Abstract
Background:
Gastric mechanistic target of rapamycin (mTOR) signaling is inversely associated with the expression and secretion of ghrelin, a 28-aa peptide hormone produced by gastric X/A-like cells. Ghrelin contributes to obesity and hepatic steatosis. We sought to control global lipid metabolism through manipulating gastric mTOR signaling in X/A-like cells.
Methods:
We established a ghrl-cre transgene in which the cre enzyme is expressed in X/A-like cells under the control of the ghrelin-promoter. mTORflox/flox and TSC1flox/flox mice were separately bred with ghrl-cre mice to generate mTOR-ghrl-cre (mG) or TSC1-ghrl-cre (TG) mice, within which mTOR signaling was suppressed or activated respectively. Lipid metabolism in liver and adipose depots was analyzed.
Results:
Under the control of the ghrelin-promoter, cre enzyme is exclusively expressed in stomach X/A-like cells in adult animals. Knockout of mTOR in X/A-like cells increased circulating acyl-ghrelin and promoted hepatic lipogenesis with effects on adipose depots. Activation of mTOR signaling by deletion of its upstream inhibitor, tuberous sclerosis 1 (TSC1), decreased ghrelin expression and secretion, altering lipid metabolism as evidenced by resistance to high fat diet-induced obesity and hepatic steatosis. Both ghrelin administration and rapamycin, an inhibitor of mTOR, altered the phenotypes of TG mice.
Conclusion:
Gastric mTOR signaling in X/A-like cells contributes to organism lipid homeostasis by regulating hepatic and adipose lipid metabolism. Gastric mTOR signaling may provide an alternative strategy for intervention in lipid disorders.
Keywords: TSC1, ghrelin, liver, adipose, lipid metabolism
Mechanistic target of rapamycin (mTOR), a highly conserved serine-threonine kinase, is an intracellular energy sensor(1, 2). mTOR activity is dynamically regulated by nutrients, energy supply and various hormones. Complex relationships between mTOR and food intake, obesity and glucose metabolism have been demonstrated in hypothalamus, liver and skeletal muscle using various approaches including genetic manipulation, diet and pharmacological methods(3–6). Less attention has been focused on the possibility of fuel sensing by the gastrointestinal (GI) tract, despite its critical role in the regulation of food intake, and glucose and lipid homeostasis.
Ghrelin, the only known peripherally circulating orexigenic hormone, is produced by gastric X/A-like cells, a distinct population that composes 20-30% of all endocrine cells in the oxyntic gland(7). This peptide is composed of 28 amino acids. Encoded by 5 exons, preproghrelin undergoes endoproteolytic processing and posttranslational modification to yield des-acyl ghrelin and acyl-ghrelin. For acyl-ghrelin, acylation of the 3rd serine (Ser3) is required for stimulation of growth hormone release and feeding effects(8). In addition to its orexigenic effects, ghrelin also demonstrates a lipogenic effect through a mechanism independent of energy intake. Although low dose ghrelin (12nmol/kg/day) injection has no effect on food intake, it still increases lipogenesis in white- and brown- adipose tissues(9). Our previous studies also demonstrated that acyl-ghrelin contributes to rapamycin induced glucose metabolic disorders(10), and promotes high fat diet induced hepatic steatosis(11). Conversely, ghrelin receptor (ghsr) ablation protects mice from high fat diet (HFD) induced obesity and steatosis, and also increases thermogenic capacity(11–13). Therefore, targeting ghrelin production might provide a novel strategy for improving global lipid metabolism.
Production of ghrelin from gastric X/A-like cells is tightly linked with organism energy supply(14, 15). Studies using pharmacological approach suggest that gastric mTOR may serve as a critical molecule mediating the effect of energy supply on the production of ghrelin(10, 14, 16). Xu et al. have demonstrated that 1) HFD feeding activates gastric mTOR signaling activity while inhibiting ghrelin production; 2) fasting inhibits gastric mTOR activity while stimulating ghrelin production; 3) rapamycin, an inhibitor of mTOR, increases gastric and circulating ghrelin levels and subsequently stimulates food intake(10, 14, 16). These observations suggest that mTOR signaling functions as a fuel sensing mechanism in the gastric mucosa to link the production of ghrelin with organism energy supply. Because of the limits of pharmacological approaches, the mechanisms by which gastric mTOR signaling contributes to energy metabolism are not well defined.
To better define the specific roles of gastric mTOR signaling on ghrelin production and its control on organism lipid metabolism, we established novel transgenic animal models in which mTOR signaling is specifically altered in gastric X/A-like cells. Our studies demonstrate that gastric mTOR signaling in X/A-like cells contributes to the regulation of hepatic and adipose lipid metabolism partially via regulation of acyl-ghrelin.
Materials and Methods
Animals
Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). All experimental protocols were approved by the Animal Care and Use Committee of Peking University (Permit Number: LA2012-60) and University of Michigan. Ghrl-mTOR−/− mice (mG mice) or Ghrl-TSC1−/− mice (TG mice) were generated by crossing Ghrl-Cre mice with mTORloxp/loxp mice or TSC1loxp/loxp mice, both of which were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in standard plastic rodent cages and maintained in a regulated environment (24 °C, 12-hour light and 12-hour dark cycle with lights on at 7:00 AM). Regular chow and water were available ad libitum unless specified otherwise. Interventions were done during the light cycle.
Diets
Four-week-old male mice were assigned to receive standard laboratory chow (control diet, D12450H; Research Diets) or a high-fat diet (60% fat, D12492; Research Diets) for 12 weeks.
Surgery and implantation of osmotic mini-pumps
Mice were anesthetized with ketamine (100mg/kg bodyweight) and xylazine (6mg/kg bodyweight) by intraperitoneal injection. A 1-cm incision was made in the back skin and mice were implanted subcutaneously with an Alzet osmotic minipump (model 1002) filled with vehicle (saline) or acyl-ghrelin (11 nmol·kg−1·d−1), or insulin (0.5U/d) for 14 days. Before implantation, pumps were filled with the test agent and placed in a petri dish with sterile 0.9% saline at 37 °C for at least 4 hours before implantation to prime the pumps.
Cold exposure
Mice were placed in 4°C cold-room for 6 hours and rectal temperature measured every hour during cold challenge with a Rectal Probe (Braintree Scientific, Braintree, MA).
Tissue sample preparation and immunofluorescent staining
C57BL/6J mice were deeply anaesthetized using pentobarbital (0.07g·kg−1). Tissues were quickly removed and rinsed thoroughly with PBS, then fixed in 4% paraformaldehyde (wt/vol.), dehydrated, embedded in tissue OCT compound or paraffin, and sectioned at 6 μm. Immunofluorescent staining on paraffin embedded sections was performed as previously described(14).
Western blotting and quantitative RT-PCR
Tissues were homogenized in RIPA buffer. Proteins were extracted, separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted using the specific antibodies provided in materials. Total RNA was isolated using Trizol from Invitrogen. Reverse transcription and quantitative PCR were performed as previously described(11).
Statistical analysis
All values were expressed as mean±SEM. Statistical differences were evaluated by two-way ANOVA and Newman–Student–Keuls test. Comparisons between two groups involved use of the Student’s t test. P<0.05 denotes statistical significance.
Results
Activation of gastric mTOR signaling by TSC1 knockdown decreases ghrelin, food intake, body weight and hepatic lipid content
Since ghrelin is known for its orexigenic and lipogenic effects, and is regulated by mTOR (14, 15) and its downstream molecular, ribosomal protein S6 kinase beta-1 (S6K1) (Supplemental Figure 1), we hypothesized that mTOR signaling in X/A-like cell impacts organism-level metabolism by manipulating ghrelin production. To test this hypothesis, we first used local injection via the left gastric artery to deliver cre adenovirus into TSC1flox/flox mice in order to knockdown gastric TSC1, an upstream inhibitor of mTOR activity. The feasibility of local injection was validated by typan-blue injection (Figure 1a), which showed that only the body of stomach was affected without leak to other organs around the stomach. Knockdown of gastric TSC1 validated by Western blot was associated with activation of mTOR signaling evidenced by the phosphorylation of its downstream modeluce-S6 (Figure 1b). Further, activation of gastric mTOR signaling by knocking down TSC1 inhibited ghrelin production (Figure 1c) and food intake, also showed a trend of decrease in body weight (Figure 1d). Hepatic triglyceride content was decreased by activation of mTOR signaling in the stomach (Figure 1e). These observations indicate that it is feasible to deliver modified genes relevant to mTOR signaling to the stomach with local expression. Crucially, activation of gastric mTOR signaling inhibits ghrelin mRNA production, food intake and hepatic lipid content. A limitation of this approach is that mTOR signaling exists in various cell populations in stomach, and knockdown of TSC1 gene by left gastric artery injection is not cell specific. To overcome this difficulty, and to assess the specific effects of mTOR signaling in X/A like cells, we established a transgenic mouse in which genes related to mTOR signaling are manipulated specifically in X/A like cells.
Figure 1. Effects of local manipulation of gastric mTOR signaling by Cre adenovirus.
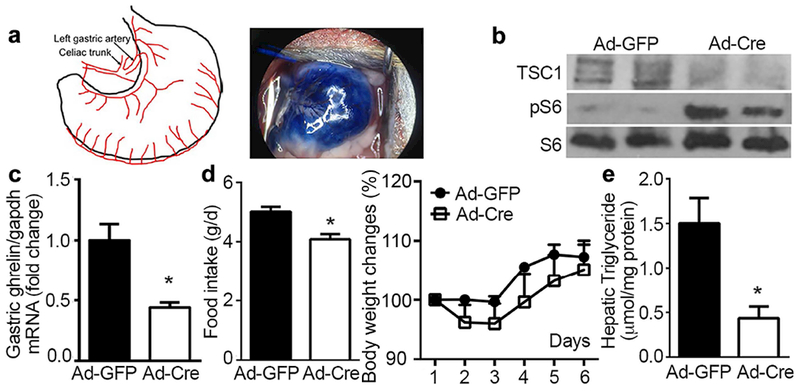
Laparotomy was performed on 12 weeks old TSC1flox/flox mice to expose the left gastric artery, through which Cre adenovirus (1×109 pfu) was delivered locally to the stomach. GFP adenovirus (Ad-GFP) was used as control. All values are expressed as mean ±SEM. * indicates p<0.05 vs. Ad-GFP group, n=12 mice for each condition.
a. Schematic diagram of blood supply for stomach and location of left gastric artery for injection. Tracing of typan-blue injected through the left gastric artery at a dose of 50μl showed the feasibility of injection.
b. Knockdown of TSC1 and activation of mTOR signaling in gastric mucosa were validated by Western blotting.
c. Levels of ghrelin mRNA analyzed by RT-qPCR. Glyceraldehyde phosphate dehydrogenase (gapdh) was used as reference to normalize ghrelin expression.
d. Food intake and percentage of body weight change were recorded daily for 1 week.
e. One week after injection, hepatic lipid was extracted and assayed for triglyceride.
Generation of X/A-like cell-specific Cre transgenic mice
To specially modulate mTOR signaling in X/A-like cells, we used the ghrelin promotor to drive Cre enzyme expression (Figure 2a). First generation pups of different founders were sacrificed to screen for cre high-expressing lines. Representative lines are shown in Figure 2b. Only transgenic lines with high-expression of cre mRNA in stomach were selected to breed with Gt(Rosa)26Sortmlsor/J mice, which carry the loxP-flanked DNA STOP sequence preventing expression of the downstream LacZ gene. In these ROSA-Ghrl-cre (RG) mice, ghrelin-cre positive cells express β-galactosidase because the STOP sequence is removed by Cre enzyme and lacZ is expressed. By detecting β-galactosidase, we evaluated the efficiency of cre enzyme, and also the specificity of different lines. No positive signal was shown in the hypothalamus (Figure 2c). Since ghrelin is expressed in both pancreas and stomach during development(17, 18), we monitored gastric and pancreatic β-galactosidase positive signals at different ages of RG mice (Supplemental Figure 2). Consistent with ghrelin expression levels, β-galactosidase signal was negligible in neonatal stomach (Supplemental Figure 2a), then significantly increased after weaning (Supplemental Figure 2a) until adulthood (Figure 2d). Modest levels of Cre activity was detected in neonatal islets (Supplemental Figure 2b). Further, co-staining β-galactosidase with chromogranin A (marker for endocrine cells) and insulin (marker for β-cells) demonstrated that all β-galactosidase positive cells are also positive for chromogranin A and most are also insulin positive (Supplemental Figure 2c and d). This observation indicates that all ghrl-cre cells are endocrine cells. After weaning, both ghrelin and β-galactosidase decreased significantly (Supplemental Figure 2b) in pancreatic islets. At age of 12 weeks, most β-galactosidase-positive cells disappeared from pancreas (Figure 2e). Of note, no differences in body weight, food intake, serum glucose, plasma ghrelin levels, liver and epididymal white adipose tissue (eWAT) weights (Supplemental Figure 2e) were detected between ghrl-cre positive animals and their wild-type littermates.
Figure 2. Establishment of ghrelin-cre (Ghrl-Cre) transgenic mice.
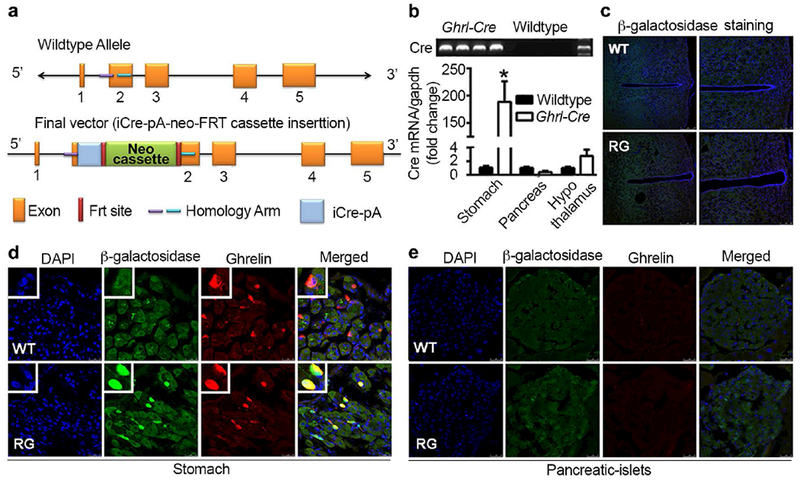
a. Schematic diagram for the generation of ghrelin-cre mice. A ghrelin BAC construct containing the 59.36kb sequence upstream the ATG code was used. The first 29-bp of the ghrelin coding sequence was replaced by the coding sequence of iCre gene followed by an SV40 polyadenylation signal (pA).
b. Screening for the transgenes with high expression of cre gene. Primers were designed to target the cre sequence. mRNAs of stomach, pancreas and hypothalamus from wild-type and ghrelin-cre animals were used for cDNA preparation.
c-e. Validation of cre enzyme in hypothalamus (c), stomach (d) and pancreatic islets (e). Ghrl-cre mice were bred with Gt(Rosa)26Sortmlsor/J mice (RG), which carry the β-galactosidase reporter gene. RG mice and wild-type (WT) littermates 12 weeks-old were perfused with 4% paraformaldehyde. Brain, stomach and pancreas were harvested, sectioned and stained for β-galactosidase (green) and ghrelin (red). Nuclei were stained with DAPI (blue). n=12 and 15 for wild-type littermates and transgene, respectively.
X/A-like cell-specific knockout of mTOR increases triglyceride accumulation in liver
After validating the cell-specificity of ghrl-cre, we bred these mice with mTORflox/flox mice to generate a X/A-like cell-specific mTOR knockout animal model. Only ghrl-cre positive and mTOR flox/flox homozygous mice were selected as mTOR-Ghrl-cre (mG) (Supplemental Figure 3a). Immunofluorescent staining of stomach showed co-localization of phospho-p70 S6k1 (pS6k1), a downstream molecule of mTOR signaling, and ghrelin in wild-type mice. Significant reduction of pS6K1 was observed in ghrelin positive X/A-like cells of mG mice, indicating decrease in mTOR signaling in these cells (Figure 3a). Consistent with our previous finding that inhibition of mTOR signaling by systemic rapamycin injection increases ghrelin secretion(14), circulating active-ghrelin (acyl-ghrelin) was significantly increased in mG mice relative to wild-type littermates (Figure 3b). Phenotype analysis of this animal model showed that overall body weight and most tissue weights were similar between mG mice and wild-type littermates. Stomach weight and epididymal white adipose tissue (eWAT) weights were higher (Supplemental Figure 3b). Interestingly, hepatic triglyceride content was increased in mG mice (Supplemental Figure 3c-d, and Figure 3c), whereas circulating triglyceride and cholesterol remained virtually unaltered. Associated with the alteration in hepatic triglyceride content was significant increase in mRNA levels of lipogenesis-related genes, such as sterol regulatory element-binding protein 1 (srebf1), peroxisome proliferator-activated receptor gamma (pparγ), fatty acid synthase (fasn), glycerol-3-phosphate acyltransferase, mitochondrial (gpam) and diacylglycerol O-acyltransferase 1 (dgat1). Genes related to β-oxidation and lipid transport remained largely unchanged (Figure 3d). Moreover, genes related to lipid absorption in small intestine remained largely unaltered (Supplemental Figure 3e) except the gene of FABP4, indicating that the increase of lipid content in transgenic mice may not due to increased absorption of lipids.
Figure 3. Effects of mTOR suppression in X/A-like cells.
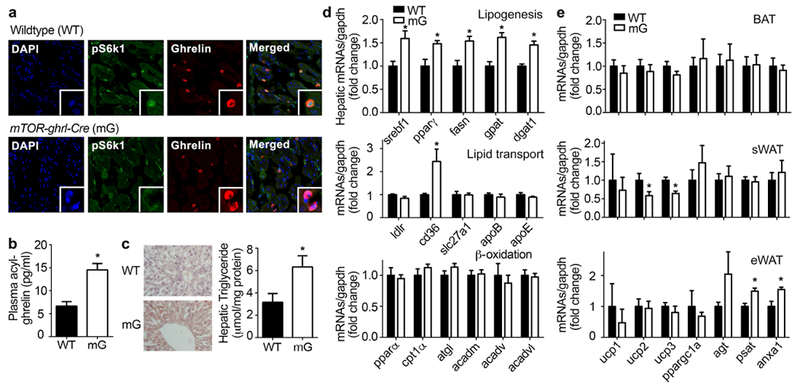
Ghrelin-cre mice were bred with mTOR flox/flox mice to generate X/A-like cells-specific knockout of mTOR, mTOR-ghrl-cre (mG). Eight-week old animals were used to assess mTOR signaling in X/A-like cells, and analysis of lipid metabolism in liver and adipose tissues. Results were expressed as mean±SEM. n=8 for each group. *P < 0.05 vs. WT.
a. Co-localization of pS6k1 (green) and ghrelin (red) in stomach of WT and mG mice. Nuclei were stained with DAPI (blue).
b. Plasma levels of acyl-ghrelin.
c. Oil-red O staining, and hepatic triglyceride content were measured by colorimetric assay and normalized by protein level.
d. mRNA levels of lipogenesis-, lipid transport- and β-oxidation-related genes were determined by RT-qPCR and normalized by gapdh.
e. mRNAs from BAT, sWAT and eWAT were analyzed for expression levels of brown (ucp1-3 and ppargc1α) or white adipose (agt, psat and anxa1) markers; gapdh was used to normalize results.
To further examine therapeutic potential, hepatic enzymes, and genes relative to proliferation and senescence, apoptosis and fibrosis were assayed. Hepatic ALT content was markedly increased in mG mice fed with HFD relative to the obese WT littermates (Supplemental Figure 3f). Immuno-staining of p16 and PCNA, as well as TUNEL staining showed no obvious change in mG mice and wild-type littermates (Supplemental Figure 3g). There were no significant changes in genes related to proliferation and senescence. Interestingly, genes related to fibrosis such as αSMA and TGFβ1 were increased in mG mice fed HFD (Supplemental Figure 3h). However, ghrelin only demonstrated a mild effect on proliferation- and fibrosis-related genes in cultured LX2 cells, a hepatic stellate cell (HSC) line (Supplemental Figure 3i). Together with our previous studies showing the presence of functional ghrelin receptor and its direct stimulation of de novo lipogenesis in hepatocytes, this observation focused subsequent experiments on lipid metabolism.
No distinguishable alteration in adipose tissue markers was observed in brown adipose tissue (BAT). mRNA levels for uncoupling protein (ucp) 2 and ucp3 decreased significantly in subcutaneous WAT (sWAT) from mG mice. White adipose tissue development-related genes such as psat and anxa1 were increased in eWAT from mG mice versus wild-type littermates (Figure 3e). Since fat depots are highly innervated, sympathetic tone significantly affects the adipose phenotype. We recorded heart rate and mean blood pressure as a measurement of the sympathetic activity; neither showed any difference between mG and wild-type littermates (Supplemental Figure 3j).
X/A-like cell-specific activation of mTOR signaling alters organism lipid metabolism
In order to activate mTOR signaling in X/A-like cells, we knocked-out TSC1, an upstream inhibitor of mTOR signaling, by breeding the TSC1flox/flox with ghrl-cre (TG) mice (Supplemental Figure 4a). Activation of mTOR signaling in X/A-like cells was validated by the co-staining of phospho-p70 S6k1 (pS6k1) and ghrelin. Relative to wild-type littermates, nuclear translocation of pS6k1 was observed (Figure 4a) in X/A-like cells of TG mice. S6K1 has been reported to exist in both cytoplasmic and nuclear compartments under basal conditions and to translocate into nuclei upon activation of mTOR signaling(19). Strikingly, TG mice showed lower body weights (Supplemental Figure 4b), and less epididymal and subcutaneous WAT weight relative to wild-type littermates under both normal chow diet (NCD) and high fat diet (HFD) conditions (Supplemental Figure 4c). There was a trend of decreasing food intake in TG mice compared with the WT littermates, but not statistically significant. Hypothalamic genes related to regulation of food intake (NPY, AgRP and POMC) were not altered in TG mice (Supplemental Figure 4d).
Figure 4. Activation of mTOR signaling in X/A-like cells alters HFD-induced hepatic steatosis.
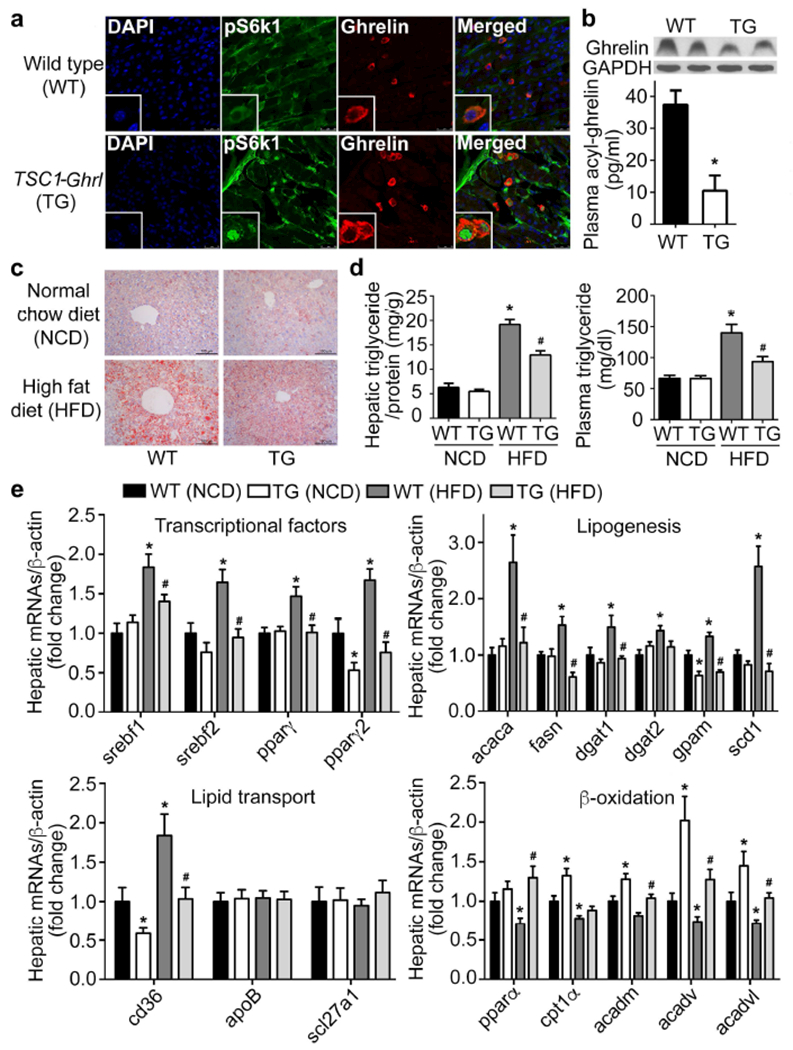
TSC1flox/flox mice were bred with ghrelin-cre mice to generate TSC1 knockout animals (TG), in which mTOR signaling is specifically activated in X/A-like cells. Results were expressed as mean±SEM. n = 20 for each group. *P < 0.05 vs. WT (NCD); # P < 0.05 vs. WT (HFD).
a. Co-localization of pS6k1 (green) and ghrelin (red) in stomach of 12 weeks-old wild-type littermates and TG mice. Nuclei were stained with DAPI (blue).
b. Gastric and plasma ghrelin were measured by western blot and ELISA.
c-e. Oil-red O staining (c), hepatic and circulating triglyceride contents were measured by colorimetry (d) and levels of lipogenesis-, lipid transport- and β-oxidation-related genes were determined by RT-qPCR and normalized to β-actin (e).
To explore the mechanisms by which a lean phenotype is induced by X/A-cell specific activation of mTOR signaling, production of ghrelin in stomach was examined. Protein expression and secretion of ghrelin were significantly decreased in TG mice compared with WT littermates (Figure 4b). Consistent with the pro-lipogenesis function of ghrelin in both liver and adipose tissues, decreased circulating ghrelin was associated with lower hepatic lipid content as shown by oil-red O staining in both NCD and HFD animals (Figure 4c). We detected lower hepatic and circulating triglyceride levels in TG mice fed a HFD for 12 weeks (Figure 4d). Levels of lipogenesis-related transcriptional factors (srebf1, srebf2, pparγ, pparγ2), enzymes (acaca, fasn, dgat1, dgat2, gpam, scd1) and lipid transporter (cd36) were significantly decreased in liver from TG mice fed HFD. In addition, levels of β-oxidation-related genes were markedly increased in TG mice fed either NCD or HFD (Figure 4e). No change in either hepatic or plasma cholesterol contents was observed (Supplemental Figure 4e). Transcription of genes related to cholesterol metabolism demonstrated no statistical difference (Supplemental Figure 4f). Intestinal lipid absorption-related genes were not changed between TG mice and their littermates fed either NCD or HFD (Supplemental Figure 4g), indicating that the lipid-lowering effects are not due to malabsorption.
To further examine therapeutic potential, mRNA levels of genes related to proliferation, senescence and fibrosis were examined (Supplemental Figure 4h). Both cyclinD1 and myc were significantly increased in TG mice under the condition of NCD. For genes related to senescence, only p21 showed a significant reduction in TG mice fed NCD. No obvious change of fibrosis-related genes was detected.
In addition to amelioration of HFD-induced liver steatosis, TG mice were resistant to HFD-induced hypertrophy in WAT depots. Histology of BAT, sWAT and eWAT showed that adipocyte sizes in TG mice were smaller than those in WT littermates under either NCD or HFD conditions (Figure 5a, c, e). De novo lipogenesis related genes were significantly decreased under the HFD condition, while lipid uptake related gene expression remained unchanged in TG mice (Supplemental Figure 5a). To further investigate whether lipolysis also contributes to the lean phenotype of TG mice, phosphorylation of hormone sensitive lipase (HSL) was detected in eWAT. Although both phosphor-HSL and HSL protein levels increased significantly in TG mice, the ratio of its phosphorylation level remained unaltered (Supplemental Figure 5b). Adipocyte marker genes related to browning, including ucp 1-3 and ppargc1a, were significantly increased, while genes related to WAT development, such as agt, psat, ednra and anxa1, were decreased in TG mice fed NCD or HFD relative to wild-type littermates (Figure 5b, d, f). Moreover, the thermoregulatory gene (Cox8b) and mitochondrial ATP synthase (ATP5B) were also elevated in sWAT of TG mice relative to WT littermates (Supplemental Figure 5c). At room temperature, the respiratory quotient (RQ) was decreased in TG mice fed with HFD, indicating that TG mice consume more lipids (Supplemental Figure 5d). Moreover, the activity of TG mice was also higher than WT littermates under the HFD condition (Supplemental Figure 5d). Consistently, TG mice were resistant to 4°C cold exposure-induced body temperature drop relative to WT littermates with larger extent in HFD-induced obese animals (Figure 5g). Body temperature was lower in HFD-induced obese mice (39.05°C in NCD vs. 38.39°C in HFD) (Supplemental Figure 5e), which is consistent with previous report (20, 21). Since oleic acid (18:1) has been reported to activate oxidation of fatty acids in the differentiated C2C12 cells(22), we next examined the free fatty acid profile to determine whether mono-unsaturated fatty acids contribute to cold-resistance in TG mice. As shown in supplemental Figure 5f, oleic acid (18:1) and several saturated fatty acid species, e.g. 18:0, 20:0 and 22:0, were significantly increased in TG mice. Of note, free fatty acids 18:1 and 22:0 in TG mice fed HFD demonstrated no significant difference relative to wild-type littermates fed HFD. Consistent with the elevation of β-oxidation-related gene expression in liver, these genes were increased as well in skeletal muscle of TG mice fed HFD, indicating an increase in the global fatty acid utilization in these transgenic mice (Supplemental Figure 5g). Also, heart rate and mean blood pressure were unchanged in TG mice (Supplemental Figure 5h), indicating the lipid-lowering effects may not act through effects of the sympathetic system.
Figure 5. Activation of mTOR signaling in X/A-like cells increases brown phenotype of BAT and WAT depots.
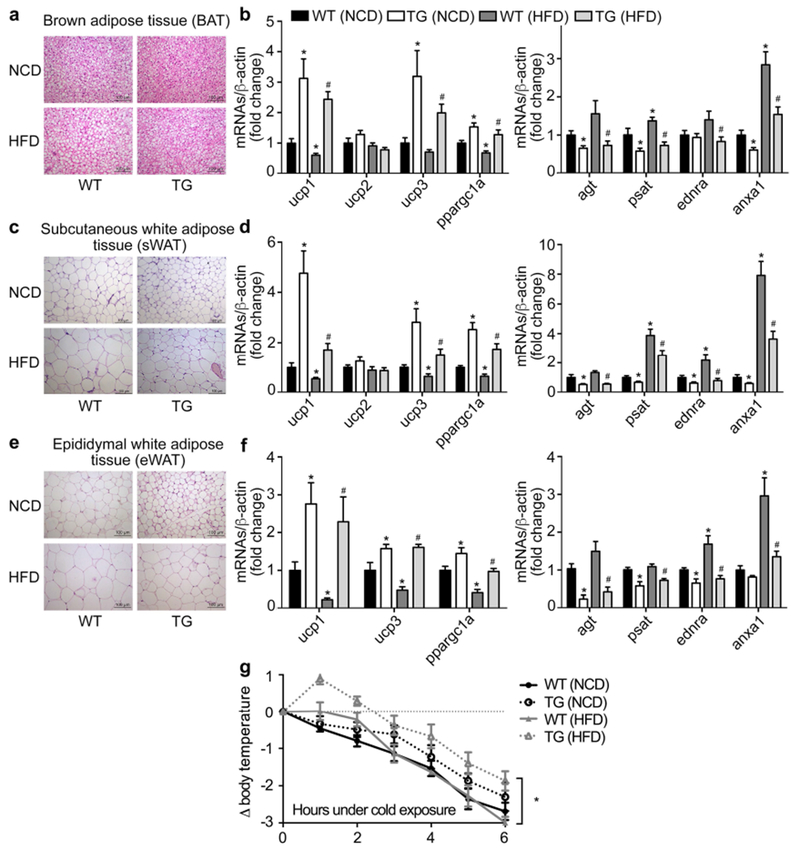
Histology of BAT (a), sWAT (c) and eWAT (e) shown by HE staining. mRNA levels of brown- and white- adipocyte marker genes were detected by RT-qPCR in BAT (b), sWAT (d) and eWAT (f), and normalized to β-actin. Fold changes were expressed as mean±SEM. n=20 for each group. *P<0.05 vs. wild-type mice fed NCD; # P<0.05 vs. wild-type littermates fed HFD (Student’s t test). Mice were placed in cold room (4°C) for 6 hours and rectal temperature was measured every hour (g). n=6 per group. *P<0.05 indicates TG vs. wild-type mice fed HFD.
Global lipid-lowering effects of mTOR activation in X/A like cells are independent of insulin deficiency
Unexpectedly, we observed a significant reduction in insulin levels, impaired glucose tolerance and no change in insulin sensitivity in the TG mice. Neither change was observed in ghrelin positive cells at neonatal stage nor pS6 staining at adult stage in islets of TG mice (Supplemental Figure 6a, b). To rule out that the lipid-lowering phenotypes in TG mice is due to the insulin deficiency, we supplemented TG mice with exogenous insulin (0.5U/d) for 2 weeks by subcutaneous osmotic mini-pump and assayed the glucose and lipid metabolism. Though insulin administration normalized the serum insulin levels and glucose in WT littermates (Supplemental Figure 6c, d), genes related to lipogenesis and lipid transportation in liver, browning- and whiting- markers in sWAT and BAT were not affected, remaining similar to TG mice receiving saline treatment (Supplemental Figure 6e-g). These data demonstrate that the lipid-lowering effects in TG mice are independent of insulin levels.
Exogenous acyl-ghrelin partially eliminates the leaner phenotypes in TG mice
The observation that ghrelin production and secretion in TG mice were significantly reduced prompted us to examine whether resistance to HFD-induced obesity and steatosis in these transgenes relies on the suppression of ghrelin. We rescued plasma acyl-ghrelin in TG mice to levels observed in WT littermates by infusing acyl-ghrelin peptide via osmotic min-pump implantation. Administration of acyl-ghrelin demonstrated no significant effect on the activation of mTOR signaling in X/A-like cells as measured by co-immunostaining of phosphor-mTOR and ghrelin (Supplemental figure 7a-b) in TG mice. Consistent with our previous study(11), ghrelin directly stimulated lipogenesis in primary hepatocytes (Supplemental Figure 7c), leading to the increase of hepatic lipid accumulation in wild-type animals. Further, the decrease of hepatic lipid storage in TG mice was partially reversed by exogenous acyl-ghrelin (Figure 6a). Administration of acyl-ghrelin partially reversed the reduction of hepatic and circulating triglyceride in TG mice (Figure 6b). Exogenous ghrelin also reversed the suppression of lipogenesis-related genes including srebfs, pparγs, fasn, dgats and gpam in TG mice to levels similar to WT littermates (Figure 6c). Application of exogenous acyl-ghrelin demonstrated little effect on the expression of lipid transport- and β-oxidation- related genes. Similarly, the decrease of adipocyte size was partially reversed by acyl-ghrelin administration (Figure 6d, Supplemental Figure 7d and f). Elevation of browning marker genes in sWAT of TG mice were also reversed by acyl-ghrelin. Among the white adipocyte marker genes, anxa1 was recovered (Figure 6e). The up-regulation of brown marker genes in BAT from TG mice were partially reversed by acyl-ghrelin administration (Supplemental Figure 7e), whereas agt was the only white adipocyte genes reversed by acyl-ghrelin (Supplemental Figure 7e). In eWAT of TG mice, ucp1 was significantly suppressed by acyl-ghrelin, while the decrease of white adipocyte marker genes in TG mice was recovered by acyl-ghrelin (Supplemental Figure 7g).
Figure 6. Effects of ghrelin supplementation in TG mice.
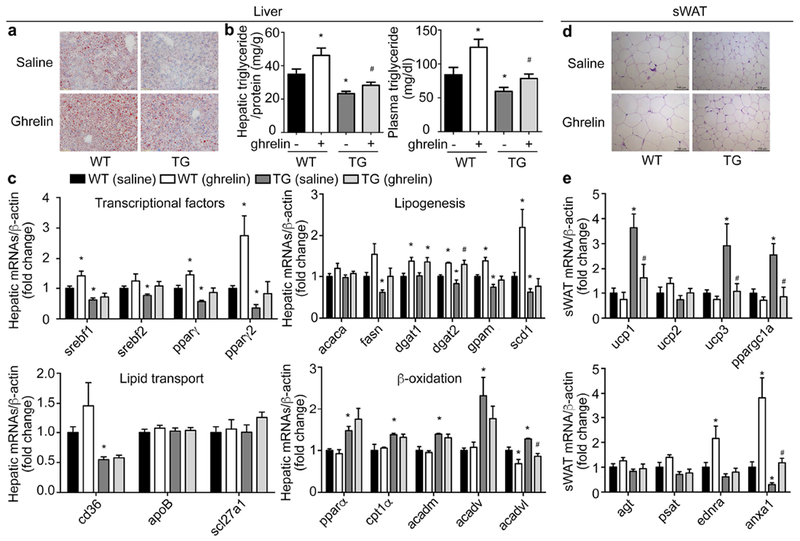
Wild-type littermates (WT) and TG mice were fed HFD for 12 weeks and administered acyl-ghrelin (11nmol/kg/day) for 2 weeks through a subcutaneous osmotic mini-pump. Levels of mRNAs were determined by RT-qPCR and normalized to β-actin. Fold changes were expressed as means±SEM. n = 10 for each group. *P<0.05 vs. WT (saline), # P<0.05 vs. TG (saline)).
a. Oil-red O staining.
b. Hepatic and circulating triglyceride content measured by colorimetric assay.
c. Hepatic mRNA levels of lipogenesis-, lipid transport- and β-oxidation-related genes.
d. Representative H&E staining of subcutaneous white adipose tissue (sWAT).
e. mRNAs levels of brown- or white- marker genes in sWAT.
Inhibition of mTOR signaling by rapamycin eliminates metabolic benefit on lipids in TG mice
To further confirm that mTOR signaling in X/A-like cells contributes to the improvement in lipid metabolism in TG mice, rapamycin, an inhibitor of mTOR activity, was intraperitoneally injected for 6 days into TG mice and WT littermates. This approach has been demonstrated to suppress gastric mTOR signaling in previous reports(10, 14) and Supplemental Figure 8a in current study. As shown in Figure 7a, rapamycin injection in TG mice increased the circulating acyl-ghrelin to levels identical to WT littermates. The decrease in levels of hepatic and circulating triglyceride in TG mice was totally reversed to levels observed in WT littermates (Figure 7b). mRNA levels of hepatic lipid metabolism-related genes were also restored by rapamycin (Figure 7c). Similarly, elevation of most brown adipocyte marker genes in sWAT and BAT from TG mice were reversed to levels of WT littermates (Figure 7 d and e, Supplemental Figure 8 b and c). The decrease of white adipocyte marker genes in eWAT was reversed by rapamycin injection (Supplemental Figure 8d and e).
Figure 7. Rapamycin reverses the lipid metabolic effects in TG mice.
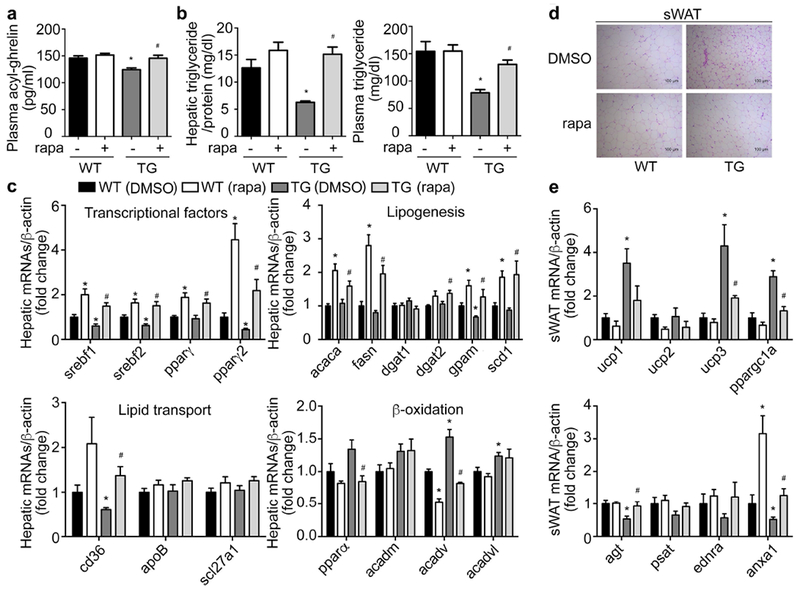
Rapamycin (1mg/kg/day) was intraperitoneally injected into 16-week old wild-type littermates (WT) or TG mice for 2 weeks. Levels of mRNAs were measured by RT-qPCR and normalized to β-actin. Fold changes were expressed as mean±SEM. n=9 for each group. *P<0.05 vs. WT (DMSO), # P<0.05 vs. TG (DMSO).
a. Circulating levels of acyl-ghrelin in WT and TG mice treated with rapamycin (rapa) or control DMSO.
b. Hepatic and circulating triglyceride content measured by colorimetric assay.
c. Hepatic mRNA levels of lipogenesis-, lipid transport- and β-oxidation-related genes.
d. Representative H&E staining of subcutaneous WAT (sWAT).
e. mRNAs levels of brown- or white- marker genes in sWAT.
Discussion
By this gain- or loss-of-function genetic approach, our studies provide evidence that mTOR signaling pathway in X/A-like cells plays a role in the regulation of organism lipid homeostasis. Using gastric targets to inhibit orexigenic hormonal signal may thus provide a novel therapeutic strategy for intervention in obesity and lipid disorders. This concept is supported by following findings: 1) establishment of transgenic mice in which mTOR signaling gene in gastric X/A-like cells is specifically manipulated under the control of the ghrelin promoter allows us to explore the role of mTOR signaling pathway in the metabolic function of these cells; 2) deletion of mTOR genes in X/A-like cells increases acyl-ghrelin levels and subsequently induces hepatic steatosis in mice fed NCD; 3) deletion of TSC1 activates mTOR signaling in X/A-like cells, decreases ghrelin production and protects animals from HFD-induced obesity and hepatic steatosis; 4) the metabolic effects of activation of mTOR signaling in X/A-like cell partially rely on suppression of ghrelin; 5) inhibition of mTOR signaling by rapamycin reverses the phenotype of TG mice.
In ghrl-cre transgenic mice, the specificity of cre expression in gastric X/A-like cells and pancreatic islets are age-dependent.
Though we detected exclusive expression of cre enzyme in X/A-like cells in adult animals, there were cre-positive cells in pancreas during early stages of development (Figure 2 and Supplemental Figure 2b). This observation is consistent with previous studies demonstrating that ghrelin is transiently expressed in pancreatic islets during embryonic development(17). The physiological significance of the transient expression of this hormone in pancreatic islets is unknown. Previous studies have shown that deficiency of Nkx2.2 or Pax4 transcriptional factors critical for the development of beta cells, leads to expansion of ghrelin-producing cells at the expense of beta cells in pancreatic islets(23, 24). Since the cre enzyme is constitutively activated in our animal model, we cannot exclude the possibility that ghrelin might influence organism lipid metabolism indirectly by altering the development and differentiation of endocrinal cells in pancreas. Our observation that neither ghrelin positive cells at neonatal stage nor mTOR signaling activity at adult stage was changed in the islets of TG mice (Supplemental Figure 6a, b) does not support this concept. Though TG mice demonstrate insulin deficiency due to loss of β-cells, the lipid-lowering effects in these transgenic mice are independent of insulin. Supplementation of exogenous insulin normalizes the glucose metabolism, but demonstrates no effects on lipid metabolism (Supplemental Figure 6c-g). further studies could use inducible cre activation to exclude indirect effects of pancreatic endocrine cells during early developmental stages.
Gastric mTOR signaling in X/A-like cells influences hepatic lipid metabolism.
The existence of gut-liver communication has been well-studied in terms of regulating hepatic glucose metabolism either through the vagal-vagal reflex or endocrine hormones(25–27). Intestinal mucosa is able to sense luminal nutrients such as lipids and glucose, and alter hepatic gluconeogenesis and glycogenolysis, leading to change in net hepatic glucose output through neuronal network (25–27). Intestinal hormones, like CCK from I cells and glucagon-like peptide 1 (GLP1) from L cells, are well-known for their regulatory effects on glucose metabolism as well(28–30). Our studies extend the gut-liver axis to gastric X/A-like cells. Alteration of mTOR signaling in X/A-like cells contributes to the regulation of hepatic lipid metabolism. Together with our previous studies demonstrating that mTOR activity in X/A-like cells is closely related to organism energy levels, we propose mTOR signaling as a fuel sensing mechanism in the stomach whose activity coordinates organism energy levels with hepatic lipid metabolism.
In the present study, we also demonstrate that mTOR activity in X/A-like cells regulate hepatic lipid metabolism partially through ghrelin. Ghrelin, a 28 amino acid peptide hormone, is the only circulating orexigenic hormone(31). The primary action site for ghrelin has been proposed to be within the hypothalamus(32). Interestingly, no significant alteration in hypothalamic genes relative to appetite control is detected in TG mice. This observation indicates that alternative pathway may contribute to the regulation of global lipid metabolism by mTOR signaling in gastric X/A like cells. Recent studies have also suggested a direct peripheral action of ghrelin in the regulation of hepatic lipogenesis and adipogenesis(11, 33). Consistently, GLP1 has been found to regulate hepatic lipid metabolism by its direct activation of the GLP1 receptor on hepatocytes (34). These observations indicate an alternative pathway for ghrelin, and a direct gut-liver interaction instead of relay through the central nervous system.
Our previous study demonstrated that acyl-ghrelin infusion promotes lipogenesis, but has little effect on lipid transport and β-oxidation(11). In line with this observation, reduction of acyl-ghrelin in TG mice increases lipogenesis whereas increase of acyl-ghrelin in mG mice is associated with an up-regulation in expression of lipogenesis-related genes. Interestingly, activation of mTOR signaling in X/A-like cells not only suppresses hepatic lipogenesis but also stimulates β-oxidation. Further, supplementation of acyl-ghrelin partially reverses the improvement of lipid metabolism in TG mice. Taken together, these findings suggest that in addition to acyl-ghrelin, other signals from X/A-like cells may contribute to the metabolic effects observed in these transgenic mice. Nesfatin-1, an 82-aa peptide derived from a 396-aa precursor protein nucleobindin 2 (NUCB2), is identified in X/A-like cells, but in different vesicles than ghrelin. Although secreted from the same cell, nesfatin-1 often exerts opposing metabolic functions than ghrelin. Previous studies have demonstrated that activation of mTOR signaling increases the production of nesfatin-1 in a manner opposite to the ghrelin. Consistently, increase of nesfatin-1 has been detected in TG mice in which mTOR signaling is activated specifically in X/A-like cells (data not shown). In addition, we have demonstrated that nesfatin-1 promotes the differentiation of brown adipocytes(35). However, until the receptor for nesfatin-1 is characterized, it is not possible to efficiently block the action of nesfatin-1, and thus to confirm that increase of nesfatin-1 in TG mice contributes to metabolic effects in TG mice. In addition to ghrelin and nesfatin-1, there might exist other unknown secretory factors from gastric X/A-like cells which contribute to the regulation of global lipid metabolism. Future investigation will focus on seretome profiling of TG and mG mice to characterize these molecules.
Gastric mTOR signals adipose tissue browning.
Emerging evidence supports the concept that “brown conversion” of white fat is an inherent property of most or all white fat cells(36, 37). Because of great potential in the therapy of obesity and metabolic diseases, attention has been focused on the identification of molecules critical for browning. While a range of transcriptional factors such as FoxC2, IκB kinase ε, PGC-1a, PRDM16, RIP140, and secreted molecules including cardiac natriuretic peptides, fibroblast growth factor 21, irisin and BMPs have been identified(37), little attention has been focused on the possibility of gastrointestinal control of browning, despite its critical role in the regulation of food intake and energy balance. Our studies suggest that mTOR activity in gastric X/A-like cells contributes to the browning of white adipose tissue. Suppression of mTOR signaling in X/A-like cells reduces, whereas activation of its activity increases the expression of browning genes. More importantly, our functional test demonstrated that activation of mTOR signaling in X/A-like cells attenuates the decline of body temperature induced by cold exposure. Our studies also indicate that ghrelin may mediate the effects of gastric mTOR signaling on browning of white adipose tissue. There exists a negative relation between circulating levels of ghrelin and gastric mTOR activity and levels of browning genes. Suppression of gastric mTOR signaling in mG mice increases circulating ghrelin, which is associated with a decrease of browning genes. Activation of gastric mTOR signaling in TG mice reduces circulating ghrelin, leading to the subsequent increase in browning. The effects of mTOR activation is blocked by rapamycin, a mTOR inhibitor. Importantly, the effect of mTOR activation in X/A-like cells on browning are reversed by exogenous ghrelin. Ghrelin might act in a concentration-specific manner. In high acyl-ghrelin concentration models such as mG mice (Figure 3e) and exogenous acyl-ghrelin infusion (Figure 6 d, e and Supplemental Figure 7b-g), ghrelin demonstrates only modest effects on either brown- or white- marker genes. However, in a low acyl-ghrelin animal model (TG mice), the brown-marker genes are significantly increased and white-marker genes decreased. Intriguingly, almost all brown-marker genes in sWAT and BAT, as well as white marker genes in eWAT from TG mice are affected by acyl-ghrelin infusion. These observations suggest that ghrelin is important for maintenance of phenotypes of adipose tissue, and that the effects of ghrelin may be tissue-specific.
In conclusion, this study demonstrates that the mTOR signaling in X/A-like cells is important for organism lipid homeostasis by regulating hepatic and adipose tissue lipid metabolism. These effects occur partially through ghrelin (Figure 8). These findings support the endocrine functions of stomach and highlights potential strategies for dyslipidemia treatment by targeting this organ.
Figure8.
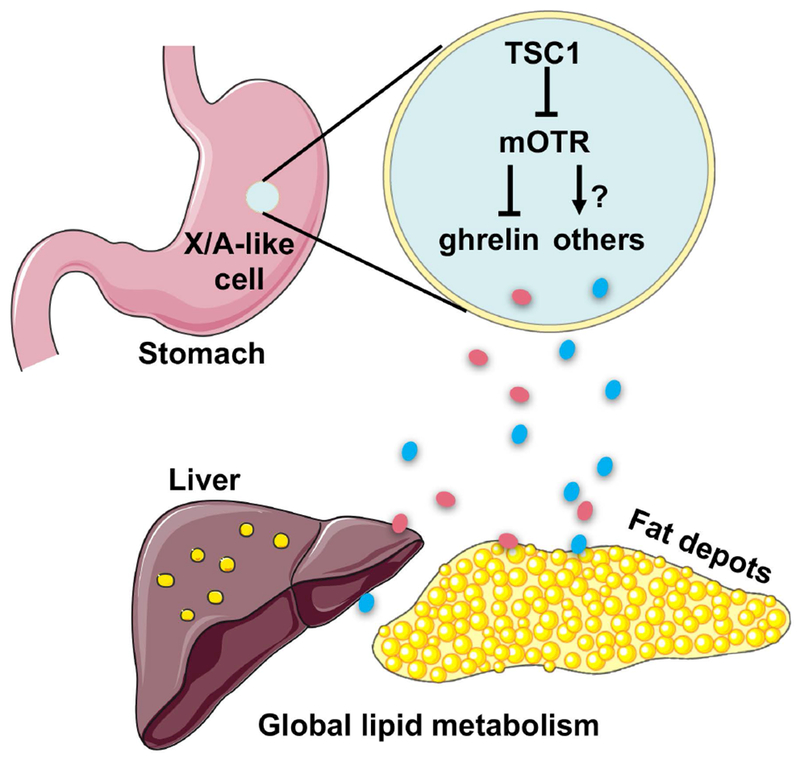
Graphic highlight of findings.
Supplementary Material
Acknowledgments
Financial support: The National Key R&D Program of China (2017YFC0908900), the National Natural Science Foundation of China (81730020, 81330010, 81390354), and the National Institutes of Health Grant 1R01DK110273-01A1.
Abbreviations:
- mTOR
mechanistic target of rapamycin
- TSC1
tuberous sclerosis 1
- DMSO
dimethyl sulfoxide
- mG
mTOR-ghrl-cre
- TG
TSC1-ghrl-cre
- RG
ROSA-Ghrl-cre
- WT
wild-type
- eWAT
epididymal white adipose tissue
- BAT
brown adipose tissue
- sWAT
subcutaneous white adipose tissue
- srebf1
sterol regulatory element-binding protein 1
- pparγ
peroxisome proliferator-activated receptor gamma
- fasn
fatty acid synthase
- gpam
glycerol-3-phosphate acyltransferase, mitochondrial
- dgat1
diacylglycerol O-acyltransferase 1
- ucp
uncoupling protein
- NCD
normal chow diet
- HFD
high fat diet
- rapa
rapamycin
- GLP1
glucagon-like peptide 1
Footnotes
Conflicts of interest: All authors declare no conflict of interests.
Reference
- 1.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 2005;69:79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science 2001;294:1102–1105. [DOI] [PubMed] [Google Scholar]
- 3.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930. [DOI] [PubMed] [Google Scholar]
- 4.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 2005;146:1473–1481. [DOI] [PubMed] [Google Scholar]
- 5.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431:200–205. [DOI] [PubMed] [Google Scholar]
- 6.Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 2008;19:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stengel A, Tache Y. Ghrelin - a pleiotropic hormone secreted from endocrine x/a-like cells of the stomach. Front Neurosci 2012;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 9.Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul Pept 2005;130:97–103. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Wang Z, Li Y, Li Z, Tang H, Zhao J, Xiang X, et al. Ghrelin contributes to derangements of glucose metabolism induced by rapamycin in mice. Diabetologia 2012;55:1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, Xiang X, et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway. Proc Natl Acad Sci U S A 2014;111:13163–13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005;115:3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, Buras ED, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 2011;10:996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, et al. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology 2009;150:3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Tang H, Yin Y, Yu R, Zhao J, Li Y, Mulholland MW, et al. HDAC5-mTORC1 Interaction in Differential Regulation of Ghrelin and Nucleobindin 2 (NUCB2)/Nesfatin-1. Mol Endocrinol 2015;29:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu G, Li Y, An W, Zhao J, Xiang X, Ding L, Li Z, et al. Regulation of gastric hormones by systemic rapamycin. Peptides 2010;31:2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS One 2012;7:e52026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, Kangawa K, et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol 2002;173:239–245. [DOI] [PubMed] [Google Scholar]
- 19.Rosner M, Hengstschlager M. Nucleocytoplasmic localization of p70 S6K1, but not of its isoforms p85 and p31, is regulated by TSC2/mTOR. Oncogene 2011;30:4509–4522. [DOI] [PubMed] [Google Scholar]
- 20.Carlisle HJ, Dubuc PU. Temperature preference of genetically obese (ob/ob) mice. Physiol Behav 1984;33:899–902. [DOI] [PubMed] [Google Scholar]
- 21.Reitman ML. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Lett 2018;592:2098–2107. [DOI] [PubMed] [Google Scholar]
- 22.Lim JH, Gerhart-Hines Z, Dominy JE, Lee Y, Kim S, Tabata M, Xiang YK, et al. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1alpha complex. J Biol Chem 2013;288:7117–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A 2004;101:2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordowich S, Collombat P, Mansouri A, Serup P. Arx and Nkx2.2 compound deficiency redirects pancreatic alpha- and beta-cell differentiation to a somatostatin/ghrelin co-expressing cell lineage. BMC Dev Biol 2011;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 2009;10:99–109. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Wang J, Wu S, Yuan L, Zhao X, Liu C, Xie J, et al. Duodenal GLP-1 signaling regulates hepatic glucose production through a PKC-delta-dependent neurocircuitry. Cell Death Dis 2017;8:e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duca FA, Bauer PV, Hamr SC, Lam TK. Glucoregulatory Relevance of Small Intestinal Nutrient Sensing in Physiology, Bariatric Surgery, and Pharmacology. Cell Metab 2015;22:367–380. [DOI] [PubMed] [Google Scholar]
- 28.Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, et al. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 2011;60:2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002;51 Suppl 3:S434–442. [DOI] [PubMed] [Google Scholar]
- 30.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–386. [DOI] [PubMed] [Google Scholar]
- 31.Hameed S, Dhillo WS, Bloom SR. Gut hormones and appetite control. Oral Dis 2009;15:18–26. [DOI] [PubMed] [Google Scholar]
- 32.Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol 2009;7:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, et al. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 2003;144:754–759. [DOI] [PubMed] [Google Scholar]
- 34.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 2011;31:1285–1297. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Li Z, Zhang X, Xiang X, Li Y, Mulholland MW, Zhang W. Nesfatin-1 promotes brown adipocyte phenotype. Sci Rep 2016;6:34747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cinti S Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest 2002;25:823–835. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013;27:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


