | 25.6 ± 1.2 | 25.4 ± 1.1 | 25.7 ± 1.3 | <0.001 | 0.003 |
| BW, grams | 808 ± 155 | 776 ± 152 | 791 ± 157 | 766 ± 148 | <0.001 | 0.017 |
| SGA1, n (%) | 28 (8.9) | 119 (13.0) | 33 (9.4) | 86 (15.4) | 0.056 | 0.009 |
| Length at birth, cm | 32.4 ± 2.3 | 32.9 ± 2.4 | 32.8 ± 2.4 | 33.0 ± 2.5 | 0.001 | 0.365 |
| HC at birth, cm | 23.1 ± 1.6 | 22.9 ± 1.6 | 22.9 ± 1.6 | 22.8 ± 1.6 | 0.016 | 0.419 |
| BMI at birth, kg/m2 | 7.7 ± 0.9 | 7.1 ± 0.8 | 7.3 ± 0.8 | 7.0 ± 0.8 | <0.001 | <0.001 |
| DOL of first feed, median (IQR) | 4 (2–5) | 3 (2–5) | 3 (2–5) | 4 (3–5) | 0.518 | 0.130 |
| DOL full feed, median (IQR) | 20 (15–29) | 21 (15–32) | 20 (15–28) | 22 (16–35) | 0.330 | 0.001 |
| Days received parenteral nutrition, median (IQR) | 21 (15–30) | 23 (15–36) | 21 (14–32) | 24 (16–39) | 0.034 | 0.002 |
Data shown are mean ± SD unless otherwise noted.
– SGA defined as birth weight z-score < −1 using Fenton growth curve
Infant anthropometrics at status are shown in Table 2. Infants with and without LGR were of similar age at status, but LGR infants had lower weight, length and head circumference, and higher BMI compared to infants without LGR. Among infants with restricted linear growth, those with a high BMI at status were older, had a larger HC, and, as expected, had a significantly greater weight at status; the difference in mean weight at status was nearly 600 grams.
Table 2.
Infant Characteristics at Status
| Variable | No LGR | LGR | Bivariate Analyses | |||
|---|---|---|---|---|---|---|
| N = 315 | AllN = 912 | BMI at status ≥ 75th centileN = 353 (39%) | BMI at status < 75th centileN = 559 (61%) | p, no LGR vs. LGR | p, high vs. low BMI, LGR | |
| Measures | ||||||
| PMA, weeks | 40.0 ± 2.6 | 40.0 ± 2.5 | 40.3 ± 2.4 | 39.8 ± 2.6 | 0.845 | 0.003 |
| Weight, grams | 3209 ± 660 | 2922 ± 632 | 3288 ± 595 | 2692 ± 539 | <0.001 | <0.001 |
| Length, cm | 49.1 ± 3.2 | 45.6 ± 3.2 | 45.6 ± 3.4 | 45.6 ± 3.1 | <0.001 | 0.753 |
| HC, cm | 34.2 ± 2.4 | 33.3 ± 2.3 | 34.1 ± 2.4 | 32.9 ± 2.0 | <0.001 | <0.001 |
| BMI, kg/m2 | 13.2 ± 2.4 | 13.9 ± 2.5 | 15.8 ± 2.0 | 12.8 ± 1.3 | <0.001 | <0.001 |
| Outcomes | ||||||
| Culture-positive sepsis, n (%) | 58 (18.4) | 233 (25.6) | 92 (26.1) | 141 (25.2) | 0.011 | 0.815 |
| NEC requiring surgery, n (%) | 5 (1.6) | 17 (1.9) | 3 (0.8) | 14 (2.5) | 1.000 | 0.082 |
| IVH (grade 3–4), n (%) | 32 (10.2) | 142 (15.6) | 62 (17.6) | 80 (14.4) | 0.015 | 0.223 |
| PVL, n (%) | 15 (4.8) | 47 (5.2) | 21 (6.0) | 26 (4.7) | 0.882 | 0.443 |
| Postnatal steroids, n (%) | 51 (18.3) | 182 (22.5) | 82 (26.9) | 100 (19.9) | 0.150 | 0.024 |
| BPD, n (%) | 168 (53.5) | 450 (49.5) | 198 (56.2) | 252 (45.2) | 0.239 | 0.001 |
| ROP requiring surgery, n (%) | 13 (4.2) | 80 (8.8) | 23 (6.6) | 57 (10.3) | 0.006 | 0.071 |
| PDA requiring surgery, n (%) | 27 (8.6) | 112 (12.3) | 34 (9.6) | 78 (14.0) | 0.080 | 0.062 |
Data shown are mean ± SD unless otherwise noted.
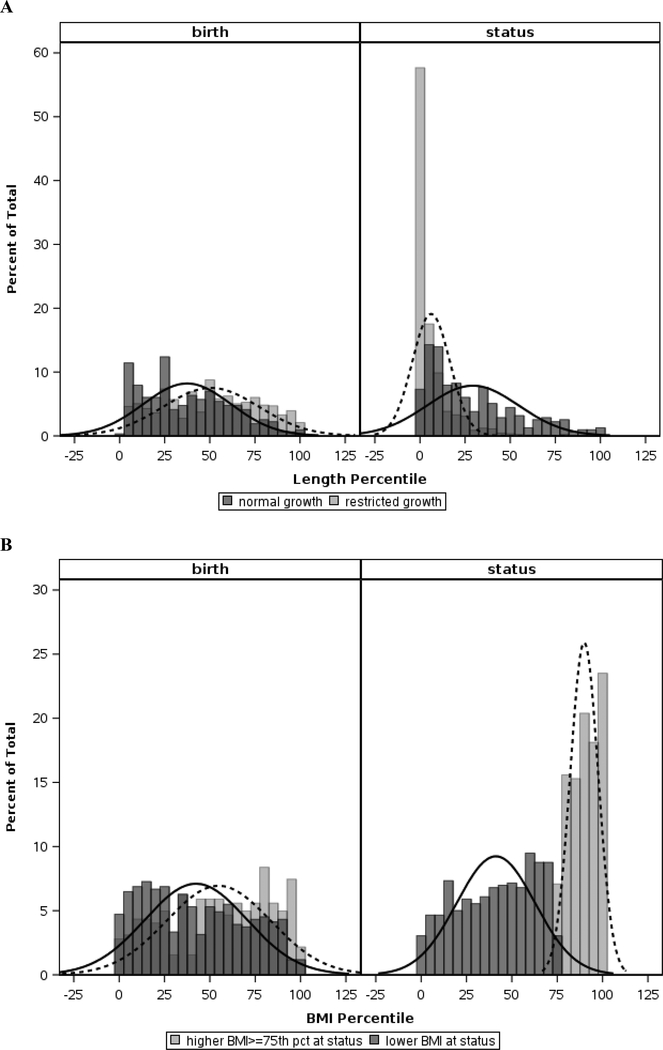
The distributions of length (for all infants) and BMI curves (for LGR infants only) at birth and status are shown as density curves with histograms in Figure 1. As expected for infants with LGR, the distribution of length percentiles shifted leftward from birth to status, representing a negative change in z-score from birth to status, while the distribution in length percentiles for infants without LGR was preserved (Figure 1A). The distribution of BMI at birth for LGR infants with high versus low-normal BMI was relatively similar, and at status the difference noted in these distributions, shown in Figure 1B, represented weight gain out of proportion to linear growth (i.e. high vs low-normal BMI).
Figure 1.
Graphical depiction of length percentiles at birth and status for infants with (light gray bars, dotted line) and without (dark gray bars, solid line) LGR (A), and of BMI percentiles at birth and status for LGR infants with elevated (light gray bars, dotted line) versus low-normal (dark gray bars, solid line) BMI (B). The bars are constructed as histograms and the curves represent smoothed density curves. The third shade of gray noted is where the histogram bars overlap for each of the two groups. Note the leftward shift in length percentiles from birth to status in infants with LGR (A), and the separation of BMI curves from birth to status in LGR infants (B) suggesting different patterns of weight gain relative to linear growth.
Results of bivariate analyses comparing in-hospital morbidities between groups are shown in Table 2. Compared to infants without LGR, those with LGR had higher rates of several morbidities including sepsis, grade 3–4 IVH and ROP requiring surgery. Among infants with linear growth restriction, those with an elevated BMI at status were more likely to be diagnosed with BPD and receive postnatal steroids than those infants with low-normal BMI.
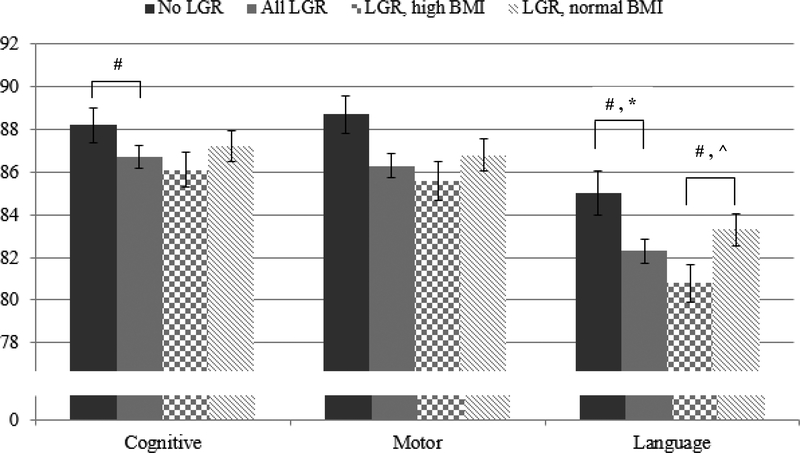
Bayley-III composite scores at 22–26 months are shown in Figure 2 for all groups. In unadjusted analyses, mean Bayley-III motor and language scores were significantly lower in infants with LGR versus those without LGR (86.3 vs 88.7 and 82.3 vs 85.0, respectively, p<0.05 for both comparisons). Among infants with LGR, mean Bayley-III language score was significantly lower in infants with a high BMI at status compared to those with a low-normal BMI (80.8 vs 83.3, p=0.03). In the initial multivariable analysis, only the difference in Bayley-III language score between infants with and without LGR remained significantly different (adjusted mean difference −2.35, 95% CI −4.49 to −0.21, p=0.03) (Figure 2). Results of secondary outcomes are shown in Table 3. As noted, there were no significant differences in these outcomes between infants with and without LGR, and for LGR infants with high versus low-normal BMI in the initial multivariate analysis. However, after controlling for postnatal head growth, LGR infants with high BMI had lower observed Bayley-III language scores (adjusted mean difference −2.33, 95% CI −4.56 to −0.10, p=0.04) and higher odds of GMFCS level ≥ 2 (OR 1.69, 95% CI 1.05 to 2.72, p=0.03) and NDI (OR 1.50, 95% CI 1.02 to 2.20, p=0.04) compared with LGR infants with low-normal BMI. After addition of postnatal head growth to the multivariate model the difference in Bayley-III language scores between infants with and without LGR was no longer statistically significant (adjusted mean difference −2.07, 95% CI −4.25 to 0.11, p=0.06).
Figure 2.
Bar graph demonstrating the observed mean composite Bayley scores for infants without LGR (dark solid gray columns), with LGR (light solid gray columns), with LGR and elevated BMI (checkered gray columns) and with LGR and low-normal BMI (striped gray columns). The black bars represent the standard error. The # denotes a p-value < 0.05 between groups on unadjusted analysis. After multivariate analyses, only the observed differences in language scores between infants with and without LGR (*, Model 1, p<0.05) and LGR infants with high versus low-normal BMI (^, Model 2, p<0.05) remained significant.
Table 3.
Neurodevelopmental Outcomes at 22–26 Months
| Measure | No LGR | LGR | Multivariate Analyses | |||||
|---|---|---|---|---|---|---|---|---|
| N = 315 | AllN = 912 | BMI at status ≥ 75th centileN = 353 (39%) | BMI at status < 75th centileN = 559 (61%) | p, no LGR vs. LGR | p, high vs. low BMI, LGR | |||
| Model 11 | Model 22 | Model 11 | Model 22 | |||||
| Moderate/severe cerebral palsy, n (%) | 17 (5.4) | 73 (8.0) | 35 (9.9) | 38 (6.8) | 0.367 | 0.538 | 0.247 | 0.111 |
| GMFCS level 2+, n (%) | 23 (7.3) | 95 (10.4) | 45 (12.8) | 50 (8.9) | 0.479 | 0.588 | 0.058 | 0.030 |
| NDI, n (%) | 45 (14.3) | 169 (18.5) | 73 (20.7) | 96 (17.2) | 0.348 | 0.592 | 0.115 | 0.037 |
– Model 1 variables: GA, birth weight, gender, SGA, maternal insurance, and center
– Model 2 variables: All in Model 1 plus postnatal head sparing
Discussion
In this study of premature infants born at <29 weeks’ gestation, we have shown that acquired LGR between birth and hospital discharge is common, present in three quarters of infants in the data set. LGR is associated with poorer language outcomes at 2 years’ corrected age. Among infants with LGR, additional weight gain alone does not ameliorate the neurodevelopmental deficits associated with linear growth restriction, and in fact may confer a disadvantage.
While the association between postnatal growth failure and poorer neurodevelopmental outcome in preterm infants has been well-established,1, 2, 4 growth failure has been largely described using weight gain and sometimes head circumference. As a result, greater attention is often paid to achieving weight gain, and perhaps head growth, with relatively little emphasis placed on achieving or maintaining linear growth. For example, the New York State Perinatal Quality Collaborative, working with 18 regional perinatal centers, has focused on reducing extrauterine growth restriction among infants < 31 weeks gestation since 2010.15 However, the collaborative focused on reducing the percent of infants discharged with weight < 10th percentile and improving head growth (measured as the difference in HC z-scores between birth and discharge), with no stated goal to improve linear growth.15
In a cohort of 62 very low birthweight infants, Ramel et al. reported a positive correlation between length z-score at NICU discharge and Bayley-III language score at 24 months’ CA, as well as a correlation between greater length z-score at 4 and 12 months’ CA and improved cognitive scores at 24 months’ CA after controlling for weight and head circumference z-scores.6 Similarly, more rapid linear growth between term and 4 months’ CA was associated with improved neurodevelopmental scores at 18 months in infants born < 33 weeks.1 Although these studies suggest that improved linear growth both before and after term-equivalent age is linked with neurodevelopment, to our knowledge ours is the first study to report on linear growth during hospitalization and neurodevelopmental outcomes in a large cohort comprised exclusively of extremely low gestational age newborns.
Whether greater weight gain relative to linear growth, reflected by an increase in BMI, confers neurodevelopmental benefit is still not clear. In infants born < 33 weeks, an increase in BMI from birth to term has been associated with better neurodevelopmental outcomes at 18 months’ CA, and BMI gains later in infancy have been associated with lower odds of IQ < 85 later into childhood.1, 7 Moreover, the study by Belfort et al1 noted that the association between increasing BMI and improved neurodevelopmental outcomes was isolated to infants with birth weight < 1250 grams. In our study, LGR infants who experienced greater weight gain relative to linear growth, and thus an increase in BMI, did not experience improved neurodevelopmental outcomes at 2 years of age when compared with LGR infants with weight gain more proportional to linear growth (low-normal BMI). The exact reason for these conflicting results regarding BMI and neurodevelopment is not entirely clear, but may be due to the fact that our study focused specifically on outcomes in LGR infants. An increase in BMI, by definition, indicates a greater increase in weight relative to length. Anthropometric curves suggest an increase in BMI from birth to term in preterm infants is to be expected.11 The pattern of BMI increase associated with optimal outcomes however, such as weight gain with appropriate linear growth versus weight gain in the setting of linear growth restriction, is not well-established. In fact, weight gain out of proportion to linear growth may be undesirable for long-term health. On multivariable analysis, LGR infants with high BMI at status had lower Bayley-III language scores and were more likely to have GMFCS ≥ 2 and NDI when compared with LGR infants with low-normal BMI. Additionally, greater gains in BMI both before and after term have been associated with greater risk of obesity at 8 and 18 years of age in preterm infants.7 Identifying and promoting patterns of growth that optimize outcomes in preterm infants, particularly those born extremely premature, is critical to provide the highest level of care for these infants.
While the exact reasons for poor linear growth, as well as different patterns of weight gain in the setting of poor linear growth, remain unknown, it is reasonable to consider potential mechanisms. Certainly, nutritional mediators impact infant growth, including gains in length. Both longer time to reach full enteral feeds and greater caloric deficit during hospitalization have been associated with lower length z-score at discharge in VLBW infants.6 Nutritional interventions that promote early enteral feeding and target macronutrient recommendations have been successful in improving linear growth in preterm infants.16 Linear growth, a surrogate for lean body mass accrual, and thus organ growth, may better represent brain growth and development in premature infants. Specific nutrient and energy deficits can impact the growth and development of different brain regions, resulting in altered brain structure and function.17, 18, 19 More specifically, these nutrient and energy deficits, which occur commonly in premature infants, may result in regionalized effects on brain development. We speculate that critical windows of development likely exist (before term, early infancy, and later infancy/childhood), which may help explain why certain growth parameters, such as linear growth, do not consistently correlate with neurodevelopmental outcomes across all previously investigated “windows”. Our database (GDB) does not have comprehensive nutritional information for these infants from which to draw conclusions about the role of nutrition in our findings.
In our population, LGR infants with elevated BMI at status were more likely to have BPD compared with LGR infants with low-normal BMI, and all LGR infants had a higher rate of culture-positive sepsis compared with infants without LGR. Thus, the role inflammation may play in poor linear growth should be considered. The accrual of lean body mass, measured indirectly by linear growth, is altered by systemic inflammation. A sepsis model in rats, mediated by TNF-α, results in decreased protein synthesis in skeletal muscle.20, 21 Better outcomes in anthropometrics and bone growth have been observed in preterm infants with lower levels of inflammatory mediators.22, 23 While the incidence of BPD was significantly higher in LGR infants with high BMI, due to this hypothesis that the same underlying mechanism (i.e. systemic inflammation) may contribute to both LGR with high BMI and BPD, we have chosen not to additionally adjust models for BPD, as doing so would detract from the effect of interest. Moreover, though BPD has been associated with poorer neurodevelopmental outcomes in extremely preterm infants, conflicting data on the true influence of BPD on neurodevelopment exist, with some studies failing to demonstrate BPD as an independent influencer of neurodevelopmental impairment.25, 26, 27, 28
This study has several limitations. The definitions of both LGR and low-normal versus elevated BMI, although established a priori, were arbitrary. The definition of LGR does, however, parallel a similar cut-off defining postnatal head sparing (change in head circumference z-score ≥ −1 from birth to status) among growth-restricted premature infants that we have found to be associated with improved neurodevelopmental outcome.9 Length itself is inherently difficult to measure, and was not consistently measured using a length board, considered the most accurate method.24 Preliminary evaluation from the University of Rochester NICU has shown poor agreement between length measured serially by tape measure and length board, with only 62% of 97 paired measurements agreeing to within 1 cm (unpublished data). However, the same evaluation showed no skew of tape measure measurements toward higher or lower values relative to length board determinations. Thus, tape measure lengths, while imprecise, were accurate (i.e., showed no systematic bias). As a result, while there may have been some misclassification of infants as having or lacking LGR at the borders of the current data, we would not expect any systematic misclassification. Another potential limitation exists with the choice of growth curves used to measure weight, length, HC and BMI. Slight differences do exist in the predicted percentiles for weight and length between the Fenton and Olsen curves, which may have introduced systematic bias. The Fenton curves, rather than Olsen, were chosen to calculate weight, length and HC z-scores because they were derived from a more comprehensive and diverse patient population and include infants born as young as 22 weeks gestation.10 However, the Fenton anthropometric data do not include a weight-for-length comparison (such as BMI), and thus the Olsen BMI curves were used to calculate BMI percentiles for those infants ≤ 41 weeks CA. In this study, we expect any systematic bias to be slight, and thus believe the benefits of using the chosen growth curves outweigh this risk. Poor linear growth in our population was associated with poor HC growth, which we and others have shown to predict poorer neurodevelopmental outcome.1, 2, 3, 4, 9 However, secondary analyses accounting for HC growth showed little difference in outcomes, suggesting that linear growth is an independent risk factor for poor neurodevelopmental outcome. Finally, the retrospective nature of the dataset and the limited nutritional data restrict both the ability to draw causal conclusions and the possibility of identifying potential mediators of LGR. On the other hand, conclusions drawn from this large dataset representing multiple centers is likely to be generalizable to other groups of similar infants.
Conclusion
Among extremely preterm infants with LGR, weight gain out of proportion to linear growth did not result in improved neurodevelopmental outcomes, and was associated with poorer performance on some measures. Improved linear growth during hospitalization is associated with improved language development; this finding deserves further exploration.
Supplementary Material
Acknowledgements
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Advancing Translational Sciences (NCATS) provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies. NICHD staff provided input into the study design, conduct, analysis, and manuscript drafting; NCATS cooperative agreements provided infrastructure support to the NRN. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data included in this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Sylvia Tan (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904) – Abbot R. Laptook, MD; Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS RNC-NIC; Elisa Vieira, RN BSN; Emilee Little, RN BSN; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Elisabeth C. McGowan, MD; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Anna Maria Hibbs, MD MSCE; Nancy S. Newman, BA RN; Allison H. Payne, MD MS; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (UG1 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (UG1 HD27853) – Brenda B. Poindexter, MD MS; Kurt Schibler, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Teresa L. Gratton, PA; Lenora Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS; Stacey Tepe, BS; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (UG1 HD40492, UL1 TR1117, UL1 TR1111) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; William F. Malcolm, MD; Patricia L. Ashley, MD PHD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Joanne Finkle, RN JD; Kathryn E. Gustafson, PhD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Janice Wereszczak CPNP-AC/PC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC; Ira Adams-Chapman, MD; Yvonne Loggins, RN; Sheena L. Carter, PhD; Maureen Mulligan LaRossa, RN; Lynn C. Wineski, RN MS; Diane I. Bottcher, RN MSN; Colleen Mackie, BS RRT.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (UG1 HD27856) – Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; Lu-Ann Papile, MD; Heidi M. Harmon, MD MS; Abbey C. Hines, PsyD; Leslie Dawn Wilson, BSN CCRC; Dianne E. Herron, RN CCRC; Susan Gunn, NNP CCRC; Lucy Smiley CCRC.
McGovern Medical School at the University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Memorial Hermann Southwest (UG1 HD87229, U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Elizabeth Allain, MS; Julie Arldt-McAlister, RN BSN; Allison G. Dempsey, PhD; Carmen Garcia, RN CCRP; Janice John, CPNP; Patrick M. Jones, MD; Layne M. Lillie, RN BSN; Karen Martin, RN; Sara C. Martin, RN; Georgia E. McDavid, RN; Shawna Rodgers, RN; Saba Siddiki, MD; Daniel Sperry, RN; Sharon L. Wright, MT (ASCP).
Nationwide Children’s Hospital and the Ohio State University Medical Center (UG1 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Christine A. Fortney, PhD RN; Patricia Luzader, RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – Abhik Das PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret Crawford, BS; Jenna Gabrio, BS CCRP; Marie G. Gantz, PhD; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children’s Hospital (UG1 HD27880, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; M. Bethany Ball, BSc CCRC; Melinda S. Proud, RCP; Barbara Bentley, PsychD MSEd; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Casey E. Krueger, PhD; Ryan Lucash, PhD; Hali E. Weiss, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of Iowa and Mercy Medical Center (UG1 HD53109) – Tarah T. Colaizy, MD MPH; Jane E. Brumbaugh, MD; Dan L. Ellsbury, MD; Karen J. Johnson, RN BSN; Jacky R. Walker, RN; Donia B. Campbell, RNC-NIC; Diane L. Eastman, RN CPNP MA.
University of New Mexico Health Sciences Center (UG1 HD53089, UL1 TR41) – Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Andrea F. Duncan, MD; Tara Dupont, MD; Elizabeth Kuan, RN BSN; Sandra Sundquist Beauman, MSN RNC-NIC.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Noah Cook, MD; Dara M. Cucinotta, RN.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, UL1 TR42) – Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD MPH; Rosemary L. Jensen; Joan Merzbach, LMSW; Gary J. Myers, MD; Ashley Williams, MSEd; Kelley Yost, PhD; William Zorn, PhD; Karen Wynn, RN; Deanna Maffett, RN; Diane Prinzing; Julianne Hunn, BS; Stephanie Guilford, BS; Farooq Osman, MD; Mary Rowan, RN; Michael G. Sacilowski, BS; Holly I.M. Wadkins, MA; Melissa Bowman, MSN; Cait Fallone, MA; Kyle Binion, BS; Constance Orme; Ann Marie Scorsone, MS CCRC; Michelle Andrews-Hartley, MD.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689) – Myra H. Wyckoff, MD; Pablo J. Sánchez, MD; Luc P. Brion, MD; Diana M. Vasil, MSN BSN; Lijun Chen, PhD RN; Roy J. Heyne, MD; Sally S. Adams, MS RN CPNP; Elizabeth Heyne, PsyD PA-C; Alicia Guzman; Lizette E. Lee, RN; Catherine Twell Boatman, MS CIMI.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (UG1 HD21385) – Seetha Shankaran MD; Athina Pappas, MD; Girija Natarajan, MD; Sanjay Chawla, MD; Monika Bajaj, MD; Melissa February, MD; Prashant Agarwal, MD; Kirsten Childs, RN BSN; Eunice Woldt, RN MSN; Rebecca Bara, RN BSN; Laura A. Goldston, MA; John Barks MD; Mary Christensen, RT; Stephanie Wiggins, MS; Diane White, RT.
Footnotes
Conflict of Interest
The authors have no disclosures or conflicts of interest to resolve.
Disclosures: The authors have no disclosures or conflicts of interest to resolve
References
- 1.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P. et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011, 128(4): e899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117(4): 1253–1261. [DOI] [PubMed] [Google Scholar]
- 3.Kan E, Roberts G, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study G. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev 2008, 84(6): 409–416. [DOI] [PubMed] [Google Scholar]
- 4.Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 2003, 143(2): 163–170. [DOI] [PubMed] [Google Scholar]
- 5.Modi M, Saluja S, Kler N, Batra A, Kaur A, Garg P. et al. Growth and neurodevelopmental outcome of VLBW infants at 1 year corrected age. Indian Pediatr 2013, 50(6): 573–577. [DOI] [PubMed] [Google Scholar]
- 6.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 7.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr 2013, 163(6): 1564–1569 e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes GB. Relation of lean body mass to height in children and adolescents. Pediatr Res 1972, 6(1): 32–37. [DOI] [PubMed] [Google Scholar]
- 9.Meyers JM, Bann CM, Stoll BJ, D’Angio CT, Bell EF, Duncan AF. et al. Neurodevelopmental outcomes in postnatal growth-restricted preterm infants with postnatal head-sparing. J Perinatol 2016, 36(12): 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013, 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen IE, Lawson ML, Ferguson AN, Cantrell R, Grabich SC, Zemel BS. et al. BMI curves for preterm infants. Pediatrics 2015, 135(3): e572–581. [DOI] [PubMed] [Google Scholar]
- 12.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD, Follow-Up Study Group of Eunice Kennedy Shriver National Institute of Child H. et al. Improving the Neonatal Research Network annual certification for neurologic examination of the 18–22 month child. J Pediatr 2012, 161(6): 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayley N Bayley scales of infant and toddler development. San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 14.Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP. et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 2000, 80(10): 974–985. [PubMed] [Google Scholar]
- 15.Stevens TP, Shields E, Campbell D, Combs A, Horgan M, La Gamma EF. et al. Variation in Enteral Feeding Practices and Growth Outcomes among Very Premature Infants: A Report from the New York State Perinatal Quality Collaborative. Am J Perinatol 2016, 33(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 16.Stefanescu BM, Gillam-Krakauer M, Stefanescu AR, Markham M, Kosinski JL. Very low birth weight infant care: adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum Dev 2016, 94: 25–30. [DOI] [PubMed] [Google Scholar]
- 17.Cooke RW. Are there critical periods for brain growth in children born preterm? Arch Dis Child Fetal Neonatal Ed 2006, 91(1): F17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugelstad ARR, Georgieff M. The Role of Nutrition in Cognitive Development Handbook in Developmental Cognitive Neuroscience, 2nd edn. MIT Press: Cambridge, MA, 2008. [Google Scholar]
- 19.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007, 85(2): 614S–620S. [DOI] [PubMed] [Google Scholar]
- 20.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med 2006, 12(11–12): 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 2007, 56(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad I, Zaldivar F, Iwanaga K, Koeppel R, Grochow D, Nemet D. et al. Inflammatory and growth mediators in growing preterm infants. J Pediatr Endocrinol Metab 2007, 20(3): 387–396. [DOI] [PubMed] [Google Scholar]
- 23.Eliakim A, Nemet D, Ahmad I, Zaldivar F, Koppel R, Grochow D. et al. Growth factors, inflammatory cytokines and postnatal bone strength in preterm infants. J Pediatr Endocrinol Metab 2009, 22(8): 733–740. [DOI] [PubMed] [Google Scholar]
- 24.Wood AJ, Raynes-Greenow CH, Carberry AE, Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J Paediatr Child Health 2013, 49(3): 199–203. [DOI] [PubMed] [Google Scholar]
- 25.Karagianni P, Tsakalidis C, Kyriakidou M, Mitsiakos G, Chatziioanidis H, Porpodi M. et al. Neuromotor outcomes in infants with bronchopulmonary dysplasia. Pediatr Neurol 2011, 44(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 26.Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M. et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed 2017, 102(3): F235–F234. [DOI] [PubMed] [Google Scholar]
- 27.Brumbaugh JE, Colaizy TT, Patel NM, Klein JM. The changing relationship between bronchopulmonary dysplasia and cognition in very preterm infants. Acta Paediatr 2018, 107(8): 1339–1344. [DOI] [PubMed] [Google Scholar]
- 28.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ. et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics 2009, 124(2): 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.