Summary
Introduction:
Blood donation has been proposed as a potential therapy to reduce risk of cardiovascular disease, but the effects of phlebotomy on vascular function in human subjects have not been well characterized.
Aims:
We conducted a prospective randomized double-blind study to determine the effects of serial phlebotomy on vascular endothelial function in the brachial artery. Eighty-four iron-replete, non-anemic subjects were randomly assigned to one of three study treatment groups: (a) four serial phlebotomy procedures each followed by intravenous infusion of placebo normal saline; (b) four serial phlebotomy procedures each followed by intravenous infusion to replete lost iron; and (c) four serial sham phlebotomy procedures each followed by intravenous infusion of placebo normal saline. Assigned phlebotomy procedures were conducted at 56-day intervals. We measured brachial artery reactivity (BAR, %) in response to transient oxidative stress induced by oral methionine with high-resolution duplex ultrasound imaging before and one week after the fourth study phlebotomy.
Results:
Before phlebotomy, oral methionine decreased BAR by −2.04% (95% CI −2.58%, −1.50%), P < 0.001) with no significant difference between groups (P = 0.42). After phlebotomy, the BAR response to oral methionine did not significantly change between groups (P = 0.53). Brachial artery nitroglycerin-mediated dilation did not change in response to phlebotomy.
Conclusions:
Four serial phlebotomy procedures over six months with or without intravenous iron supplementation did not alter vascular endothelial function in the brachial artery when compared with sham phlebotomy.
Keywords: endothelial function, iron therapy, physiology, vascular biology
1 |. INTRODUCTION
Sullivan first proposed in 1981 that blood donation might be associated with reduced risk of coronary heart disease.1 Although this hypothesis was initially posed based solely on epidemiological observations, substantial evidence has accumulated to support plausible mechanistic links between the biological effects of blood donation and progression of atherosclerosis.2 A standard blood donation procedure removes between 200 and 250 mg of elemental iron (Fe), decreases serum ferritin levels by approximately 30%−40% and decreases hemoglobin levels by approximately 8%.3–5 Reduction in body Fe stores in response to blood donation might slow atherosclerosis progression via reduction in Fe-dependent oxygen free radical production within the vascular wall and/or hepcidin-induced changes in macrophage phenotype.6–12 Reduction in red blood cells (RBC) in response to blood donation might also slow atherosclerosis progression independently of Fe stores. Potential RBC-dependent pathways include alterations in blood rheology and vascular endothelial cell shear stress, changes in nitric oxide-hemoglobin interactions, and/or increased synthesis of hypoxia-induced hematopoietic and angiogenic growth factors.13–17
The current study was undertaken to determine the effects of Fe loss and RBC loss in response to serial phlebotomy procedures on vascular endothelial function in the intact human circulation. To accomplish this aim, we performed a prospective randomized study of experimental serial phlebotomy procedures to determine the effects of Fe loss and RBC loss on brachial artery reactivity (BAR), a physiological measure of vascular endothelial function associated with cardiovascular risk,18–20 in Fe-replete, non-anemic human subjects.
2 |. METHODS
2.1 |. Subject selection
Men and post-menopausal women age 40–70 years were recruited from the list of blood donors deferred from active donation ≥2 years for non-medical administrative reasons at the New York Blood Center and from the general population of New York County between January 2009 and August 2012. The most common reason for administrative deferral was previous residence or travel in the United Kingdom or Europe that exceeded FDA guidelines to reduce risk of transmission of Creutzfeldt-Jakob disease in blood products.
Other inclusion criteria were hemoglobin levels >13.5 g/dL for men, or >12.5 g/dL for women, and serum ferritin 50–400 ng/mL. The study protocol was approved by the institutional review boards of New York University Langone Medical Center and the New York Blood Center. All subjects provided written informed consent prior to participation in study procedures. All study procedures were performed on the main campus of New York University Langone Medical Center in New York City, NY, USA.
2.2 |. Study design
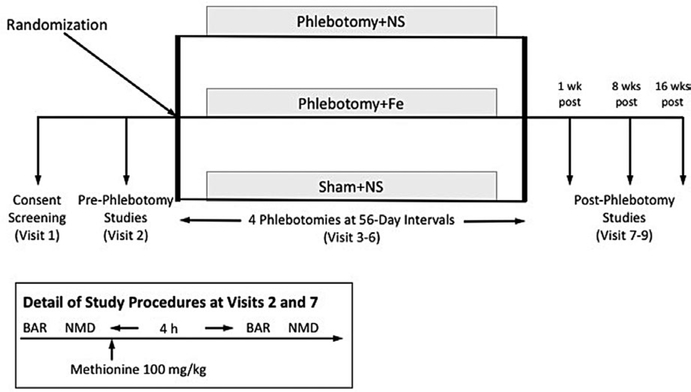
The study is registered on the clinicaltrials.gov website (ID: NCT02762422) (Figure 1). To minimize bias and the potential impact of unmeasured confounders, the study was designed as a prospective randomized double-blind interventional study with three study phlebotomy arms: (a) four serial phlebotomy procedures as described below, each followed by intravenous infusion of 250 mL normal saline (Phlebotomy+NS); (b) four serial phlebotomy procedures as described below, each followed by intravenous infusion of Fe sucrose (Venofer®, American Regent Inc, Shirley NY) in 250 mL normal saline, dose adjusted to match the Fe content of the blood removed during phlebotomy (Phlebotomy+Fe); and (c) four serial sham phlebotomy procedures as described below, each followed by infusion of 250 mL of normal saline (Sham+NS). Assigned study phlebotomy procedures were conducted at 56-day intervals (visits 3–6). BAR testing before and after oral methionine administration and blood sampling for biochemical markers was performed by blinded research staff before phlebotomy (visit 2) and one week after the fourth study phlebotomy procedure (visit 7). BAR testing without oral methionine administration was repeated at 8 weeks (visit 8) and 16 weeks (visit 9) after the fourth study phlebotomy procedure.
FIGURE 1.
Schematic of study design. Eligible subjects were randomly assigned to one of three study phlebotomy groups: (1) four serial phlebotomy procedures as described in text, each followed by intravenous infusion of 250 mL normal saline (Phlebotomy+NS); (2) four serial phlebotomy procedures, each followed by intravenous infusion of Fe sucrose (Venofer®, American Regent Inc, Shirley NY), dose adjusted to match the Fe content of the blood removed during phlebotomy (Phlebotomy+Fe); and (3) four serial sham phlebotomy procedures as described below, each followed by intravenous infusion of 250 mL of normal saline (Sham+NS). Serial phlebotomy procedures were conducted at approximately 56-day intervals. Brachial artery reactivity (BAR) and nitroglycerin-mediated dilation (NMD) were determined before and after oral methionine 100 mg/kg (inset) before study phlebotomy (visit 2) and one week after completion of the experimental phlebotomy procedures (visit 7). BAR was repeated without oral methionine administration at 8 wk (visit 8) and 16 wk (visit 9) after completion of experimental phlebotomy procedures
2.3 |. Randomization and blinding procedures
Study treatment allocation was determined with a variable size blocked randomization scheme (1:1:1 ratio), stratified by sex and the total number of previous blood donations, generated by an independent biostatistician and held by the staff of the New York University Research Pharmacy. The blinding of treatment assignment was maintained by the nursing staff of the New York University Clinical and Translational Science Institute (NYU CTSI), who performed all assigned study phlebotomy procedures as described below to prevent knowledge of treatment assignment for subjects and investigators.
2.4 |. Phlebotomy procedure
After sterile preparation of the skin over the antecubital fossa, a 16-gauge sterile steel cannula was placed into a suitable subcutaneous vein. For subjects randomized to either of the two phlebotomy arms, the cannula was connected to tubing for gravity drainage of 500 mL over 10–15 minutes. For subjects randomized to sham phlebotomy, the same procedures for placement of the venous cannula were followed, but the drainage tube remained clamped for 10–15 minutes to prevent blood removal. After the phlebotomy or sham phlebotomy procedure was completed, the blood collection tubing was disconnected, and subjects received either 250 mL normal saline or Fe sucrose in 250 cc normal saline, according to assigned study phlebotomy group. The dose of Fe sucrose was calculated to replace the amount of Fe removed during the phlebotomy procedure according the formula: Fe sucrose dose (mg) = blood volume removed (dL) × hemoglobin (g/dL) × 3.47 mg Fe/g hemoglobin. To maintain subject blinding, all blood removal and intravenous line equipment were shielded from subject view with curtains.
2.5 |. Brachial artery reactivity testing
Brachial artery reactivity in response to increased blood flow was determined non-invasively with a duplex ultrasound vascular imaging system connected to a 7–11 MHz linear array probe (Sonosite Inc, Bothell WA), with an imaging protocol adapted from guideline recommendations as previously described.21,22 Brachial artery diameter (trailing edge of anterior intima-lumen interface to leading edge of posterior lumen-intima interface) was measured in mm with a semi-automated edge detection image analysis system (Artery Measurement Systems, Gothenburg, Sweden) by an investigator blinded to treatment assignment.23 Internal and external landmarks were used to identify the same brachial artery segment for measurements at each study visit. The percent change in brachial artery diameter before cuff occlusion to brachial artery diameter 60–90 seconds after release of cuff occlusion was calculated as an index of endothelium-dependent, flow-mediated vasodilation (BAR).
Fifteen minutes after deflation of the forearm occlusion cuff, nitroglycerin 0.4 mg was administered sublingually to test the vascular smooth muscle response to an endothelium-independent exogenous source of nitric oxide. The percent change from brachial diameter before nitroglycerin administration to brachial artery diameter five minutes after nitroglycerin administration was calculated as nitroglycerin-mediated dilation. BAR and nitroglycerin-mediated dilation were measured before and 4 hours after oral administration of methionine at a dose of 100 mg/kg (Figure 1, inset). This dose of methionine is known to transiently increase plasma homocysteine levels to 15–30 μmol/L (300%−400% over normal baseline levels) and is associated with transient oxidant stress-mediated decrease in endothelium-dependent flow-mediated vasodilation.24–26
2.6 |. Estimated brachial artery shear stress
Mean blood flow velocity (cm/s, derived from auto-traced velocity-time integral with software correction for incident angle ≤60° was measured with a 1 mm pulsed Doppler sample volume placed at the center of the brachial artery at supine rest before cuff occlusion and for 15 seconds immediately after release of cuff occlusion. Estimated brachial artery shear rate (s−1) before and after cuff occlusion was calculated as 4* mean blood flow velocity/pre-occlusion brachial artery diameter.21 Estimated brachial artery shear stress (dynes/cm2) before and after cuff occlusion was calculated as estimated shear rate X whole blood viscosity.
2.7 |. Laboratory analysis
Venous blood samples were collected before study phlebotomy procedures at visit 2 and 1 week after completion of the study phlebotomy procedures at visit 7 for determination of blood biomarkers that might be associated with Fe loss: Fe stores (serum ferritin (ng/mL), Associated Regional and University Pathologists (ARUP) Laboratory, Salt Lake City UT), oxidative stress (serum nitrotyrosine (nM), ELISA Cell Biolabs Inc, San Diego CA) and red blood cell size (mean corpuscular volume (MCV, fL), NYU Hospital Laboratory), and blood biomarkers that might be associated with RBC loss: hemoglobin level (g/dL, NYU Hospital Laboratory), biomarker of hypoxia-inducible factor-1 signaling (serum erythropoietin (mIU/mL), NYU Hospital Laboratory), and whole blood viscosity (cone-plate viscometer, dynes•s/cm2, NYU Hospital Laboratory).
2.8 |. Data analysis
All analyses were conducted based on the intent-to-treat principle, for which all available data were analyzed based on the randomized treatment assignment. The primary outcome variable was the change in BAR in response to oral methionine. For the primary outcome variable analyses, the null hypotheses to be tested was that the change in BAR in response to oral methionine before and after study phlebotomy procedures would not differ between study phlebotomy groups. To test this hypothesis, we used mixed models with predictor variables study phlebotomy group, study visit, the inter-action term group*visit, and outcome variable of change in BAR in response to methionine (SAS version 9.4 statistical software, SAS Institute, Cary, NC). For secondary endpoints, nitroglycerin-mediated dilation, estimated brachial artery shear stress, and biomarkers were substituted for change in BAR in response to methionine as the outcome variable in the same mixed models. For all reported analyses, a two-tailed P-value of <0.05 was used to infer statistical significance.
2.9 |. Sample size calculation
Based on our initial assumptions (mean baseline post-methionine change in BAR 2.4% ± 1.5%, two-tailed α = 0.05), 30 subjects in each randomized study phlebotomy group provided 76%−98% power to detect 33%−50% differences between analysis groups. The 33%−50% relative difference (corresponding to an anticipated 0.8%−1.2% absolute post-methionine change in BAR) was selected based on empiric designation of a physiological meaningful change in BAR (1%) and previous reports of the effects of Vitamin C and dexrazoxane on the BAR response to oral methionine.
3 |. RESULTS
3.1 |. Study participants
Study subject participation is summarized in Figure 2. A total of 169 subjects were screened for participation and 84 eligible subjects were randomized. The most common reasons for exclusion were serum ferritin levels, hemoglobin levels, and fasting blood glucose levels that did not meet entry criteria. Selected characteristics at study entry (means±SD, median (25th, 75th percentile), or percentage) for the 84 randomized subjects are summarized in Table 1. There were no withdrawals due to development of anemia or other adverse events.
FIGURE 2.
CONSORT study flow diagram. The 3-arm study allocation scheme randomized eligible subjects on a 1:1:1 basis to one of three study phlebotomy groups. The numbers of subjects in each arm at time of randomization and at end of study are provided for each group
TABLE 1.
Selected characteristics at study entry (mean ± SD, median (25th, 75th percentile), or percentage) for all randomized subjects and subjects by study phlebotomy group
| All subjects (n = 84) | Phlebotomy+NS (n = 30) | Phlebotomy+Fe (n = 27) | Sham+NS (n = 27) | |
|---|---|---|---|---|
| Age (y) | 54 ± 8 | 55 ± 9 | 54 ± 7 | 55 ± 7 |
| Sex (% female) | 22.9 | 20.0 | 26.9 | 22.2 |
| Race (%white) | 91.4 | 91.7 | 95.8 | 86.4 |
| Hypertension (%) | 22.1 | 25.9 | 25.0 | 15.4 |
| Hyperlipidemia (%) | 22.1 | 26.9 | 20.0 | 19.2 |
| Body mass index (kg/m2) | 28.2 ± 5.3 | 29.4 ± 6.2 | 27.9 ± 4.5 | 27.1 ± 4.8 |
| Systolic Blood Pressure (mm Hg) | 123.1 ± 11.2 | 123.5 ± 11.2 | 122.6 ± 11.1 | 123.0 ± 11.7 |
| Diastolic Blood Pressure (mm Hg) | 75.0 ± 10.1 | 75.6 ± 8.4 | 76.8 ± 10.3 | 72.7 ± 11.4 |
| Fasting blood glucose (mg/dL) | 94.1 ± 7.2 | 94.6 ± 7.5 | 92.1 ± 7.5 | 95.5 ± 6.2 |
| Serum creatinine (mg/dL) | 0.92 ± 0.14 | 0.93 ± 0.15 | 0.92 ± 0.15 | 0.91 ± 0.13 |
| Total serum cholesterol (mg/dL) | 194.6 ± 36.2 | 197.5 ± 36.9 | 200.5 ± 32.9 | 185.5 ± 38.0 |
| Serum ferritin (ng/mL) | 134.3 (86.4, 198.0) | 159.5 (91.7, 209.4) | 131.1 (66.9, 215.5) | 135.3 (91.3, 189.4) |
| Hemoglobin (g/dL) | 14.5 ± 0.9 | 14.6 ± 0.9 | 14.4 ± 0.8 | 14.4 ± 0.9 |
3.2 |. Study phlebotomy procedures
About 98% of the assigned experimental phlebotomy procedures were completed per protocol. The median interval between phlebotomy procedures was 59 (57, 63) days and did not differ between randomized study phlebotomy groups. Serial fingerstick hemoglobin values measured before each study phlebotomy procedure did not change from baseline and did not differ between groups (data not shown). One phlebotomy procedure was deferred for 4 weeks for reduced hemoglobin level per safety protocol in a female subject assigned to the Phlebotomy+Fe group. There were no adverse reactions during the study phlebotomy procedures.
3.3 |. Pre-phlebotomy bar testing
Pre-phlebotomy BAR testing results at visit 2 by study phlebotomy group are summarized in Table 2. There were no differences between study phlebotomy groups for these baseline vascular measurements. Consistent with prior studies, oral methionine administration at visit 2 was associated with a significant reduction in BAR when compared with that before oral methionine administration. The estimated mean decrease in BAR in response to methionine for all subjects was −2.04% (95% CI 0.−1.51%, −2.58%), P < 0.001) with no significant difference between study phlebotomy groups (P = 0.25). Oral methionine administration did not change brachial artery nitroglycerin-mediated dilation (estimated mean decrease for all subjects −0.73% (95% CI (−1.82, 0.36%), P = 0.19) and there was no significant difference between study phlebotomy groups (P = 0.87).
TABLE 2.
Pre-Phlebotomy duplex ultrasound derived variables for brachial artery reactivity (BAR) testing and nitroglycerin-mediated dilation (mean ± SD) at visit 2 before and after oral methionine administration reported for all subjects and subjects grouped by study phlebotomy group
| All subjects (n = 84) | Phlebotomy+NS (n = 30) | Phlebotomy+Fe (n = 27) | Sham+NS (n = 27) | |
|---|---|---|---|---|
| Pre-Phlebotomy: Before oral methionine administration | ||||
| Pre-occlusion BA diameter (cm) | 3.73 ± 0.62 | 3.78 ± 0.58 | 3.56 ± 0.68 | 3.84 ± 0.59 |
| Post-occlusion BA diameter (cm) | 3.86 ± 0.62 | 3.91 ± 0.59 | 3.72 ± 0.69 | 3.96 ± 0.58 |
| Post-NTG BA diameter (cm) | 4.62 ± 0.66 | 4.74 ± 0.65 | 4.47 ± 0.72 | 4.64 ± 0.62 |
| BAR (%) | 3.77 ± 2.47 | 3.57 ± 2.18 | 4.43 ± 2.45 | 3.34 ± 2.75 |
| NTG-mediated dilation (%) | 24.73 ± 6.87 | 25.72 ± 5.55 | 26.37 ± 7.88 | 21.96 ± 6.56 |
| Pre-phlebotomy: After oral methionine administration | ||||
| Pre-occlusion BA diameter (cm) | 3.77 ± 0.59 | 3.85 ± 0.52 | 3.61 ± 0.67 | 3.85 ± 0.56 |
| Post-occlusion BA diameter (cm) | 3.84 ± 0.60 | 3.93 ± 0.53 | 3.68 ± 0.68 | 3.91 ± 0.57 |
| Post-NTG BA diameter (cm) | 4.67 ± 0.65 | 4.76 ± 0.55 | 4.53 ± 0.75 | 4.71 ± 0.66 |
| BAR (%) | 1.73 ± 2.27 | 1.65 ± 2.23 | 1.91 ± 2.09 | 1.65 ± 2.54 |
| NTG-mediated dilation (%) | 23.83 ± 6.62 | 24.94 ± 5.46 | 25.38 ± 7.43 | 21.16 ± 6.38 |
BA, Brachial artery; BAR, Brachial artery reactivity; Fe, Fe; NS, normal saline; NTG, nitroglycerin.
3.4 |. Post-phlebotomy bar testing
There were no significant differences in the change of the BAR response to oral methionine from visit 2 to visit 7 among the three study phlebotomy groups (Figure 3). Post-phlebotomy brachial artery nitroglycerin-mediated dilation at visit 7 did not differ between analysis groups or study phlebotomy groups or analysis groups (data not shown). The change in pre-methionine BAR testing from visit 2 (pre-phlebotomy) to visit 7 (1 week post-phlebotomy), visit 8 (8 weeks post-phlebotomy) and visit 9 (16 weeks post-phlebotomy) did not differ between study phlebotomy groups (data not shown).
FIGURE 3.
Brachial artery reactivity (BAR, %) response to oral methionine (mean ± standard error of mean) before study phlebotomy (black bars) and one week after completion of study phlebotomy (gray bars) by study phlebotomy groups. The estimated difference in the post-phlebotomy BAR response to oral methionine administration from visit 2 to visit 7 did not differ between study phlebotomy groups (treatment by visit interaction P = 0.53). The change in BAR in response to oral methionine administration from visit 2 to visit 7 did not differ for pairwise comparisons between the phlebotomy+NS vs Sham+NS groups (0.83%, 95% CI (−0.72%, 2.38%), P = 0.29) and the phlebotomy+Fe vs Sham+NS groups (0.77%, 95% CI (−0.81%, 2.37%), P = 0.34)
3.5 |. Post-occlusion estimated brachial artery shear stress
Brachial artery mean blood flow velocity, estimated shear rate, and estimated shear stress after release of cuff occlusion did not differ between randomized study phlebotomy groups (data not shown, P-values for treatment by visit interaction: mean brachial artery blood flow velocity P = 0.90, estimated brachial artery shear rate P = 0.75, estimated brachial artery stress P = 0.26).
3.6 |. Biomarker results
Biomarker results are summarized in Figure 4 and Table 3. Serum ferritin levels significantly decreased between visit 2 and visit 7 in the Phlebotomy+NS group when compared with the Sham Phlebotomy group (estimated mean difference −95.87 ng/mL, 95% CI (−125.62, −66.11 ng/mL), P < 0.001) and significantly increased between visit 2 and visit 7 in the Phlebotomy+Fe group when compared with the Sham Phlebotomy group (estimated mean difference 45.85 ng/mL, 95% CI (13.23, 78.48 ng/mL), P = 0.01, Figure 4). Hemoglobin levels significantly decreased between visit 2 and visit 7 in the Phlebotomy+NS group (estimated mean difference −0.75 g/dL, 95% CI (−1.13, −0.38 g/dL), P < 0.001, Figure 4) and the Phlebotomy+Fe group (estimated mean difference −0.86 g/dL, 95% CI (−1.25, −0.46 g/dL), P < 0.001, Figure 4) when compared with the Sham Phlebotomy group. Serum erythropoietin significantly increased between visit 2 and visit 7 in the Phlebotomy+NS group when compared with Sham Phlebotomy (estimated mean difference 3.55 mIU/mL, 95% CI (−0.0005, 7.10 mIU/mL), P = 0.05). There were no significant changes between randomized study phlebotomy groups for the other biomarkers listed in Table 3.
FIGURE 4.
Mean ± standard error of mean change in post-phlebotomy biomarker values by study phlebotomy group for serum ferritin (ng/mL, panel A), hemoglobin (gm/dL, panel B), and serum erythropoietin (mIU/mL, panel C). P-values for comparison with sham group are shown
TABLE 3.
Biomarkers by study phlebotomy group (mean ± SD or median (interquartile range)). before phlebotomy (visit 2) and after phlebotomy (visit 7)
| Phlebotomy+NS | Phlebotomy+Fe | Sham+NS | |
|---|---|---|---|
| Serum Ferritin (ng/mL) | |||
| Before Phlebotomy | 123.00 (86.00, 197.00) | 111.00 (90.00, 190.00) | 152.00 (89.00, 214.00) |
| After Phlebotomy | 35.00 (22.00, 46.00) | 153.00 (114.00, 203.00) | 150.00 (75.00, 191.00) |
| Serum Nitrotyrosine (nM) | |||
| Before Phlebotomy | 8.00 (6.00, 12.00) | 10.00 (5.50, 13.00) | 8.00 (6.00, 11.00) |
| After Phlebotomy | 9.50 (5.00, 18.00) | 8.00 (2.50, 12.00) | 9.50 (3.50, 17.50) |
| Mean Corpuscular Volume (fL) | |||
| Before Phlebotomy | 90.47 ± 3.85 | 91.12 ± 3.41 | 91.19 ± 4.97 |
| After Phlebotomy | 90.03 ± 4.19 | 90.48 ± 3.92 | 91.85 ± 4.87 |
| Hemoglobin (g/dL) | |||
| Before Phlebotomy | 14.38 ± 0.98 | 14.21 ± 0.96 | 14.33 ± 1.02 |
| After Phlebotomy | 13.60 ± 1.02 | 13.14 ± 1.00 | 14.20 ± 1.04 |
| Serum Erythropoietin (mlU/mL) | |||
| Before Phlebotomy | 8.34 (5.86, 13.40) | 8.82 (6.97, 11.80) | 9.59 (7.13, 11.80) |
| After Phlebotomy | 11.40 (9.41, 14.50) | 10.10 (8.53, 16.60) | 8.99 (5.68, 13.40) |
| Whole Blood Viscosity (dynes•s/cm2) | |||
| Before Phlebotomy | 4.97 ± 0.34 | 5.07 ± 0.47 | 5.34 ± 0.58 |
| After Phlebotomy | 4.84 ± 0.46 | 4.70 ± 0.42 | 5.08 ± 0.53 |
4 |. DISCUSSION
The salient finding of this prospective randomized double-blind study is that serial phlebotomy with or without intravenous iron replacement did not significantly modify the change in BAR in response to oral methionine when compared with the sham phlebotomy control group.
The impetus for the current study was the ongoing controversy surrounding the hypothesis that lower Fe stores might confer decreased risk of coronary heart disease.1 This hypothesis was based on epidemiological data demonstrating a temporal coincidence between the age- and sex-dependent increases in serum ferritin levels and risk of coronary heart disease events. Blood donation was proposed as a natural experiment to test this hypothesis, but subsequent observational studies on the association between blood donation and coronary heart disease risk have yielded conflicting findings.27–31 Interpretation of these past reports is limited by methodological issues related to selection bias (healthy blood donor effect), misclassification in ascertainment of blood donation exposure and/or cardiovascular outcomes (recall bias), and missing data on important potential confounding variables (reproductive history, inflammation, cancer, and bleeding). These observational studies are also potentially confounded by the effects of reverse causation, since the presence of occult or overt cardiovascular disease may reduce blood donation frequency.
The current prospective randomized study used BAR testing, a physiological measure of vascular endothelial function with a previously established association with risk of cardiac events, as a surrogate outcome.18–20 The change in BAR in response to oral methionine was selected as the primary endpoint, as previous studies have demonstrated that the BAR response to oral methionine is associated with increased transient oxidative stress and is modifiable by acute changes in iron bioavailability.24,25,32,33 The study phlebotomy interventions were associated with the expected changes in serum ferritin, hemoglobin, and serum erythropoietin, but did not significantly modify the BAR response to oral methionine, or the pre-methionine BAR. Accordingly, the current findings do not support a causal link between the biological responses to phlebotomy and vascular endothelial function over the 6-month study intervention. The current findings are discordant with our previous cross-sectional study that reported significantly increased BAR in higher frequency blood donors (≥8 blood donations in past two years) when compared with lower frequency blood donors (1–2 blood donations in past two years).34 The discordance could be attributable to unmeasured confounders in the cross-sectional study sample, differences in the degree of depletion of iron stores (median post-phlebotomy serum ferritin 35 ng/mL in the current study vs 17 ng/mL in the higher frequency donor group of the cross-sectional study), and/or differences in the magnitude and duration of phlebotomy exposure (four phlebotomies over 6 months in the current study vs a mean of 35 blood donations over 10 years in the higher frequency donor group of the cross-sectional study). The potential importance of a longer duration of phlebotomy exposure is also supported by the findings of a previous single-blinded randomized clinical trial of standard care plus serial phlebotomy vs standard care control without phlebotomy in subjects with peripheral arterial disease. The serial phlebotomy intervention decreased serum ferritin to 80 ng/mL over an average follow-up of 4.5 years, with evidence of reduced ferritin levels associated with improved cardiovascular outcomes.35 Taken together, the current findings and previous reports suggest that a reduction of iron stores sustained over many years may be necessary to impact the biological effects of iron on progression of atherosclerosis and coronary risk.
Strengths of the study include the randomized double-blind design to minimize bias and the effects of unmeasured confounders, and the high degree of adherence to the study protocol. There are several caveats to consider in the interpretation of the study findings. The selected target ferritin levels for the experimental phlebotomy intervention was empiric, as the optimal levels of iron stores for cardiovascular health remain uncertain.36,37 Due to ethical and budgetary considerations, the study design used a surrogate endpoint rather than cardiovascular events and a limited duration of phlebotomy over six months. Accordingly, the current findings can not be directly linked to risk of cardiovascular events. Recruitment of blood donors deferred from active donation may have introduced selection bias that limits generalizability of the study findings. The predominance of white men in the study population is consistent with national demographic data for blood donors, and may also be attributable to the entry criteria for hemoglobin and serum ferritin levels that were selected to minimize risk of iron-deficiency anemia in response to study phlebotomy procedures.38 Since Fe and RBC homeostasis are known to differ by sex and race, additional work is needed to confirm the current findings in more diverse populations.39 A slight shortfall in subject accrual (7% less than the intended minimal sample size) reduced study power from our a priori assumptions, but since the original power calculation provided 98% power to detect a 50% difference in the BAR response to methionine between groups, the absence of a significant difference between groups is not likely attributable to beta error.
In conclusion, four serial phlebotomy procedures over 6 months with or without intravenous iron supplementation did not significantly alter vascular endothelial function in the brachial artery when compared with sham phlebotomy.
ACKNOWLEDGMENTS
The investigators thank the New York Blood Center and the study participants for their contributions to the study.
Funding information
Supported by grant number 1R01HL086932 from the National Heart, Lung, and Blood Institute, National Institutes of Health and grant number NIH/NCATS UL1 TR000038 from the National Center for Research Resources, National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan JL. Do hemochromatosis mutations protect against ironmediated atherogenesis? Circ Cardiovasc Genet. 2009;2:652–657. [DOI] [PubMed] [Google Scholar]
- 3.Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50:441–447. [PubMed] [Google Scholar]
- 4.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pottgiesser T, Specker W, Umhau M, Dickhuth HH, Roecker K, Schumacher YO. Recovery of hemoglobin mass after blood donation. Transfusion. 2008;48:1390–1397. [DOI] [PubMed] [Google Scholar]
- 6.Araujo JA, Romano EL, Brito BE, et al. Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1172–1180. [DOI] [PubMed] [Google Scholar]
- 7.Boyle JJ, Johns M, Kampfer T, et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res. 2012;110:20–33. [DOI] [PubMed] [Google Scholar]
- 8.Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99:1222–1229. [DOI] [PubMed] [Google Scholar]
- 9.Li JJ, Meng X, Si HP, et al. Hepcidin destabilizes atherosclerotic plaque via overactivating macrophages after erythrophagocytosis. Arterioscler Thromb Vasc Biol. 2012;32:1158–1166. [DOI] [PubMed] [Google Scholar]
- 10.Saeed O, Otsuka F, Polavarapu R, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson DR, Lane DJ, Becker EM, et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cliville X, Bofill C, Joven J, et al. Hemorheological, coagulative and fibrinolytic changes during autologous blood donation. Clin Hemorheol Microcirc. 1998;18:265–272. [PubMed] [Google Scholar]
- 14.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L529–L533. [DOI] [PubMed] [Google Scholar]
- 15.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. [DOI] [PubMed] [Google Scholar]
- 16.Lorentz A, Jendrissek A, Eckardt KU, Schipplick M, Osswald PM, Kurtz A. Serial immunoreactive erythropoietin levels in autologous blood donors. Transfusion. 1991;31:650–654. [DOI] [PubMed] [Google Scholar]
- 17.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348: 1483–1485. [DOI] [PubMed] [Google Scholar]
- 18.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelani QU, Norcliffe-Kaufmann L, Kaufmann H, Katz SD. Vascular endothelial function and blood pressure regulation in afferent autonomic failure. Am J Hypertens. 2015;28:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28:2195–2200. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy MF, McDowell IF, Ramsey MW, et al. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–1852. [DOI] [PubMed] [Google Scholar]
- 25.Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation. 1999;99:1156–1160. [DOI] [PubMed] [Google Scholar]
- 26.Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999;100:1161–1168. [DOI] [PubMed] [Google Scholar]
- 27.Ascherio A, Rimm EB, Giovannucci E, Willett WC, Stampfer MJ. Blood donations and risk of coronary heart disease in men. Circulation. 2001;103:52–57. [DOI] [PubMed] [Google Scholar]
- 28.Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion. 2002;42:1135–1139. [DOI] [PubMed] [Google Scholar]
- 29.Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuomainen TP, Salonen R, Nyyssonen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314:793–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germain M, Delage G, Blais C, Maunsell E, Decary F, Gregoire Y. Iron and cardiac ischemia: a natural, quasi-random experiment comparing eligible with disqualified blood donors. Transfusion. 2013;53:1271–1279. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Dimayuga C, Hudaihed A, Katz SD. Effect of dexrazoxane on homocysteine-induced endothelial dysfunction in normal subjects. Arterioscler Thromb Vasc Biol. 2002;22:E15–E18. [DOI] [PubMed] [Google Scholar]
- 33.Zheng H, Huang X, Zhang Q, Katz SD. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int. 2006;69:679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol. 2005;25:1577–1583. [DOI] [PubMed] [Google Scholar]
- 35.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007;297:603–610. [DOI] [PubMed] [Google Scholar]
- 36.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. [DOI] [PubMed] [Google Scholar]
- 37.Zacharski LR, Shamayeva G, Chow BK, DePalma RG. Ferritin and percent transferrin saturation levels predict type 2 diabetes risk and cardiovascular disease outcomes. Curr Diabetes Rev. 2017;13:428–436. [DOI] [PubMed] [Google Scholar]
- 38.Murphy EL, Shaz B, Hillyer CD, et al. Minority and foreign-born representation among US blood donors: demographics and donation frequency for 2006. Transfusion. 2009;49:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thal-assemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]