Abstract
Objective:
To investigate the relationship between acute exposure to air pollutants and spontaneous pregnancy loss.
Design:
Case-crossover study.
Setting:
An academic emergency department (ED) in the Wasatch Front area of Utah.
Patient(s):
A total of 1,398 women who experienced a spontaneous pregnancy loss events from 2007– 2015.
Intervention(s):
None
Main Outcome Measure(s):
Odds of spontaneous pregnancy loss.
Result(s):
We found that a 10 ppb increase in 7-day average levels of nitrogen dioxide (NO2) was associated with a 16% increase in the odds of spontaneous pregnancy loss (odds ratio (OR)= 1.16; 95% CI 1.01–1.33; p=0.04). A 10 μg/m3 increase in 3-day and 7-day averages of fine particulate matter (PM2.5) were associated with increased risk of spontaneous pregnancy loss, but the associations did not reach statistical significance (OR3-day average=1.09; 95 CI 0.99–1.20; p=0.05) (OR7-day average=1.11; 95 CI 0.99–1.24; p=0.06). We found no evidence of increased risk for any other metrics of NO2 or PM2.5 or any metric for ozone (O3).
Conclusions:
We found that short term exposure to elevated levels of air pollutants was associated with higher risk for spontaneous pregnancy loss.
Keywords: Air pollution, female reproductive effects, adverse pregnancy outcomes, PM10-PM2.5-ultrafine, NO2
Capsule
This case-crossover study found that short-term exposure to ambient NO2 increased the risk of spontaneous pregnancy loss by 16% in 1398 women in Utah from 2007–2015.
Introduction
Ambient air pollution poses a significant risk to population health, increasing risk for both morbidity and mortality at all ages (1–3). Currently, air pollution is associated with multiple adverse obstetric outcomes, including pregnancy induced hypertensive disorders, neonates small for gestational age, preterm birth, low birth weight, and stillbirth (4–7). During early gestation, both placental and fetal developments are established, and exposure to deleterious agents can lead to significant damage. The underlying mechanism for adverse pregnancy outcomes is hypothesized to be a result of oxidative stress, systemic inflammation, (8–10) and compromised placental growth and function (11, 12). Despite biological and epidemiological evidence of such effects, however, a limited number of studies have investigated the relationship between air pollution and spontaneous pregnancy loss. Most (13–20), but not all (21) of the available literature has found an increased risk of spontaneous pregnancy loss and air pollutant exposure and results vary by pollutant and demographic factors. Additionally, many studies have been ecological in nature or limited by small sample size. Supplementary Table 1 shows a summary of results of published studies examining this association. The heterogeneity of results may be due to unobservable personal factors or misclassification of exposure and further investigation is needed to elucidate the effect of air pollutants on spontaneous pregnancy loss, particularly the effects during acute exposures (22).
The University of Utah Emergency Department (UUED) services a large urban area known as the Wasatch Front. This region is an area of unique topography where temperature inversions create high concentrations of air pollutants in the winter for limited periods of time, at levels deemed unhealthy by the United States Environmental Protection Agency (EPA)(23). Physicians practicing in UUED noted anecdotal increases in incidence of spontaneous pregnancy loss during these inversion events. Given these observations, we conducted a case-crossover study to examine the risk of spontaneous pregnancy loss among women who presented to the UUED from 2007–2015 with short-term exposures to fine particulate matter (PM2.5, <2.5 μm in aerodynamic diameter), nitrogen dioxide (NO2), and ozone (O3). Because the underlying mechanisms linking air pollution and early pregnancy loss have not been identified, we performed an exploratory analysis examining various exposure time windows and pollutant metrics. Due to known differences in pollutant exposure by sociodemographic factors (24), we investigated effect modification by Hispanic ethnicity as supplementary analyses.
Material and Methods
We identified cases of spontaneous pregnancy loss diagnosed in the UUED by extracting data from the University of Utah Enterprise Data Warehouse (UU EDW) using the following diagnosis codes (ICD-9-CM: 634.xx, 632.xx, 637.9; ICD-10: O03.4, O03.6, O03.9). We identified a total of 1,577 events in the UU EDW from 2007–2015. We excluded 73 events that occurred to women residing outside the state of Utah at the time of spontaneous pregnancy loss. For women who experienced multiple pregnancy loss events, we were not able to determine if a second observation was truly another pregnancy loss event or related to the first event. Therefore, we included only the first event if the two events occurred within 14 weeks; we excluded 106 events that occurred within 14 weeks of a previous event. Our final sample consisted of 1,398 spontaneous pregnancy loss events that occurred prior to 20 weeks gestation. This study was approved by the Institutional Review Board of the University of Utah (IRB_00104032). Sample inclusion criteria are found in Figure 1. County-level daily average temperature data was obtained from the United States National Centers for Environmental Information Climate Data Online (25).
Figure 1:
Sample selection schematic of University of Utah (UU) Emergency Department (ED) patients who experienced a spontaneous pregnancy loss (2007–2015).
Study Design
We used a case-crossover study design to analyze the acute effects of short term exposure to air pollution. The case-crossover design is characterized by selecting a case or event date and each subject then serving as her own control. In this design, acute exposure before an event is compared to a similar window of exposure on days not associated with the event (26, 27). This allows for an increase in efficiency and minimizes time-invariant confounding (such as, genetic predisposition, age, race/ethnicity, and birth cohort) as well as confounding by time-variant factors that do not change within a single month (for example, socioeconomic status or chronic health conditions), making it useful for examining acute exposures to high levels of air pollution. We considered case dates as the day of presentation to the UUED. Controls, i.e. other “referent” times, days for the individual were then selected and risk was estimated by comparing the date of the event to the referent dates.
Referent Selection
We used a time-stratified approach for referent period selection. We selected referent days for each individual as the same day of the week as the event for the calendar month and year, which resulted in 3–4 referent periods per event day. We selected this strategy to control for any bias associated with time trends, overlap bias, increase efficiency (26), and control for season and day of the week by design.
Air Quality Measures
We obtained air pollution data from the EPA’s Air Quality System (AQS) Data Mart (28). We determined population-weighted centroids for every residential zip code based Census 2010 block group population totals. Using topographic features, we delineated 6 air basins within the Wasatch Front as areas where lateral air movement would be reduced due to mountain ranges and basin, and assigned each monitoring station to the air basin where it was located using ArcGIS © (ver 9.3, Redlands, CA). We estimated daily PM2.5, NO2, and O3 levels for each zip code centroid using inverse distance weighting of all observations from monitoring stations located in the same air basin as the zip code centroid. The benefit of this method is that we were able to assign values at the zip code level, rather than county-level measurements from the raw data.
We then calculated exposure measurements as the average daily concentrations of the day of the spontaneous pregnancy loss and the preceding two days of PM2.5, NO2, and O3. We calculated the average of the 3-day averages for the case/referent date and 2 days prior (i.e. lag0, lag1, and lag2) 7-day averages for the case/referent date and 6 days prior (i.e. lag0, lag1 – lag6), 3-day maximum value, and 7-day maximum value. Descriptive statistics for each pollutant and metric can be found in Table 1. Pearson correlation coefficients among air pollutant metrics can be found in Supplementary Table 2.
Table 1:
Descriptive Statistics of Ambient Air Pollutants
| Generated Metric | Min | Mean | Max | Std |
|---|---|---|---|---|
| Average 3 day PM2.5 (μg/m3) | 0.0 | 9.0 | 80.0 | 0.8 |
| Average 7 day PM2.5 (μg/m3) | 0.3 | 9.0 | 73.0 | 0.8 |
| Maximum value 3 day PM2.5 (μg/m3) | 0.0 | 12.0 | 84.0 | 1.1 |
| Maximum value 7 day PM2.5 (μg/m3) | 2.0 | 16.0 | 84.0 | 1.3 |
| Average 3 day O3 (ppb) | 2 | 40 | 90 | 1.6 |
| Average 7 day O3 (ppb) | 4 | 40 | 80 | 1.5 |
| Maximum value 3 day O3 (ppb) | 2 | 40 | 90 | 1.6 |
| Maximum value 7 day O3 (ppb) | 8 | 50 | 90 | 1.6 |
| Average 3 day NO2 (ppb) | 0.8 | 18 | 67 | 1.1 |
| Average 7 day NO2 (ppb) | 0.5 | 18 | 65 | 1.0 |
| Maximum value 3 day NO2 (ppb) | 0.8 | 23 | 74 | 1.3 |
| Maximum value 7 day NO2 (ppb) | 3.0 | 27 | 76 | 1.3 |
Statistical Analysis
We estimated the association between a 10 unit change, measured continuously, in pollutant concentrations and spontaneous pregnancy loss using conditional logistic regression clustered at the event level. Effects were estimated as odds ratios (OR). All analyses controlled for daily average temperature and were completed using SAS 9.4.
Results
Table 2 displays the descriptive statistics of the sample (Nevents=1398). The sample was largely comprised of non-Hispanic White (53.6%) and Hispanic women (38.0%). The average age at admission was 28 years. The average daily temperature during the study period was 23 degrees Fahrenheit (range −1.5– 48.0).
TABLE 2:
Descriptive Statistics of Sample
| No. of Events | 1,398 |
| No. of Referent Days | 4,353 |
| Age at Admission | |
| Mean (years) | 28 |
| Range (years) | 12–46 |
| Ethnicity | |
| White | 750 (53.6%) |
| Hispanic | 531 (38.0%) |
| Other or Missing | 117 (8.4%) |
| County | |
| Salt Lake | 1219 (87.2%) |
| All others | 179 (12.8%) |
| Average Daily Temperature | |
| Mean (degrees F) | 23.3 |
| Range (degrees F) | −1.5–48.0 |
Figure 2 shows the results of the conditional logistic regression when controlling for daily mean temperature. We found a 16% statistically significant increase in the odds of spontaneous pregnancy loss per 10 ppb increase in 7-day average NO2 (OR= 1.16; 95% CI 1.01–1.33; p=0.04). As an example, this increase in risk means that in our data, an increase from the 25th (10.3 ppb) to 75th (24.7 ppb) percentile of 7-day average NO2 increases the risk of spontaneous pregnancy loss by 11.1%. A 10 μg/m3 increase in 3-day and 7-day averages of PM2.5 were associated with increased risk of spontaneous pregnancy loss, however the associations did not reach significance at the p<0.05 threshold (OR3-day average=1.09; 95 CI 0.99–1.20; p=0.05) (OR7-day average=1.11; 95 CI 0.99–1.24; p=0.06). A similar pattern was observed for the 3-day maximum value of PM2.5 (OR=1.07; 95 CI 0.99– 1.14; p=0.08). We did not find a statistical association between other metrics of NO2 or PM2.5 or any metric of O3. We found that 7-day averages or maximums provided the best model fit for NO2. We did not find any substantive differences in 7-day or 3-day metrics for PM2.5 or for O3.
Figure 2:
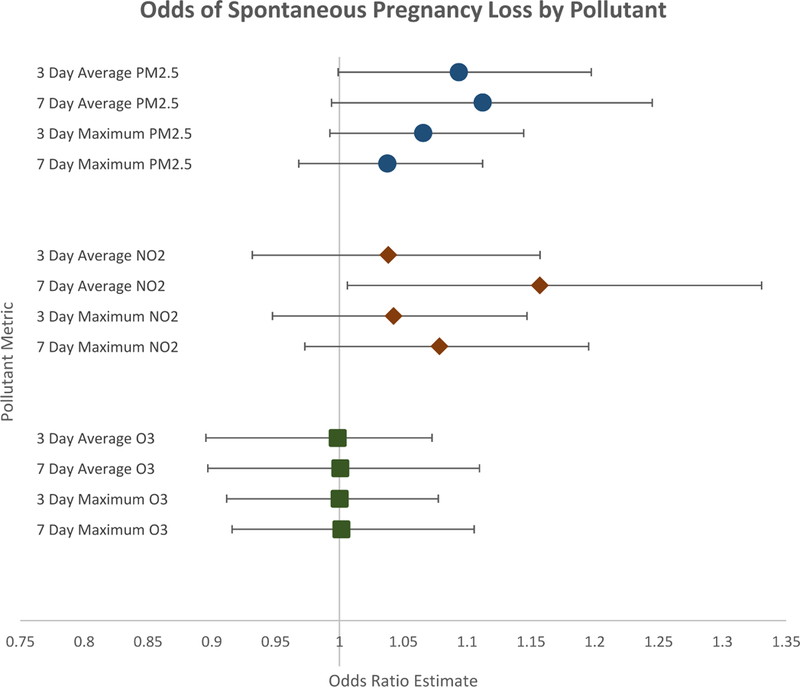
Forest plot of the effect of ambient air pollutants on spontaneous pregnancy loss by pollutant metric (2007–2015). Results of conditional logistic regression. Error bars indicate 95% Confidence Intervals. All models control for average daily temperature.
Supplemental Analysis
We tested for effect modification of Hispanic ethnicity via interaction term. Results are presented in Supplementary Table 3. We did not find statistically significant effect modification by Hispanic ethnicity.
Discussion
The results of this study provide evidence that acute elevated levels of ambient air pollutants, specifically NO2, are associated with spontaneous pregnancy loss. There are several possible biological mechanisms by which air pollution could contribute to spontaneous pregnancy loss including oxidative stress to the developing fetus, maternal endocrine disruption, and systemic maternal inflammation leading to abnormal placentation and growth abnormalities. Approximately 50% of early pregnancy spontaneous pregnancy loss are attributed to non-chromosomal abnormalities (29) and maternal exposure to combustion particles is associated with oxidative damage to DNA and lipids (30) which could be detrimental to growing fetuses. Exposure to air pollution has also been shown to inhibit embryo implantation, which is a risk factor for spontaneous pregnancy loss (31). We found the highest risk for spontaneous pregnancy loss occurred with a high 7-day average exposure to NO2. Previous meta-analyses have shown NO2 exposures were related to an increased risk in cardiac defects including coarctation of the aorta (OR = 1.17; 95 CI 1.00–1.36) and Tetralogy of Fallot (OR = 1.20; 95 CI 1.02–1.42) (32), which supports a DNA damage mediated pathway of embryonic disruption. We also found an increased risk for spontaneous pregnancy loss with exposure to PM2.5, though the estimates did not reach statistical significance. Because both PM2.5 and NO2 are emitted from mobile sources, however, the results of our study add to a growing body of evidence that primary emissions contribute to spontaneous pregnancy loss.
Interestingly, results from previous studies have been mixed. Ha and colleagues found chronic exposures to O3 and PM2.5 during pregnancy were positively associated with the risk of pregnancy loss (HRO3 = 1.12, 95 CI 1.07–1.17; HRPM2.5 = 1.13, 95 CI 1.13–1.24), but not NO2 (HRNO2 = 1.03; 95 CI = 0.98–1.08) (16). In two recent ecological studies, Dastoorpoor and colleagues found a significant relationship between NO2 and premature birth, but not spontaneous pregnancy loss (13) and Enkhmaa and colleagues found a strong correlation between NO2 and spontaneous pregnancy loss (r > 0.8) (15). The observed differences between studies may be due to underlying differences in the composition of air pollution in different geographical regions or due to methodological differences between our studies. Ha and colleagues used a prospective cohort from Michigan and Texas, and while their study did examine risk at varying time points, the exposures were measured through the entire pregnancy and thus were largely chronic in nature. In contrast, we tested for associations between spontaneous pregnancy loss and exposures to pollutants over a much shorter duration in time (3 days, 7 days) in an urban area where air pollution is highly varied based on weather patterns and temperature inversions. Because Utah has the lowest smoking rates in the US and unique topography, our study area provided for a unique place to conduct this natural experiment. While the two aforementioned ecological studies show a relationship between pregnancy outcomes and NO2, our study benefits from self-matching and, thus allowing us to find an effect at the individual patient level.
This study has some notable limitations and strengths. Air pollution exposure was assigned at zip code of residence and thus we were unable to measure air pollution at a smaller level of aggregation or total exposure across all daily activities. We were not able to determine the exact gestational age of the fetuses in our study and therefore we could not test for differences in the effect by exact gestational age at time of exposure. Additionally, this study only captured women who presented to the UUED for care. Many other women may have sought outpatient care through their Obstetric or Primary Care providers. Spontaneous pregnancy loss that occurs within the first several weeks of gestation may not be documented if a woman is unaware of the pregnancy and perceives the event as a normal menstrual cycle or if the non-viable pregnancy is not detected until the patient’s first ultrasound. These factors will limit the absolute number of cases documented in our study period. Furthermore, we were able to document time of symptom presentation, but not time of actual embryonic or fetal demise. Because we cannot ascertain the exact time of spontaneous pregnancy loss, we explored 3-day and 7-day exposure windows. Strengths of this study include the large sample size (N=1398) and study design. The case-crossover design allows us to control for unobservable personal characteristics that do not change over the period of study including other risk factors for spontaneous pregnancy loss such as maternal age at conception, smoking behaviors, and previous spontaneous pregnancy loss (33).
Our results provide important insights for clinicians and patients making health care decisions and further study is needed in order to establish medical recommendations. Women who have other risk factors for spontaneous pregnancy loss may be a target group for future interventions. Additionally, the health effects of air pollution disproportionately affect some sub-populations over others. For example, NO2 exposure in the US has been shown to be higher for nonwhites, individuals living below the poverty level, and individuals with less than a high school education (24). While our results did not show effect modification by Hispanic ethnicity, future studies should test for differences in effect of NO2 and other air pollutants in this and other potentially sensitive subpopulations. As more evidence emerges that sperm epigenetics are essential for not only initiation, but also maintenance of a successful pregnancy (34), the association between air pollution and spermatogenesis should also be explored. Importantly, because we found increased risk with pollutants directly related to industrial and auto emissions, the results of this study can be utilized for potential public policy changes as well as personal behavior modification when particular environmental pollutants are high.
Conclusions
Our findings suggest that pregnant women may be at higher risk for spontaneous pregnancy loss during short periods of increased air pollution.
Supplementary Material
Acknowledgements
The authors wish to thank Bianca Rich and Ashley Allen for valuable assistance in managing data. This work was supported by the National Institutes of Health (Grant Number 1K12HD085852-01). This project has been also been supported by Consortium for Families & Health Research (C-FAHR) at the University of Utah.
Footnotes
Conflicts of Interest
Dr. Madsen reports grants from Roche Diagnostics, grants from Bristol-Myers Squibb, outside the submitted work. All other authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the Medicare population. N Engl J Med 2017; 376:2513–2522. doi: 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Yadama GN, et al. The Lancet Commission on pollution and health. Lancet 2018; 391:462–512. doi: 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- 3.Gentile D, Hegde GG, Shang J, Kekre S, Presto A, Venkat A. An evaluation of the relationship between outdoor airborne pollutants and emergency department presentations for acute respiratory conditions. Ann Emerg Med 2017; 66:S133–S135. doi: 10.1016/j.annemergmed.2015.07.406 [DOI] [Google Scholar]
- 4.Olsson D, Mogren I, Eneroth K, Frosberg B. Traffic pollution at the home address and pregnancy outcomes in Stockholm, Sweden. BMJ Open 2015; 5:e07034. doi: 10.1136/bmjopen-2012-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson D, Mogren I, Forsberg B. Air pollution exposure in early prengnacy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open 2013; 3:e001955. doi: 10.1136/bmjopen-2014-007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int 2011; 37:498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Tan Y, Mei H, Wang F, Li N, Zhao J, et al. Ambient air pollution the risk of stillbirth: a prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health 2018; 221:502–509. doi: 10.1016/j.ijheh.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Dadvand P, Nieuwenhuijsen MJ, Agustí À, de Batlle J, Benet M, Beelen R, et al. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. Eur Respir J 2014; 44:603–613. doi: 10.1183/09031936.00168813. [DOI] [PubMed] [Google Scholar]
- 9.Lanki T, Hampel R, Tiittanen P, Andrich S, Beelen R, Brunekreef B, et al. Air pollution from road traffic and systemic inflammation in adults: a cross-sectional analysis in the European ESCAPE Project. Environ Health Perspect 2015; 123:785–791. doi: 10.1289/ehp.1408224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, et al. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc 2016; 5:e002742. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, et al. Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Perspect 2012; 120:1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum JL, Chen LC, Zelikoff JT. Exposure to ambient particulate matter during specific gestational periods produces adverse obstetric consequences in mice. Environ Health Perspect 2017; 125:077020. doi: 10.1289/EHP1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dastoorpoor M, Idani E, Goudarzi G, Khanjani N. Acute effects of air pollution on spontaneous abortion, premature delivery, and stillbirth in Ahvaz, Iran: a time-series study. Environ Sci Pollut Res Int 2018; 25:5447–5458. doi: 10.1007/s11356-017-0692-9. [DOI] [PubMed] [Google Scholar]
- 14.Di Ciaula A, Bilancia M. Relationships between mild PM10 and ozone urban air levels and spontaneous abortion: clues for primary prevention. Int J Environ Health Res 2015; 25:640–255. doi: 10.1080/09603123.2014.1003041. [DOI] [PubMed] [Google Scholar]
- 15.Enkhmaa D, Warburton N, Javzandulam B, Uyanga J, Khishigsuren Y, Lodoysamba S, et al. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth 2014; 14:146. doi: 10.1186/1471-2393-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha S, Sundaram R, Buck Louis GM, Nobles C, Seeni I, Sherman S, et al. Ambient air pollution and the risk of pregnancy loss: a prospective cohort study. Fertil Steril 2018; 109:148–153. doi: 10.1016/j.fertnstert.2017.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou HY, Wang D, Zou XP, Yang ZH, Li TC, Chen YQ. Does ambient air pollutants increase the risk of fetal loss? A case–control study. Arch Gynecol Obstet 2014; 289:285–291. doi: 10.1007/s00404-013-2962-1. [DOI] [PubMed] [Google Scholar]
- 18.Moridi M, Ziaei S, Kazemnejad A. Exposure to ambient air pollutants and spontaneous abortion. J Obstet Gynaecol Res 2014; 40:743–748. [DOI] [PubMed] [Google Scholar]
- 19.Pereira LA, Loomis D, Conceição GM, Braga AL, Arcas RM, Kishi HS, et al. Association between air pollution and intrauterine mortality in São Paulo, Brazil. Environ Health Perspect 1998; 106:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green RS, Malig B, Windham GC, Fenster L, Ostro B, Swan S. Residential exposure to traffic and spontaneous abortion. Environ Health Perspect 2009; 117:1939–1944. doi: 10.1289/ehp.0900943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki K, Niemi ML. Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide. Int Arch Occup Environ Health 1982; 51:55–63. [DOI] [PubMed] [Google Scholar]
- 22.Mahalingaiah S Is there a common mechanism underlying air pollution exposures and reproductive outcomes noted in epidemiologic and in vitro fertilization lab-based studies? Fertil Steril 2018; 109:68. doi: 10.1016/j.fertnstert.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 23.United States Environmental Protection Agency. Air Now Updated 2018, May; Cited 2018, May. https://www.airnow.gov/.
- 24.Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One 2014; 9:e94431. doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Oceanic and Atmospheric Administration. Climate Data Online Search Updated 2018. Cited 2018, September https://www.ncdc.noaa.gov/cdo-web/search.
- 26.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 2005;16(6):717–726. [DOI] [PubMed] [Google Scholar]
- 27.United States Environmental Protection Agency. AQS Data Mart Updated 2015, Feb. Cited 2018, May https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html.
- 28.van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta 2012; 1822:1951–1959. doi: 10.1016/j.bbadis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Møller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 2010; 118:1126–1136. doi: 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Checa Vizcaíno MA, González-Comadran M, Jacquemin B. Outdoor air pollution and human infertility: a systematic review. Fertil Steril 2016; 106:897–904. doi: 10.1016/j.fertnstert.2016.07.1110. [DOI] [PubMed] [Google Scholar]
- 31.Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect 2011; 119:598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000; 320:1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Census Bureau. American Fact Finder Revised 2015, Feb, cited 2018, Jan https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t.
- 34.Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction 2012; 143:727–734. doi: 10.1530/REP-11-0450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


