Summary
In response to low levels of magnesium (Mg2+), the PhoQP two component system induces the transcription of two convergent genes, one encoding a 31-amino acid protein denoted MgtS and the second encoding a small, regulatory RNA (sRNA) denoted MgrR. Previous studies showed that the MgtS protein interacts with and stabilizes the MgtA Mg2+ importer to increase intracellular Mg2+ levels, while the MgrR sRNA base pairs with the eptB mRNA thus affecting lipopolysaccharide modification. Surprisingly, we found overexpression of the MgtS protein also leads to induction of the PhoRB regulon. Studies to understand this activation showed that MgtS forms a complex with a second protein, PitA, a cation-phosphate symporter. Given that the additive effect of ΔmgtA and ΔmgtS mutations on intracellular Mg2+ concentrations seen previously is lost in the ΔpitA mutant, we suggest that MgtS binds to and prevents Mg2+ leakage through PitA under Mg2+-limiting conditions. Consistent with a detrimental role of PitA in low Mg2+, we also observe MgrR sRNA repression of PitA synthesis. Thus, PhoQP induces the expression of two convergent small genes in response to Mg2+ limitation whose products act to modulate PitA at different levels to increase intracellular Mg2+.
Keywords: PhoP, PhoR, Hfq
Graphical Abstract
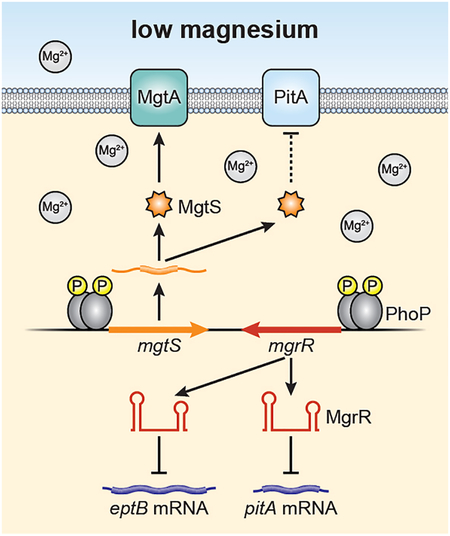
In response to limiting magnesium (Mg2+), the PhoQP two component system induces the transcription of two convergent genes that encode the 31-amino acid MgtS protein and the regulatory RNA MgrR. These small gene products were previously shown to regulate the MgtA Mg2+ importer and the eptB mRNA, respectively. We now find both also modulate the PitA phosphate symporter to increase intracellular Mg2+, pointing to a detrimental role of PitA under limiting Mg2+ conditions.
Introduction
When bacteria experience changing environmental conditions, they must enact a suitable physiological response to adapt and survive. Signal transduction systems allow environmental changes sensed extracellularly to be converted into an intracellular response, which can be an alteration in the transcription of specific genes or changes in the stability or translation of specific mRNAs, consequently leading to changes in protein levels or activity. Mediators of many of the post-transcriptional responses are small RNAs (sRNA) and small proteins whose expression is upregulated in response to altered environmental conditions such as cell envelope damage or fluctuations in metal ion or carbon source availability.
The sRNAs are approximately 50–300 nucleotides long and act either via base pairing with their mRNA target(s) to affect translation, stability and/or transcription, or by binding to protein targets to modify their functions (reviewed in (Storz et al., 2011, Gottesman & Storz, 2011, Wagner & Romby, 2015)). Since the discovery of the first sRNAs, hundreds have been characterized across bacterial species. Many of these sRNAs regulate protective responses, for instance reduction of synthesis of outer membrane proteins upon cell envelope damage or repression of select iron-containing proteins upon iron limitation, and thus help bacteria adapt to changes in their environment.
Comparatively little is known about small proteins, which are defined as proteins of ≤50 or ≤100 amino acids (aa) in bacteria or eukaryotes, respectively, that are encoded by a specific open reading frame (ORF) rather than being derived from cleavage of a larger protein (Storz et al., 2014). In the past decade, genome-wide-searches in bacterial and eukaryotic organisms have identified many previously unannotated small proteins (Hemm et al., 2008, Slavoff et al., 2013). However, despite the increasing number of newly discovered small proteins, very few of their targets have been identified and their mechanisms of action generally remain unclear. Nevertheless, it is evident that small proteins are induced in response to specific environmental conditions, and initial studies indicate they are also important regulators of bacterial stress responses (reviewed in (Storz et al., 2014, Duval & Cossart, 2017)).
One environmental factor that has significant impact on bacterial cells is Mg2+ levels. This divalent cation is essential in living organisms as it serves as a cofactor for critical enzymes such as RNA and DNA polymerases, stabilizes polyphosphate molecules such as nucleic acids, is important for membrane stability, and impacts the rate of protein synthesis (reviewed in (Groisman et al., 2013, Pontes et al., 2015)). Thus, maintenance of Mg2+ homeostasis is necessary despite the wide range of Mg2+ concentrations encountered by bacteria.
The major sensor of extracytoplasmic Mg2+ in Escherichia coli and a number of other bacteria is the sensor kinase PhoQ of the PhoQP two component signal transduction system (Véscovi et al., 1997). In addition to being activated by low Mg2+ (Kato et al., 1999), PhoQ in Salmonella enterica has been shown to be activated by other environmental stresses such as acidic pH (Prost et al., 2007) and cationic antimicrobial peptides (Bader et al., 2005) as well as by a decrease in cytoplasmic pH (Choi & Groisman, 2016). When activated, PhoQ promotes phosphorylation of the response regulator PhoP. Phosphorylated PhoP subsequently binds its target promoters, resulting in increased transcription of the PhoP regulon.
In E. coli, the PhoQP system upregulates two small membrane proteins, MgrB and MgtS, to affect Mg2+ homeostasis (Lippa & Goulian, 2009, Wang et al., 2017). MgrB interacts with the sensor kinase PhoQ to repress autophosphorylation, resulting in feedback inhibition that modulates PhoP target gene expression (Salazar et al., 2016). MgtS stabilizes the Mg2+ transporter MgtA to increase Mg2+ import. In addition to these small proteins, PhoP induces the sRNA MgrR (Moon et al., 2013, Moon & Gottesman, 2009), which has been shown to reduce transcription of the lipopolysaccharide (LPS)-modifying phosphoethanolamine transferase EptB to increase antimicrobial peptide sensitivity.
The PhoQP-regulated genes encoding the sRNA MgrR and the small protein MgtS are convergently transcribed and conserved in many enterobacterial species (Moon & Gottesman, 2009, Wassarman et al., 2001). Here we report that both small gene products regulate the phosphate symporter PitA to increase intracellular Mg2+ levels for cells in limiting Mg2+. We first found that overexpression of the MgtS protein induces the PhoRB regulon in a pitA-dependent manner for cells grown in rich medium. Further exploration of this effect led us to discover that the small protein co-purifies with PitA symporter and increases intracellular Mg2+ in a PitA-dependent manner in Mg2+ -limiting media. We also suggest that the MgrR sRNA base pairs with the pitA mRNA to reduce mRNA and protein levels and increase intracellular Mg2+. Together, these results indicate that PitA activity impacts bacterial growth in low Mg2+ conditions.
Results
MgtS overexpression activates the PhoRB signaling pathway
In the course of characterizing the 31-aa MgtS protein, we examined the consequences of a 7.5 min pulse of MgtS overexpression on the whole genome transcription profile using microarray analysis. Among the seven genes that were most strongly upregulated by MgtS overexpression (transcription increased over 5-fold in two independent experiments) under LB growth conditions, four (psiE, phoR, phoB, pstS) are known to be in the PhoRB regulon, which is activated by low phosphate (reviewed in (Hsieh & Wanner, 2010)) (Table 1). With a less stringent 2-fold cut-off, even more PhoRB targets were found to be upregulated by MgtS overexpression in both arrays (Table S1).
Table 1.
Microarray data for top induced genes
| Gene | Description | Fold change (array 1) | Fold change (array 2) | PhoRB regulation |
|---|---|---|---|---|
| psiE | phosphate-starvation inducible protein | 55.1 | 10.2 | + |
| phoR | sensor histidine kinase | 23.3 | 8.5 | + |
| phoB | DNA-binding response regulator | 20.9 | 9.7 | + |
| ycgB | hypothetical protein (RpoS regulon) | 9.9 | 8.5 | |
| pstS | phosphate ABC transporter | 8.1 | 5.5 | + |
| malS | periplasmic amylase, maltodextrin metabolism | 5.7 | 7.2 | |
| ycjF | heat shock protein (RpoH regulon) | 5.3 | 8.9 |
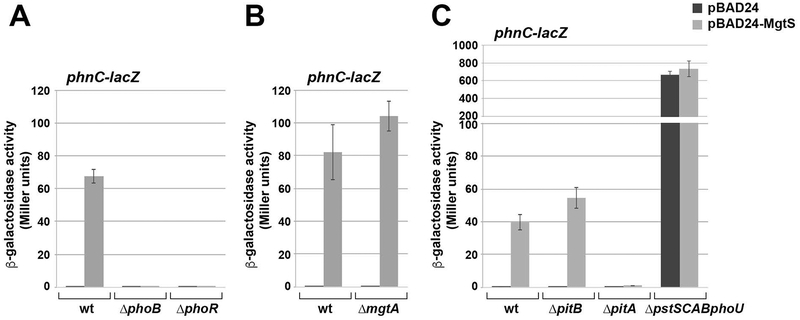
The activation of the PhoRB two-component system by MgtS was confirmed by assays of a transcriptional lacZ fusion to phnC, a well characterized PhoRB target gene (reviewed in (Hsieh & Wanner, 2010)). The expression of the phnC-lacZ fusion as measured by β-galactosidase activity was extremely low during exponential and early stationary growth in LB media but was induced greater than 200-fold within 15 min of MgtS overexpression (Fig. 1A and Fig. S1A). The induction of the phnC-lacZ fusion was abolished when either phoB or phoR were deleted from the chromosome (Fig. 1A), indicating that this effect is dependent on the PhoRB two-component system. We also examined induction of another PhoB target, the phoA gene encoding alkaline phosphatase by measuring phosphatase activity and observed similar results (Fig. S1B). Together these results indicate that high levels of MgtS rapidly induce the PhoRB regulon for E. coli cells are grown in LB medium.
Fig. 1. MgtS induction of the PhoRB target gene phnC is pitA-dependent.
A. MgtS acts on the PhoRB two-component system to induce phnC expression. Strains with ΔmgtS (GSO797), ΔmgtS ΔphoB (GSO798) and ΔmgtS ΔphoR (GSO799) with a chromosomal PphnC-lacZ fusion and carrying the pBAD24 vector control or pBAD24-MgtS plasmid were inoculated into LB medium, grown to OD600 ~0.5 and treated with 0.2% arabinose for 30 min. Culture aliquots (1 mL) were collected for measurement of β-galactosidase activity.
B. The induction of phnC expression by MgtS is not dependent on mgtA. Strains with ΔmgtS (GSO797) and ΔmgtS ΔmgtA (GSO800) with a chromosomal PphnC-lacZ fusion and carrying the pBAD24 vector control or pBAD24-MgtS plasmid were assayed for β-galactosidase activity as described above.
C. The induction of phnC expression by MgtS requires pitA. Strains with ΔmgtS (GSO797), ΔmgtS ΔpitB (GSO802), ΔmgtS ΔpitA (GSO801), and ΔmgtS ΔpstSCAB-phoU (GSO803) with a chromosomal PphnC-lacZ fusion and carrying the pBAD24 vector control or pBAD24-MgtS plasmid were assayed for β-galactosidase activity as described above.
For each strain, the enzyme activity reported is the average of three independent trials, and the error bars represent one SD.
MgtS-dependent activation of the PhoRB signaling pathway requires the PitA symporter
Previous studies showed MgtS interacts with and stabilizes the Mg2+ importer, MgtA (Wang et al., 2017). To determine whether the effects of MgtS on phnC-lacZ induction were via MgtA, we assayed the fusion in an ΔmgtA background (Fig. 1B). The mgtA deletion did not reduce MgtS-dependent induction of phnC-lacZ, suggesting MgtS has a second target in the cell.
We next wondered whether any of the known phosphate transport activities were required for MgtS activation of the PhoRB system. Three transporters for inorganic phosphate have been characterized in E. coli, the high-affinity transporter PstSCAB and two homologous low-affinity transporters PitA and PitB (reviewed in (Hsieh & Wanner, 2010)). To examine if MgtS acts through any of these three transporters, we deleted the corresponding genes and monitored the effects of MgtS overexpression in these backgrounds. For the pstSCAB deletion, the phoU gene, which encodes a regulatory protein and lies in the same operon, was also deleted. As expected, given the dominant role played by PstSCAB and PhoU in phosphate transport and sensing, we observed very high induction of the phnC-lacZ fusion in the ΔpstSCABphoU strain and were unable to observe a statistically significant increase in β-galactosidase activity upon MgtS overexpression. MgtS-dependent PhoRB induction was still present in the ΔpitB mutant; however, it was abolished in the ΔpitA mutant (Fig. 1C). This result indicates MgtS acts through PitA to activate PhoRB.
We do not know the mechanisms by which MgtS and PitA impact PhoRS activity. Interestingly, low Mg2+ was recently found to activate the PhoRB response in S. enterica due to low free cytoplasmic phosphate levels brought about by decreased protein synthesis associated with reduced ATP consumption, though induction of the reporter construct was only observed after 120 min (Pontes & Groisman, 2018). Independent of the mechanisms, we followed up our observation that MgtS acts through PitA to test the effect of different MgtS alleles and explore the consequences for growth on low Mg2+.
An MgtS D30A mutant, but not a D31A mutant, is defective for phnC-lacZ induction
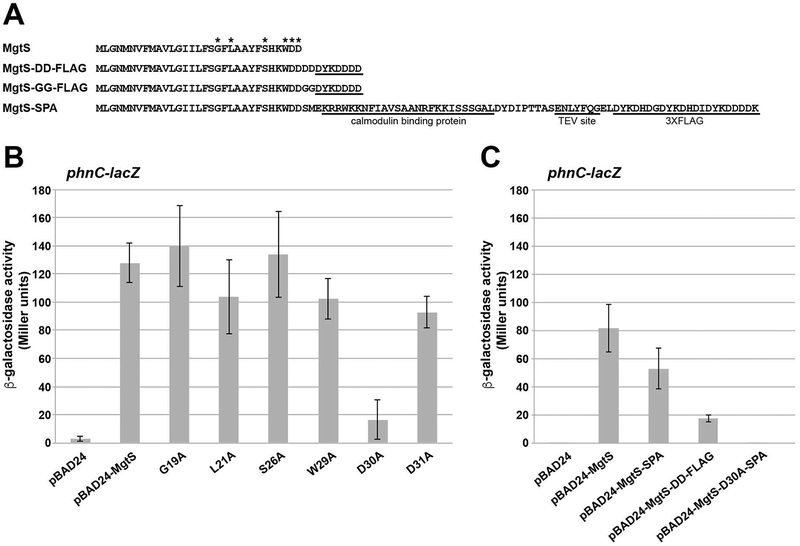
We previously found that mutations of either of the two C-terminal aspartate residues (D30A or D31A) abolished the MgtS-mediated increase in intracellular Mg2+, as reflected in decreased activity of lacZ fusions with a Mg2+-responsive riboswitch from the mgtA leader (Wang et al., 2017). To determine if the same MgtS derivatives were defective at phnC-lacZ induction, we next tested the effects of the mutants (Fig. 2A) on induction of this second fusion. Intriguingly, while the D30A mutant was unable to induce expression of the phnC-lacZ reporter, the D31A mutant had almost wild type activity (Fig. 2B).
Fig. 2. D30A and MgtS-DD-FLAG derivatives of MgtS are defective for phnC-lacZ induction.
A. The sequence of MgtS and the different tagged derivatives. Asterisks indicate the mutated residues.
B. A strain with ΔmgtS (GSO797) and a chromosomal PphnC-lacZ fusion carrying pBAD24, pBAD24-MgtS or indicated mutant derivatives were assayed for β-galactosidase activity as described for Fig. 1A.
C. A strain with ΔmgtS (GSO797) and a chromosomal PphnC-lacZ fusion carrying pBAD24, pBAD24-MgtS or indicated tagged derivatives were assayed for β-galactosidase activity as described for Fig. 1A. Note the pBAD24 and pBAD-MgtS samples were the same as those in Fig 1B.
For each strain, the enzyme activity reported is the average of three independent trials, and the error bars represent one SD.
Our previous study also showed that while MgtS C-terminally tagged with a modified FLAG tag (containing a DD linker and D-rich FLAG tag) was functional for maintaining high intracellular Mg2+ concentrations as assayed by the riboswitch reporter, MgtS C-terminally tagged with an SPA tag (containing the calmodulin binding protein followed by three conventional FLAG tags) was not (Wang et al., 2017). The opposite was true for the effect of tagged MgtS (Fig. 2A) on phosphate levels as assayed by the phnC-lacZ fusion (Fig. 2C). Overexpression of MgtS-SPA led to significant induction of the fusion, while overexpression of MgtS-DD-FLAG had a lesser effect. As expected from the results of the mutational analysis, MgtS-D30A-SPA also was unable to induce expression of the phnC-lacZ reporter. Based on these experiments, MgtS-SPA was used to investigate a potential PitA-MgtS interaction, with MgtS-D30A and MgtS-D30A-SPA serving as controls.
MgtS forms a complex with PitA
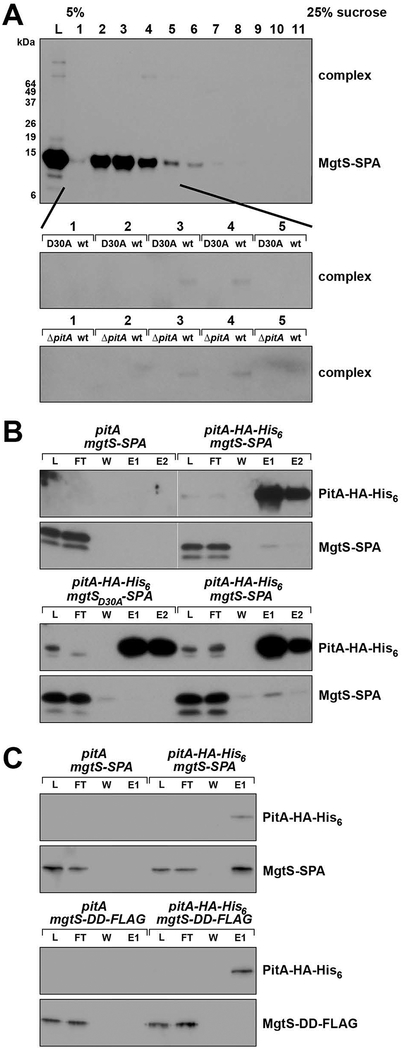
To determine whether the pitA-dependent induction of phnC-lacZ by MgtS overexpression was due to a direct interaction between MgtS and PitA, we tested MgtS association with PitA in biochemical assays using various epitope-tagged derivatives of MgtS and PitA. First, we performed sucrose density gradient sedimentation and chemical crosslinking to test if plasmid-expressed MgtS-SPA forms complex(es) with other proteins in LB medium when MgtA is not expressed. When treated with a disuccinimidyl suberate (DSS) crosslinker, one band of around 70 kDa appears in fraction 4, representing MgtS-SPA covalently linked to a larger complex (Fig. 3A). This crosslinked band was absent when the non-functional MgtS-D30A-SPA mutant allele, which does not induce phnC-lacZ, was expressed instead of wild type MgtS-SPA. Additionally, the band was absent when the pitA gene is deleted, indicating the band corresponds to a complex between PitA and MgtS. The apparent molecule weight of the complex (~70 kDa) suggests a stoichiometry of a one MgtS-SPA (12 kDa) to one PitA (53 KDa).
Fig. 3. MgtS-SPA associates with the PitA phosphate transporter.
A. Chemical crosslinking reveals a high molecular weight complex containing MgtS-SPA. The MgtS-D30A-SPA mutant does not form the complex. The complex also is not detected in the absence of PitA. Strains that were either pitA+ (MG1655) or ΔpitA (GSO815) and carried pBAD24-MgtS-SPA or pBAD24-MgtS-D30A-SPA plasmids were grown in LB medium to OD600 ~0.5 and treated with 0.2% arabinose for 7.5 min. Cells were then lysed and the whole cell lysate was separated by 5%−25% sucrose gradient sedimentation. Fractions recovered from gradient sedimentation were crosslinked with 25 mM DSS for 30 min prior to SDS-PAGE analysis and detection with α-FLAG antibody.
B. Wild type MgtS-SPA but not MgtS-D30A-SPA mutant co-purifies with PitA-HA-His6. Strains carrying chromosomal alleles of mgtS-SPA (GSO767), mgtS-SPA pitA-HA-His6 (GSO804), MgtS-D30A-SPA (GSO805), or mgtS-D30A-SPA pitA-HA-His6 (GSO806) were grown in LB medium to OD600 ~0.5, collected, and lysed. The whole cell lysate (L) was applied onto a Ni-NTA column and flow-through (FT), wash (W) and two step-wise eluates (E1 and E2) were collected and analyzed by SDS-PAGE. MgtS-SPA and PitA-HA-His6 were detected by using α-FLAG antibody and α-HA antibody, respectively.
C. MgtS-SPA but not MgtS-DD-FLAG co-purifies with PitA-HA-His6. Wild type E. coli (MG1655) carrying pBAD24 derivatives expressing PitA-HA-His6, MgtS-DD-FLAG or MgtS-SPA were grown in LB medium to OD600 ~0.5 and induced with 0.2% arabinose for 30 min, collected, and resuspended in lysis buffer. Wild type or PitA-HA-His6-expressing cells were mixed with those expressing MgtS-DD-FLAG or MgtS-SPA in a 2:3 ratio, homogenized, and applied to a Ni-NTA column for co-purification. After analysis by SDS-PAGE, MgtS-SPA and MgtS-DD-FLAG were detected using α-FLAG antibody and PitA-HA-His6 was detected using α-HA antibody.
We further examined this interaction in a reciprocal co-purification assay that employed PitA as the bait protein in a strain with chromosomally-encoded PitA-HA-His6 and MgtS-SPA. Cells were lysed and applied to a nickel-nitrilotriacetic acid (Ni-NTA) column for co-purification. As shown in Fig. 3B, the HA-His6-tagged PitA was efficiently purified on Ni-NTA beads and enriched in the elution fraction. The small fraction of MgtS-SPA that copurifies with PitA-HA-His6 (particularly in the first elution) is not observed for the strain with untagged PitA. Consistent with the results of the crosslinking experiment, the non-functional MgtS-D30A-SPA mutant did not co-purify with PitA-HA-His6.
We also investigated the ability of PitA-HA-His6 to co-purify with MgtS-SPA (activates phnC-lacZ) or MgtS-DD-FLAG (does not activate phnC-lacZ but interacts with MgtA) to determine whether the loss of activity observed in the assays of the phnC-lacZ fusion correlates with the lack of protein-protein interaction. In cell mixing experiments, similar to those carried out to examine the interaction between MgtS-DD-FLAG and MgtA-HA-His6 (Wang et al., 2017), cells expressing plasmid-encoded MgtS-SPA or MgtS-DD-FLAG were mixed with wild type cells or cells expressing plasmid-encoded PitA-HA-His6. These samples were then homogenized, incubated in the detergent n-dodecyl-β-maltoside (DDM) to facilitate mixing of the membranes, and applied to Ni-NTA columns (Fig. 3C). Immunoblotting the eluate revealed that MgtS-SPA co-purified with PitA-HA-His6. In contrast, MgtS-DD-FLAG did not co-purify with PitA-HA-His6. Thus, the loss of phnC induction activity by the FLAG-tagged derivative of MgtS likely is due to the loss of the PitA interaction. Together the biochemical experiments indicate that MgtS physically associates with the PitA phosphate transporter. We hypothesized that this interaction could alter PitA activity or levels and thereby decrease phosphate availability in LB, leading to PhoRB activation at the membrane.
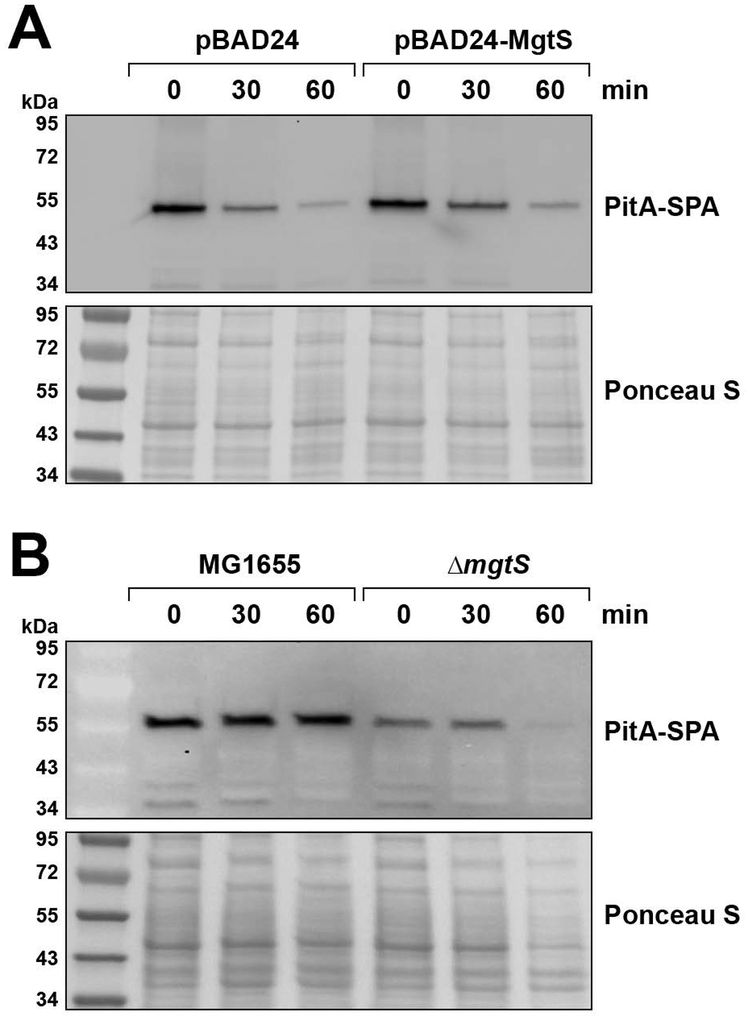
MgtS has a mild effect on PitA levels
Given that MgtS was shown to increase the stability of MgtA (Wang et al., 2017), we wanted to test whether MgtS impacts PitA levels. We thus monitored chromosomally-encoded PitA-SPA in LB-grown cells in the presence or absence of MgtS overexpression (Fig. 4A). The amount of PitA-SPA protein detected decreased over the time period of the assay for both the pBAD24 vector control and pBAD24-MgtS cells suggesting that another mechanism regulates PitA protein levels as cells enter stationary phase or the PitA-SPA construct becomes unstable. Overexpression of MgtS was associated with slightly higher levels of the phosphate transporter in the pBAD24-MgtS cells compared to the pBAD24 cells.
Fig. 4. MgtS slightly increases PitA-SPA levels.
A. Strains expressing a chromosomally-encoded SPA-tagged PitA (GSO807) and carrying pBAD24 or pBAD24-MgtS plasmids were grown in LB medium to OD600 ~0.1 whereupon an aliquot was taken (0 min). The remaining cells were induced with 0.2% arabinose and aliquots were taken at the indicated times.
B. Strains expressing a chromosomally-encoded SPA-tagged PitA in a wild type (GSO807) or ΔmgtS (GSO820) background grown to OD600 ~0.1 in N-minimal medium with 500 μM Mg2+, was washed with and resuspended in N-minimal medium without added Mg2+, whereupon samples were taken at the indicated times. We found the ΔmgtS pitA-SPA strain grew poorly in N-minimal medium without added Mg2+, which likely accounts for the reduced Ponceau staining at the 60 min time point.
For all time points, aliquots were pelleted, resuspended to OD600 ~10 in SDS loading buffer, and separated by SDS-PAGE for immunoblot analysis using anti–FLAG antibodies.
All of our experiments up to this point were conducted by overexpressing MgtS in LB media during exponential growth. However, we have previously observed that MgtS is only poorly expressed during exponential growth in LB (Hemm et al., 2008). In contrast, the small protein is robustly expressed in N minimal media without added Mg2+ (Wang et al., 2017). Given that we also wanted to assay the effect of chromosomally-expressed MgtS on PitA, we next examined PitA-SPA levels after wild type and ΔmgtS cells were shifted to N-minimal medium without added Mg2+ (Fig. 4B). The ΔmgtS strain had decreased PitA-SPA compared to the wild type strain, although the reduction of detectable PitA-SPA at 60 min is partially due to a reduction in total protein associated with slowed growth of the ΔmgtS pitA-SPA strain.
MgtS increases phosphate levels in limiting Mg2+
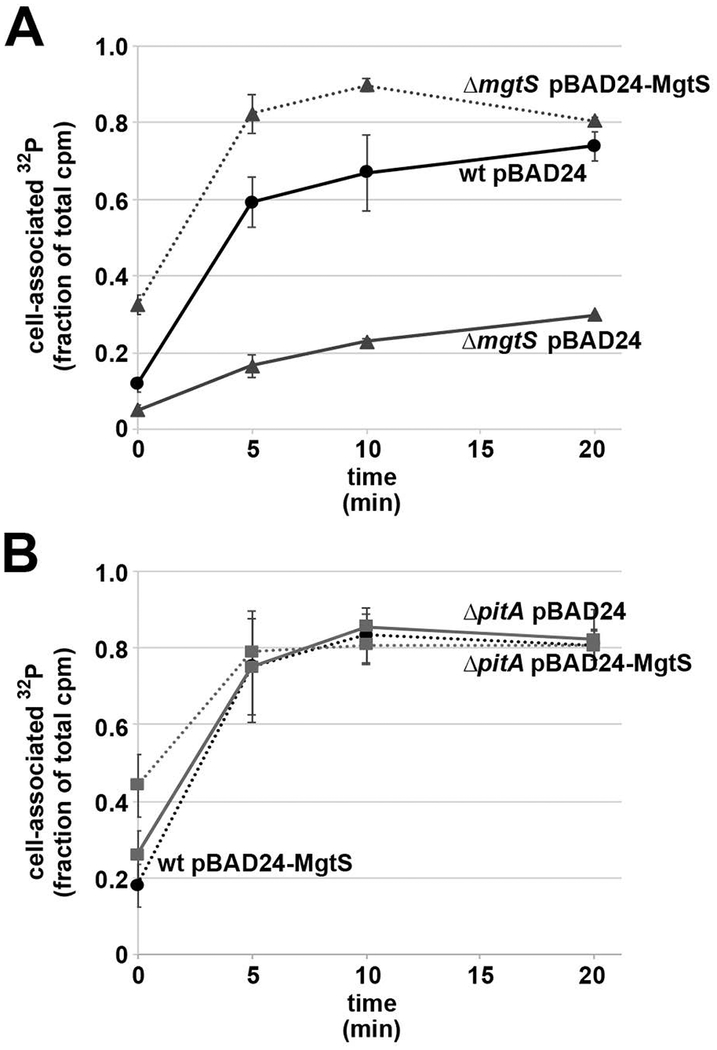
The PitA symporter has been shown to both import and export metal phosphate complexes (MeHPO4) with various cations including Mg2+ (van Veen et al., 1994). The rate and direction of transport is impacted by pH and likely other factors. The overall positive effect of MgtS on PitA levels is milder than the impact of MgtS on MgtA levels but is counterintuitive to the presumed negative effect of MgtS on phosphate levels as reported by the phnC-lacZ fusion for LB-grown cells. We thus sought to directly determine the consequences of MgtS for intracellular phosphate levels by measuring 32P in cells incubated with the radioactive potassium phosphate. Wild type and ΔmgtS strains carrying the pBAD24 control and the ΔmgtS strain complemented with pBAD24-MgtS were grown in N minimal media without added Mg2+, washed and resuspended in Tris-buffered saline to which 32P-potassium phosphate was added. At various time points, aliquots were applied to a vacuum filter disc, washed, and the radioactivity associated with the cells was quantified. Compared to the wild type strain, the ΔmgtS mutant accumulated less 32P over time, while overexpression of MgtS increased the amount of 32P associated with the bacteria (Fig. 5A). Overexpression of MgtS in the ΔpitA mutant background did not lead to increased 32P (Fig. 5B). Together these results suggest that MgtS affects phosphate transport through PitA, by enhancing phosphate import, changing PitA specificity or preventing phosphate export.
Fig. 5. MgtS increases cell-associated 32P levels.
A. Wild type (GSO770) carrying pBAD24 and ΔmgtS (GSO772) carrying pBAD24 or pBAD24- MgtS plasmids were transferred to in N minimal media without added Mg2+, induced for 30 min, washed and resuspended in Tris-buffered saline.
B. Wild type (GSO770) carrying pBAD24 and ΔpitA (GSO816) carrying pBAD24 or pBAD24- MgtS plasmids were transferred to in N minimal media without added Mg2+, induced for 30 min, washed and resuspended in Tris-buffered saline.
For both sets of strains, 32P-potassium phosphate was added, and an aliquot removed (0 min). The samples were incubated at 37°C, and at the indicated time points, aliquots were removed for the determination of CPM/mL.
Additive effect of ΔmgtA and ΔmgtS mutations is lost in a ΔpitA background
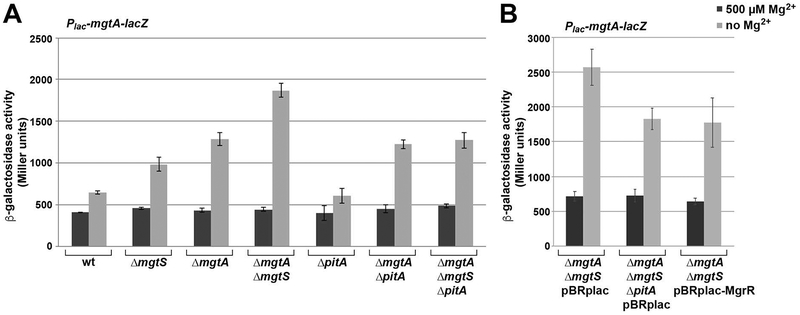
We next examined the consequences of the MgtS-PitA interaction on intracellular Mg2+ levels. Our previous study showed that the ΔmgtS strain is defective for intracellular Mg2+ accumulation as monitored by assays of the mgtA riboswitch fusion to lacZ. These defects were exacerbated in a ΔmgtA ΔmgtS double mutant strain, which lacks the MgtA Mg2+ importer target of MgtS. The ΔmgtA ΔmgtS double mutant strain had higher β-galactosidase activity associated with low Mg2+ levels than the ΔmgtA single mutant strain (Wang et al., 2017). This difference between the ΔmgtA and ΔmgtA ΔmgtS strains also suggested that MgtS might have a second partner. To test whether this partner was PitA, we examined the effects of deleting the pitA gene in the wild type background as well as in the strain lacking both MgtA and MgtS (Fig. 6A). As previously, β-galactosidase activity was measured for cultures grown in N-minimal medium with 500 μM Mg2+ or without added Mg2+. In low Mg2+, the levels of β-galactosidase activity in the triple mutant were reduced to that of the ΔmgtA single mutant consistent with the hypothesis that MgtS prevents PitA-mediated efflux of Mg2+ ions in addition to stabilizing the MgtA Mg2+ importer.
Fig. 6. ΔpitA and MgrR sRNA overexpression eliminate additional induction of Mg2+-dependent fusion in ΔmgtA ΔmgtS background.
A. β-galactosidase activity was assayed for cultures of wild type (GSO770), ΔmgtS (GSO772), ΔmgtA (GSO808), ΔmgtA ΔmgtS (GSO809), ΔpitA (GSO810), ΔmgtA ΔpitA (GSO811), and ΔmgtA ΔmgtS ΔpitA (GSO812) carrying the chromosomal Plac-leadermgtA-lacZ fusion grown in N medium with 500 μM Mg2+ or without added Mg2+ and assayed for β-galactosidase as described (Wang et al., 2017).
B. ΔmgtA ΔmgtS (GSO809) and ΔmgtA ΔmgtS ΔpitA (GSO812) carrying the chromosomal Plac-leadermgtA-lacZ fusion and containing pBR-plac or pBR-plac-MgrR as indicated were assayed for β-galactosidase activity as described for Fig. 5A.
For each strain, the enzyme activity reported is the average of three independent trials, and the error bars represent one SD.
MgrR small RNA encoded convergent to mgtS represses PitA synthesis
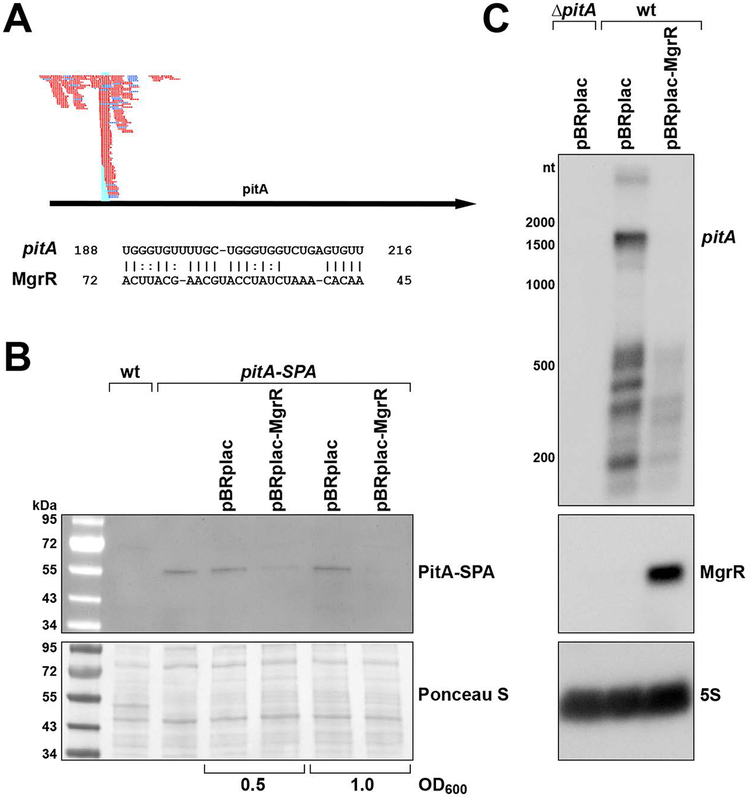
In the first study of the PhoQP-regulated MgrR sRNA encoded convergent to MgtS, the levels of the pitA mRNA were observed to decrease upon pulse MgrR overexpression in microarray assays, but it was reported that there were no discernable changes in pitA mRNA levels by northern analysis (Moon & Gottesman, 2009). However, a recent experimental analysis of all targets of Hfq-binding sRNAs again provided evidence that MgrR base pairs with the pitA mRNA (Melamed et al., 2016). Unlike the case for sRNAs that regulate protein translation by base pairing with and occluding the ribosome binding site, the predicted MgrR binding site lies ~200 nt into the coding sequence of pitA (Fig. 7A) overlapping the pitA-MgrR chimeric fragments identified by the global analysis (Melamed et al., 2016).
Fig. 7. pitA is repressed by the MgrR sRNA.
A. Chimeras (indicated in red and dark blue) found in RIL-Seq experiments (Melamed et al., 2016) encompass region (light blue) of base pairing predicted between pitA mRNA and MgrR using IntaRNA (Mann et al., 2017) in December 2017. Numbering is with respect to start codon of the pitA ORF and +1 of MgrR.
B. MgrR overexpression leads to reduced PitA-SPA levels. Strains containing a chromosomally-encoded PitA-SPA (GSO807) and pBRplac or pBRplac-MgrR plasmids were grown in LB medium with 1 mM IPTG to OD600 ~0.5 and ~1.0, whereupon aliquots were taken. A strain expressing untagged PitA (MG1655) and GSO807 without a plasmid grown to OD600 ~0.5 were used as negative and positive controls, respectively. For all samples, aliquots were pelleted, resuspended to OD600 ~10 in SDS loading buffer, and separated by SDS-PAGE for immunoblot analysis using anti–FLAG antibodies.
C. MgrR overexpression leads to reduced pitA mRNA levels. Wild type MG1655 carrying pBRplac or pBRplac-MgrR plasmids and ΔpitA (GSO816) carrying pBRplac were grown in LB medium with 1 mM IPTG to OD600 ~0.5 whereupon total RNA was isolated and separated on an agarose gel for northern blot detection with probes against the pitA mRNA, MgrR sRNA, and 5S rRNA.
We asked whether MgrR could repress PitA synthesis and thus examined the levels of PitA-SPA without and with MgrR overexpression (Fig. 7B). MgrR expression caused a decrease in PitA-SPA detected compared to empty vector. A similar decrease in the levels of the ~1,500 nt pitA mRNA and its degradation products was observed upon MgrR overexpression (Fig. 7C). A fragment of pitA encompassing the 5′ UTR and the sequences where most pitA-MgrR chimeras are found was fused to lacZ. This construct was also repressed by wt MgrR overexpression but not a MgtR derivative with two mutations in the region of predicted pairing (Fig. S2). A pitA-lacZ fusion carrying the compensatory mutations was less well regulated by wt MgrR. However, regulation was not restored by mutant MgrR.
Since MgrR acts to negatively regulate PitA levels, we expected that overexpression of MgrR should phenocopy the effect of ΔpitA on intracellular Mg2+ accumulation in the ΔmgtA ΔmgtS double mutant background. Thus, we assayed the effects of overexpressing MgrR in ΔmgtA ΔmgtS. (Fig. 6B). Induction of MgrR reduced β-galactosidase activity to the levels observed in the ΔmgtA ΔmgtS ΔpitA strain, consistent with the observation that MgrR reduces PitA levels to prevent Mg2+ efflux.
The findings presented here demonstrate that in Mg2+ limiting conditions, the PhoQP-regulated small protein MgtS and sRNA MgrR both regulate the PitA transporter, leading to increased intracellular Mg2+. The results also show that the 31-aa MgtS protein has a second protein partner (PitA) in addition to the Mg2+ importer (MgtA), which MgtS was previously shown to stabilize.
Discussion
In recent years, re-examination of genome annotation and proteomics approaches have led to the identification of an increasing number of small proteins (reviewed in (Storz et al., 2014, Duval & Cossart, 2017)). However, very few of their functions have been characterized. We previously showed that the 31-aa protein MgtS, which is induced by PhoQP in response to Mg2+ limitation, binds to and stabilizes the MgtA Mg2+ importer to increase intracellular Mg2+ in E. coli (Wang et al., 2017). In the present study, we observed that overexpression of MgtS activated the PhoRB regulon via the cation-phosphate symporter PitA. We found that MgtS binds to PitA and that this interaction contributes to an increase in intracellular Mg2+ content. Reinforcing the connection between Mg2+ homeostasis and PitA, we also showed that PitA expression is repressed by the PhoQP-regulated MgrR sRNA. Together our work suggests that the convergent mgtS-mgrR locus promotes intracellular Mg2+ accumulation in cells with MgtS stabilizing the MgtA Mg2+ transporter and modulating PitA activity and with MgrR reducing PitA levels.
31-aa MgtS targets two different membrane proteins
Although multiple different small proteins have been shown to bind to and regulate the mammalian sarcoplasmic reticulum Ca2+-ATPase (SERCA) (Anderson et al., 2016), to our knowledge, a single small protein has not previously been reported to interact with more than one larger protein. The targets of MgtS are from two different transporter families, the P-type ATPase and inorganic phosphate transporter families, although both are predicted to have 10 transmembrane domains. Although MgtS is only 31 amino acids long, the determinants of the MgtS interactions with PitA and MgtA differ. While the C-terminus appears to be important for the binding to both transporters, the penultimate aspartic acid residue (D30) is required for the MgtS effect on both phosphate sensing and Mg2+ import but the terminal aspartic acid (D31) was only required for the effect on Mg2+ import. A broad alignment of MgtS homologs revealed that the C-terminal SHKWDD sequence is nearly invariable, including the two terminal aspartic acid residues (Fig. S3A). The only exceptions are D30N substitutions in some Salmonella, Dickeya and Kluyvera strains and D31N substitutions in some Escherichia albertii and Klebsiella strains. Further experiments should address whether the asparagine substitutions selectively affect MgtS activity on MgtA and/or PitA and whether other small proteins possess a similarly conserved motif.
In line with the C-terminal amino acids being important for function, we observed that different C-terminal tags used for biochemical studies of MgtS affected its ability to interact with MgtA or PitA. The MgtS-DD-FLAG-tagged version could co-purify with MgtA but was unable to interact with PitA. In contrast, the MgtS-SPA-tagged version could interact with PitA but was unable to interact with MgtA. It is possible that the additional aspartic acids provided near the C-terminus by the short FLAG tag sequence (DYKDDDD) allows the interaction with MgtA but prevents the interaction with PitA. The PitA transporter structure is not known and the mechanism by which MgtS affects PitA function remains an open question. To further characterize the determinants that drive the MgtS interaction with its targets, it will be interesting to identify additional variants that are specific to either MgtA or PitA.
Co-regulated and co-conserved MgtS and MgrR have the same target
At first glance, the two syntenic and convergently-transcribed PhoQP targets, mgtS and mgrR, appear to represent two distinct functions within the PhoQP pathway: MgrR increases resistance to the cationic antibiotic polymyxin B by downregulating eptB transcript levels to affect lipopolysaccharide modification (Moon & Gottesman, 2009), while MgtS promotes survival under low Mg2+(Wang et al., 2017). However, the co-regulation and co-conservation of these two genes is consistent with the observation that MgrR and MgtS act in concert in low Mg2+ to regulate PitA activity by repressing synthesis and affecting function, respectively. Another example of a sRNA and small protein pair regulating the same gene product is SgrST regulation of PtsG (Lloyd et al., 2017). The SgrS sRNA base pairs with the ptsG transcript to promote degradation while the SgrT small protein blocks the activity of already synthesized PtsG. However, in contrast to MgrR and MgtS, which are encoded by different genes, SgrT is encoded on the SgrS sRNA.
Unlike most characterized sRNA-mRNA interactions in enteric bacteria, where base pairing near the ribosome binding site occludes 30S access and blocks translation initiation, the chimeras observed in the RIL-Seq data (Melamed et al., 2016) indicate MgrR base pairs well within the coding sequence of pitA. For the few sRNA interactions within coding sequences that have been characterized thus far, the base pairing either leads to the recruitment of the Hfq chaperone, which in turn blocks ribosome binding as in the case of DicF binding to the manX mRNA (Azam & Vanderpool, 2018), or promotes cleavage of the target mRNA as observed for SdsR regulation of ompD (Fröhlich et al., 2012). Given the reduction in pitA mRNA levels, we think MgrR is acting to promote cleavage.
MgtS and MgrR are highly co-conserved; however, while Cedecea, Yokenella, Cronobacter, Pectobacterium, Dickeya and Serratia species encode MgtS, they do not have reliable hits for MgrR homologs (Table 2 and Fig. S3). Conversely, Leclercia, Lelliottia and Pluralibacter species encode MgrR but lack reliable MgtS hits (Table 2 and Fig. S4). Given the broader conservation of MgtS, it is likely that the small protein is more evolutionarily ancient, with MgrR being acquired later (indicated by a star, Fig. S3B). Alternatively, MgtS may have been lost from the species encoding the sRNA but lacking the small protein. The mgtS and mgrR are syntenic in most species that encode both regulators (Fig. S5). The exceptions are Enterobacter, Raoultella, and Klebsiella, where there may have been insertions between the two genes. An interesting question for further research is how regulatory small proteins and sRNAs that target the same pathway evolved.
Table 2.
Co-conservation of MgtS and MgrR*
| Species | MgtS | MgrR | Synteny |
|---|---|---|---|
| Salmonella | + | + | + |
| Cedecea | + | ||
| Citrobacter | + | + | + |
| Escherichia | + | + | + |
| Shigella | + | + | + |
| Kosakonia | + | + | + |
| Enterobacter | + | + | |
| Yokenella | + | ||
| Klebsiella | + | + | |
| Raoultella | + | + | + |
| Kluyvera | + | + | |
| Cronobacter | + | ||
| Pectobacterium | + | ||
| Dickeya | + | ||
| Serratia | + | ||
| Leclercia | + | ||
| Lelliottia | + | ||
| Pluralibacter | + |
MgtS and MgrR alignments and descriptions of applied approaches and procedures are presented in the Supporting Information. The plus sign (+) denotes reliable hits for MgtS or MgrR genes that are found in at least some species/strains of a marked genus of the family Enterobacteriaceae.
Intersection of Mg2+ and phosphate homeostasis
While the impact of phosphate on Mg2+ homeostasis and vice versa is not fully understood, our findings contribute to the growing body of knowledge pointing to intersections between the homeostasis of these two ions. For instance, the PhoU protein is known to interact with both PstB and PhoR, and a deletion of phoU locks PhoR in its kinase state, resulting in elevated expression of the PhoRB regulon (Gardner et al., 2014). The divalent cations Mg2+ and Mn2+ affect the function of PhoU, possibly by driving its localization to the membrane, which in turn causes PhoR to favor phosphatase activity, reducing PhoB activation. When intracellular Mg2+ concentrations are below the Kd (1.5 ± 0.68 mM) of PhoU, the protein no longer localizes to the membrane. PhoR is converted to its kinase state, thereby mimicking low phosphate conditions and resulting in the activation of the PhoRB regulon. Consistent with this, it was recently found that low intracellular Mg2+ in Salmonella enterica strains lacking Mg2+ transporters led to induction of the PhoRB regulon (Pontes & Groisman, 2018). It also is interesting to note that mutations in pitA, mgrB and phoQ all were found in screens for long-term adaptation to the antibiotic trimethoprim in LB medium (Baym et al., 2016). In the context of our results, we suggest that pitA loss could arise as an adaptation to low Mg2+, as might occur during growth in LB (Papp-Wallace & Maguire, 2008).
The initial observation that implicated MgtS in phosphate homeostasis was the induction of PhoRB-regulated targets upon MgtS overexpression for LB-grown cells. This induction suggested that MgtS might be causing a decrease in phosphate levels. However, our direct measurement of 32PO4 associated with cells after growth in Mg2+-limited media showed that MgtS increases the amount of phosphate. These seemingly conflicting results could be explained by differences between the effects of MgtS overexpression in LB medium versus chromosomally-expressed MgtS in N minimal medium with limited Mg2+ as well as incomplete understanding of PhoRB activation. Indeed, there are conflicting reports of whether PhoRB detects extracellular phosphate at the periplasm (Hsieh and Wanner, 2010) or intracellular phosphate at the cytoplasm (Pontes and Groisman, 2018). Regardless, our observations that two Mg2+-responsive small gene products can promote intracellular Mg2+ accumulation by modulating the PitA phosphate symporter adds yet another area of regulatory crosstalk between Mg2+ and phosphate homeostasis. This dual regulation together with the fact that pitA mutants have been detected as random mutants under a number of experimental conditions where limiting Mg2+ could be a problem (Baym et al., 2016, Malykh et al., 2018) suggest that further studies of the PitA protein and its interaction with the small MgtS protein are warranted.
Experimental procedures
Strain construction
All strains are derivatives of a Storz laboratory stock of E. coli K-12 MG1655 and are listed in Table S2. The plasmids employed in the study are listed in Table S3 and oligonucleotides employed are listed in Table S4. All deletion strains were generated by λ-Red–mediated recombineering (Datsenko & Wanner, 2000, Yu et al., 2000) using the indicated oligonucleotides, some of which had barcodes (Hobbs et al., 2010). Antibiotic markers were moved between strains by P1 transduction (Thomason et al., 2007). When necessary, resistance cassettes were excised from the chromosome by FLP-mediated recombination (Cherepanov & Wackernagel, 1995). To generate a strain harboring a chromosomal phnC-lacZ fusion (GSO814), we amplified a fragment from −529 to +27 nt with respect to the phnC ORF, encompassing the phnC promoter region, ribosome binding site and first nine amino acids. A mini-λ cassette (tet) and standard recombineering techniques were used to replace a counter-selectable cat-sac cassette (Mandin & Gottesman, 2009) on the MG1655 chromosome with this fragment. The phnC fragment ultimately replaced a genomic region that extends from −60 to +27 nt with respect to the lacI and lacZ ORFs, respectively; the first nine codons of phnC thus replace the first nine codons of lacZ. To generate a strain with the chromosomal pitA-lacZ fusion (GSO821), we amplified a fragment from −30 to +375 nt with respect to the pitA ORF, encompassing the 5′ UTR and the sequences of the majority of the MgrR chimeras. This fragment was used to replace the cat-sac cassette by recombination as above. To generate the compensatory mutant, the pitA region was cloned into pBAD24, and the mutation was introduced using the Q5 Site-Directed Mutagenesis kit (New England Biolabs). The resulting plasmid was used as a template for amplification, and the product was used for recombination as above. All mutations and fusions were confirmed by sequencing.
Growth conditions
All cells were grown in liquid or solid LB medium (10 g of tryptone, 5 g of yeast extract, 10 g of NaCl per liter) or in N-minimal medium (pH 7.4) (Hmiel et al., 1986) at 37°C, except for any strains carrying mini-λ cassettes, which were grown in LB at 30°C. Liquid cultures were all shaken at 250 rpm. Glucose and arabinose were used at 0.2% (w/v), and IPTG was used at 1 mM. Antibiotics were used at the following concentrations: kanamycin, 30 μg/mL; chloramphenicol, 25 μg/mL; ampicillin, 100 μg/mL; tetracycline, 12.5 μg/mL.
Microarray analysis
MG1655 ΔmgtS araEcon (kan) (GSO818) cells harboring pBAD24 or pBAD24-MgtS grown to OD600 ~0.5 in LB were induced with arabinose for 7.5 min, after which cells were harvested and total RNA was prepared as described previously (Durand & Storz, 2010). The preparation of the cDNA and hybridization to the Affymetrix E. coli Genome 2.0 array were performed as described in Affymetrix manual Section 3: Prokaryotic Sample and array Processing (www.affymetrix.com/support/downloads/manuals/expression_s3_manual.pdf).
β-galactosidase assays
For assays of the phnC-lacZ reporter fusion, overnight cultures grown in LB medium with ampicillin were diluted 1:100 into 3 mL of the same medium in 50 mL conical tubes. After 90 min of growth (OD600 ~0.5), arabinose was added, and cells were grown another 30 min before aliquots (1 mL) were harvested. For assays of the Mg2+ riboswitch-lacZ reporter fusion, overnight cultures grown in LB medium with ampicillin were diluted 1:50 into 6 mL of N minimal medium (Hmiel et al., 1986) with ampicillin and 500 μM MgSO4, grown to OD600 ~0.4. Cultures were split and each half was washed twice with 5 mL of N media with ampicillin and no added MgSO4 or 500 μM added MgSO4, and then resuspended in 3 mL of N media with 1 mM IPTG and no additional MgSO4 or 500 μM MgSO4. Cell were harvested after 45 min. For both fusions, β-galactosidase activity was assayed as previously described (Miller, 1992).
Sucrose gradient separation and chemical crosslinking
LB (150 mL in a 1 L flask) was inoculated 1:100 with an overnight culture and incubated for 90 min (OD600 ~0.5). After induction (30 min) with arabinose, cells were collected by centrifugation (4,650 × g, 20 min) and washed with 25 mL of resuspension buffer containing 1X PBS and 20% glycerol. The cells were collected again (3,700 × g, 20 min) and resuspended in 25 mL of PBS lysis buffer containing 1X PBS, 10% glycerol, 20 mM imidazole, 1.5 mM dodecyl β-D-maltoside (DDM) and protease inhibitor cocktail (Roche). Cells were lysed by using an M-110P microfluidizer (Microfluidics) set at 20k Psi. Insoluble cellular debris was removed by centrifugation (20,000 × g, 20 min). The cleared lysate was immediately layered onto 2-ml 5–25% sucrose gradients in lysis buffer. Centrifugation was carried out for 5 h at 55,000 rpm at 4°C in a TLS-55 rotor (Beckman), after which 11 fractions (200 μL each) were removed consecutively from the top of the gradient. For the chemical crosslinking reaction, freshly prepared disuccinimidyl suberate (DSS, Pierce) was added to a 25 mM final concentration to all gradient fractions, which were then incubated at room temperature for 30 min. Aliquots (30 μL) of each reaction were quenched by adding 1 μL of 1M Tris-HCl, pH 7.5. To detect MgtS-SPA, 5 μL of 4X loading buffer (4X stacking buffer, 25% glycerol, 0.1% bromophenol blue, 5% β-ME) was added to 15 μL of each quenched sample. After a 10 min incubation at 95°C, the samples were subjected to SDS-PAGE in a 10–20% Tris-glycine gel (Invitrogen) at 12V/cm and electro-transferred to a nitrocellulose membrane (0.2 μm pore size; Invitrogen) at 100 V for 50 min.
PitA-MgtS co-purification
For purification from chromosomally tagged loci, LB (150 ml in a 1 L flask) was inoculated with an overnight culture (1:100) and incubated for 90 min of growth (OD600 ~0.5). Cells were harvested, washed and lysed as described above. The cleared cell lysate was twice passed through 150 μL of Ni2+-NTA resin (Qiagen). The column was washed 3X with 1 mL of PBS lysis buffer. Elution buffer (1X PBS, 10% glycerol, 1.5 mM DDM and protease inhibitor cocktail) with 20 mM imidazole (75 μL) was added, and then the proteins were eluted from the column with 2X 300 μL of elution buffer containing 250 mM imidazole. Each purification fraction was concentrated 5:1 by TCA precipitation before 5 μL of 4X loading buffer was added to 15 μL of each sample and incubated at room temperature for 10 min. The samples were subjected to SDS-PAGE in an Any kD™ Mini-PROTEAN® TGX™ precast gel (Biorad) at 12V/cm and electro-transferred to a nitrocellulose membrane (0.2 μm pore size; Invitrogen) at 100 V for 1 h.
For cell mixing experiments, LB supplemented with ampicillin (1 L in a 2.8 L flask) was inoculated an overnight culture (1:50), grown to OD600 ~0.4, and induced with 1 mM IPTG for 30 min. Cells were harvested by centrifugation, and the pellets were resuspended in Tris lysis buffer (10 mM Tris, pH 8.0, 100 mM NaCl, 10% glycerol) supplemented with EDTA-free cOmplete protease inhibitor cocktail tablets at 1 tablet/50 mL (Roche). The cells were mixed in a 2:3 v:v ratio (6 mL PitA-HA-His6 or 6 mL buffer with 8 mL MgtS-tagged derivatives), lysed by using an M-110P microfluidizer (Microfluidics) set at 20k Psi, and incubated with 50 mM DDM at 4°C with nutation for 2.5 h. Insoluble cellular debris was removed by centrifugation (20,000 × g, 20 min), and the cleared cell lysate was twice passed through 600 μL Ni-NTA resin (Qiagen). The column was washed with 25 mL (5X 5 mL) of wash buffer (10 mM Tris pH8, 100 mM NaCl, 10% glycerol, 2 mM DDM, 20 mM imidazole). The proteins were eluted from the column in 2X 300 μL of elution buffer (10 mM Tris pH 8, 100 mM NaCl, 10% glycerol, 2 mM DDM, 250 mM imidazole) and pooled for TCA precipitation. The load, flow through, wash, and elution were mixed with sample buffer, heated for 2 min at 99°C and loaded (2 μL) onto 4–15% Mini-PROTEAN® TGX™ precast gel (Biorad) for SDS-PAGE separation. SDS-PAGE and transfer were as described above.
Immunoblot analysis
All membranes were blocked for 1 h with 5% milk in PBST buffer. To detect FLAG or SPA-tagged proteins, either monoclonal M2 α-FLAG-AP (Sigma, RRID: AB_539699) at a dilution of 1:1000 or α-FLAG-HRP (Sigma, RRID: AB_439702) at a dilution of 1:2000 was added to PBST-5% milk blocking buffer and incubated for 1 h at room temperature. The membrane was then washed 4X with PBST (15 min each) and incubated with either Lumi-Phos WB (ThermoScientific) for 5 min or with SuperSignal West Pico PLUS Chemiluminescent Substrate (Pierce) prior to exposure.
To detect PitA-HA-His6, either monoclonal α-HA antibody from mouse (received from A. Sharma) diluted 1:10,000 with PBST-3% BSA blocking buffer or monoclonal α-HA antibody from rabbit (CHIP grade, Abcam, AB_307019) diluted 1:2000 in PBST-5% milk was added to the membrane and incubated at 4°C overnight. The membrane was briefly washed with PBST after incubation with primary antibody. For the mouse α-HA antibody, α-mouse IgG antibody conjugated to horseradish peroxidase (received from A. Sharma) was diluted 1:10,000 with PBST-5% milk blocking buffer and incubated with the membrane for 1 h. For the rabbit α-HA antibody, ECL donkey α-rabbit IgG conjugated to horseradish peroxidase (GE Health, AB_772206) was diluted 1:5,000 with PBST-5% milk blocking buffer and incubated with the membrane for 2 h. After four 15-min washes, membranes were incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce) for 5 min prior to exposure.
To detect PhoR-His6, rabbit α-His6 antibody (received from A. Sharma) diluted 1:2500 with PBST-5% milk blocking buffer was added to the membrane and incubated 1 h. The membrane was briefly washed with PBST. Then goat α -rabbit antibody conjugated to horseradish peroxidase (received from A. Sharma) was added to PBST-5% milk at a dilution of 1:10,000 and incubated with membrane for 1 h. The membrane was washed and incubated with SuperSignal West Pico Chemiluminescent Substrate as described above.
Phosphate assay
Overnight cultures grown in LB medium with ampicillin were diluted 1:100 into 5 mL of N minimal medium (Hmiel et al., 1986) with ampicillin and 500 μM MgSO4 and grown to OD600 ~0.4. Cultures were washed 2X with 5 mL of N media with ampicillin and no added MgSO4 and then resuspended in the same volume with N media with ampicillin, arabinose, and no added MgSO4. After 30 min, cells were washed 2X in 5 mL of Tris-buffered saline and resuspended to an OD600 ~0.5. 32P-potassium phosphate (1 μL) at 1100 mCi/mmol (Perkin Elmer) was added to 1 mL of resuspended bacteria and incubated at 37°C. At the times indicated, a 200 μL-aliquot was applied to 0.45 μm 25 mm diameter mixed cellulose esters filter discs (Millipore) on a 1225 sampling manifold (Millipore) and washed 2X with 500 μL of phosphate-buffered saline. The filter discs were transferred to scintillation vials containing 10 mL of scintillation fluid and counted on an LS 6500 liquid scintillation counter (Beckman Coulter).
Northern blot analysis
Overnight cultures grown in LB medium with ampicillin were diluted 1:100 into the same medium with 1 mM IPTG (30 mL in 125 mL baffled flasks) and grown to OD600 ~0.5. RNA was extracted using TRIzol Reagent (Invitrogen) followed by isopropanol precipitation. Total RNA (20 μg) was separated using a formaldehyde-MOPS agarose gel as previously described (Adams et al., 2017). Briefly, RNA was denatured in 3.7% formaldehyde (Fisher), 1X MOPS (20 mM MOPS, 5 mM NaOAC, 1 mM EDTA, pH 7.0) (Fisher), and 1X RNA loading dye (ThermoScientific) for 10 min at 70°C and incubated on ice. The RNA was loaded onto a 2% NuSieve 3:1 agarose (Lonza), 1X MOPS, 2% formaldehyde gel and separated at 125V at 4°C for approximately 3 h and then transferred to a Hybond XL membrane (Amersham) via capillary action overnight (Streit et al., 2009). The RNA was cross-linked to UV irradiation, and the membrane was blocked in ULTRAhyb-Oligo Hybridization Buffer (Ambion) for 2 h at 45°C prior to probing overnight with the appropriate oligonucleotide probes. Probes were 5´−32P-end-labeled with γ−32P ATP (Perkin Elmer) by T4 polynucleotide kinase (NEB) at 37°C for 1 h. The membrane was rinsed two times with 2X SSC/0.1% SDS at room temperature, one time with 0.2X SSC/0.1% SDS at room temperature, washed 25 min with 0.2 × SSC/0.1% SDS at 45°C, followed by a final rinse with 0.2X SSC/0.1% SDS at room temperature prior to exposure. The probed membrane was stripped and reprobed for each gene indicated.
Supplementary Material
Acknowledgments
We thank S. Melamed for pointing out the MgrR-pitA interaction, P. Adams for help with the northern analysis, A. Sharma for sharing antisera, and A. Kouse and S. Melamed for comments on the manuscript. Work in the G.S. laboratory was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. E.C.H. was additionally supported by the Pharmacology Research Associate Program of the National Institute of General Medical Sciences. This work was also supported by the Intramural Research Program of the National Library of Medicine (S.A.S.). The authors declare no conflict of interest.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
References
- Adams PP, Flores Avile C, Popitsch N, Bilusic I, Schroeder R, Lybecker M, and Jewett MW (2017) In vivo expression technology and 5’ end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Res. 45: 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Makarewich CA, Anderson KM, Shelton JM, Bezprozvannaya S, Bassel-Duby R, and Olson EN (2016) Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci. Signal 9: ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam MS, and Vanderpool CK (2018) Translational regulation by bacterial small RNAs via an unusual Hfq-dependent mechanism. Nucleic Acids Res. 46: 2585–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, and Miller SI (2005) Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122: 461–472. [DOI] [PubMed] [Google Scholar]
- Baym M, Lieberman TD, Kelsic ED, Chait R, Gross R, Yelin I, and Kishony R (2016) Spatiotemporal microbial evolution on antibiotic landscapes. Science 353: 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov PP, and Wackernagel W (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158: 9–14. [DOI] [PubMed] [Google Scholar]
- Choi J, and Groisman EA (2016) Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol Microbiol. 101: 1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, and Storz G (2010) Reprogramming of anaerobic metabolism by the FnrS small RNA. Mol. Microbiol 75: 1215–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, and Cossart P (2017) Small bacterial and phagic proteins: an updated view on a rapidly moving field. Curr. Opin. Microbiol 39: 81–88. [DOI] [PubMed] [Google Scholar]
- Fröhlich KS, Papenfort K, Berger AA, and Vogel J (2012) A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD. Nucleic Acids Res. 40: 3623–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SG, Johns KD, Tanner R, and McCleary WR (2014) The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J. Bacteriol 196: 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, and Storz G (2011) Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect. Biol 3: pii: a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Hollands K, Kriner MA, Lee EJ, Park SY, and Pontes MH (2013) Bacterial Mg2+ homeostasis, transport, and virulence. Annu. Rev. Genet 47: 625–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm MR, Paul BJ, Schneider TD, Storz G, and Rudd KE (2008) Small membrane proteins found by comparative genomics and ribosome binding site models. Mol. Microbiol 70: 1487–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel SP, Snavely MD, Miller CG, and Maguire ME (1986) Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol 168: 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs EC, Astarita JL, and Storz G (2010) Small RNAs and small proteins involved in resistance to cell envelope stress and acid shock in Escherichia coli: analysis of a bar-coded mutant collection. J. Bacteriol 192: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YJ, and Wanner BL (2010) Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol 13: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Tanabe H, and Utsumi R (1999) Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J. Bacteriol 181: 5516–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa AM, and Goulian M (2009) Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 5: e1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CR, Park SY, Fei J, and Vanderpool CK (2017) The small protein SgrT controls transport activity of the glucose-specific phosphotransferase system. J. Bacteriol 199: pii: e00869–00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykh EA, Butov IA, Ravcheeva AB, Krylov AA, Mashko SV, and Stoynova NV (2018) Specific features of L-histidine production by Escherichia coli concerned with feedback control of AICAR formation and inorganic phosphate/metal transport. Microb. Cell Fact 17: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandin P, and Gottesman S (2009) A genetic approach for finding small RNAs regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol 72: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M, Wright PR, and Backofen R (2017) IntaRNA 2.0: enhanced and customizable prediction of RNA–RNA interactions. Nucleic Acids Res. 45: W435–W439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed S, Peer A, Faigenbaum-Romm R, Gatt YE, Reiss N, Bar A, Altuvia Y, Argaman L, and Margalit H (2016) Global mapping of small RNA-target interactions in bacteria. Mol. Cell 63: 884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH, (1992) A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- Moon K, and Gottesman S (2009) A PhoQ/P-regulated small RNA regulates sensitivity of Escherichia coli to antimicrobial peptides. Mol. Microbiol 74: 1314–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Six DA, Lee HJ, Raetz CR, and Gottesman S (2013) Complex transcriptional and post-transcriptional regulation of an enzyme for lipopolysaccharide modification. Mol. Microbiol 89: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, and Maguire ME (2008) Magnesium transport and magnesium homeostasis. EcoSal Plus 3: 10.1128. [DOI] [PubMed] [Google Scholar]
- Pontes MH, and Groisman EA (2018) Protein synthesis controls phosphate homeostasis. Genes Dev. 32: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MH, Sevostyanova A, and Groisman EA (2015) When too much ATP Is bad for protein synthesis. J. Mol. Biol 427: 2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, and Miller SI (2007) Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26: 165–174. [DOI] [PubMed] [Google Scholar]
- Salazar ME, Podgornaia AI, and Laub MT (2016) The small membrane protein MgrB regulates PhoQ bifunctionality to control PhoP target gene expression dynamics. Mol. Microbiol 102: 430–445. [DOI] [PubMed] [Google Scholar]
- Slavoff SA, Mitchell AJ, Schwaid AG, Cabili MN, Ma J, Levin JZ, Karger AD, Budnik BA, Rinn JL, and Saghatelian A (2013) Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat. Chem. Biol 9: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Vogel J, and Wassarman KM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43: 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Wolf YI, and Ramamurthi KS (2014) Small proteins can no longer be ignored. Annu. Rev. Biochem 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit S, Michalski CW, Erkan M, Kleeff J, and Friess H (2009) Northern blot analysis for detection and quantification of RNA in pancreatic cancer cells and tissues. Nat. Protoc 4: 37–43. [DOI] [PubMed] [Google Scholar]
- Thomason LC, Costantino N, and Court DL (2007) E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol 1: 1.17. [DOI] [PubMed] [Google Scholar]
- van Veen HW, Abee T, Kortstee GJ, Konings WN, and Zehnder AJ (1994) Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 33: 1766–1770. [DOI] [PubMed] [Google Scholar]
- Véscovi EG, Ayala YM, Di Cera E, and Groisman EA (1997) Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+. J. Biol. Chem 272: 1440–1443. [DOI] [PubMed] [Google Scholar]
- Wagner EGH, and Romby P (2015) Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv. Genet 90: 133–208. [DOI] [PubMed] [Google Scholar]
- Wang H, Yin X, Wu Orr M, Dambach M, Curtis R, and Storz G (2017) Increasing intracellular magnesium levels with the 31-amino acid MgtS protein. Proc. Natl. Acad. Sci. USA 114: 5689–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman KM, Repoila F, Rosenow C, Storz G, and Gottesman S (2001) Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15: 1637–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, and Court DL (2000) An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97: 5978–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.