SUMMARY
Ail, a multifunctional outer membrane protein of Yersinia pestis, confers cell binding, Yop delivery, and serum resistance activities. Resistance to complement proteins in serum is critical for survival of Y. pestis during the septicemic stage of plague infections. Bacteria employ a variety of tactics to evade the complement system, including recruitment of complement regulatory factors, such as factor H, C4b-binding protein (C4BP), and vitronectin (Vn). Y. pestis Ail interacts with the regulatory factors Vn and C4BP, and Ail homologs from Y. enterocolitica and Y. pseudotuberculosis recruit factor H. Using co-sedimentation assays, we demonstrate that two surface-exposed amino acids, F80 and F130, are required for interaction of Y. pestis Ail with Vn, factor H, and C4BP. However, although Ail-F80A/F130A fails to interact with these complement regulatory proteins, it still confers 10,000-fold more serum resistance than a Δail strain and prevents C9 polymerization, potentially by directly interfering with MAC assembly. Using site-directed mutagenesis we further defined this additional mechanism of complement evasion conferred by Ail. Finally, we find that at Y. pestis concentrations reflective of early-stage septicemic plague, Ail weakly recruits Vn and fails to recruit factor H, suggesting that this alternative mechanism of serum resistance may be essential during plague infection.
GRAPHICAL ABSTRACT
ABBREVIATED SUMMARY
To survive in humans, Yersinia pestis must prevent killing by the human complement system. We show hydrophobic residues F80 and F130 in the extracellular loops of Ail are required to recruit the complement regulatory proteins Factor H and vitronectin, but their recruitment is largely dispensable for survival in serum. Thus, we propose an additional mechanism of Ail-mediated serum resistance involving interference with C8 or C9 in the final steps of membrane attack complex assembly.
INTRODUCTION
Yersinia pestis, a gram-negative rod, is the causative agent of plague, a rapidly progressing, often fatal disease (Perry & Fetherston, 1997). The bacterium is primarily transmitted to humans through the bite of infected fleas (Perry & Fetherston, 1997, Sebbane et al., 2005, Hinnebusch et al., 1996, Hinnebusch, 2005), where it enters the tissue and travels to the nearest regional lymph node (bubonic plague) (Perry & Fetherston, 1997). After growing to high numbers in the regional lymph node, Y. pestis can enter the bloodstream (septicemic plague), and spread to other blood-filtering organs including the liver and spleen. Once in the blood, Y. pestis can also spread to the lungs progressing to secondary pneumonic plague. At this point the infection can be spread human to human via respiratory droplets resulting in primary pneumonic plague, a rapidly fatal disease (Perry & Fetherston, 1997).
For host-host transmission via fleas, progression of a plague infection from buboes to the blood, and human to human transmission via respiratory droplets, it is critical that Y. pestis survive in human blood. Human complement, an innate immune defense mechanism against bacterial infections, is present in blood. Thus, Y. pestis must be able to evade complement to grow and survive in the host. The human complement system consists of three pathways: the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP). CP and LP, upon activation by antibodies or ficolins/mannose-binding lectins respectively, both lead to C4 cleavage to C4b. After forming an ester linkage with a cellular target, C4b can initiate formation of the CP/LP C3 convertase (C4b2a). The CP/LP C3 convertase cleaves C3 into C3a, an anaphylatoxin that induces a proinflammatory response (Klos et al., 2009), and C3b. C3b can either act as an opsonin for complement receptors on phagocytes or become incorporated into the CP/LP C5-convertase (C4b2a3b). C5 convertase is a serine protease that cleaves C5 into C5a and C5b. C5a is an additional anaphylatoxin and C5b can initiate assembly of the membrane attack complex (MAC) by interacting with C6 (Merle et al., 2015). C5bC6 then interacts with C7. Upon interaction with C8, the complex becomes membrane embedded and then finally C9 is recruited (Merle et al., 2015). Interaction of C5b-8 with C9 initiates polymerization of 12–18 C9 monomers into a ring structure called the membrane attack complex (MAC) (Tschopp et al., 1984, Serna et al., 2016). Assembly of the MAC in membranes of gram-negative bacteria leads to disruption of the bacterial membrane and osmotic lysis (Merle et al., 2015, Tegla et al., 2011, Serna et al., 2016).
The alternative pathway (AP) of complement differs from the CP/LP in the steps of initiation. The AP begins with spontaneous hydrolysis of C3 in the blood plasma (Pangburn & Müller‐Eberhard, 1983). This leads to the formation of a covalent ester bond between C3b and cellular target molecules, followed by interaction of C3b with Factor B (Law et al., 1979, Müller-Eberhard & Götze, 1972). A conformational change in Factor B after binding C3b allows for cleavage by Factor D, resulting in two fragments, Ba and Bb, and creating the active AP C3-convertase, C3bBb (Lesavre et al., 1979). Positive feedback and cleavage of more C3 into C3a and C3b by the AP C3-convertase leads to formation of the AP C5 convertase, (C3bBbC3b). From there, the AP follows the same pathway for MAC formation as CP/LP (Pangburn & Müller-Eberhard, 1983).
Regulatory factors of the complement system exist to prevent uncontrolled or inadvertent progression of the cascade on host cells. The fluid-phase regulator of the CP/LP, C4b-binding protein (C4BP), exists in the blood plasma and regulates activation of the cascade by acting as a cofactor for factor I-mediated inactivation of C4b, accelerating the decay of the CP/LP C3 convertase, and preventing assembly of the convertase by competitively binding C4b, thus preventing binding of C2 (Blom et al., 2004). Analogous to C4BP, factor H regulates the alternative pathway by binding surface molecules (often on host cells) and facilitating factor I-mediated cleavage and inactivation of C3b, as well as accelerating decay and preventing assembly of the AP C3 convertase before it can become an active component of the C3 convertase and drive assembly of the MAC (Pangburn & Müller-Eberhard, 1983, Whaley & Ruddy, 1976, Weiler et al., 1976). An additional regulator of complement activation is vitronectin. Vitronectin can inhibit formation of the MAC by binding the C5b-C7 complex (and C5b-C8 and C5b-C9 complexes) and sequestering it away from participation in the final steps of MAC assembly, thus halting progression of the MAC assembly pathway (Podack et al., 1977, Milis et al., 1993, Preissner et al., 1989). Vitronectin also has been implicated in direct interference with C9 polymerization (Milis et al., 1993, Podack et al., 1984).
Resistance of Y. pestis to human serum and complement has been attributed to the outer membrane protein Ail (Kolodziejek et al., 2007, Bartra et al., 2008). Ail is a transmembrane protein belonging to the Ail/Lon family, consisting of eight transmembrane β-strands and four extracellular loops. Previous studies have demonstrated that Y. pestis Ail interacts with C4BP (Ho et al., 2014) and vitronectin (Bartra et al., 2015). Other members of the Ail/Lon family: Y. enterocolitica Ail, Y. pseudotuberculosis Ail, and Salmonella enterica Rck confer varying degrees of serum resistance to their host strains (Heffernan et al., 1992, Bliska & Falkow, 1992, Yang et al., 1996). Each has the ability to recruit functional C4BP and factor H to the surface of the bacteria, providing potential mechanisms of serum resistance (Biedzka-Sarek et al., 2008a, Biedzka-Sarek et al., 2008b, Ho et al., 2012b, Ho et al., 2011, Ho et al., 2010, Ho et al., 2012a). Specific amino acids in the extracellular loops of Y. enterocolitica Ail and S. enterica Rck are required for serum resistance in these bacteria (Miller et al., 2001, Cirillo et al., 1996).
In addition to serum resistance, previous studies have demonstrated the importance of Y. pestis Ail in host cell binding, extracellular matrix (ECM) binding, and Yop (cytotoxin) delivery, (Felek et al., 2010, Tsang et al., 2010, Yamashita et al., 2011, Felek & Krukonis, 2009). Tsang et al. recently described the contribution of surface exposed hydrophobic residues, F80, S128, and F130 to these functions (Tsang et al., 2017). Cumulative mutation of these residues (Ail-F80A/F130A and Ail-F80A/S128A/F130A) resulted in substantial defects in cell adhesion, ECM binding, Yop delivery, and auto-aggregation, while Ail-F94 played a particularly critical role in fibronectin binding (Tsang et al., 2017). Despite these defects in binding to multiple substrates, Ail-F80A/F130A and Ail-F80A/S128A/F130A maintained strong serum resistance (10,000-fold higher than a Δail strain). Since Ail-S128A contributes minimally to Ail-mediated serum resistance (Tsang et al., 2017), studies presented here utilize Ail-F80A/F130A to assess mechanisms of serum resistance.
In this study, we found Ail-F80A/F130A also failed to interact with vitronectin, factor H, and C4BP, despite providing 10,000-fold greater serum resistance activity than a Δail mutant. Cumulative substitutions in Ail residues, along with F80A/F130A, identified an Ail molecule completely defective in conferring serum resistance. Thus, Ail can provide serum resistance via multiple mechanisms and recruitment of vitronectin, factor H, and C4BP is largely dispensable for Ail-mediated serum resistance.
RESULTS
The alternative pathway of complement is responsible for killing Y. pestis Δail.
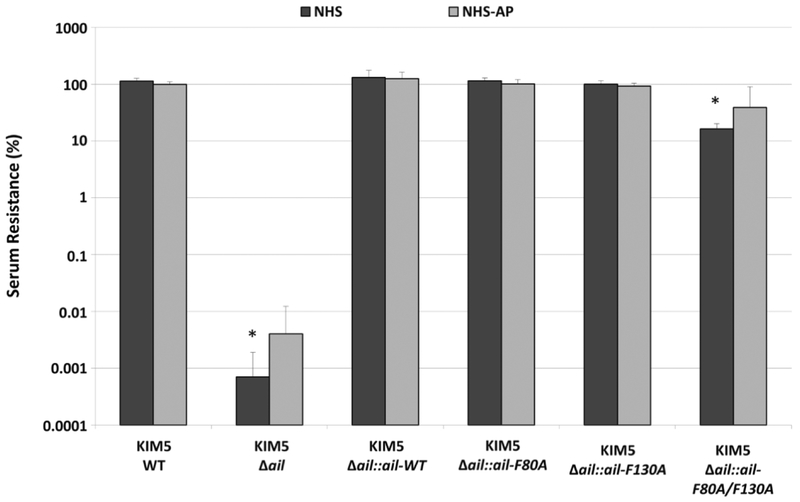
Y. pestis is resistant to high levels of human serum and this resistance is dependent completely on Ail (Kolodziejek et al., 2007, Bartra et al., 2008). To determine the pathway of complement, CP, LP, or AP, responsible for killing a Δail mutant, we assessed serum resistance under conditions that inhibit specific pathways of complement killing. Y. pestis strains were incubated with normal human serum (NHS) or NHS treated with 5mM EGTA and 10mM MgCl2 (NHS-AP) which eliminates any contribution of the classical (CP) or lectin pathways (LP) of complement killing (Fig. 1)(Des Prez et al., 1975). While the Δail strain was ~100,000-fold defective for survival in NHS, chromosomally expressed wild-type Ail, Ail-F80A, or Ail-F130A conferred 100% serum resistance similar to previous findings (Tsang et al., 2017). Furthermore, Ail-F80A/F130A had only a modest (6-fold) survival defect in NHS compared to the wild-type Ail as previously reported (Tsang et al., 2017). Serum inactivated for CP and LP (NHS-AP) had no statistically significant difference in killing of Δail or Ail-F80A/F130A compared to NHS, as determined by two-way ANOVA analysis (Fig. 1). Thus, killing of Δail and Ail-F80A/F130A is mediated by the alternative pathway of complement. To further demonstrate that the AP system of complement is the main mediator of Δail killing, C4-depleted serum, which lacks activity of only the CP and LP, retained >1,000-fold greater bactericidal activity against Y. pestis Δail than cells expressing wild-type Ail (Fig. S1A), while addition of 5mM EDTA, which prevents the function of all three complement pathways, prevented killing of the Δail mutant (Fig. S1B).
Figure 1. Killing of Y. pestis Δail by human serum is mediated by the alternative pathway of complement.
~7.5 × 105 CFU of mid-log cultures of Y. pestis strains containing wild-type Ail, a chromosomal deletion of ail (Δail), or chromosomal ail recombinants were treated with 80% NHS, 80% HIS (Heat-inactivated serum), or 80% NHS-AP (NHS treated with 5mM EGTA and 10mM MgCl2 to inactivate CP/LP) for one hour at 37°C. Surviving bacteria were plated and enumerated by colony counting. Percent serum resistance was calculated as the number of surviving colonies in NHS/HIS or NHS-AP/HIS x 100 and is displayed on a logarithmic scale. Strains were tested a minimum of 3 times for each condition in separate experiments. Significance was determined using the two-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to the parental KIM5 wild-type (WT) strain in the same serum condition.
Ail-dependent recruitment of the complement regulators, factor H and Vitronectin, requires residues F80 and F130.
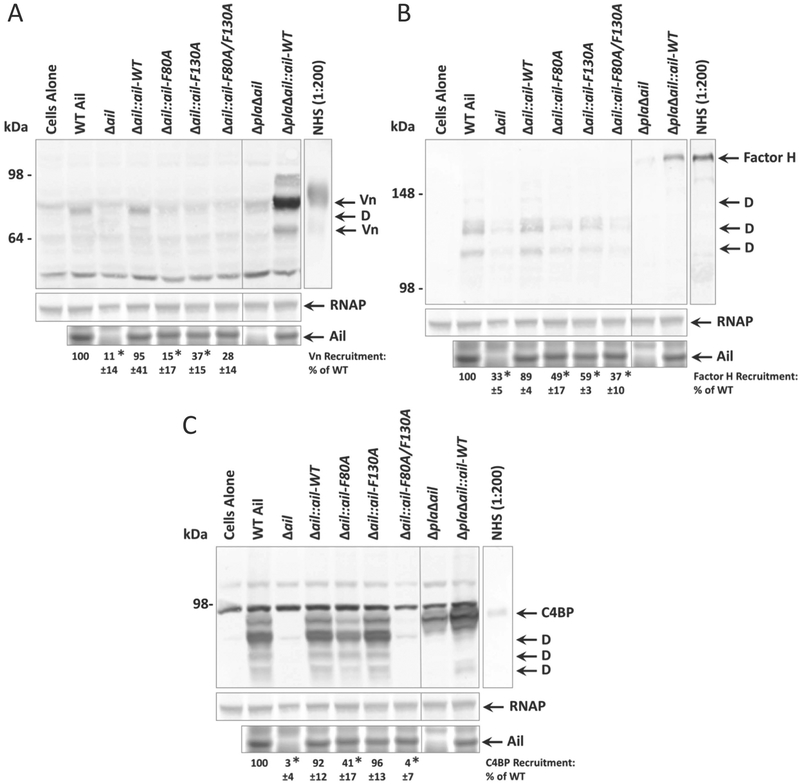
Y. pestis Ail has previously been shown to recruit the complement regulatory factors C4BP and vitronectin (Ho et al., 2014, Bartra et al., 2015) and the closely related Ail protein from Y. pseudotuberculosis recruits C4BP and factor H (Ho et al., 2012a, Ho et al., 2012b). Since a Y. pestis strain expressing Ail-F80A/F130A exhibited only six-fold less serum resistance than a strain expressing wild-type Ail, while remaining ~10,000-fold more serum resistant than Δail (Fig.1, (Tsang et al., 2017)), we determined whether residues F80 and F130 contribute to Y. pestis Ail-mediated recruitment of complement regulatory proteins as a mechanism of serum resistance. Co-sedimentation assays were performed with strains expressing Ail-F80A, Ail-F130A, and Ail-F80A/F130A to assess vitronectin and factor H binding. Y. pestis at a final OD620 = 50 (~1.5 × 1010 CFU/mL) were incubated with 50% NHS and evaluated for serum protein binding by co-sedimentation of complement proteins and Western blotting. Vitronectin co-sedimented with strains expressing wild-type Ail, but was only minimally bound in bacterial pellets of strains expressing Ail containing mutations to either F80 or F130 (Fig. 2A). Statistical analysis, adjusting for multiple comparisons using Tukey’s post hoc test, revealed that Ail-F80A/F130A showed a strong trend towards decreased vitronectin recruitment without reaching statistical significance (p=0.0576) compared to wild-type. However, in a pairwise comparison, Ail-F80A/F130A recruited significantly less vitronectin (p<0.05). Given that much more vitronectin is recruited by Ail in the absence of the outer membrane protease plasminogen activator (Pla) (Fig. 2A, lane 9), we hypothesize vitronectin is cleaved by Pla, as previously demonstrated (Bartra et al., 2015). Furthermore, co-sedimentation with the Δpla mutant reveals that Ail can bind full length and degraded forms of vitronectin (Fig. 2A, lane 9).
Figure 2. Co-sedimentation of complement regulatory factors with Y. pestis is mediated by Ail extracellular loop residues F80 and F130.
Overnight cultures of Y. pestis KIM5 strains containing wild-type Ail, a chromosomal deletion of ail, or specific chromosomal alleles of ail were mixed with 50% NHS to a final OD620 = 50. Mixtures were shaken vigorously at 37°C for 30 minutes. Cells were centrifuged, washed, and analyzed by Western blot for the presence of membrane-associated complement regulators: A) vitronectin (Vn) B) factor H C) C4b-binding protein (C4BP). Levels of expressed Ail were determined by Coomassie staining. ΔailΔpla strains are included to show full-length, un-degraded complement regulatory proteins. The cells alone lane indicates Y. pestis KIM5 cross-reactive bands recognized in the absence of NHS. Blots are one representative of at least three independent experiments and are shown with the Coomassie-stained gel showing Ail expression from the same experiment, as well as the loading control anti-E. coli RNA polymerase alpha. Molecular weight markers are indicated on the left of the blot. Quantification of band intensity was performed using at least 3 independent experiments with ImageJ software (NIH). Intensity of bands corresponding to complement regulator recruitment is shown as a percentage of WT recruitment (normalized to 100%) in each individual blot. Significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to the wild-type strain of Y. pestis KIM5. Abbreviations: Vn=vitronectin, C4BP=C4b-binding protein, D=degraded form of protein (degraded by Pla).
Y. pestis also exhibited Ail-dependent recruitment of factor H, which was also cleaved by Pla into discrete bands (Fig. 2B). Previous studies have also indicated Pla is capable of cleaving factor H (Riva et al., 2015). A strain lacking Ail recruited 33% of the factor H recruited by a strain expressing wild-type Ail. Ail-F80A and Ail-F130A had significantly decreased levels of factor H recruitment, while a double mutant Ail-F80A/F130A had levels of recruitment (37%) comparable to the Δail strain.
C4BP had a co-sedimentation profile similar to that of factor H, having a partial defect in C4BP recruitment with the Ail-F80A mutant and a complete loss of binding with the Ail-F80A/F130A mutant (Fig. 2C). Furthermore, C4BP was cleaved by Pla (Fig. 2C). While C4BP contributes primarily to regulation of CP and LP, which are not involved in killing Δail (Fig. 1), it also can play a minor role in control of C3 activation in the AP of complement, albeit to a much lesser extent than factor H (Seya et al., 1995, Blom et al., 2003).
These data demonstrate that strains expressing Ail-F80A/F130A lose the ability to recruit three complement regulatory factors vitronectin, factor H, and C4BP, comparable to recruitment by Δail. Despite the inability to recruit those factors, the Ail-F80A/F130A mutant maintains ~10,000 fold serum resistance (Fig.1). Therefore, an alternate Ail-dependent mechanism of complement evasion exists.
Additional amino acid substitutions to Ail-F80A/F130A result in serum sensitivity comparable to Δail.
Several Ail/Lon family members in other bacterial pathogens confer serum resistance including Y. enterocolitica Ail, Y. pseudotuberculosis Ail, and S. enterica Rck (Heffernan et al., 1992, Bliska & Falkow, 1992, Yang et al., 1996). Extensive studies on the involvement of Y. enterocolitica Ail residues in adhesion and serum resistance revealed the contribution of D90 and V91 (numbered according to the unprocessed form) to serum resistance (Miller et al., 2001). Expressing double mutants, Ailent-D90A/V91R and Ailent-D90G/V91G both resulted in serum sensitivity in Y. enterocolitica (Miller et al., 2001).
Therefore, we mutated the homologous residues (D93/F94) in combination in Y. pestis Ail (Fig. 3A). Strains expressing Ail-D93A/F94R or Ail-D93G/F94G from the plasmid pMMB207 had no significant difference in serum resistance compared to wild-type Ail (Fig. 3B). Ail-D93A/F94R resulted in slight decreases in recruitment of vitronectin to 58% of wild-type Ail (Fig. 4A), compared to 14% recruitment exhibited by Ail-F80A/F130A and 13% by Δail. Similarly, Ail-D93A/F94R was defective for factor H recruitment (48% relative to wild type Ail) while Ail-D93G/F94G displayed no significant decrease in recruitment of factor H (Fig. 4B). Ail-F80A/F130A recruited only 7% of factor H relative to wild-type Ail (Fig. 4B). When combined with the F80A/F130A mutation, the D93/F94 mutations reduced Ail-mediated serum resistance to levels statistically indistinguishable from an ail mutant (Fig. 3B). Additionally, recruitment of vitronectin and factor H was eliminated in strains expressing Ail-D93/F94 mutations in combination with the F80A/F130A mutations (Fig. 4A), as expected due to dependence on F80 and F130 for binding (Fig. 2AB).
Figure 3. Multiple Ail substitutions required to reveal a serum sensitivity phenotype comparable to Δail deletion.
A) Amino acid substitutions of Y. pestis Ail residues corresponding to homologous residues that no longer confer full serum resistance in Yersinia enterocolitica Ail (Miller et al., 2001) and Salmonella enterica Rck (Cirillo et al., 1996). B) Resistance of Y. pestis KIM5 Δail expressing plasmid-borne Ail or Ail derivatives, to killing by normal human serum (NHS). ~7.5 × 105 CFU of mid-log culture grown with 500μM IPTG (to induce Ail expression) was added to 80% heat-inactivated serum (HIS) or 80% NHS for one hour at 37oC. Surviving bacteria were plated and enumerated by colony counting. Percent serum resistance was calculated by (number of surviving colonies in NHS or NHS-AP/HIS) x 100 and is displayed on a logarithmic scale. Strains were tested a minimum of 3 times in separate experiments. Ail expression and stability was determined by Coomassie staining shown beneath the graph. Significance was assessed using the one-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to serum resistance of a strain expressing wild-type Ail.
Figure 4. Co-sedimentation of alternative pathway regulatory factors is mediated by various extracellular loop residues of Y. pestis.
Overnight cultures grown in the presence of 100μM IPTG (to induce Ail expression) were mixed with 50% NHS to a final OD620 = 50. Mixtures were shaken vigorously at 37°C for 30 minutes. Mixtures were centrifuged and cell pellets were washed, then subjected to Western blotting for complement regulatory factors: A) vitronectin (Vn) and B) factor H. All Western blots are accompanied by Coomassie-stained gel showing Ail expression in the same samples. Molecular weight markers are indicated on the left. The cells alone lane represents Y. pestis in the absence of NHS. Quantification of band intensity was performed using at least 3 independent experiments with ImageJ software (NIH). Intensity of bands corresponding to complement regulator recruitment is shown as a percentage of WT recruitment (normalized to 100%) in each individual blot. Significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to a strain expressing wild-type Ail.
Cell adhesion/invasion and serum resistance activities are conferred upon S. enterica by the protein Rck (Cirillo et al., 1996, Heffernan et al., 1992). Amino acids D43 and G118 of Rck, when mutated to D43K and G118D respectively, caused decreases in serum resistance. The greatest drop in resistance was revealed when both residues were mutated in combination (Cirillo et al., 1996). To address the contribution of homologous residues in Y. pestis Ail, we generated the variants E43K and G122D (Fig. 3A). Individually, these substitutions in Ail led to 2 to 3-fold decreases in serum resistance compared to wild-type Ail (Fig. 3B). A strain expressing the double mutant, Ail-E43K/G122D, had a much larger defect in serum resistance (1000-fold decrease in survival), and when combined with F80A/F130A, the quadruple mutant, Ail-F80A/F130A/E43K/G122D, had an additional 10-fold decrease in serum resistance, comparable to Δail containing empty pMMB207, although after adjusting the statistical analysis for multiple comparisons, the difference in serum resistance between Ail-E43K/G122D and Ail-F80A/F130A/E43K/G122D was not significant (Fig. 3B).
Regarding recruitment of complement regulatory proteins, Ail-E43K/G122D maintains similar levels of vitronectin and factor H recruitment as Ail-D93A/F94R (Fig. 4), yet Ail-E43K/G122D loses 1,000-fold serum resistance activity, while the Ail-D93A/F94R mutant maintains ~100% serum resistance (Fig. 3B). Together, these data show that residues E43 and G122 play a minor role in co-sedimentation of complement regulators, but an important role in Y. pestis serum resistance by an additional mechanism.
It should be noted that all Ail mutants reported have been shown to be stably expressed in the outer membrane of Y. pestis (Fig. S2).
Co-sedimentation of complement components at lower bacterial concentration affects Ail-dependent recruitment of Vn and Factor H.
Our initial serum co-sedimentation assays were performed at a bacterial concentration based on assays in previous studies (Biedzka-Sarek et al., 2008a, Bartra et al., 2015, Kirjavainen et al., 2008, Ho et al., 2012b). This high bacterial concentration (OD620 = 50, 1.5 × 1010 CFU/mL), while useful to assess binding of serum components to Y. pestis, is closer to levels seen in late stage plague infection (Sebbane et al., 2005, Lorange et al., 2005). Given that in some cases recruitment of complement regulatory proteins did not reflect serum bactericidal activity (e.g. Ail-E43K/G122D), we assessed recruitment of complement regulatory proteins in Y. pestis expressing wild-type Ail, Ail-F80A/F130A, Ail-E43K/G122D, Ail-F80A/F130A/E43K/G122D, or pMMB207 (empty vector) at a bacterial concentration more closely reflecting early-stage, septicemic plague infection (OD620 = 0.25, 7.5 × 107 CFU/mL) (Sebbane et al., 2005). This is also a bacterial density closer to how serum resistance assays are routinely performed.
At the lower bacterial concentration, the level of Ail-dependent recruitment of vitronectin dropped to 2-fold above the background of the Δail mutant (Fig. 5A). This modest level of vitronectin recruitment was lost with the Ail-F80A/F130A mutant. Additionally, Ail-E43K/G122D and Ail-F80A/F130A/E43K/G122D failed to recruit Vn at the reduced bacterial density (Fig. 5A). Similarly, Ail-dependent binding of factor H was completely lost at the lower bacterial concentration (Fig. 5B). Cleavage of recruited serum factors by Pla was determined to be a result of the high bacterial density used in the previous experiments, as at lower bacterial density the recruited proteins vitronectin and factor H were mostly full length with little to no degradation (Fig. 5). This suggested Pla cleavage of Vn and factor H may be due to interbacterial cleavage, not cleavage by Pla on the same bacterial surface. Additionally, the higher concentration of Pla at OD620=50 may allow for cleavage of poorly-recognized substrates. These trends in recruitment further indicate that another mechanism of serum resistance is utilized by Y. pestis under bacterial concentrations achieved during plague infections.
Figure 5. Loss of Ail-mediated recruitment of complement regulatory factors at lower bacterial concentration.
Overnight cultures grown in the presence of 100μM IPTG (to induce Ail expression) were mixed with 50% NHS to a final OD620 = 0.25. Mixtures were shaken vigorously at 37°C for 30 minutes. Samples were centrifuged and cell pellets were washed, then subjected to Western blotting for complement regulatory factors: A) vitronectin (Vn) and B) factor H. Western blots are accompanied by Coomassie-stained gel showing Ail expression in the same samples, as well as the loading control anti-E. coli RNA polymerase alpha. Molecular weight markers are indicated on the left. The cells alone lane represents Y. pestis in the absence of NHS. Quantification of band intensity was performed using at least 3 independent experiments with ImageJ software (NIH). Intensity of bands corresponding to complement regulator recruitment is shown as a percentage of wild-type Ail-mediated recruitment (normalized to 100%) in each individual blot. Significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to a strain expressing wild-type Ail.
MAC assembly occurs to a higher extent in serum sensitive mutants.
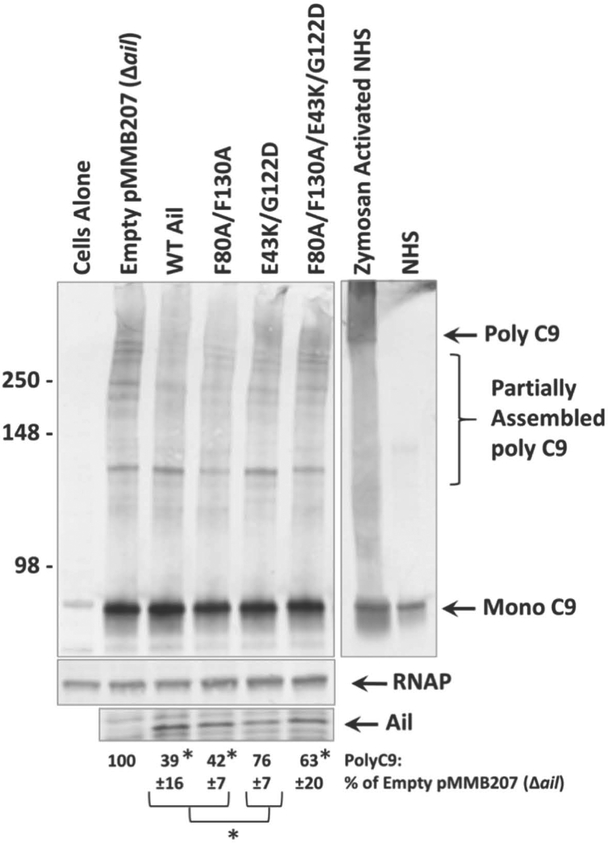
Δail exhibits minimal recruitment of complement regulatory factors and we observed similarly low levels of recruitment in Ail-F80A/F130A and Ail-F80A/F130A/E43K/G122D regardless of bacterial concentrations used during co-sedimentation. However, Ail-F80A/F130A confers 10,000-fold greater serum resistance than Ail-F80A/F130A/E43K/G122D. Thus, Ail-F80A/F130A must maintain a mechanism of serum resistance disrupted by the E43K/G122D mutations. In fact, even the Ail- E43K/G122D double mutant (with F80 and F130 intact) has a 500-fold defect in serum resistance relative to Ail-F80A/F130A. In S. enterica, D43K and G118D mutations in Rck disrupt the ability of Rck to prevent C9 polymerization, the last step in MAC formation (Cirillo et al., 1996, Heffernan et al., 1992). Thus, we assessed levels of C9 polymerization on the surface of Y. pestis in the presence of various Ail derivatives. Strains were mixed at low concentration (OD620 = 0.25, 7.5 × 107 CFU/mL) with NHS and subjected to non-reducing SDS-PAGE followed by Western blotting, as polymerized-C9 is SDS-resistant (Podack & Tschopp, 1982). An anti-C9 antibody was used to assess levels of polymerized C9. Zymosan-activated NHS was used as a positive control for C9 polymerization and untreated NHS was shown as a control for monomeric C9. Serum sensitive Δail with empty vector displayed the highest level of high molecular weight polymerized C9 as expected (Fig. 6, lane 2). Serum resistant wild-type Y. pestis and Ail-F80A/F130A exhibited the lowest levels of polymerized C9 (Fig. 6, lanes 3 and 4), 39% and 42% relative to Δail, respectively. The levels of C9 polymerization were increased in strains expressing Ail-E43K/G122D and Ail-F80A/F130A/E43K/G122D, however the levels in Ail-F80A/F130A/E43K/G122D remained significantly lower than Δail. This finding suggests that there may be a threshold level of polymerized C9 that correlates with serum resistance. The serum sensitive strain, Ail-F80A/F130A/E43K/G122D, has less polymerized C9 incorporated than Δail, but allows enough MAC assembly to render it serum sensitive.
Figure 6. Membrane-association of polymerized C9 is increased in strains lacking ail or containing multiple mutations to extracellular loops.
Overnight cultures grown in the presence of 100μM IPTG (to induce Ail expression) were mixed with 50% NHS to a final OD620 = 0.25. Mixtures were shaken vigorously at 37°C for 30 minutes. Samples were centrifuged, cell pellets were washed, and subjected to Western blotting under non-reducing conditions using an anti-C9 polyclonal antibody. Western blot is one representative of at least three independent experiments. Ail expression was determined by Coomassie staining and is shown beneath the blot. Molecular weight markers are indicated on the left of the blot. Cells alone lane represents Y. pestis in the absence of NHS. Zymosan activated NHS is a positive control for the formation of polymerized C9 compared to NHS alone (monomeric C9). Quantification of band intensity from at least 3 independent experiments was performed using ImageJ software (NIH). Intensity of bands corresponding to C9 polymerization is shown as a percentage of the Δail mutant (a strain with no ability to inhibit C9 polymerization), which was normalized to 100%. Significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p-value < 0.05 when compared to a strain expressing wild-type Ail.
Additional experiments were attempted to confirm C9 polymerization defects using the anti-C9 neo-antigen antibody aE11 (Life Technologies), that only recognizes fully polymerized poly C9 (Kolb & Muller-Eberhard, 1975). Unfortunately, to obtain complete MAC assembly (and C9 polymerization) as reflected by bacterial killing, such a low bacterial density was required in the presence of 50–80% human serum that we could not reliably precipitate so few bacteria to enable processing of the neo-antigen Ab binding studies with any consistency by ELISA assay.
DISCUSSION
Ail is a multi-functional outer membrane protein involved in cell adhesion, binding to ECM components, Yop delivery, and serum resistance, thus delivering important functions during various stages in Y. pestis infection (Felek & Krukonis, 2009, Felek et al., 2010, Tsang et al., 2010, Yamashita et al., 2011, Bartra et al., 2008, Kolodziejek et al., 2007, Kolodziejek et al., 2010). In late-stage plague infection, Y. pestis survive in blood and grow to a high-level septicemia approaching 1010CFU/mL (Sebbane et al., 2005) despite the bactericidal effects of complement in serum. High level septicemia allows for transmission to a new flea vector during a blood meal (Lorange et al., 2005). Ail is necessary and sufficient to confer resistance to serum to Y. pestis, providing 100,000-fold greater evasion of complement-mediated killing than a strain lacking ail (Tsang et al., 2017, Bartra et al., 2015, Kolodziejek et al., 2007). In this study, we show that a Δail mutant is efficiently killed in 80% NHS during in vitro serum resistance assays and this killing is attributed to the alternative pathway of complement (Fig. 1).
Two residues of Ail, F80 and F130, mediate cell-binding, binding to extracellular matrix proteins, and facilitate Yop delivery (Tsang et al., 2017). Mutation of both residues to alanine leads to only a modest decrease (three to six-fold) in serum resistance compared to a wild-type Y. pestis strain, while remaining 10,000-fold more resistant to serum than an ail deletion mutant (Figs. 1, 3B (Tsang et al., 2017)). However, Ail-F80A/F130A is unable to recruit alternative pathway complement regulatory factors, such as vitronectin (Vn) and factor H, at both high cell density (Fig. 2A,B) and lower cell concentration (Fig. 5A,B) the latter being more physiologically relevant to bacterial levels in blood during early-stage septicemic plague (Perry & Fetherston, 1997, Lorange et al., 2005, Sebbane et al., 2005). Recruitment of complement regulatory proteins is a complement evasion tactic employed by a multitude of pathogens (Hovingh et al., 2016). However, recruitment of complement regulators by Ail-F80A/F130A is indistinguishable from recruitment by a Δail mutant. Thus, Ail-F80A/F130A must be providing serum resistance via a different mechanism.
Site-directed mutagenesis targeting amino acids in Y. pestis Ail, based on previous studies with Ail homologs in Y. enterocolitica (Ail) and S. enterica (Rck), was performed to determine essential residues in Y. pestis Ail that contribute to serum resistance. One mutant, Ail-E43K/G122D, based on studies on S. enterica Rck (Cirillo et al., 1996), had a large defect in serum resistance, even when F80 and F130 were intact. Alternatively, mutations of D93/F94 (based on studies with Y. enterocolitica Ail (Miller et al., 2001)) had no effect on Y. pestis serum resistance and required being combined with the F80A/F130A mutations to result in a loss of serum resistance. Based on the dramatic loss in serum resistance activity of Ail-F80A/F130A/D93A/F94R and Ail-F80A/F130A/D93G/F94G compared to Ail-F80A/F130A (Fig. 3B), residues D93 and F94 clearly contribute to serum resistance activity. However, defects associated with mutations in D93 and F94 are masked by wild-type F80 and F130 residues. In contrast to the Ail-F80A/F130A mutant, mutations of E43, G122, D93, and F94 had little impact on recruitment of Vn and factor H, similar to studies done on homologous residues in Y. enterocolitica Ail (Biedzka-Sarek et al., 2008b).
At bacterial concentrations reflective of late-stage septicemic plague, we saw a 10-fold increase in Vn recruitment and a 3-fold increase in factor H recruitment in wild-type Y. pestis relative to a Δail mutant (Fig. 2AB). At a lower bacterial concentration, we saw only a 2-fold increase in Vn recruitment relative to the Δail mutant (Fig. 5A) and wild-type Ail mutant actually showed less membrane-associated factor H than the Δail mutant (Fig. 5B, blots were over-developed to detect weak factor H binding). These data indicate a potential disparity in complement regulator recruitment depending on the bacterial concentration at various stages of plague infection. These findings suggest the major alternative pathway complement regulators Vn and factor H may not play a role in serum resistance during early stages of septicemic plague and instead Y. pestis relies on our newly described alternative mechanism of Ail-mediated serum resistance for survival in blood during this critical stage of infection.
Studies showing Ail-dependent recruitment of C4BP and factor H by Y. pseudotuberculosis (Ho et al., 2012a, Ho et al., 2012b) and Y. enterocolitica (Kirjavainen et al., 2008, Biedzka-Sarek et al., 2008a), as well as C4BP in Y. pestis (Ho et al., 2014), revealed that regulators were bound and facilitated cofactor-dependent inactivation of C4b and C3b, respectively. Based on these regulatory protein binding and functionality studies in other Yersinia spp., it is plausible that Y. pestis Ail also recruits fully functional factor H (Fig. 2B) that retains the ability to inactivate C3b. However, it should be noted that at high bacterial concentrations, Pla, which is unique to Y. pestis, degrades factor H, C4BP and Vn (Fig. 2, (Bartra et al., 2015)). The fact that the Δail strain and strains expressing Ail-F80A/F130A and Ail-F80A/F130A/E43K/G122D recruited similar levels of vitronectin at lower bacterial concentration (Fig. 5A), but only Ail-F80A/F130A conferred serum resistance (Fig. 3B) indicates serum resistance of Ail-F80A/F130A is conferred by an alternate Ail-dependent mechanism.
Finally, we analyzed the extent of MAC maturation in Y. pestis by measuring levels of C9 polymerization by Western blotting. Y. pestis Δail (containing the empty vector pMMB207), which is highly serum sensitive, had the greatest degree of polymerized C9, as expected. When wild-type Ail was expressed, the amount of C9 in the polymerized form was drastically decreased (39% of Δail, Fig. 6). Y. pestis expressing Ail-F80A/F130A also inhibited the maturation of the MAC (Fig. 6), reflecting its serum resistance activity (Figs. 1 and 3B). Rck of S. enterica confers serum resistance via inhibition of C9 polymerization (Cirillo et al., 1996, Heffernan et al., 1992) and mutation of D43 and G118 in Rck eliminates serum resistance activity (Cirillo et al., 1996, Heffernan et al., 1992). Y. pestis expressing the homologous mutant Ail-E43K/G122D had reduced serum resistance (Fig. 3B), approaching the levels of a Δail mutant. These mutations have little effect on the recruitment of vitronectin and factor H (Figs. 4, 5), but Y. pestis expressing Ail-E43K/G122D display significantly more polymerized C9 compared to wild-type Ail and Ail-F80A/F130A (Fig. 6). Therefore, it is plausible that E43 and G122 of Y. pestis Ail may mediate direct inhibition of C9 polymerization similar to the proposed mechanism of serum resistance conferred by Rck.
It should be noted that due to the bacterial densities used for these studies, C9 was not fully assembled to the MAC and what is observed is partially polymerized C9 (Fig. 6; compare sizes of poly-C9 with Y. pestis to the fully assembled MAC in the zymosan-activated sample). Attempts to reduce the bacterial concentration to allow for more complete MAC assembly on each Y. pestis membrane surface were hampered by the inability to reproducibly precipitate so few bacteria. This limitation also prevented us from assessing C9 polymerization by a secondary assay of MAC assemble, neo-antigen exposure, which is dependent on complete MAC assembly (Kolb & Muller-Eberhard, 1975).
Various mechanisms beyond recruitment of host complement regulatory proteins and inhibition of C9 polymerization, are employed by pathogens to evade complement. One such mechanism is sequestration of C7 by Borrelia burgdorferi (Hallstrom et al., 2013). CspA of B. burgdorferi, similar to Y. pestis Ail, is involved in many facets of complement evasion. CspA binds factor H, while also binding C7 and C9, primarily interfering with maturation of the MAC at the C7 step (Hallstrom et al., 2013). We found Y. pestis Ail also mediated binding to C7 at both high and low bacterial concentration, (Fig. S3AB). Ail may play a role in binding C7 to inhibit MAC maturation at the step of C7, however this recruitment C7 may also be attributed to vitronectin-associated C5b-C7, C5b-C8 and C5b-C9 complexes (Podack et al., 1977, Preissner et al., 1989). Ail can also mediate binding to C6 at low cell density, whereas, at high bacterial concentration C6 recruitment is only seen in the absence of Pla (Fig. S3AB). Expression of Ail-F80A/F130A leads to a decrease in membrane-associated C6 and C7 compared to wild-type Ail, which is consistent with the loss of binding to vitronectin. Binding to C8 and C9 remains consistent regardless of the bacterial cell concentration or the presence of Ail. We interpret this to reflect the fact that C8 and C9 are inserted into the membrane of serum-sensitive mutants like Ail-E43K/G122D or Ail-F80A/F130A/E43K/G122D, while for serum-resistant strains like those expressing wild-type Ail or Ail-F80A/F130A, C8 and C9 would be recruited as part of the Vn/C5b-C8 and C5b-C9 complexes (Preissner et al., 1989). Our findings indicate that what distinguishes serum-sensitive strains of Y. pestis (Δail, Ail-E43K/G122D, Ail-F80A/F130A/E43K/G122D) from serum-resistant strains (expressing wild-type Ail, Ail-F80A/F130A) is the higher level of polymerized C9 in the serum-sensitive strains (Fig. 6). Additionally, Ail-F80A/F130A has less binding to vitronectin as well as less membrane-associated C6 and C7 while remaining serum resistant, suggesting other amino acids (E43 and G122) may be involved with interrupting progression to the MAC at the level of C8 and C9 interaction/polymerization.
We noted Pla was able to cleave Vn, factor H, and C4BP (Fig. 2) as has been shown previously with several substrates (Riva et al., 2015, Bartra et al., 2015, Caulfield et al., 2014, Caulfield & Lathem, 2012, Sodeinde et al., 1992, Sodeinde et al., 1988). However, for Vn and factor H, Pla-mediated cleavage required a high cell density (Figs. 2, 5), suggesting that cleavage of complement proteins occurs via Pla proteases on neighboring bacterial cells (interbacterial cleavage). Pla also plays a role in cleavage of C3, however Pla mutants remain completely resistant to high levels of human serum (Sodeinde et al., 1992), indicating the unlikelihood that Pla plays a role in in vitro complement-mediated lysis. The fact that Pla cleaves multiple complement regulatory proteins calls into question the role of these proteins in serum resistance of Y. pestis. Further studies are needed to determine whether Pla-degraded forms of these proteins are still able to interrupt serum-dependent killing.
Animal studies with Δail mutants suggested that Ail interferes with the production of C3a and C5a (potentially via factor H recruitment) due to the observation of a strong influx of polymorphonuclear leukocytes (PMN) to the site of infection in a Δail mutant (Hinnebusch et al., 2011). Our assays detect factor H binding at high concentrations of Y. pestis, which may indicate that Ail-dependent recruitment of factor H in buboes may prevent production of the alarmones, C3a and C5a, thus preventing PMN infiltration. Alternatively, it is possible the lack of PMN recruitment seen during bubonic plague models of infection are due to a reduced efficiency of Yop delivery via T3SS in a Δail mutant (Marketon et al., 2005, Merritt et al., 2014). Nonetheless, our studies show Y. pestis can survive complement-mediated lysis in human serum, even without the ability to recruit factor H or vitronectin (as demonstrated by Ail-F80A/F130A).
Defining the amino acids of Ail involved in preventing complement-mediated lysis further elucidates the role of Ail during host infection. We have found residues in Ail (F80 and F130) that not only mediate cell binding, binding to ECM, and Yop delivery (Tsang et al., 2017), but also facilitate the binding/recruitment of complement regulatory proteins (Vn, factor H, and C4BP) to the bacterial surface. We found that Ail residues (E43 and G122), when mutated in combination decrease serum resistance, while regulatory protein binding remains relatively unchanged, implicating an alternative mechanism of serum resistance in addition to/instead of complement regulatory protein recruitment. This alternative mechanism may be direct interference with C9 polymerization. The role of serum resistance during plague infection is not well defined, however, Y. pestis must have the ability to survive in blood to reach the high levels of bacteremia needed to be transmitted to a new flea host during feeding (Lorange et al., 2005). Experiments comparing the role of Ail during mouse infections (mouse serum is not bactericidal for a Δail mutant, (Bartra et al., 2008)) compared to rat studies (rat serum is bactericidal for a Δail mutant, (Bartra et al., 2008)) suggest an important role for Ail-mediated serum resistance during human plague infections (reflective of rat infections, (Hinnebusch et al., 2011, Kolodziejek et al., 2010)), but this hypothesis has yet to be tested. Future studies will assess the contribution of specific Ail residues defined in this study to serum resistance in vivo, and will clarify the role of Ail during the course of Y. pestis infection.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions.
Y. pestis KIM5 strains were cultured overnight in heart infusion broth (HIB) or on heart infusion agar (HIA) for 48 hours at 28°C. Escherichia coli strains were grown overnight in Luria-Bertani (LB) broth or LB agar at 37°C. Antibiotics were used at the following concentrations: chloramphenicol (25μg/mL), ampicillin (100μg/mL), and kanamycin (30μg/mL). Isopropyl-β-D-thiogalactopyranoside (IPTG) was used at concentrations of 100μM or 500μM depending on assay. Characteristics of strains and plasmids used in this study are listed in Table S1.
Strain and plasmid construction.
Strains containing mutated ail alleles recombined into the ail locus were created in a previous study (Tsang et al., 2017). These strains were subjected to λ-RED recombination to knockout pla as in (Felek et al., 2010). Deletions were confirmed by PCR and plasminogen activator assays.
Site-directed mutagenesis of Ail was conducted using whole-plasmid replication using primers designed according to the protocol in (Liu & Naismith, 2008). Briefly, primers (listed in Table S2) were designed to incorporate desired mutation/s to ail using pSK-Bluescript-ail plasmid as a template (Tsang et al., 2017). PCR reactions were conducted using Phusion High-Fidelity DNA Polymerase (Thermo) and the following cycle settings: 94°C for 3 minutes, then 25 cycles of 94°C for 1 minute, 52°C for 1 minute, 72°C for 6 minutes, followed by a final extension at 72°C for 60 minutes. PCR reactions were then subjected to DpnI restriction digestion to degrade template DNA followed by transformation into E. coli DH5α + pREP4. Potential mutants were sequenced to confirm mutation. Sequenced clones were digested with BamHI and PstI to isolate the entire ail locus and ribosomal binding site and were ligated into pMMB207.
Serum resistance assay.
Strains were grown overnight in HIB at 28°C, subcultured 1:50 into fresh HIB and incubated while shaking for 3 hours at 28°C. Subcultures of strains containing pMMB207 derivatives were subcultured with the addition of 500μM. Cultures were resuspended in PBS to OD620 = 0.5 and further diluted 1:10 in PBS. 50μL cells was mixed with 200μL Normal Human Serum (NHS) (Sigma) or Heat-Inactivated Serum (HIS). HIS was prepared by incubating NHS at 56°C for 30 minutes. For alternative pathway only serum (NHS-AP), 5mM EGTA and 10mM MgCl2 was added to NHS. Bacterial counts were enumerated by colony counting. Percent serum resistance was calculated by the number of surviving colonies in NHS or NHS-AP/HIS x 100. Strains were tested a minimum of 3 times in separate experiments. Significance was determined using the Student’s t-test.
Serum co-sedimentation assay.
Strains were grown overnight in HIB +/− 100μM IPTG depending on experiment. Cultures were centrifuged, washed once with 1mL PBS, and resuspended to OD620 = 100 or 0.5 in PBS. For cultures resuspended to OD620 = 100, 50μL culture (~1.5 × 109 CFU) was mixed with 50μL NHS (Final OD620 = 50, ~1.5 × 1010 CFU/mL). For cultures resuspended to OD620 = 0.5, 250μL of culture (~7.5 × 106 CFU) was mixed with 250μL NHS (Final OD620 = 0.25, ~7.5 × 107 CFU/mL). Mixtures were shaken vigorously (300rpm) at 37°C for 30 minutes. Samples were then incubated for 5 minutes on ice and centrifuged at 4°C. Pellets were washed 3 times with cold PBS. Co-sedimentation mixtures at OD620 = 50 were resuspended in 200μL 1X reducing protein buffer (50mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 0.1% Bromophenol blue, 100mM dithiothreitol). Cultures at OD620 = 0.25 were resuspended in 50μL 1X reducing protein buffer. Samples were subjected to analysis by western blotting for complement factors and Coomassie blue staining for expression of Ail.
Protein expression and western blot analysis.
Cultures of Y. pestis or co-sedimentation reactions were resuspended in Laemmli sample buffer (+/− DTT) normalizing for OD620. Samples were boiled for 5 minutes and subjected to 15% SDS-polyacrylamide gel electrophoresis (PAGE) for determination of Ail expression followed by Coomassie blue staining, where Ail is identified as a band at approximately 15kDa (Felek & Krukonis, 2009). Co-sedimentation samples were run on 7.5% SDS-PAGE gel (poly-C9 detection run on 4%−15% gradient gel (Bio-Rad)), followed by blotting on polyvinylidene fluoride (PVDF) membrane for visualization of complement factors by western blotting. Primary antibodies were added at the following dilutions: polyclonal anti-human vitronectin (1:20,000) (Complement Technology-A260), polyclonal anti-human factor H (1:2,000) (Complement Technology-A237), polyclonal anti-human C4BPA (1:10,000) (Thermo PA5–42001), polyclonal anti-human C9 (1:5,000) (Complement Technology-A226), monoclonal anti-Escherichia coli RNA polymerase alpha (1:1000) (Neoclone). Anti-goat IgG (1:30,000) (Thermo) and anti-rabbit IgG (1:5,000) (Invitrogen) conjugated to alkaline phosphatase were used followed by visualization of bands using immuno-BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium liquid substrate (Sigma). Quantification of band intensity was performed using ImageJ software (NIH). Complement factor recruitment was calculated/displayed as a % of the factor recruited by Y. pestis expressing wild-type Ail.
Data analysis and statistics.
Statistical analyses were conducted using GraphPad Prism Software (GraphPad, La Jolla, CA, USA). Two-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed to analyze the levels of serum resistance in strains containing various ail mutations in both NHS and NHS-AP (Figure 1). One-way ANOVA with Tukey post hoc test was used for comparisons of resistance to NHS between Y. pestis strains expressing Ail variants from a plasmid (Figure 3B). One-way ANOVA with Tukey post hoc test was performed on all densitometric analyses of western blots for comparisons of recruited complement proteins between Y. pestis strains (Figures 2, 4, 5, 6). Data are presented as mean ± standard deviation. * were used to denote significance (p<0.05) as determined by post hoc test.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by R21 AI133570 to ESK. We would also like to thank the University of Detroit Mercy School of Dentistry for financial support of this work. We would like to thank Jamal Alhabeil and Dr. Sanjay Ram for helpful discussions of experiments and results.
REFERENCES
- Bartra SS, Ding Y, Fujimoto LM, Ring JG, Jain V, Ram S, Marassi FM & Plano GV, (2015) Yersinia pestis uses the Ail outer membrane protein to recruit vitronectin. Microbiology 161: 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra SS, Styer KL, O’Bryant DM, Nilles ML, Hinnebusch BJ, Aballay A & Plano GV, (2008) Resistance of Yersinia pestis to Complement-Dependent Killing Is Mediated by the Ail Outer Membrane Protein. Infect. Immun 76: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedzka-Sarek M, Jarva H, Hyytiainen H, Meri S & Skurnik M, (2008a) Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3. Infect Immun 76: 4100–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedzka-Sarek M, Salmenlinna S, Gruber M, Lupas AN, Meri S & Skurnik M, (2008b) Functional mapping of YadA- and Ail-mediated binding of human factor H to Yersinia enterocolitica serotype O:3. Infect Immun 76: 5016–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J & Falkow S, (1992) Bacterial Resistance to Complement Killing Mediated by the Ail Protein of Yersinia enterocolitica. Proc Natl Acad Sci USA 89: 3561–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom AM, Kask L & Dahlbäck B, (2003) CCP1–4 of the C4b-binding protein α-chain are required for factor I mediated cleavage of complement factor C3b. Mol Immunol 39: 547–556. [DOI] [PubMed] [Google Scholar]
- Blom AM, Villoutreix BO & Dahlbäck B, (2004) Complement inhibitor C4b-binding protein—friend or foe in the innate immune system? Mol Immunol 40: 1333–1346. [DOI] [PubMed] [Google Scholar]
- Caulfield AJ & Lathem WW, (2012) Substrates of the plasminogen activator protease of Yersinia pestis In: Advances in Yersinia Research. Springer, pp. 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield AJ, Walker ME, Gielda LM & Lathem WW, (2014) The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation. Cell Host Microbe 15: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo D, Heffernan E, Wu L, Harwood J, Fierer J & Guiney D, (1996) Identification of a domain in Rck, a product of the Salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect Immun 64: 2019–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Prez R, Bryan C, Hawiger J & Colley D, (1975) Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun 11: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S & Krukonis ES, (2009) The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence. Infect Immun 77: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S, Tsang TM & Krukonis ES, (2010) Three Yersinia pestis Adhesins Facilitate Yop Delivery to Eukaryotic Cells and Contribute to Plague Virulence. Infect Immun 78: 4134–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom T, Siegel C, Morgelin M, Kraiczy P, Skerka C & Zipfel PF, (2013) CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan EJ, Reed S, Hackett J, Fierer J, Roudier C & Guiney D, (1992) Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella typhimurium virulence plasmid gene rck. J Clin Invest 90: 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch BJ, (2005) The evolution of flea-borne transmission in Yersinia pestis. Curr Issues Mol Biol 7: 197–212. [PubMed] [Google Scholar]
- Hinnebusch BJ, Jarrett CO, Callison JA, Gardner D, Buchanan SK & Plano GV, (2011) Role of the Yersinia pestis Ail protein in preventing a protective polymorphonuclear leukocyte response during bubonic plague. Infect Immun 79: 4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch BJ, Perry RD & Schwan TG, (1996) Role of the Yersinia pestis Hemin Storage (hms) Locus in the Transmission of Plague by Fleas. Science 273: 367–370. [DOI] [PubMed] [Google Scholar]
- Ho DK, Jarva H & Meri S, (2010) Human complement factor H binds to outer membrane protein Rck of Salmonella. J Immunol 185: 1763–1769. [DOI] [PubMed] [Google Scholar]
- Ho DK, Riva R, Kirjavainen V, Jarva H, Ginstrom E, Blom AM, Skurnik M & Meri S, (2012a) Functional recruitment of the human complement inhibitor C4BP to Yersinia pseudotuberculosis outer membrane protein Ail. J Immunol 188: 4450–4459. [DOI] [PubMed] [Google Scholar]
- Ho DK, Riva R, Skurnik M & Meri S, (2012b) The Yersinia pseudotuberculosis outer membrane protein Ail recruits the human complement regulatory protein factor H. J Immunol 189: 3593–3599. [DOI] [PubMed] [Google Scholar]
- Ho DK, Skurnik M, Blom AM & Meri S, (2014) Yersinia pestis Ail recruitment of C4b-binding protein leads to factor I-mediated inactivation of covalently and noncovalently bound C4b. Eur J Immunol 44: 742–751. [DOI] [PubMed] [Google Scholar]
- Ho DK, Tissari J, Jarvinen HM, Blom AM, Meri S & Jarva H, (2011) Functional recruitment of human complement inhibitor C4B-binding protein to outer membrane protein Rck of Salmonella. PLoS One 6: e27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh ES, van den Broek B & Jongerius I, (2016) Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion. Front Microbiol 7: 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen V, Jarva H, Biedzka-Sarek M, Blom AM, Skurnik M & Meri S, (2008) Yersinia enterocolitica serum resistance proteins YadA and Ail bind the complement regulator C4b-binding protein. PLoS Pathog 4: e1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich K-O, Ager RR, Reis ES & Köhl J, (2009) The role of the anaphylatoxins in health and disease. Mol Immunol 46: 2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb WP & Muller-Eberhard HJ, (1975) Neoantigens of the membrane attack complex of human complement. Proc Natl Acad Sci U S A 72: 1687–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek AM, Schnider DR, Rohde HN, Wojtowicz AJ, Bohach GA, Minnich SA & Hovde CJ, (2010) Outer membrane protein X (Ail) contributes to Yersinia pestis virulence in pneumonic plague and its activity is dependent on the lipopolysaccharide core length. Infect Immun 78: 5233–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek AM, Sinclair DJ, Seo KS, Schnider DR, Deobald CF, Rohde HN, Viall AK, Minnich SS, Hovde CJ, Minnich SA & Bohach GA, (2007) Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 153: 2941–2951. [DOI] [PubMed] [Google Scholar]
- Law S, Lichtenberg N & Levine R, (1979) Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol 123: 1388–1394. [PubMed] [Google Scholar]
- Lesavre PH, Hugli TE, Esser AF & Müller-Eberhard HJ, (1979) The alternative pathway C3/C5 convertase: chemical basis of factor B activation. Journal Immunol 123: 529–534. [PubMed] [Google Scholar]
- Liu H & Naismith JH, (2008) An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC biotechnology 8: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorange EA, Race BL, Sebbane F & Joseph Hinnebusch B, (2005) Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J Infect Dis 191: 1907–1912. [DOI] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, Jabri B & Schneewind O, (2005) Plague bacteria target immune cells during infection. Science 309: 1739–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle NS, Church SE, Fremeaux-Bacchi V & Roumenina LT, (2015) Complement system part I–molecular mechanisms of activation and regulation. Frontiers in immunology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt PM, Nero T, Bohman L, Felek S, Krukonis ES & Marketon MM, (2014) Yersinia pestis targets neutrophils via complement receptor 3. Cell Microbiol 17: 666–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milis L, Morris CA, Sheehan MC, Charlesworth JA & Pussell BA, (1993) Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b-7 and C9. Clin Exp Immunol 92: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VL, Beer KB, Heusipp G, Young BM & Wachtel MR, (2001) Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol Microbiol 41: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard HJ & Götze O, (1972) C3 proactivator convertase and its mode of action. J Exp Med 135: 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn MK & Müller‐Eberhard HJ, (1983) Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Annal New York Acad Sci 421: 291–298. [DOI] [PubMed] [Google Scholar]
- Perry RD & Fetherston JD, (1997) Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev 10: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E & Tschopp J, (1982) Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem 257: 15204–15212. [PubMed] [Google Scholar]
- Podack ER, Kolb WP & Muller-Eberhard HJ, (1977) The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol 119: 2024–2029. [PubMed] [Google Scholar]
- Podack ER, Preissner KT & Muller-Eberhard HJ, (1984) Inhibition of C9 polymerization within the SC5b-9 complex of complement by S-protein. Acta Pathol Microbiol Immunol Scand Suppl 284: 89–96. [PubMed] [Google Scholar]
- Preissner KP, Podack ER & Muller-Eberhard HJ, (1989) SC5b-7, SC5b-8 and SC5b-9 complexes of complement: ultrastructure and localization of the S-protein (vitronectin) within the macromolecules. Eur J Immunol 19: 69–75. [DOI] [PubMed] [Google Scholar]
- Riva R, Korhonen TK & Meri S, (2015) The outer membrane protease PgtE of Salmonella enterica interferes with the alternative complement pathway by cleaving factors B and H. Front Microbiol 6: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F, Gardner D, Long D, Gowen BB & Hinnebusch BJ, (2005) Kinetics of disease progression and host response in a rat model of bubonic plague. Am J Pathol 166: 1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna M, Giles JL, Morgan BP & Bubeck D, (2016) Structural basis of complement membrane attack complex formation. Nature comm 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Nakamura K, Masaki T, Ichihara-Itoh C, Matsumoto M & Nagasawa S, (1995) Human factor H and C4b-binding protein serve as factor I-cofactors both encompassing inactivation of C3b and C4b. Mol Immunol 32: 355–360. [DOI] [PubMed] [Google Scholar]
- Sodeinde O, Subrahmanyam Y, Stark K, Quan T, Bao Y & Goguen J, (1992) A surface protease and the invasive character of plague. Science 258: 1004–1007. [DOI] [PubMed] [Google Scholar]
- Sodeinde OA, Sample AK, Brubaker RR & Goguen JD, (1988) Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun 56: 2749–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegla CA, Cudrici C, Patel S, Trippe R, Rus V, Niculescu F & Rus H, (2011) Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunologic research 51: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Felek S & Krukonis ES, (2010) Ail binding to fibronectin facilitates Yersinia pestis binding to host cells and Yop delivery. Infect Immun 78: 3358–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Wiese JS, Alhabeil JA, Usselman LD, Thomson JJ, Matti R, Kronshage M, Maricic N, Williams S, Sleiman NH, Felek S & Krukonis ES, (2017) Defining the Ail Ligand-Binding Surface: Hydrophobic Residues in Two Extracellular Loops Mediate Cell and Extracellular Matrix Binding To Facilitate Yop Delivery. Infect Immun 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Engel A & Podack E, (1984) Molecular weight of poly (C9). 12 to 18 C9 molecules form the transmembrane channel of complement. J Biol Chem 259: 1922–1928. [PubMed] [Google Scholar]
- Weiler JM, Daha MR, Austen KF & Fearon DT, (1976) Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci 73: 3268–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K & Ruddy S, (1976) Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med 144: 1147–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Lukacik P, Barnard TJ, Noinaj N, Felek S, Tsang TM, Krukonis ES, Hinnebusch BJ & Buchanan SK, (2011) Structural Insights into Ail-Mediated Adhesion in Yersinia pestis. Structure 19: 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Merriam J, Mueller J & Isberg R, (1996) The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect Immun 64: 2483–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.